Abstract
Objectives
Apoptosis-inducing factor mitochondria-associated 1 (AIFM1) gene encodes a mitochondrial flavoprotein that mediates caspase-independent programmed cell death. We report a novel AIFM1 variant in 2 siblings with early-onset hearing loss and progressive cerebellar ataxia.
Methods
We evaluated the clinical features, brain MRI scans, EMG studies, and whole genome sequencing (WGS).
Results
Sibling A is a 19-year-old man with auditory neuropathy at age 15 years, who subsequently developed optic atrophy, progressive gait and limb ataxia, peripheral neuropathy, and ambulation with cane by age 17 years. Brain MRI was normal. Sibling B is a 13-year-old boy with auditory neuropathy diagnosed at 7 and gait instability at 13, with rapid development of peripheral neuropathy, cerebellar ataxia, muscle weakness and atrophy needing wheelchair for mobility, and neuromuscular respiratory failure requiring noninvasive ventilation. Brain MRI showed mild cerebellar atrophy. Initial EMGs showed axonal neuropathy in both and diffuse chronic and active anterior horn cell disorder later in Sibling B. WGS revealed an X-linked, maternally inherited novel AIFM1 variant (c.1299C>G p. Ile433Met).
Discussion
AIFM1 variants should be considered in patients with hereditary cerebellar ataxia and auditory neuropathy. We highlight a novel AIFM1 variant and its phenotypic intrafamilial variability expanding the knowledge of the genetic spectrum of AIFM1-related diseases.
Introduction
The X-linked apoptosis-inducing factor mitochondria-associated 1 (AIFM1) gene encodes a flavin adenine dinucleotide (FAD)-dependent oxidoreductase that mediates caspase-independent programmed cell death and is essential for redox metabolism.1 AIFM1 mutations are rare and associated with variable phenotypes, including severe encephalomyopathy with combined oxidative phosphorylation deficiency 6 (COXPD6),2-4 auditory and peripheral neuropathy with or without cerebellar ataxia (also known as Cowchock Syndrome or X-linked Charcot Marie Tooth Type 4),5-8 X-linked deafness 5,9 and spondyloepimetaphyseal dysplasia with hypomyelinating leukodystrophy.10 We report a novel AIFM1 variant and its phenotypic variability in 2 siblings with early-onset hearing loss and progressive cerebellar ataxia.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This study was deemed exempt from institutional review board review. Written informed consent was obtained from the patients and patient's guardians.
Whole Genome Sequencing Analysis
Clinical-based Whole genome sequencing (WGS) and its subsequent analysis were performed by Variantyx laboratory Framingham, MA, USA.
Data Availability
Anonymized data are available on reasonable request.
Results
Sibling A is a 19-year-old man who developed sensorineural hearing loss at age 13 requiring cochlear implant. At age 15, he developed slowly progressive gait dysfunction, ataxia, and recurrent falls leading to necessity of a cane for ambulation by age 17. Examination at age 18 revealed normal cognition, end-gaze nystagmus, bilateral sensorineural hearing loss, areflexia, severe truncal ataxia, appendicular dysmetria, dysdiadochokinesia, and ataxic gait without motor or sensory changes or pes cavus.
EMG showed a length-dependent predominantly sensory peripheral neuropathy (eFigure 1). Brain MRI was normal. Optical coherence tomography (OCT) showed generalized thinning of the ganglion cell layer and optic atrophy. He received riboflavin, idebenone, and l-carnitine supplementation and showed stable disease course at his 20-month follow-up.
Sibling B is a 13-year-old boy who developed sensorineural hearing loss at age 5 years requiring right cochlear implant placement at age 8. At age 13, he developed progressive gait dysfunction and recurrent falls. Neurologic examination revealed normal cognition, end-gaze nystagmus, and abnormal tandem gait. Four months later, he reported difficulty swallowing, weight loss, dyspnea, and functional decline requiring wheelchair for mobility. Examination revealed mild symmetric distal upper extremity weakness, generalized hyporeflexia, diminished sensation to vibration and proprioception in lower extremities distally, appendicular dysmetria, and ataxic gait limited by dyspnea.
EMG showed length-dependent, axonal sensorimotor peripheral neuropathy affecting distal upper extremities (eFigure 2A), without phrenic neuropathy. Brain MRI showed mild atrophy of the superior aspect of the cerebellum and vermis. Swallow study was normal. Pulmonary function test showed a restrictive pattern, and direct laryngoscopy and flexible fiberoptic bronchoscopy were negative for airway obstruction. Overnight capnography showed hypercarbia, leading to implementation of noninvasive ventilation (NIV) with improvement of hypoventilation. OCT showed thinning of the ganglion cell layer.
Riboflavin, idebenone, and l-carnitine supplementation were prescribed but not administered consistently given subjective dysphagia and unpleasant taste. After 1.5 months of stability, he had rapid decline in functional and respiratory status with poor oral intake and malnutrition. Swallow evaluation showed aspiration of thin liquids. Repeat EMG, 2 months after initial EMG, showed diffuse, chronic, and active anterior horn cell disorder affecting the bulbar and whole spine and mild superimposed axonal peripheral neuropathy (eFigure 2B). Diaphragm ultrasound revealed left hemidiaphragm weakness with paradoxical breathing. At last follow-up, 7 months after rapid decline, he had flaccid dysarthria, end-gaze nystagmus, moderate symmetric distal > proximal weakness in upper extremities, bilateral hand atrophy, mild proximal lower extremity weakness, generalized hyporeflexia, and severe truncal ataxia with appendicular dysmetria. No fasciculations were noticed. The patient remained on continuous NIV and wheelchair for mobility in the setting of prominent ataxia.
Genomic Data
WGS identified a novel AIFM1 hemizygous variant, c.1299C>G p.Ile433Met (NM_004208.4), which was present in both siblings and in their unaffected mother (heterozygous). Ile433Met was absent in gnomAD11 but is predicted to be disease causing by MutationTaster, probably damaging with a score of 1.000 by PolyPhen-2, and affect protein function with a score of 0.03 by SIFT. The patients' phenotype of the auditory neuropathy and ataxia is highly specific to AIFM1. While this variant meets criteria for a Variant of Uncertain Significance under ACMG criteria,12 the phenotypic specificity, presence in 2 siblings, and predicted to be disease causing by in silico analysis makes it a variant of interest. No other variants were reported for independent genome sequencing for each sibling.
Family History
Family history was notable for 2 paternal uncles with intellectual disability.
Discussion
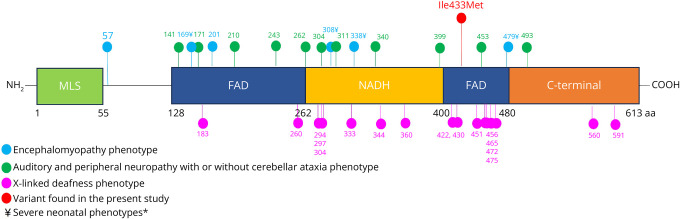
We report 2 siblings with a maternally inherited novel hemizygous AIFM1 variant c.1299C>G, p.Ile433Met. AIFM1 protein participates in regulation of reactive oxygen species, maintenance of electron transport chain function, programmed cell death, and neurodegeneration.13 AIFM1 protein is highly expressed in the inner ear, specifically within hair cells and spiral ganglion, indicating its role in normal auditory function.9 Data from a Harlequin mouse, spontaneous Aifm1 mutant, demonstrated that mutations causing decreased protein expression cause cerebellar granular cell apoptosis,14 explaining why cerebellar degeneration and atrophy can be seen in these disorders. AIFM1 is composed of one nicotinamide adenine dinucleotide (NAD/NADH)-binding domain and 2 flanking FAD-binding motifs.15 Our patients' variant Ile433 is located in the FAD-dependent oxidoreductase region of the protein and is highly conserved (Figure). Ile433Met is predicted to be pathogenic in different databases and is not reported in the gnomAD database.
Figure. Scheme of Human AIFM1 Protein and Reported Mutations Associated With Neuromuscular Involvement and Auditory Neuropathy.
Scheme of human AIFM1 protein showing its components including the mitochondrial localization sequence (MLS), flavin adenine dinucleotide (FAD) and nicotinamide adenine dinucleotide (NAD/NADH) binding domains, and C-terminal and the reported pathogenic variants identified in patients presenting with encephalomyopathy (blue), auditory and peripheral neuropathy with or without cerebellar ataxia (green), and X-linked deafness (purple). In red we show the variant found in our patients. Symbol ¥ denote severe neonatal phenotypes. Numbers at the bottom indicate the location of each component in the amino acid sequence.*Peng et al. 2022 detailed in eTable 1, reported a severe neonatal phenotype which was not included given that gene location was not described.
Both siblings' presentations with early-onset sensorineural hearing loss, axonal sensorimotor peripheral neuropathy, and cerebellar ataxia overlap with previous reports of AIFM-associated disease. eTable 1 summarizes published AIFM1-related phenotypes including auditory neuropathy and/or neuromuscular involvement.
Our patients' phenotypes are most consistent with Cowchock Syndrome. In this phenotype, hearing loss characterized by auditory neuropathy often presents in childhood with subsequent development of peripheral neuropathy and gait instability over years. Optic atrophy and retinopathy have been reported in some cases,6,7 which can be seen in other mitochondrial disorders (e.g., Leber optic atrophy). Although the exact underlying mechanism for AIFM1 to contribute to nerve pathology is not clear, its involvement in the mitochondrial OXPHOS system supports the predilection for metabolically active tissues and pathogenicity. Intellectual disability is commonly described, but this was not observed in our patients, possibly because of phenotypic variability with mitochondrial disease. Despite the same AIFM1 mutation, the disease course differed in our patients with the younger sibling exhibiting a rapid disease progression after developing cerebellar ataxia. Such rapidly progressive and severe presentation with motor neuronopathy and respiratory insufficiency has not been previously documented with Cowchock Syndrome. However, similar symptoms have been reported with COXPD6 presenting with mitochondrial encephalopathy, ventriculomegaly, and electrophysiologic signs of motor neuron involvement.4 We hypothesize that it may be due to environmental factors, such as earlier metabolic stress (e.g., malnutrition) or potentially due to overlapping features within the spectrum of AIFM-associated diseases.
Although not consistent throughout the literature, riboflavin has shown benefit in slowing the disease progression in some patients because it is a FAD precursor, and supplementation can improve AIFM1 protein levels and mitochondrial respiratory function.2,6,16 Two patients with AIFM1 mutations presenting with progressive ataxia were treated with 200 mg/d of riboflavin for 12 months and demonstrated reduction in the International Cooperative Ataxia Rating Scale (ICARS) scores by 44% and 20%, respectively, at 6 months compared with baseline.16 Sibling A received riboflavin, however, association between ataxia stability and riboflavin administration cannot be established due limited follow-up time and no clear change from baseline examination. As more AIFM1 variants are identified, implementation of quantitative assessments and longer follow-up times could be helpful to monitor disease progression and response to riboflavin in future larger cohorts.
We highlight a novel AIFM1 variant, c.1299C>G p.Ile433Met, and its phenotypic intrafamilial variability expanding the knowledge of the genetic spectrum of AIFM1-related diseases.
Acknowledgment
The authors thank the patients and family for their collaboration.
Appendix. Authors
| Name | Location | Contribution |
| Alejandra Vasquez, MD | Department of Neurology, Mayo Clinic | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Lisa A Schimmenti, MD | Department of Clinical Genomics, Mayo Clinic | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Nadir Demirel, MD, MS | Division of Pediatric Pulmonology, Department of Pediatrics and Adolescent Medicine, Mayo Clinic | Drafting/revision of the manuscript for content, including medical writing for content |
| Amy E. Rabatin, MD | Division of Pediatric Rehabilitation Medicine, Department of Physical Medicine and Rehabilitation; Department of Pediatrics and Adolescent Medicine, Mayo Clinic | Drafting/revision of the manuscript for content, including medical writing for content |
| Callie R. Fischer, MD | Department of Pediatrics and Adolescent Medicine, Mayo Clinic | Drafting/revision of the manuscript for content, including medical writing for content |
| Marcus V. Pinto, MD, MS | Department of Neurology, Mayo Clinic | Drafting/revision of the manuscript for content, including medical writing for content |
| Richard Paul Boesch, DO | Division of Pediatric Pulmonology, Department of Pediatrics and Adolescent Medicine, Mayo Clinic | Drafting/revision of the manuscript for content, including medical writing for content |
| Duygu Selcen, MD | Department of Neurology, Mayo Clinic | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/NG for full disclosures.
References
- 1.Joza N, Pospisilik JA, Hangen E, et al. AIF: not just an apoptosis-inducing factor. Ann N Y Acad Sci. 2009;1171:2-11. doi: 10.1111/j.1749-6632.2009.04681.x [DOI] [PubMed] [Google Scholar]
- 2.Ghezzi D, Sevrioukova I, Invernizzi F, et al. Severe X-linked mitochondrial encephalomyopathy associated with a mutation in apoptosis-inducing factor. Am J Hum Genet. 2010;86(4):639-649. doi: 10.1016/j.ajhg.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger I, Ben-Neriah Z, Dor-Wolman T, et al. Early prenatal ventriculomegaly due to an AIFM1 mutation identified by linkage analysis and whole exome sequencing. Mol Genet Metab. 2011;104(4):517-520. doi: 10.1016/j.ymgme.2011.09.020 [DOI] [PubMed] [Google Scholar]
- 4.Diodato D, Tasca G, Verrigni D, et al. A novel AIFM1 mutation expands the phenotype to an infantile motor neuron disease. Eur J Hum Genet. 2016;24(3):463-466. doi: 10.1038/ejhg.2015.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinaldi C, Grunseich C, Sevrioukova IF, et al. Cowchock syndrome is associated with a mutation in apoptosis-inducing factor. Am J Hum Genet. 2012;91(6):1095-1102. doi: 10.1016/j.ajhg.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ardissone A, Piscosquito G, Legati A, et al. A slowly progressive mitochondrial encephalomyopathy widens the spectrum of AIFM1 disorders. Neurology. 2015;84(21):2193-2195. doi: 10.1212/WNL.0000000000001613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogdanova-Mihaylova P, Alexander MD, Murphy RP, et al. Clinical spectrum of AIFM1-associated disease in an Irish family, from mild neuropathy to severe cerebellar ataxia with colour blindness. J Peripher Nerv Syst. 2019;24(4):348-353. doi: 10.1111/jns.12348 [DOI] [PubMed] [Google Scholar]
- 8.Pandolfo M, Rai M, Remiche G, Desmyter L, Vandernoot I. Cerebellar ataxia, neuropathy, hearing loss, and intellectual disability due to AIFM1 mutation. Neurol Genet. 2020;6(3):e420. doi: 10.1212/NXG.0000000000000420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zong L, Guan J, Ealy M, et al. Mutations in apoptosis-inducing factor cause X-linked recessive auditory neuropathy spectrum disorder. J Med Genet. 2015;52(8):523-531. doi: 10.1136/jmedgenet-2014-102961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyake N, Wolf NI, Cayami FK, et al. X-linked hypomyelination with spondylometaphyseal dysplasia (H-SMD) associated with mutations in AIFM1. Neurogenetics. 2017;18(4):185-194. doi: 10.1007/s10048-017-0520-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnomad. Accessed September 15, 2024. gnomad.broadinstitute.org/
- 12.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polster BM. AIF, reactive oxygen species, and neurodegeneration: a “complex” problem. Neurochem Int. 2013;62(5):695-702. doi: 10.1016/j.neuint.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein JA, Longo-Guess CM, Rossmann MP, et al. The harlequin mouse mutation downregulates apoptosis-inducing factor. Nature. 2002;419(6905):367-374. doi: 10.1038/nature01034 [DOI] [PubMed] [Google Scholar]
- 15.Bano D, Prehn JHM. Apoptosis-inducing factor (AIF) in physiology and disease: the tale of a repented natural born killer. eBioMedicine. 2018;30:29-37. doi: 10.1016/j.ebiom.2018.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heimer G, Eyal E, Zhu X, et al. Mutations in AIFM1 cause an X-linked childhood cerebellar ataxia partially responsive to riboflavin. Eur J Paediatr Neurol. 2018;22(1):93-101. doi: 10.1016/j.ejpn.2017.09.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data are available on reasonable request.