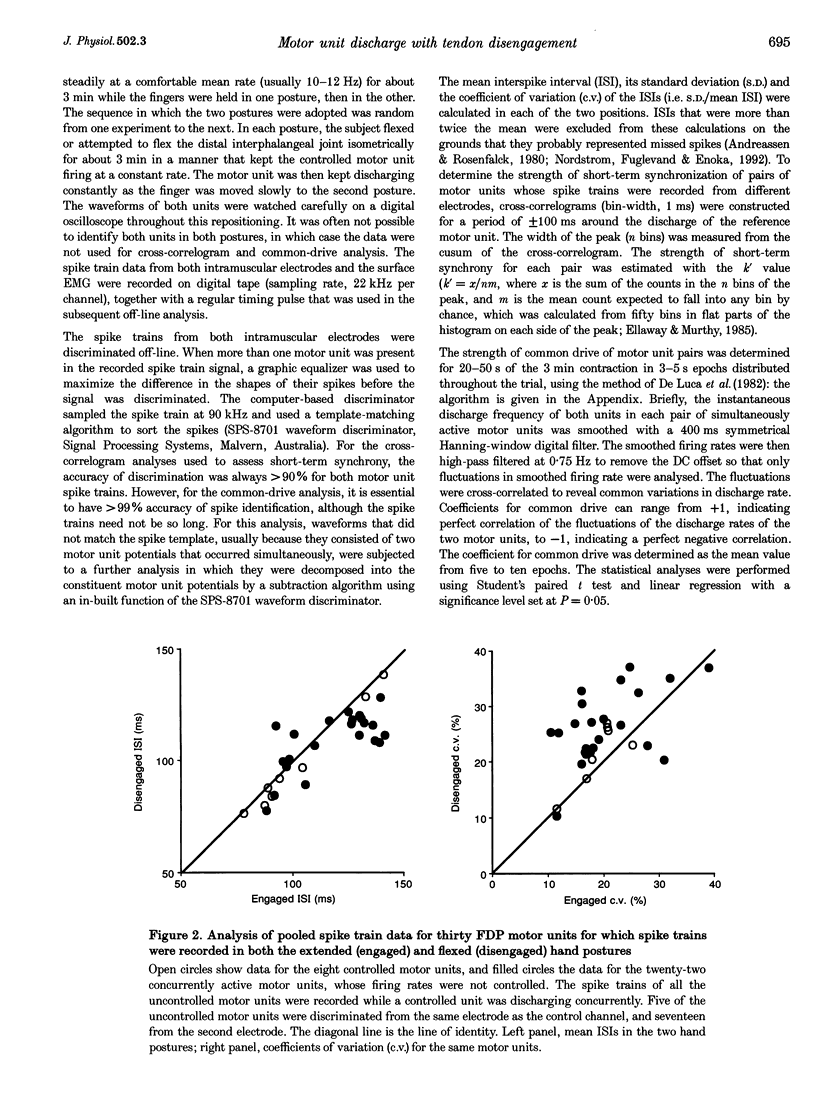
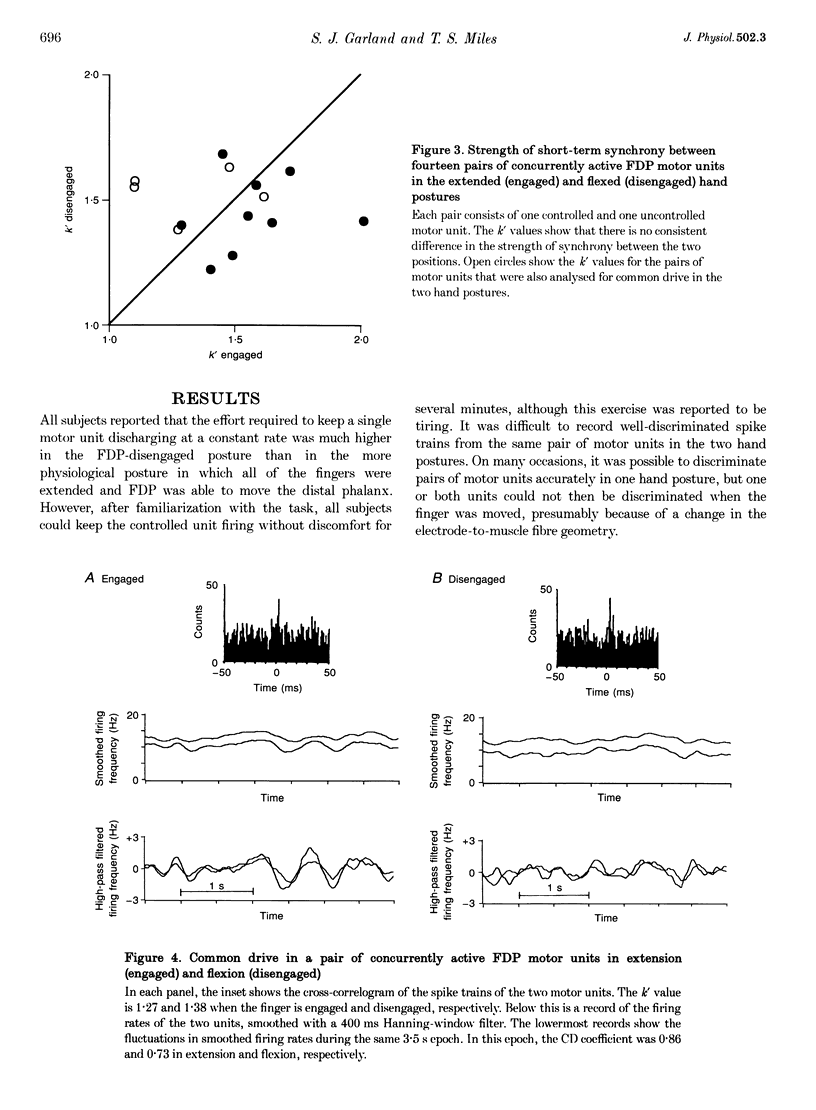
Abstract
1. Changing the posture of the human fingers can functionally 'disengage' the deep finger flexor muscle from its normal action on the terminal phalanx of the fourth (or third) finger. This enables the activity of the muscle to be studied both with and without its normal proprioceptive inputs. 2. Spike trains of long duration from pairs of concurrently active motor units in this muscle were recorded in both the engaged and disengaged hand postures. Subjects voluntarily kept one of the motor units (the 'controlled' unit) discharging at the same target frequency in both postures. The strength of short-term synchrony, the strength of common drive, and the variability of discharge of these pairs of motor units were determined in both postures. 3. All subjects reported that the effort required to activate the motor units in the disengaged hand posture was substantially greater than in the normal engaged posture. 4. Short-term synchrony, which is a function of common corticospinal inputs to pairs of motor units, was similar in both hand postures. However, the strength of common drive was significantly decreased when the muscle was disengaged. Although the neural substrate for common drive is not known, this observation suggests that proprioceptive feedback is involved either directly or indirectly. 5. Although the discharge rate of the 'uncontrolled' motor units increased when the muscle was disengaged, the variability of discharge of these and the 'controlled' motor units increased significantly. This supports the idea that the precision with which fine motor tasks can be performed is improved when proprioceptive feedback is intact.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L., Datta A. K., Guz A. Synchronization of motor unit firing during different respiratory and postural tasks in human sternocleidomastoid muscle. J Physiol. 1989 Jun;413:213–231. doi: 10.1113/jphysiol.1989.sp017650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen S., Rosenfalck A. Regulation of the firing pattern of single motor units. J Neurol Neurosurg Psychiatry. 1980 Oct;43(10):897–906. doi: 10.1136/jnnp.43.10.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner F. D., Baker J. R., Stephens J. A. Effect of task on the degree of synchronization of intrinsic hand muscle motor units in man. J Neurophysiol. 1991 Dec;66(6):2072–2083. doi: 10.1152/jn.1991.66.6.2072. [DOI] [PubMed] [Google Scholar]
- Datta A. K., Farmer S. F., Stephens J. A. Central nervous pathways underlying synchronization of human motor unit firing studied during voluntary contractions. J Physiol. 1991 Jan;432:401–425. doi: 10.1113/jphysiol.1991.sp018391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. K., Stephens J. A. Synchronization of motor unit activity during voluntary contraction in man. J Physiol. 1990 Mar;422:397–419. doi: 10.1113/jphysiol.1990.sp017991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca C. J., LeFever R. S., McCue M. P., Xenakis A. P. Control scheme governing concurrently active human motor units during voluntary contractions. J Physiol. 1982 Aug;329:129–142. doi: 10.1113/jphysiol.1982.sp014294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca C. J., Mambrito B. Voluntary control of motor units in human antagonist muscles: coactivation and reciprocal activation. J Neurophysiol. 1987 Sep;58(3):525–542. doi: 10.1152/jn.1987.58.3.525. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H., Murthy K. S. The origins and characteristics of cross-correlated activity between gamma-motoneurones in the cat. Q J Exp Physiol. 1985 Apr;70(2):219–232. doi: 10.1113/expphysiol.1985.sp002905. [DOI] [PubMed] [Google Scholar]
- Farmer S. F., Swash M., Ingram D. A., Stephens J. A. Changes in motor unit synchronization following central nervous lesions in man. J Physiol. 1993 Apr;463:83–105. doi: 10.1113/jphysiol.1993.sp019585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia S. C., McCloskey D. I. Joint sense, muscle sense, and their combination as position sense, measured at the distal interphalangeal joint of the middle finger. J Physiol. 1976 Sep;260(2):387–407. doi: 10.1113/jphysiol.1976.sp011521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia S. C., McCloskey D. I. Sensations of heaviness. Brain. 1977 Jun;100(2):345–354. doi: 10.1093/brain/100.2.345. [DOI] [PubMed] [Google Scholar]
- Garland S. J., Miles T. S. Responses of human single motor units to transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1997 Apr;105(2):94–101. doi: 10.1016/s0924-980x(97)96111-7. [DOI] [PubMed] [Google Scholar]
- Hulliger M., Nordh E., Vallbo A. B. The absence of position response in spindle afferent units from human finger muscles during accurate position holding. J Physiol. 1982 Jan;322:167–179. doi: 10.1113/jphysiol.1982.sp014030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen G., De Luca C. J. Firing rate interactions among human orbicularis oris motor units. Int J Neurosci. 1992 May-Jun;64(1-4):167–175. doi: 10.3109/00207459209000542. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. J Physiol. 1978 Feb;275:103–134. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield V. G., Gandevia S. C., Bigland-Ritchie B., Gorman R. B., Burke D. The firing rates of human motoneurones voluntarily activated in the absence of muscle afferent feedback. J Physiol. 1993 Nov;471:429–443. doi: 10.1113/jphysiol.1993.sp019908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B., Miles T. S. On the long-latency reflex responses of the human flexor digitorum profundus. J Physiol. 1988 Oct;404:515–534. doi: 10.1113/jphysiol.1988.sp017303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir R. B., Porter R. The effect of a preceding stimulus on temporal facilitation at corticomotoneuronal synapses. J Physiol. 1973 Feb;228(3):749–763. doi: 10.1113/jphysiol.1973.sp010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom M. A., Fuglevand A. J., Enoka R. M. Estimating the strength of common input to human motoneurons from the cross-correlogram. J Physiol. 1992;453:547–574. doi: 10.1113/jphysiol.1992.sp019244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person R. S., Kudina L. P. Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr Clin Neurophysiol. 1972 May;32(5):471–483. doi: 10.1016/0013-4694(72)90058-2. [DOI] [PubMed] [Google Scholar]
- Proske U., Morgan D. L., Gregory J. E. Muscle history dependence of responses to stretch of primary and secondary endings of cat soleus muscle spindles. J Physiol. 1992 Jan;445:81–95. doi: 10.1113/jphysiol.1992.sp018913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell J. C., Traub M. M., Day B. L., Obeso J. A., Thomas P. K., Marsden C. D. Manual motor performance in a deafferented man. Brain. 1982 Sep;105(Pt 3):515–542. doi: 10.1093/brain/105.3.515. [DOI] [PubMed] [Google Scholar]
- Schmied A., Ivarsson C., Fetz E. E. Short-term synchronization of motor units in human extensor digitorum communis muscle: relation to contractile properties and voluntary control. Exp Brain Res. 1993;97(1):159–172. doi: 10.1007/BF00228826. [DOI] [PubMed] [Google Scholar]
- Sears T. A., Stagg D. Short-term synchronization of intercostal motoneurone activity. J Physiol. 1976 Dec;263(3):357–381. doi: 10.1113/jphysiol.1976.sp011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo A. B. Afferent discharge from human muscle spindles in non-contracting muscles. Steady state impulse frequency as a function of joint angle. Acta Physiol Scand. 1974 Feb;90(2):303–318. doi: 10.1111/j.1748-1716.1974.tb05593.x. [DOI] [PubMed] [Google Scholar]
- Wiegner A. W., Wierzbicka M. M. A method for assessing significance of peaks in cross-correlation histograms. J Neurosci Methods. 1987 Dec;22(2):125–131. doi: 10.1016/0165-0270(87)90006-9. [DOI] [PubMed] [Google Scholar]


