Abstract
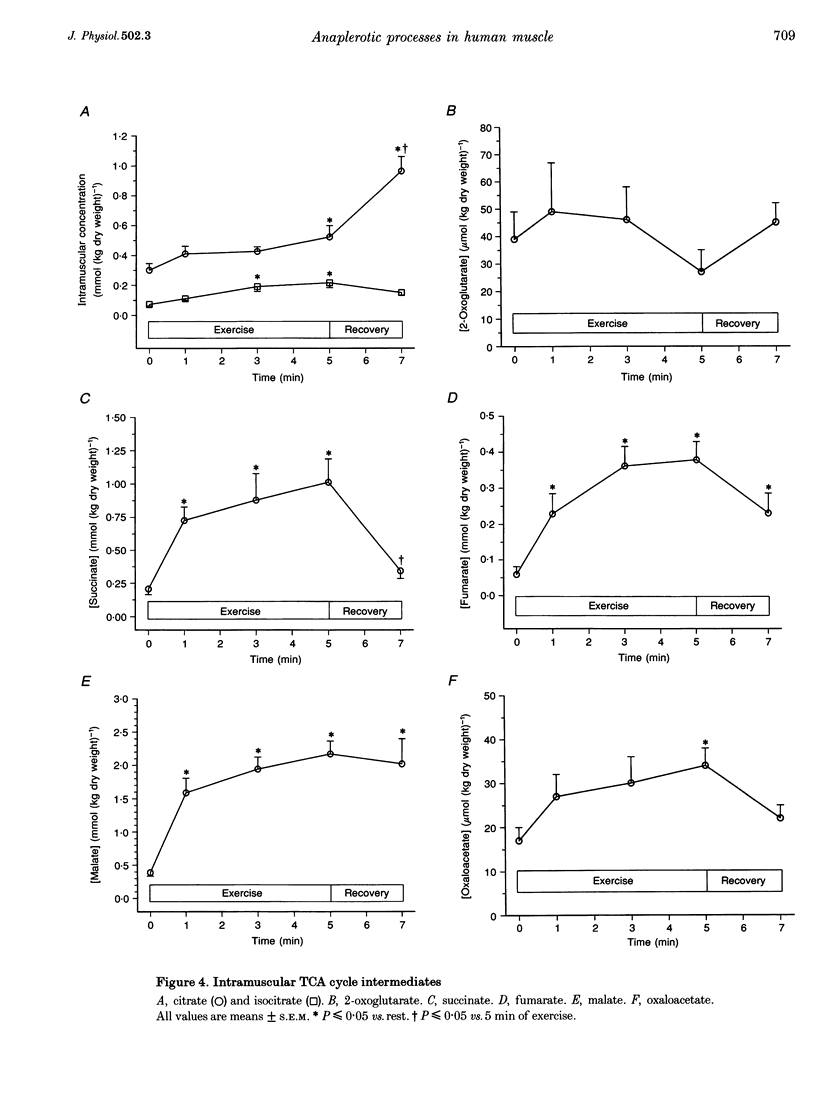
1. This study examined changes in tricarboxylic acid cycle intermediates (TCAIs) in human skeletal muscle during 5 min of dynamic knee extensor exercise (approximately 80% of maximum workload) and following 2 min of recovery. 2. The sum of the seven measured TCAIs (sigma TCAIs) increased from 1.10 +/- 0.08 mmol (kg dry weight)-1 at rest to 3.12 +/- 0.24, 3.86 +/- 0.35 and 4.33 +/- 0.30 mmol (kg dry weight)-1 after 1, 3 and 5 min of exercise, respectively (P < or = 0.05): The sigma TCAIs after 2 min of recovery (3.74 +/- 0.43 mmol (kg dry weight)-1) was not different compared with 5 min of exercise. 3. The rapid increase in sigma TCAIs during exercise was primarily mediated by large changes in succinate, malate and fumarate. These three intermediates accounted for > 90% of the net increase in sigma TCAIs during the first minute of contraction. 4. Intramuscular alanine increased after 1 min of exercise by an amount similar to the increase in the sigma TCAIs (2.33 mmol (kg dry weight)-1) (P < or = 0.05). Intramuscular pyruvate was also higher (P < or = 0.05) during exercise, while intramuscular glutamate decreased by approximately 50% within 1 min and remained low despite an uptake from the circulation (P < or = 0.05). 5. The calculated net release plus estimated muscle accumulation of ammonia after 1 min of exercise (approximately 60 mumol (kg wet weight)-1) indicated that only a minor portion of the increase in sigma TCAIs could have been mediated through the purine nucleotide cycle and/or glutamate dehydrogenase reaction. 6. It is concluded that the close temporal relationship between the increase in sigma TCAIs and changes in glutamate, alanine and pyruvate metabolism suggests that the alanine aminotransferase reaction is the most important anaplerotic process during the initial minutes of contraction in human skeletal muscle.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985 Sep;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragón J. J., Lowenstein J. M. The purine-nucleotide cycle. Comparison of the levels of citric acid cycle intermediates with the operation of the purine nucleotide cycle in rat skeletal muscle during exercise and recovery from exercise. Eur J Biochem. 1980 Sep;110(2):371–377. doi: 10.1111/j.1432-1033.1980.tb04877.x. [DOI] [PubMed] [Google Scholar]
- Brodal B., Hjelle K. Synthesis of phosphoenolpyruvate [correction of phosphoenolphosphate] from pyruvate in rat skeletal muscle. Int J Biochem. 1990;22(7):753–758. doi: 10.1016/0020-711x(90)90011-q. [DOI] [PubMed] [Google Scholar]
- Crabtree B., Higgins S. J., Newsholme E. A. The activities of pyruvate carboxylase, phosphoenolpyruvate carboxylase and fructose diphosphatase in muscles from vertebrates and invertebrates. Biochem J. 1972 Nov;130(2):391–396. doi: 10.1042/bj1300391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. J., Spydevold O., Bremer J. Pyruvate carboxylase and propionyl-CoA carboxylase as anaplerotic enzymes in skeletal muscle mitochondria. Eur J Biochem. 1980 Sep;110(1):255–262. doi: 10.1111/j.1432-1033.1980.tb04863.x. [DOI] [PubMed] [Google Scholar]
- Essén B., Kaijser L. Regulation of glycolysis in intermittent exercise in man. J Physiol. 1978 Aug;281:499–511. doi: 10.1113/jphysiol.1978.sp012436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala M. J., Tarnopolsky M. A., Graham T. E. Tricarboxylic acid cycle intermediates in human muscle at rest and during prolonged cycling. Am J Physiol. 1997 Feb;272(2 Pt 1):E239–E244. doi: 10.1152/ajpendo.1997.272.2.E239. [DOI] [PubMed] [Google Scholar]
- Graham T. E., Bangsbo J., Gollnick P. D., Juel C., Saltin B. Ammonia metabolism during intense dynamic exercise and recovery in humans. Am J Physiol. 1990 Aug;259(2 Pt 1):E170–E176. doi: 10.1152/ajpendo.1990.259.2.E170. [DOI] [PubMed] [Google Scholar]
- Graham T. E., MacLean D. A. Ammonia and amino acid metabolism in human skeletal muscle during exercise. Can J Physiol Pharmacol. 1992 Jan;70(1):132–141. doi: 10.1139/y92-020. [DOI] [PubMed] [Google Scholar]
- Graham T. E., Saltin B. Estimation of the mitochondrial redox state in human skeletal muscle during exercise. J Appl Physiol (1985) 1989 Feb;66(2):561–566. doi: 10.1152/jappl.1989.66.2.561. [DOI] [PubMed] [Google Scholar]
- Guy P. S., Snow D. H. The effect of training and detraining on muscle composition in the horse. J Physiol. 1977 Jul;269(1):33–51. doi: 10.1113/jphysiol.1977.sp011891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. C., Hultman E., Nordesjö L. O. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest. 1974 Apr;33(2):109–120. [PubMed] [Google Scholar]
- Heinrikson R. L., Meredith S. C. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984 Jan;136(1):65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Davis E. J. Carboxylation and decarboxylation reactions. Anaplerotic flux and removal of citrate cycle intermediates in skeletal muscle. J Biol Chem. 1979 Jan 25;254(2):420–430. [PubMed] [Google Scholar]
- MacLean D. A., Graham T. E., Saltin B. Branched-chain amino acids augment ammonia metabolism while attenuating protein breakdown during exercise. Am J Physiol. 1994 Dec;267(6 Pt 1):E1010–E1022. doi: 10.1152/ajpendo.1994.267.6.E1010. [DOI] [PubMed] [Google Scholar]
- MacLean D. A., Graham T. E., Saltin B. Stimulation of muscle ammonia production during exercise following branched-chain amino acid supplementation in humans. J Physiol. 1996 Jun 15;493(Pt 3):909–922. doi: 10.1113/jphysiol.1996.sp021433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molé P. A., Baldwin K. M., Terjung R. L., Holloszy J. O. Enzymatic pathways of pyruvate metabolism in skeletal muscle: adaptations to exercise. Am J Physiol. 1973 Jan;224(1):50–54. doi: 10.1152/ajplegacy.1973.224.1.50. [DOI] [PubMed] [Google Scholar]
- Peuhkurinen K. J. Regulation of the tricarboxylic acid cycle pool size in heart muscle. J Mol Cell Cardiol. 1984 Jun;16(6):487–495. doi: 10.1016/s0022-2828(84)80637-9. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Jorfeldt L., Henriksson K. G., Lewis S. F., Haller R. G. Tricarboxylic acid cycle intermediates during incremental exercise in healthy subjects and in patients with McArdle's disease. Clin Sci (Lond) 1995 Jun;88(6):687–693. doi: 10.1042/cs0880687. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Katz A., Broberg S. Tricarboxylic acid cycle intermediates in human muscle during prolonged exercise. Am J Physiol. 1990 Nov;259(5 Pt 1):C834–C841. doi: 10.1152/ajpcell.1990.259.5.C834. [DOI] [PubMed] [Google Scholar]
- Scisłowski P. W., Aleksandrowicz Z., Swierczyński J. Purine nucleotide cycle as a possible anaplerotic process in rat skeletal muscle. Experientia. 1982 Sep 15;38(9):1035–1037. doi: 10.1007/BF01955351. [DOI] [PubMed] [Google Scholar]
- Tullson P. C., Terjung R. L. Adenine nucleotide metabolism in contracting skeletal muscle. Exerc Sport Sci Rev. 1991;19:507–537. [PubMed] [Google Scholar]
- Wibom R., Hultman E. ATP production rate in mitochondria isolated from microsamples of human muscle. Am J Physiol. 1990 Aug;259(2 Pt 1):E204–E209. doi: 10.1152/ajpendo.1990.259.2.E204. [DOI] [PubMed] [Google Scholar]
- Wibom R., Hultman E., Johansson M., Matherei K., Constantin-Teodosiu D., Schantz P. G. Adaptation of mitochondrial ATP production in human skeletal muscle to endurance training and detraining. J Appl Physiol (1985) 1992 Nov;73(5):2004–2010. doi: 10.1152/jappl.1992.73.5.2004. [DOI] [PubMed] [Google Scholar]


