Abstract
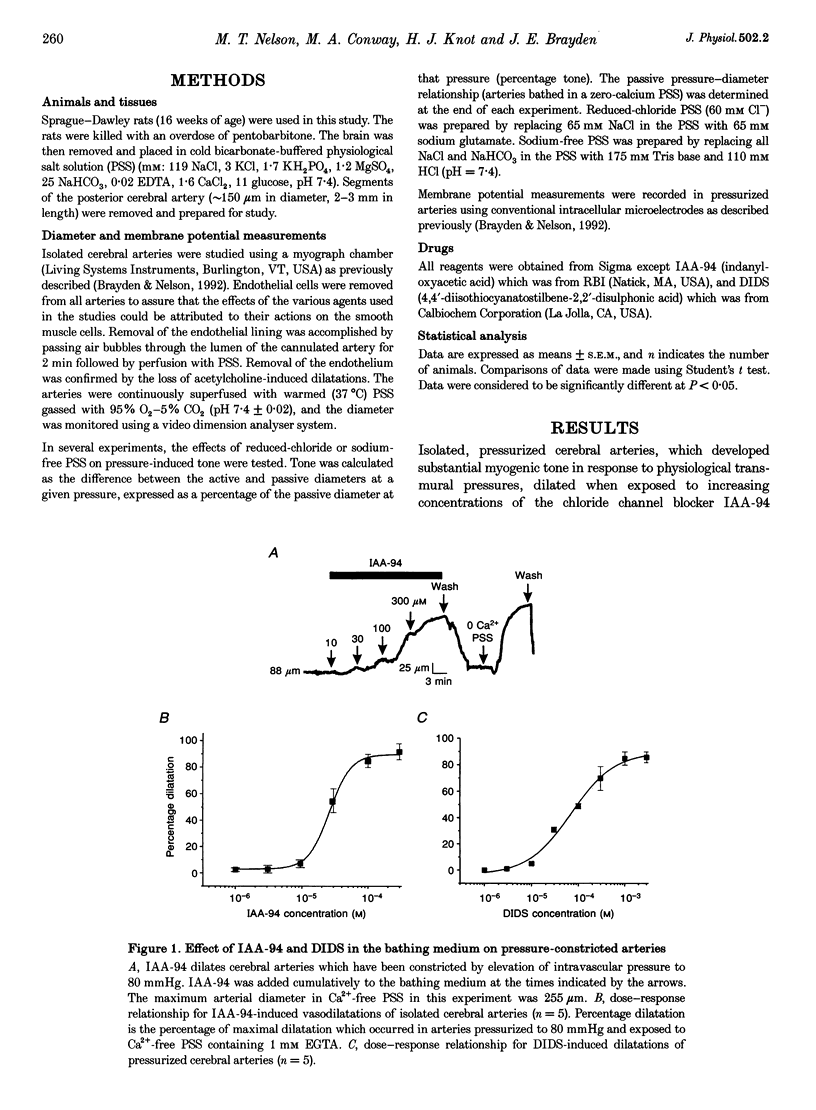
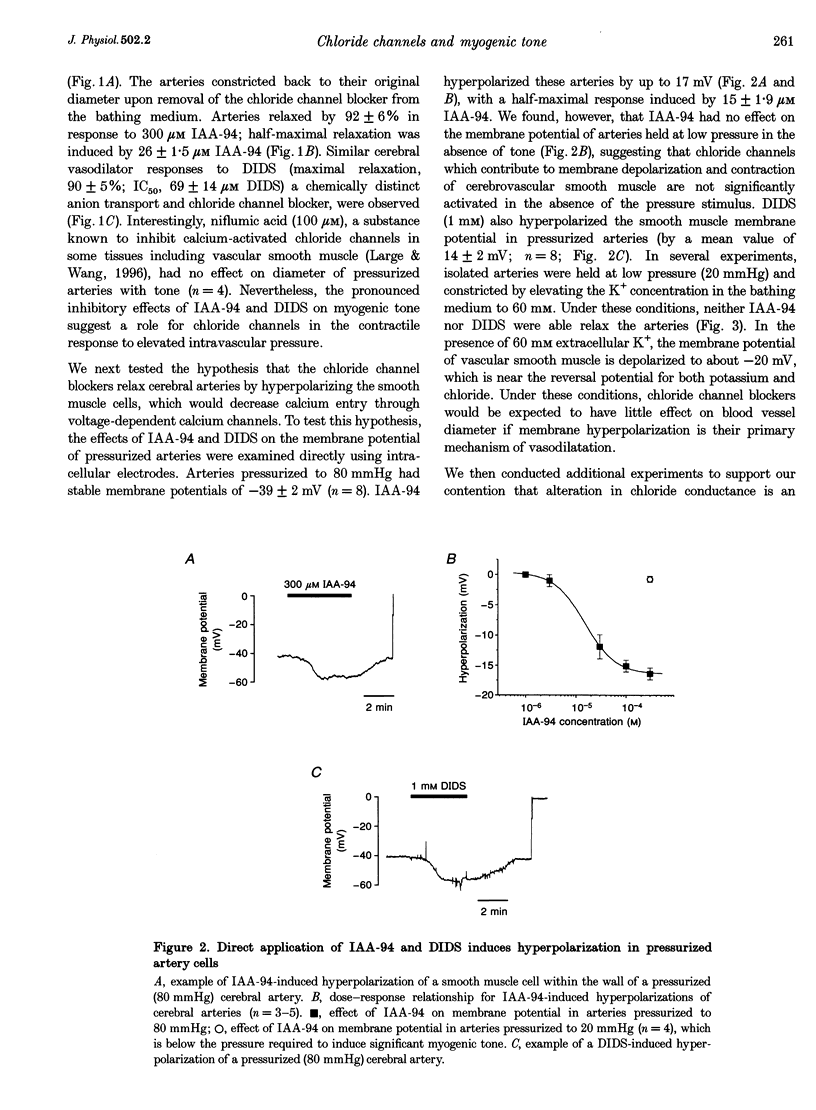
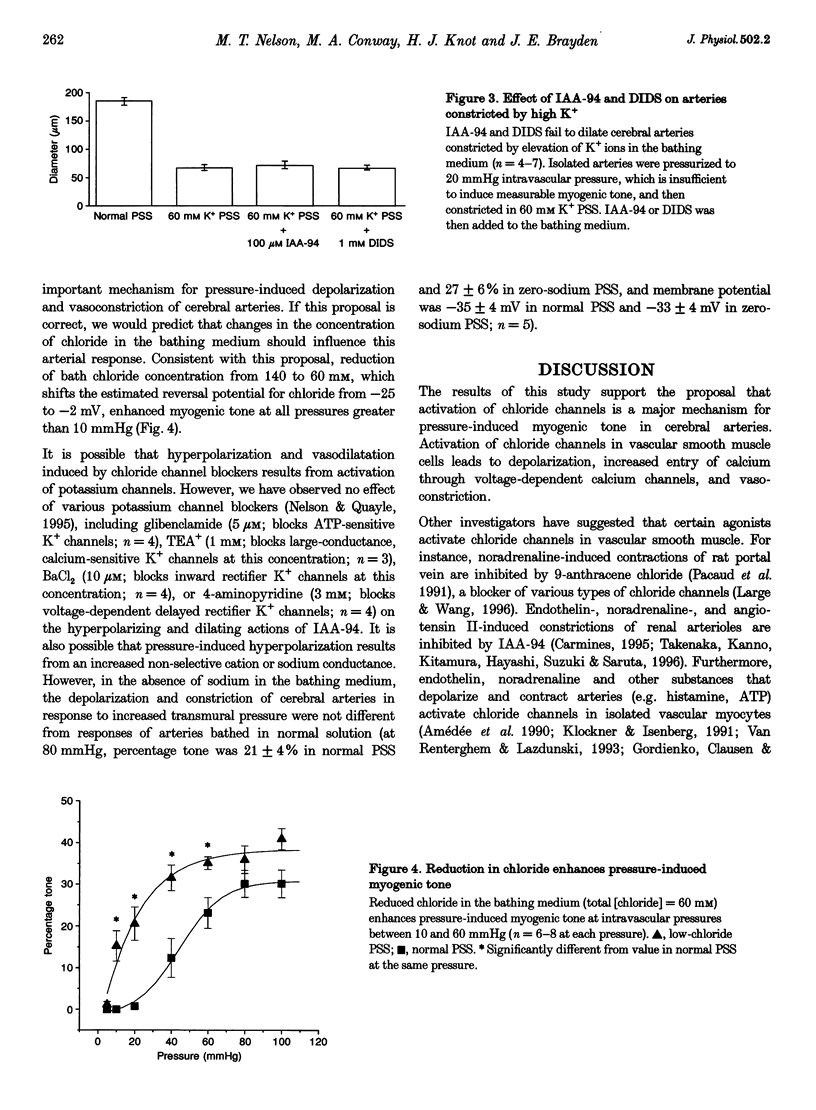
1. We have investigated the role of chloride channels in pressure-induced depolarization and contraction of cerebral artery smooth muscle cells. 2. Two chloride channel blockers, indanyloxyacetic acid (IAA-94) and 4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid (DIDS), caused hyperpolarizations (10-15 mV) and dilatations (up to 90%) of pressurized (80 mmHg), rat posterior cerebral arteries. Niflumic acid, a blocker of calcium-activated chloride channels, did not affect arterial tone. 3. Dilatations to IAA-94 and DIDS were unaffected by potassium channel blockers, but were prevented by elevated potassium. IAA-94 and DIDS had no effect on membrane potential or diameter of arteries at low intravascular pressure, where myogenic tone is absent. Reduction of extracellular chloride (60 mM Cl-) increased the pressure-induced contractions. Removal of extracellular sodium did not affect the pressure-induced responses. 4. Our results suggest that intravascular pressure activates DIDS- and IAA-94-sensitive chloride channels to depolarize arterial smooth muscle, thereby contributing to the myogenic constriction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amédée T., Large W. A., Wang Q. Characteristics of chloride currents activated by noradrenaline in rabbit ear artery cells. J Physiol. 1990 Sep;428:501–516. doi: 10.1113/jphysiol.1990.sp018224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Brayden J. E., Nelson M. T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992 Apr 24;256(5056):532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Brayden J. E., Wellman G. C. Endothelium-dependent dilation of feline cerebral arteries: role of membrane potential and cyclic nucleotides. J Cereb Blood Flow Metab. 1989 Jun;9(3):256–263. doi: 10.1038/jcbfm.1989.42. [DOI] [PubMed] [Google Scholar]
- Carmines P. K. Segment-specific effect of chloride channel blockade on rat renal arteriolar contractile responses to angiotensin II. Am J Hypertens. 1995 Jan;8(1):90–94. doi: 10.1016/0895-7061(94)00170-G. [DOI] [PubMed] [Google Scholar]
- Davis M. J., Donovitz J. A., Hood J. D. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. Am J Physiol. 1992 Apr;262(4 Pt 1):C1083–C1088. doi: 10.1152/ajpcell.1992.262.4.C1083. [DOI] [PubMed] [Google Scholar]
- Gordienko D. V., Clausen C., Goligorsky M. S. Ionic currents and endothelin signaling in smooth muscle cells from rat renal resistance arteries. Am J Physiol. 1994 Feb;266(2 Pt 2):F325–F341. doi: 10.1152/ajprenal.1994.266.2.F325. [DOI] [PubMed] [Google Scholar]
- Harder D. R. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circ Res. 1984 Aug;55(2):197–202. doi: 10.1161/01.res.55.2.197. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Edwards F. R. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989 Apr;69(2):546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- Jentsch T. J. Chloride channels: a molecular perspective. Curr Opin Neurobiol. 1996 Jun;6(3):303–310. doi: 10.1016/s0959-4388(96)80112-7. [DOI] [PubMed] [Google Scholar]
- Johnson P. C. Autoregulation of blood flow. Circ Res. 1986 Nov;59(5):483–495. doi: 10.1161/01.res.59.5.483. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Endothelin depolarizes myocytes from porcine coronary and human mesenteric arteries through a Ca-activated chloride current. Pflugers Arch. 1991 Mar;418(1-2):168–175. doi: 10.1007/BF00370467. [DOI] [PubMed] [Google Scholar]
- Knot H. J., Nelson M. T. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol. 1995 Jul;269(1 Pt 2):H348–H355. doi: 10.1152/ajpheart.1995.269.1.H348. [DOI] [PubMed] [Google Scholar]
- Landry D. W., Reitman M., Cragoe E. J., Jr, Al-Awqati Q. Epithelial chloride channel. Development of inhibitory ligands. J Gen Physiol. 1987 Dec;90(6):779–798. doi: 10.1085/jgp.90.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large W. A., Wang Q. Characteristics and physiological role of the Ca(2+)-activated Cl- conductance in smooth muscle. Am J Physiol. 1996 Aug;271(2 Pt 1):C435–C454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- Meininger G. A., Davis M. J. Cellular mechanisms involved in the vascular myogenic response. Am J Physiol. 1992 Sep;263(3 Pt 2):H647–H659. doi: 10.1152/ajpheart.1992.263.3.H647. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Quayle J. M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995 Apr;268(4 Pt 1):C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Pacaud P., Loirand G., Baron A., Mironneau C., Mironneau J. Ca2+ channel activation and membrane depolarization mediated by Cl- channels in response to noradrenaline in vascular myocytes. Br J Pharmacol. 1991 Dec;104(4):1000–1006. doi: 10.1111/j.1476-5381.1991.tb12540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange K., Emma F., Jackson P. S. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996 Mar;270(3 Pt 1):C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Takenaka T., Kanno Y., Kitamura Y., Hayashi K., Suzuki H., Saruta T. Role of chloride channels in afferent arteriolar constriction. Kidney Int. 1996 Sep;50(3):864–872. doi: 10.1038/ki.1996.386. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C., Lazdunski M. Endothelin and vasopressin activate low conductance chloride channels in aortic smooth muscle cells. Pflugers Arch. 1993 Oct;425(1-2):156–163. doi: 10.1007/BF00374516. [DOI] [PubMed] [Google Scholar]
- Wang Q., Large W. A. Action of histamine on single smooth muscle cells dispersed from the rabbit pulmonary artery. J Physiol. 1993 Aug;468:125–139. doi: 10.1113/jphysiol.1993.sp019763. [DOI] [PMC free article] [PubMed] [Google Scholar]


