Abstract
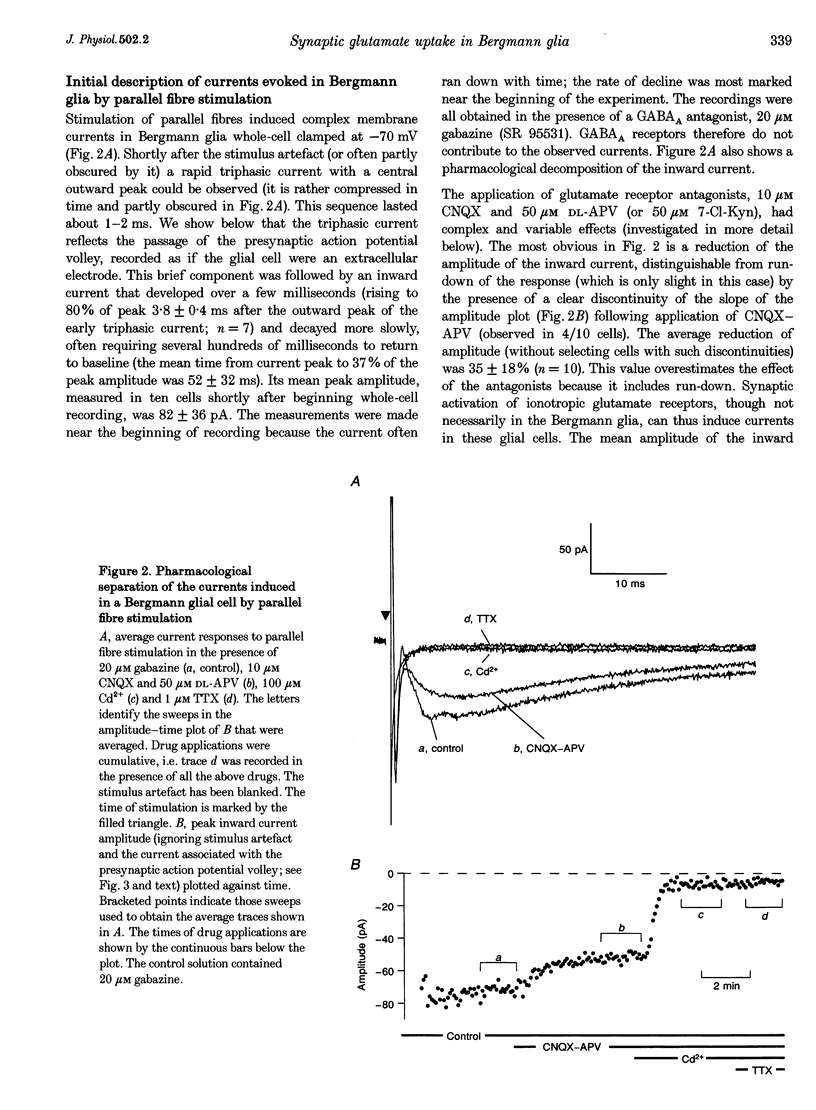
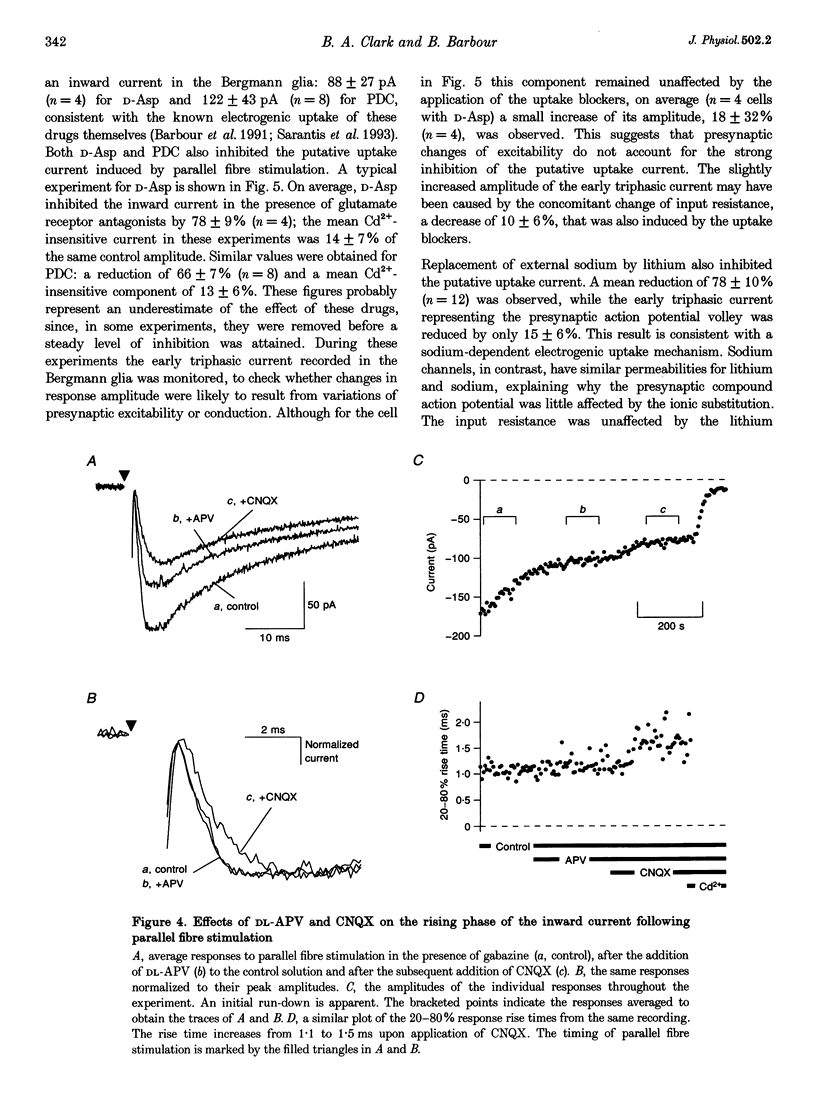
1. Whole-cell recordings were obtained from Bergmann glial cells in rat cerebellar slices. 2. The cells had low input resistances (70 +/- 38 M omega; n = 13) and a mean resting potential of -82 +/- 6 mV (n = 12) with a potassium-based internal solution. Electrical and dye coupling between Bergmann glia were confirmed. 3. Stimulation of parallel fibres induced a complex, mostly inward current which could be decomposed pharmacologically. 4. The ionotropic glutamate receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 microM), but not DL-2-amino-5-phosphonopentanoic acid (DL-APV; 100 microM) consistently blocked an early inward current component that may reflect synaptic activation of AMPA/kainate receptors in Bergmann glia. 5. Addition of cadmium ions (100 microM) to inhibit transmitter release blocked most of the CNQX-APV-insensitive current. This component probably reflects electrogenic uptake of the synaptically released glutamate. 6. Tetrodotoxin (TTX; 1 microM) blocked the remaining inward current: a slow component, possibly produced by the potassium ion efflux during action potential propagation in parallel fibres. An initial triphasic component of the response was also TTX sensitive and reflected passage of the parallel fibre action potential volley. 7. The putative glutamate uptake current was further characterized; it was blocked by the competitive uptake blockers D-aspartate (0.5 mM) and L-trans-pyrrolidine-2,4-dicarboxylic acid (PDC; 0.5 mM), and by replacement of sodium with lithium. Monitoring the triphasic TTX-sensitive component showed that this inhibition did not result from changes of action potential excitation and propagation. 8. Intracellular nitrate ions increased the putative uptake current, consistent with the effect of this anion on glutamate transporters. 9. The putative uptake current was reduced by depolarization, consistent with the voltage dependence of glutamate uptake. 10. It is concluded that a large fraction of the current induced by parallel fibre stimulation reflects the uptake of synaptically released glutamate. The uptake current activated rapidly, with a 20-80% rise time of 2.3 +/- 0.7 ms (n = 10), and decayed with a principal time constant of 25 +/- 6 ms (n = 10).
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amato A., Barbour B., Szatkowski M., Attwell D. Counter-transport of potassium by the glutamate uptake carrier in glial cells isolated from the tiger salamander retina. J Physiol. 1994 Sep 15;479(Pt 3):371–380. doi: 10.1113/jphysiol.1994.sp020302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriza J. L., Fairman W. A., Wadiche J. I., Murdoch G. H., Kavanaugh M. P., Amara S. G. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994 Sep;14(9):5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour B., Brew H., Attwell D. Electrogenic glutamate uptake in glial cells is activated by intracellular potassium. Nature. 1988 Sep 29;335(6189):433–435. doi: 10.1038/335433a0. [DOI] [PubMed] [Google Scholar]
- Barbour B., Brew H., Attwell D. Electrogenic uptake of glutamate and aspartate into glial cells isolated from the salamander (Ambystoma) retina. J Physiol. 1991 May;436:169–193. doi: 10.1113/jphysiol.1991.sp018545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour B., Keller B. U., Llano I., Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994 Jun;12(6):1331–1343. doi: 10.1016/0896-6273(94)90448-0. [DOI] [PubMed] [Google Scholar]
- Bashir Z. I., Bortolotto Z. A., Davies C. H., Berretta N., Irving A. J., Seal A. J., Henley J. M., Jane D. E., Watkins J. C., Collingridge G. L. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993 May 27;363(6427):347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- Billups B., Rossi D., Attwell D. Anion conductance behavior of the glutamate uptake carrier in salamander retinal glial cells. J Neurosci. 1996 Nov 1;16(21):6722–6731. doi: 10.1523/JNEUROSCI.16-21-06722.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier M., Szatkowski M., Amato A., Attwell D. The glial cell glutamate uptake carrier countertransports pH-changing anions. Nature. 1992 Dec 3;360(6403):471–474. doi: 10.1038/360471a0. [DOI] [PubMed] [Google Scholar]
- Brew H., Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. 1987 Jun 25-Jul 1Nature. 327(6124):707–709. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- Bruns D., Engert F., Lux H. D. A fast activating presynaptic reuptake current during serotonergic transmission in identified neurons of Hirudo. Neuron. 1993 Apr;10(4):559–572. doi: 10.1016/0896-6273(93)90159-o. [DOI] [PubMed] [Google Scholar]
- Burnashev N., Khodorova A., Jonas P., Helm P. J., Wisden W., Monyer H., Seeburg P. H., Sakmann B. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science. 1992 Jun 12;256(5063):1566–1570. doi: 10.1126/science.1317970. [DOI] [PubMed] [Google Scholar]
- Chaudhry F. A., Lehre K. P., van Lookeren Campagne M., Ottersen O. P., Danbolt N. C., Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995 Sep;15(3):711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Cerebral hypoxia: some new approaches and unanswered questions. J Neurosci. 1990 Aug;10(8):2493–2501. doi: 10.1523/JNEUROSCI.10-08-02493.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B., Mobbs P. Transmitter-operated channels in rabbit retinal astrocytes studied in situ by whole-cell patch clamping. J Neurosci. 1992 Feb;12(2):664–673. doi: 10.1523/JNEUROSCI.12-02-00664.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., JAEGER J. C. The relationship between the mode of operation and the dimensions of the junctional regions at synapses and motor end-organs. Proc R Soc Lond B Biol Sci. 1958 Jan 1;148(930):38–56. doi: 10.1098/rspb.1958.0003. [DOI] [PubMed] [Google Scholar]
- Eliasof S., Jahr C. E. Retinal glial cell glutamate transporter is coupled to an anionic conductance. Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):4153–4158. doi: 10.1073/pnas.93.9.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M., Wantorsky D., Wilson D. F. Aspartate transport in synaptosomes from rat brain. J Biol Chem. 1983 Aug 10;258(15):9069–9077. [PubMed] [Google Scholar]
- Fairman W. A., Vandenberg R. J., Arriza J. L., Kavanaugh M. P., Amara S. G. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995 Jun 15;375(6532):599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite G., Garthwaite J. Sites of D-[3H]aspartate accumulation in mouse cerebellar slices. Brain Res. 1985 Sep 16;343(1):129–136. doi: 10.1016/0006-8993(85)91166-7. [DOI] [PubMed] [Google Scholar]
- Hestrin S., Sah P., Nicoll R. A. Mechanisms generating the time course of dual component excitatory synaptic currents recorded in hippocampal slices. Neuron. 1990 Sep;5(3):247–253. doi: 10.1016/0896-6273(90)90162-9. [DOI] [PubMed] [Google Scholar]
- Isaacson J. S., Nicoll R. A. The uptake inhibitor L-trans-PDC enhances responses to glutamate but fails to alter the kinetics of excitatory synaptic currents in the hippocampus. J Neurophysiol. 1993 Nov;70(5):2187–2191. doi: 10.1152/jn.1993.70.5.2187. [DOI] [PubMed] [Google Scholar]
- Kanai Y., Hediger M. A. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992 Dec 3;360(6403):467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- Kanai Y., Nussberger S., Romero M. F., Boron W. F., Hebert S. C., Hediger M. A. Electrogenic properties of the epithelial and neuronal high affinity glutamate transporter. J Biol Chem. 1995 Jul 14;270(28):16561–16568. doi: 10.1074/jbc.270.28.16561. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Sharon I. Active transport of L-glutamate by membrane vesicles isolated from rat brain. Biochemistry. 1978 Sep 19;17(19):3949–3953. doi: 10.1021/bi00612a011. [DOI] [PubMed] [Google Scholar]
- Larsson H. P., Picaud S. A., Werblin F. S., Lecar H. Noise analysis of the glutamate-activated current in photoreceptors. Biophys J. 1996 Feb;70(2):733–742. doi: 10.1016/S0006-3495(96)79613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., Marty A., Armstrong C. M., Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991 Mar;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S., Benz A., Zorumski C. F. Components of glial responses to exogenous and synaptic glutamate in rat hippocampal microcultures. J Neurosci. 1996 Jan;16(1):55–64. doi: 10.1523/JNEUROSCI.16-01-00055.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S., Zorumski C. F. Glial contributions to excitatory neurotransmission in cultured hippocampal cells. Nature. 1994 Mar 3;368(6466):59–62. doi: 10.1038/368059a0. [DOI] [PubMed] [Google Scholar]
- Mennerick S., Zorumski C. F. Presynaptic influence on the time course of fast excitatory synaptic currents in cultured hippocampal cells. J Neurosci. 1995 Apr;15(4):3178–3192. doi: 10.1523/JNEUROSCI.15-04-03178.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T., Fritschy J. M., Grosche J., Pratt G. D., Möhler H., Kettenmann H. Developmental regulation of voltage-gated K+ channel and GABAA receptor expression in Bergmann glial cells. J Neurosci. 1994 May;14(5 Pt 1):2503–2514. doi: 10.1523/JNEUROSCI.14-05-02503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T., Möller T., Berger T., Schnitzer J., Kettenmann H. Calcium entry through kainate receptors and resulting potassium-channel blockade in Bergmann glial cells. Science. 1992 Jun 12;256(5063):1563–1566. doi: 10.1126/science.1317969. [DOI] [PubMed] [Google Scholar]
- Nelson P. J., Dean G. E., Aronson P. S., Rudnick G. Hydrogen ion cotransport by the renal brush border glutamate transporter. Biochemistry. 1983 Nov 8;22(23):5459–5463. doi: 10.1021/bi00292a030. [DOI] [PubMed] [Google Scholar]
- Nicholls D., Attwell D. The release and uptake of excitatory amino acids. Trends Pharmacol Sci. 1990 Nov;11(11):462–468. doi: 10.1016/0165-6147(90)90129-v. [DOI] [PubMed] [Google Scholar]
- Picaud S. A., Larsson H. P., Grant G. B., Lecar H., Werblin F. S. Glutamate-gated chloride channel with glutamate-transporter-like properties in cone photoreceptors of the tiger salamander. J Neurophysiol. 1995 Oct;74(4):1760–1771. doi: 10.1152/jn.1995.74.4.1760. [DOI] [PubMed] [Google Scholar]
- Pines G., Danbolt N. C., Bjørås M., Zhang Y., Bendahan A., Eide L., Koepsell H., Storm-Mathisen J., Seeberg E., Kanner B. I. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992 Dec 3;360(6403):464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- Rothstein J. D., Martin L., Levey A. I., Dykes-Hoberg M., Jin L., Wu D., Nash N., Kuncl R. W. Localization of neuronal and glial glutamate transporters. Neuron. 1994 Sep;13(3):713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Sarantis M., Ballerini L., Miller B., Silver R. A., Edwards M., Attwell D. Glutamate uptake from the synaptic cleft does not shape the decay of the non-NMDA component of the synaptic current. Neuron. 1993 Sep;11(3):541–549. doi: 10.1016/0896-6273(93)90158-n. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. L-glutamate conditionally modulates the K+ current of Müller glial cells. Neuron. 1993 Jun;10(6):1141–1149. doi: 10.1016/0896-6273(93)90062-v. [DOI] [PubMed] [Google Scholar]
- Shibuki K., Okada D. Long-term synaptic changes in rat cerebellar slices reflected in extracellular K+ activity. Neurosci Lett. 1990 May 18;113(1):34–39. doi: 10.1016/0304-3940(90)90490-z. [DOI] [PubMed] [Google Scholar]
- Stallcup W. B., Bulloch K., Baetge E. E. Coupled transport of glutamate and sodium in a cerebellar nerve cell line. J Neurochem. 1979 Jan;32(1):57–65. doi: 10.1111/j.1471-4159.1979.tb04509.x. [DOI] [PubMed] [Google Scholar]
- Storck T., Schulte S., Hofmann K., Stoffel W. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Kovalchuk Y., Attwell D. Pre- and postsynaptic determinants of EPSC waveform at cerebellar climbing fiber and parallel fiber to Purkinje cell synapses. J Neurosci. 1995 Aug;15(8):5693–5702. doi: 10.1523/JNEUROSCI.15-08-05693.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Sarantis M., Attwell D. Postsynaptic glutamate uptake in rat cerebellar Purkinje cells. J Physiol. 1996 Dec 1;497(Pt 2):523–530. doi: 10.1113/jphysiol.1996.sp021785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas N. K., Jane D. E., Tse H. W., Watkins J. C. alpha-Methyl derivatives of serine-O-phosphate as novel, selective competitive metabotropic glutamate receptor antagonists. Neuropharmacology. 1996 Jun;35(6):637–642. doi: 10.1016/0028-3908(96)84635-1. [DOI] [PubMed] [Google Scholar]
- Tong G., Jahr C. E. Block of glutamate transporters potentiates postsynaptic excitation. Neuron. 1994 Nov;13(5):1195–1203. doi: 10.1016/0896-6273(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Wadiche J. I., Amara S. G., Kavanaugh M. P. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995 Sep;15(3):721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]
- Wadiche J. I., Arriza J. L., Amara S. G., Kavanaugh M. P. Kinetics of a human glutamate transporter. Neuron. 1995 May;14(5):1019–1027. doi: 10.1016/0896-6273(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Wyllie D. J., Mathie A., Symonds C. J., Cull-Candy S. G. Activation of glutamate receptors and glutamate uptake in identified macroglial cells in rat cerebellar cultures. J Physiol. 1991 Jan;432:235–258. doi: 10.1113/jphysiol.1991.sp018383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerangue N., Kavanaugh M. P. Flux coupling in a neuronal glutamate transporter. Nature. 1996 Oct 17;383(6601):634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]



