Abstract
We have previously described enteroaggregative Escherichia coli (EAEC) strains that induce cytotoxic effects on T84 cells, ligated rat ileal loops, and human intestine in culture. Such strains secrete a 104-kDa protein termed Pet (for plasmid-encoded toxin). We have also shown previously that the Pet toxin induces rises in short-circuit current and decreases the electrical resistance in rat jejunum mounted in an Ussing chamber. The nucleotide sequence of the pet gene revealed that Pet is a member of the autotransporter class of secreted proteins. Here we show that a concentrated supernatant of E. coli HB101 harboring the minimal pet clone pCEFN1 induces temperature-, time- and dose-dependent cytopathic effects on HEp-2 cells and HT29 C1 cells in culture. The effects were characterized by release of the cellular focal contacts from the glass substratum, followed by complete rounding of the cells and detachment from the glass. Staining of the Pet-treated cells with Live/Dead viability stain revealed that >90% of rounded cells were viable. Pet-intoxicated HEp-2 and HT29 cells stained with fluorescein-labeled phalloidin revealed contraction of the cytoskeleton and loss of actin stress fibers. However, the effects of Pet were not inhibited by cytoskeleton-altering drugs, including colchicine, taxol, cytochalasin D, and phallicidin. The Pet protein induced proteolysis in zymogram gels, and preincubation with the serine protease inhibitor phenylmethylsulfonyl fluoride resulted in complete abrogation of Pet cytopathic effects. We introduced a mutation in a predicted catalytic serine residue and found that the mutant (Pet S260I) was deficient in protease activity and did not produce cytopathic effects, cytoskeletal damage, or enterotoxic effects in Ussing chambers. These data suggest that Pet is a cytoskeleton-altering toxin and that its protease activity is involved in each of the observed phenotypes.
Enteroaggregative Escherichia coli (EAEC), defined by its aggregating pattern of adherence to HEp-2 cells (28), has been associated with persistent pediatric diarrhea, especially in developing countries (2, 4, 43). Two prominent pathogenic features of EAEC histopathology have been described: (i) formation of a thick mucus gel on the intestinal mucosa (14, 41) and (ii) mucosal damage, presumably via the elaboration of a cytotoxin(s) (14, 29).
EAEC-induced mucosal cytotoxicity has been observed in several model systems (14, 29, 30, 42). Vial et al. (42) injected pathogenic EAEC strain 042 into rabbit ileal loops and described striking histopathologic changes that were characterized by shorting of the villi, hemorrhagic necrosis of the villus tips, and a mild inflammatory response with edema and mononuclear infiltration of the submucosa. Strain 042 also induced cytotoxic effects in an in vitro organ culture model, as manifested by dilatation of the crypt openings, rounding of enterocytes, and exfoliation of mucosal epithelial cells (29). Nataro et al. (29) described toxic effects in a T84 cell culture model, demonstrating that strain 042 elicited damage to the apical plasma membrane, with vesiculation and shedding of microvilli after bacterial attachment. The cytoplasm of affected T84 cells displayed subnuclear vacuolization; in some cases the nuclei became separated from the surrounding cytoplasm. Working with EAEC outbreak strain 049766, Eslava et al. (9) described mucosal damage in the ileum of children succumbing to EAEC diarrhea. 049766 induced similar effects upon injection into rat ligated ileal loops.
Supernatants from many EAEC strains express high-molecular-weight proteins (predicted molecular masses of 108 and 116 kDa) which, when injected into rat ileal loops, induce fluid accumulation and cytotoxic effects on the mucosa (9). We cloned and sequenced the ca. 108-kDa protein and found that it bears nucleotide homology to a class of serine protease autotransporters from E. coli and Shigella. We have shown that this protein, termed Pet (for plasmid-encoded toxin), produces rises in short-circuit current (Isc) and decreases in electrical resistance in rat jejunum mounted in an Ussing chamber, effects accompanied by mucosal damage, increased mucus release, exfoliation of cells, and development of crypt abscesses (30). Thus, our data suggest that Pet has enterotoxic and perhaps cytotoxic activity. Here we report that the Pet protein elicits cytoskeletal changes in both HEp-2 and HT29 cells in vitro without compromising cell viability and that these effects are dependent on serine protease activity.
MATERIALS AND METHODS
Strains and plasmids.
The minimal Pet clone pCEFN1 (previously described [10]) was constructed by cloning the pet gene of EAEC strain 042 into the BamHI/KpnI site of pSPORT1 and is expressed in E. coli HB101 (10). HB101(pCEFN1) was used to obtain Pet protein, and HB101(pSPORT1) was used as a control for all experiments. The strains were maintained on L agar or L broth containing ampicillin (100 μg/ml).
Toxin preparation.
HB101(pCEFN1) broth cultures were incubated overnight at 37°C and then centrifuged at 7,000 × g for 15 min. The culture supernatant was filtered throughout 0.22-μm-pore-size cellulose acetate membrane filters (Corning), concentrated 100-fold with an ultrafree centrifugal filter device with a 100-kDa cutoff (Millipore), filter sterilized again, and stored at −20°C for up to 3 months. One hundred milliliters of HB101(pCEFN1) overnight culture produced about 1 mg of Pet protein.
Cell culture.
HEp-2 cells were propagated in humidified 5% CO2–95% air at 37°C in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% fetal calf serum (HyClone, Logan, Utah), 1% nonessential amino acids, 5 mM l-glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml). The subcultures were serially propagated after harvesting with 10 mM EDTA and 0.25% trypsin (GIBCO BRL, Grand Island, N.Y.) in phosphate-buffered solution (PBS; pH 7.4). For experimental use, subconfluent HEp-2 cells were resuspended with EDTA-trypsin, plated into four-well LabTek slides (VWR, Bridgeport, N.J.), and allowed to grow to 60% confluence (about 2 days).
The HT29 C1 clone was obtained from Daniel Louvard (Institut Pasteur, Paris, France) and used at 20 to 32 passages. HT29 cells were grown in DMEM supplemented with 10% fetal bovine serum, 44 mM sodium bicarbonate, and 10 μg of human transferrin (Sigma, St. Louis, Mo.), 50 IU of streptomycin, and 50 μg of penicillin per ml. HT29 cells were grown at 37°C in humidified 10% CO2–90% air; medium was changed 6 days/week. For experimental use, HT29 cells were prepared in eight-well LabTek slides grown to 70% confluence (about 3 days).
Tissue culture assay.
For all experiments, Pet-containing concentrated filtrates were diluted directly into tissue culture medium on the cells (without antibiotics and serum) at a final volume of 500 μl per well (for four-well LabTek slides) or 250 μl per well (for eight-well LabTek slides). Following the specified incubation time in humidified atmosphere of 10% CO2–90% air at 37°C, the medium was aspirated, and the cells were washed twice with PBS and processed by methods described below.
(i) Giemsa.
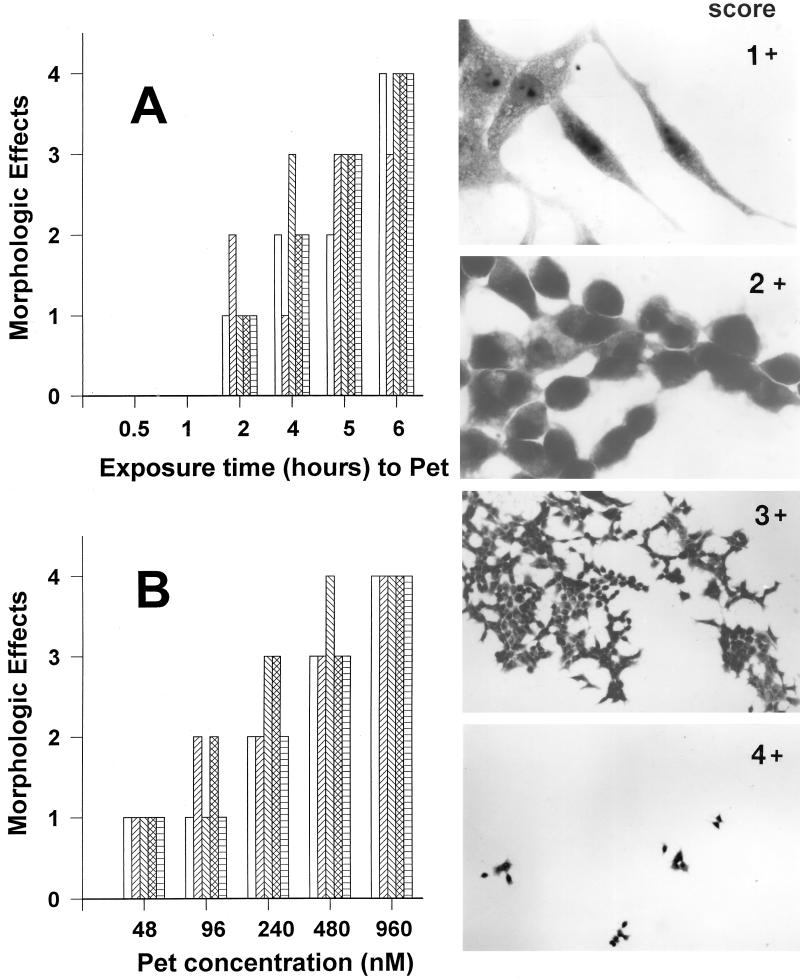
The cells were fixed with 70% methanol and stained with 10% Giemsa (Sigma). Slides were read at a magnification of ×100 with standard bright-field light microscopy. Toxic activity (defined as altered HEp-2 cell morphology) was scored on a scale modified from previous work (36, 44). A score of 1+ indicated the presence of elongated or rounded cells greater than for the control (but <50% of cells affected); 2+ indicated that >50% of the cells were rounded but detachment was <50%; 3+ indicated that >50% of the cells were detached and all remaining cells were rounded; 4+ indicated that all (or nearly all) cells were detached from the glass.
(ii) Coomassie blue.
The cells were fixed with 2% formalin in PBS, washed, permeabilized by adding 0.1% Triton X-100 in PBS, and stained with 20% Coomassie stain solution (Bio-Rad, Hercules, Calif.). Slides were read at a magnification of ×100 with standard bright-field light microscopy.
(iii) FAS assay.
Fluorescence actin staining (FAS) was performed as described by Knutton et al. (16). The cells were fixed with 2% formalin in PBS, washed, permeabilized by adding 0.1% Triton X-100 in PBS, and stained with 0.05 μg of fluorescein isothiocyanate-phalloidin per ml. Slides were mounted with 90% glycerol, covered with a glass cover slide, and examined at a magnification of ×400 under epifluorescence microscopy.
(iv) Cell viability assay.
The cells were washed with HEPES-buffered saline solution, stained with a Live/Dead reduced-biohazard viability/cytotoxicity kit as instructed by the manufacturer (Molecular Probes, Eugene, Oreg.), and fixed with 4% glutaraldehyde in HEPES-buffered saline solution. Slides were examined at a magnification of ×400 under epifluorescence microscopy. As described in the manufacturer’s literature, red cells were considered to be nonviable and the green cells were considered to be viable.
Cell treatments.
To evaluate the effect of microtubule- and actin filament-stabilizing and -destabilizing agents on Pet activity, HEp-2 cells were incubated with these drugs for 2 h in serum-free medium, followed by addition of Pet protein (to a concentration of 10 μg/ml) for 5 h at 37°C, prior to fixation and staining. The agents were used at the following concentrations: 1, 2, 4, and 10 μM taxol (Molecular Probes); 1, 2, 4, and 10 μM colchicine (Sigma); 0.5, 1, and 2 μM phallicidin (Molecular probes); and 5, 10, and 15 μg of cytochalasin D (Sigma) per ml.
Toxic neutralization.
Pet protein (10 μg/ml) was preincubated for 30 min at 37°C with a 1:10 dilution of the gamma fraction of antiserum raised against Pet protein (30) or for 15 min with 2 mM phenylmethylsulfonyl fluoride (PMSF; Boehringer, Indianapolis, Ind.); the solution was then added to wells containing HEp-2 cells in fresh medium and incubated for 5 h at 37°C prior to standard fixation and staining.
Protease assay.
Gelatinase zymogram analysis was performed by electrophoretic separation of concentrated supernatants of HB101(pCEFN1) alone or mixed with PMSF inhibitor. After electrophoresis of the supernatants in a precast zymogram gel (Novex, San Diego, Calif.), the gel was incubated for 30 min at room temperature in zymogram renaturing buffer (2.5% Triton X-100), equilibrated for 30 min with zymogram developing buffer (1.21 g of Tris base, 6.3 g of Tris HCl, 11.7 g of NaCl, and 0.74 g of CaCl2 per liter), incubated at 37°C for 4 h with fresh developing buffer, and stained with Coomassie blue R-250 for 30 min. Protease activity is detected as a clear band against a dark blue background.
Site-directed mutagenesis.
Site-directed mutagenesis was performed with a QuikChange site-directed mutagenesis kit from Stratagene. The synthetic oligonucleotides used for this purpose were 5′-CACTAATGGTGACATTGGATCAGGCGTGTA-3′ and 5′-TACACGCCTGATCCAATGTCACCATTAGTG-3′. The primers encompassed residues 1045 to 1075 of the pet sequence (accession no. AF056581) but encoded a T instead of a G at nucleotide 1059, thereby substituting an isoleucine for the serine at residue 260. Mutagenesis was performed on the minimal clone pCEFN1 according to manufacturer’s protocols. The oligonucleotide primers were extended during temperature cycling by PfuTurbo DNA polymerase according to manufacturer’s instructions. After recovering plasmid DNA from several transformants, we confirmed the DNA sequence on an Applied Biosystems model 373A automated sequencer in the Biopolymer Laboratory, Department of Microbiology and Immunology, University of Maryland School of Medicine.
Ussing chamber experiments.
The Ussing chamber experiments were performed as we have previously described (10, 30). Briefly, six pieces of Sprague-Dawley rat jejunum were cut open along the mesenteric border, washed with cold Ringer’s solution, and mounted between the circular openings of six Ussing hemichambers. Each hemichamber was filled with 10 ml of gassed Ringer’s solution and kept at 37°C under constant 95% O2–5% CO2 bubbling. After addition of the test sample, transepithelial electrical potential difference (PD) was measured at 10-min intervals under current-clamped conditions. Tissue conductance was determined at an applied current of 100 μA, and Isc was calculated by using Ohm’s law. Samples used in Ussing chamber experiments were prepared from 100-ml L-broth cultures grown overnight at 37°C. After centrifugation of the culture at 12,000 × g for 10 min, supernatants were concentrated and size fractionated (>100 kDa) by passage through Biomax-100 Ultrafree filters (Millipore). The retentate was resuspended in 1 ml of Ringer’s solution, and 100 μl of each concentrated sample was added to mucosal hemichambers.
RESULTS
Effects of Pet protein on HEp-2 and HT-29 epithelial cells.
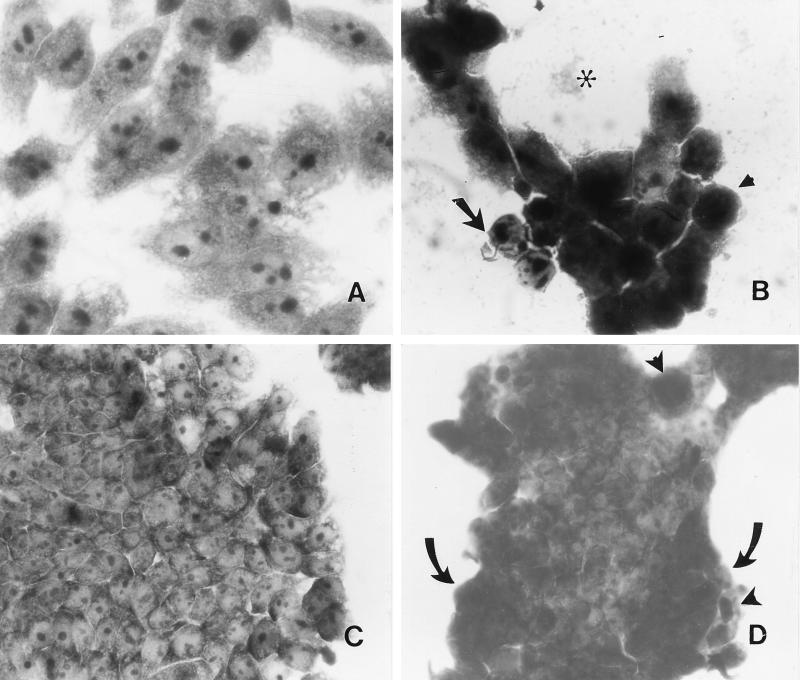
The mature Pet protein (104 kDa) was partially purified from supernatants of the minimal clone HB101(pCEFN1) by passage through a 100-kDa-retention filter device (see Fig. 4). The concentrated Pet protein was applied to HEp-2 and HT-29 epithelial cells, which had been cultured on chamber slides. The morphologies of the two lines of cells were unaltered by treatment with concentrated supernatants from HB101(pSPORT1) (Fig. 1A and C). However, supernatants containing Pet protein at 25 μg/ml (200 nM) caused extensive changes in both cell lines after 6 h of incubation. In HEp-2 cells, the damage was characterized by release of cellular focal contacts from the glass substratum, complete rounding of the cells, and detachment from the glass (Fig. 1B). HT-29 cells, which form tight junctions, revealed a dramatic change in morphology, with retraction of the tight cellular clusters, accompanied by rounding and detachment of cells from neighboring cells at the periphery of the clusters (Fig. 1D).
FIG. 4.
Proteolytic activity of Pet protein. (A) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the 104-kDa secreted Pet protein obtained from the supernatant of the minimal clone HB101(pCEFN1). An L-broth culture was grown overnight at 37°C, and then the supernatant was concentrated and fractionated with a 100-kDa-retention filter. Sizes of marker proteins are indicated in kilodaltons on the right. (B) Proteolytic activity detected by electrophoresing Pet protein alone or mixed with PMSF inhibitor in a precast gelatin zymogram gel. Protease activities are identified as clear bands against a dark blue background after staining with Coomassie blue.
FIG. 1.
Effect of Pet protein on epithelial cells. Pet protein was added to cell cultures at a final volume of 500 μl (for HEp-2 cells) or 250 μl (for HT29 cells) per well. Both untreated and treated cells were incubated for 6 h at 37°C in culture medium without antibiotics and serum. (A) HEp-2 cells treated with supernatants from HB101(pSPORT). (B) HEp-2 cells treated with 200 nM Pet protein for 6 h. Release of cellular focal contacts from the glass substratum is indicated with an arrow, rounding of the cells is indicated with an arrowhead, and cell detachment from the glass is indicated with an asterisk. (C) HT29 cells treated with supernatants from HB101(pSPORT). Cells appear normal. (D) HT29 cells treated with 200 nM Pet protein for 6 h. Retraction of the tight cluster is indicated with arrows; rounding and detachment of cells are indicated with arrowheads. Panel B shows remnant cells remaining after near total detachment of cells from the substratum (see text).
The effects of Pet on HEp-2 cells are time and dose dependent.
At 25 μg of protein/ml, the effects of Pet on the morphology of HEp-2 cells were examined after 0.5, 1, 2, 4, 5, and 6 h of exposure. Concentrated size-fractionated supernatants from HB101(pSPORT1) did not alter the morphology of HEp-2 cells at any time compared with control cells (Fig. 1A). After 30 min and 1 h of exposure, the HEp-2 cells incubated with 25 μg of Pet protein/ml appeared normal under light microscopy. The first evidence of a change in cellular morphology induced by Pet protein was noted after 2 h. At this time point, some cells became elongated or rounded (score of 1+). After 4 or 5 h of incubation in the presence of 200 nM Pet, >50% of the cells were detached and the remaining cells were rounded (score of 3+). In some cells, the cytoplasm could not be clearly defined. After 6 h of incubation, nearly all HEp-2 cells were detached from the glass (score of 4+) (Fig. 2A). After 6 h of exposure to Pet, staining of the cells with Live/Dead fluorescent viability stain (Molecular Probes) revealed that nearly all (>90%) cells which were still in contact with the substratum remained viable (not shown).
FIG. 2.
Quantitation of time- and dose-dependent effects of Pet protein on HEp-2 cells. (A) Time-dependent effects of Pet protein. The effects of Pet protein on the morphology of HEp-2 cells were examined after 0.5, 1, 2, 4, 5, and 6 h of exposure. After this time, monolayers were scored as described in the text. The bars represent individual experiments (n = 4 for each time point). (B) Dose-dependent effects of Pet protein. HEp-2 cells were exposed to different concentrations of Pet protein (48 to 960 nM). The bars represent individual experiments (n = 4 for each concentration point). The inserts show the score of the morphologic changes of HEp-2 cells produced by Pet protein described in Materials and Methods.
To assess the dose dependence of the Pet cytopathic effect on HEp-2 cells, cells were exposed to 5, 10, 25, 50, and 100 μg/ml (48 to 960 nM) for 5 h. The graded morphologic changes were similar to those observed with different times of toxin exposure: a score of 1+ was observed with 5 μg/ml, and score 4+ was seen with 100 μg/ml (Fig. 2B). However, at a low concentration, 1 μg/ml (9 nM), Pet elicited morphologic changes (score of 3+) after the incubation of standard HEp-2 assay was extended to 18 h. In subsequent experiments, 10 μg of toxin/ml and 5 h of exposure were used to produce clear (score of 3+), highly reproducible effects over a short incubation time.
Reversibility of onset and effect of temperature on HEp-2 cell intoxication by Pet.
The toxic effects of Pet on HEp-2 cells were irreversible. HEp-2 cells were treated with 10 μg of Pet/ml for 10 min, 30 min, or 1, 2, 3, or 4 h, washed with PBS, and allowed to incubate for another 5 h in fresh DMEM. Ten minutes of exposure to Pet followed by a 5-h incubation in the absence of toxin was sufficient to elicit morphologic changes. Changes were scored 2+ to 3+ and were similar to those produced by 10 μg of Pet/ml. The scored damage increased with time of exposure to the toxin.
To test the effect of incubation temperature, HEp-2 cells were inoculated with 10 μg of Pet/ml and incubated for 5 h at 4, 22, or 37°C. In contrast to the striking morphologic changes (score of 3+) seen in the standard assay at 37°C, Pet effects were completely inhibited at 4°C but not at 22°C. After a 3-h exposure at 4°C, Pet elicited morphologic changes after washing of the monolayer and incubation for a further 2 h at 37°C (score of 3+; similar to cells treated for 5 h at 37°C). Thus, once the initial interaction occurred, intoxication could not be reversed by washing but still required subsequent incubation at 37°C to become evident.
Effects of Pet on the cytoskeleton.
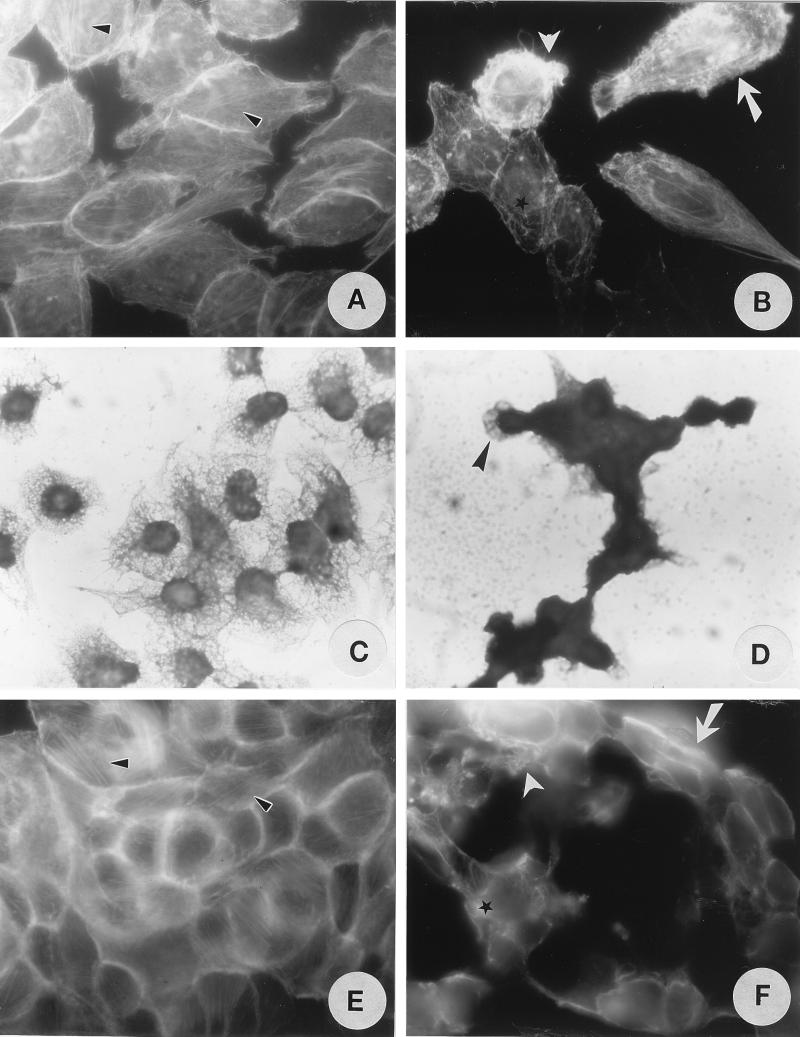
The cell rounding phenotype suggests that Pet disrupts the cytoskeleton or cytoskeleton-related proteins. To characterize these cytoskeletal effects, HEp-2 and HT29 cells incubated with Pet toxin were stained with fluorescein-labeled phalloidin and observed under fluorescence microscopy (the FAS assay). In initial experiments, the effect of 5, 10, 20, or 40 μg of Pet/ml on F-actin structure in HEp-2 cells was assessed after 3 and 6 h of exposure. Untreated control HEp-2 cells (Fig. 3A) were uniform and smooth edged, and they displayed organized, linear F-actin stress fibers. In contrast, HEp-2 cells treated with 10 μg of Pet/ml for 5 h revealed contraction of the cytoskeleton, loss of actin stress fibers, formation of surface blebs, and a globular appearance of some cells (Fig. 3B). By Coomassie blue staining of cells, alteration of the cytoskeletal web (Fig. 3D) was observed by light microscopy in Pet-treated cells but not in untreated cells (Fig. 3C).
FIG. 3.
Effects of Pet protein on the epithelial cell cytoskeleton as detected by FAS (A, B, E, and F) and Coomassie blue staining (C and D). (A and C) Untreated HEp-2 cells. (B and D) HEp-2 cells treated with 200 nM Pet protein for 6 h. (E) Untreated HT29 cells. (F) HT29 cells treated with 200 nM Pet protein for 6 h. Linear F-actin stress fibers are indicated by small arrowheads, contraction of the cytoskeleton by is indicated arrows, loss of actin stress fibers by is indicated asterisks, and formation of surface blebs by is indicated large arrowheads.
FAS of untreated HT29 cells revealed classic cytoskeletal actin structures, with linear F-actin stress fibers and staining of F-actin at the intercellular junction (Fig. 3E). After 5 h of exposure to Pet, HT29 cells revealed contraction of the cytoskeleton, loss of actin stress fibers, formation of surface blebs, and a globular appearance; increased numbers of actin filaments were observed at the edge of the cells, as was rearrangement of actin filaments associated with intercellular junctions (Fig. 3F). However, actin from Pet-treated HEp-2 cells visualized by Western blotting showed no proteolysis and appeared similar to the actin from untreated HEp-2 cells (not shown).
Using a Pet dose of 10 μg/ml, we assessed the effects of drugs which alter the structure of microtubules (i.e., the destabilizer, colchicine, and the stabilizer, taxol) or actin filaments (the inhibitor of polymerization, cytochalasin D, and the stabilizer, phallicidin). None of these agents abolished Pet-induced cytopathic changes. At high doses, both cytochalasin D and colchicine produced mild disorganization of the cellular cytoskeleton in the absence of Pet protein; also at high doses, cytochalasin D alone produced some detachment of the epithelial cells. Therefore, only experiments using doses of inhibitors below those which resulted in cytotoxicity were interpreted. Taxol and phallicidin appeared increase slightly the Pet-induced cytopathic effects (data not shown).
Inhibition of cytotoxic effects.
The cytotoxic effects of Pet on HEp-2 and HT29 cells could be inhibited by heat treatment of the Pet protein at 75°C for 15 min. Preincubation of Pet for 30 min with polyclonal antibodies raised against the Pet protein also produced a dose-dependent inhibition of cytopathic effects. The same antibodies had previously been shown to inhibit enterotoxic effects in Ussing chambers (30).
We had previously shown that Pet protein contains a serine protease motif (10). Pet protease activity was confirmed by separating concentrated HB101(pCEFN1) supernatants (Fig. 4A) on gelatin zymogram gels. Zones of clearing were evident at ca. 104 kDa, but not in control supernatants prepared from HB101(pSPORT). The proteolytic zone observed on gelatin zymograms was abolished by pretreatment of Pet with the serine protease inhibitor PMSF (Fig. 4B).
To test the role of the serine protease activity in the cytopathic effects of Pet, toxin preparations were preincubated with one of three serine protease inhibitors prior to application to HEp-2 cells. Neither leupeptin nor aprotinin inhibited the effects of Pet; however, PMSF preincubation resulted in complete abrogation of cytopathic effects. This inhibition was maintained even when Pet was preincubated with PMSF and then washed extensively on 100-kDa-retention ultrafilters. However, no inhibition was seen when HEp-2 cells were preincubated with PMSF before the addition of toxin, suggesting that the serine protease activity is conferred by the Pet protein and not the epithelial cells, as has been suggested for cholera and E. coli heat-labile toxins (19).
We suspected that the effects of Pet were fundamentally due to nonspecific serine protease activity at the cell surface. As a comparison, trypsin was added to HEp-2 cell monolayers at a molar concentration of 200 nM and incubated for 5 h. The ability of trypsin to detach cells was significantly less (1+) than that induced by a Pet concentration of 100 nM (3+). Moreover, when the detached cells treated with Pet or trypsin were recultured on chamber slides, the cells treated with trypsin were able to readhere and to grow normally, whereas cells treated with Pet did not reattach to the glass substratum (data not shown). These data suggest that although the effects of Pet may be on cell surface proteins, the effects can be clearly differentiated from those of a nonspecific serine protease.
Construction and analysis of a protease mutant.
To assess further the role of the Pet protease activity, a site-directed mutation was introduced at the predicted catalytic serine residue S260, converting this residue to isoleucine. Pet S260I was processed and secreted into the supernatants of HB101 and was detected on immunoblots with antibodies directed against Pet protein (data not shown). However, Pet S260I no longer displayed protease activity in gelatin zymogram gels.
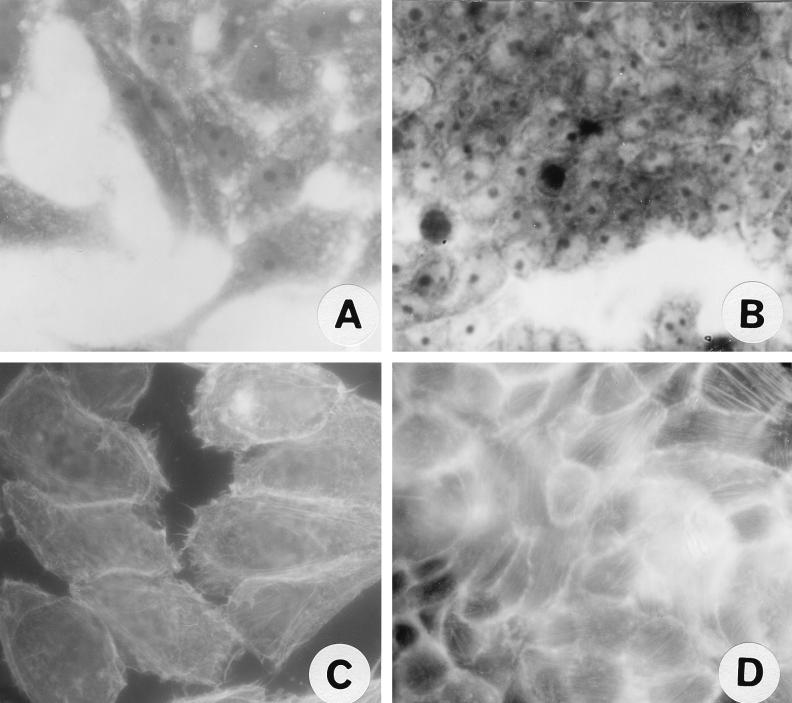
Pet S260I was found to be unable to cause rounding or other cytopathic effects on HEp-2 or HT29 cells, even at a concentration of 960 nM for 5 h (Fig. 5A and B), and was similar in activity to the negative control supernatant of HB101(pSPORT1) (Fig. 1A and C). In addition, Pet S260I did not produce cytoskeletal effects in either cell line (Fig. 5C and D), as the FAS assay results appeared similar to those for untreated cells (Fig. 3A and E).
FIG. 5.
Effects of Pet S260I mutant on epithelial cells visualized by Coomassie blue (A and B) or FAS (C and D). (A) HEp-2 cells treated with 96 nM S260I protein for 5 h. (B) HT29 cells treated with 96 nM S260I protein for 5 h. (C) HEp-2 cells treated with 96 nM S260I protein for 5 h. (D) HT29 cells treated with 96 nM S260I protein for 5 h.
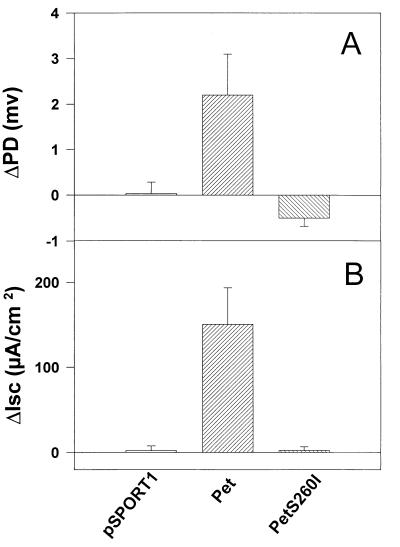
Supernatants containing Pet S260I were assessed for enterotoxic effects on rat jejunal tissue mounted in the Ussing chamber. As seen in Fig. 6, Pet S260I did not induce increases of PD or Isc or decreases in tissue conductance.
FIG. 6.
Effect of the S260I mutation on Pet enterotoxicity. Supernatants from overnight cultures were size fractionated (>100 kDa), and 25 μg of protein was added to each Ussing chamber, mounted with full-thickness rat jejunal tissue. Supernatants from HB101(pCEFN1) and HB101(pSPORT1) were used as a positive and negative controls, respectively. Data points represent the means of at least four experiments; error bars represent standard errors of the mean. The supernatant of pCEFN1 (Pet protein) generates significant rises in PD and Isc compared with the S260I mutant (P < 0.05 by Student’s t test).
DISCUSSION
EAEC has been associated with persistent diarrhea in children (2, 4, 43) and recently in adults (27, 32, 38). The main pathogenic features include formation of a mucus gel on the intestinal mucosa (14, 41), a marked degree of watery diarrhea (4, 41, 42), and mucosal damage, presumably via the elaboration of a toxin(s) (14, 29, 42). Previous data from our laboratories including animal models (9, 42), cultured cells (29), intestinal segments mounted in an Ussing chamber (30), in vitro organ culture (29), and autopsy specimens from infected patients (9) have shown that a plausible explanation for the persistent nature of EAEC disease involves intestinal mucosal damage elicited by these bacteria. However, the mechanism of this mucosal damage is not known.
We have recently shown that the plasmid-encoded Pet toxin of EAEC elicits rises in Isc on rat jejunal tissue mounted in an Ussing chamber, an effect which is accompanied by a fall in tissue resistance and damage to the tissue when examined under light microscopy (30). Thus, we hypothesize that Pet is an enterotoxin that elicits cytopathic effects on intestinal epithelial cells. Here, using HEp-2 and HT29 cells and a Pet protease-deficient mutant, we show that Pet is capable of eliciting damage to epithelial cells and that the effect is dependent on the protease activity of the protein.
Pet toxin is able to intoxicate HEp-2 and HT29 cells after 2 h at 37°C as detected under light microscopy; these effects are characterized by time- and dose-dependent cell elongation followed by rounding and ultimately release from the substratum. However, only 10 min of exposure to Pet followed by incubation for another 2 h at 37°C is sufficient to elicit the same morphologic changes. Our data suggest that the intoxication of HEp-2 cells is irreversible to washing and resembles in this respect the Bacteroides fragilis enterotoxin (BFT) (36), Clostridium difficile toxin A (21, 25), and C. perfringens enterotoxin (24).
We have previously shown that Pet is a member of the autotransporter family of secreted proteins (10) and that it belongs to a subfamily featuring a conserved serine protease motif (consensus GDSGSP). This motif is present at an analogous position in Pet, Tsh (33), EspP (3), and EspC (39) and in the Shigella proteins ShMu (34) and SepA (1). We have termed this subfamily the SPATEs (serine protease autotransporters of Enterobacteriaceae). Unlike the original autotransporter, immunoglobulin A1 protease, none of the SPATE subfamily proteases has been shown to cleave immunoglobulin A1. We have shown here that the serine protease motif of Pet protein (10) plays a role in its cytopathic and enterotoxic effects. The effects of Pet on HEp-2 cells are inhibited by a serine protease inhibitor (PMSF), which also inhibits the protease activity observed in zymogram gels. Moreover, we have shown that Pet mutated in its serine protease motif does not induce damage of either epithelial cells or enterotoxic effects on rat intestinal segments mounted in an Ussing chamber.
It is intriguing to consider that other members of the SPATE subfamily may function in similar ways, although their substrates may well be different. EspP (also called PssA) from enterohemorrhagic E. coli is capable of cleaving pepsin A and human coagulation factor V, and the proteolytic activity is similarly lost by preincubation with PMSF (3). Djafari et al. (6) have found that PssA is, like Pet, encoded on a large plasmid and that this protein induces cytopathic effects on Vero cells. PssA-induced cytoskeletal changes include loss of the stress fibers, retraction of cell bodies, and defects in cell-to-cell junctions, observed after 10 h of incubation (6). The homology of Pet with PssA is significant (54% amino acid identity of the mature proteins), and it is likely that these proteins will display at least some mechanistic similarities.
The mechanism of Pet-induced cytopathic and enterotoxic effects is not yet understood, yet it is tempting to speculate that these effects are due to alteration of the cytoskeleton. Our data reveal a contraction of the cytoskeleton and loss of actin stress fibers as early as 3 h after addition of Pet to HEp-2 and HT29 monolayers. However, the effects were not prevented by cytoskeleton-altering drugs such as taxol, colchicine, phallacidin, or cytochalasin D. Cytoskeleton altering drugs do not inhibit the action of BFT (7, 36), which is thought to act on E-cadherin at the cell surface. Our data do not permit us to assign an intra- or extracellular site of action for the Pet toxin, although the effects of Pet are different from those induced by trypsin.
Whereas the dose of Pet used in our studies (48 nM) is higher that those used with some enterotoxins such as BFT or cholera toxin (19, 31, 36), it is not inconsistent with the molar concentrations used in the study of the toxins STa and VacA (another autotransporter protein) (22, 5). Interesting, when the 5-h HEp-2 assay is prolonged to 18 or 24 h, concentrations of 9 nM Pet toxin can elicit similar morphologic changes, suggesting that even a very small amount of toxin can produce cellular intoxication. However, Ussing chamber data suggest dramatic mucosal changes after just 2 h of toxin exposure; moreover, our unpublished data suggest that a pet null mutant elicits substantially less mucosal damage to colonic tissue in culture (13a). These data suggest that normal colonic epithelial cells may be more sensitive to Pet than are HEp-2 cells and/or that the proximity of adherent bacteria results in more efficient delivery of toxin. In either case, we believe that the model described herein will be useful to study the mechanisms of action of Pet and other proteins of autransporter family, especially those from the SPATE subfamily.
Changes in the cytoskeleton of intestinal epithelial cells have been associated with disminished resistance of intestinal epithelia (12, 13, 35) as well as alteration in the function of some intestinal ion transporters (23, 40). We believe that Pet may be a member of a growing list of cytoskeleton-altering enteric toxins, which include toxin A and B of C. difficile (12, 13), the zonula occludens toxin of Vibrio cholerae (11), STa (22), and BFT (7, 17, 37). The role of Pet and other SPATE proteins in enteric disease requires further elucidation.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI33096 and TW00499 (from the Fogarty Center) to J.P.N. C.E. was supported by Consejo Nacional de Ciencia y Tecnología de México (CONACYT 25846M).
REFERENCES
- 1.Benjelloun-Touimi Z, Tahar M S, Montecucco C, Sansonetti P J, Parsot C. SepA, the 110 kDa protein secreted by Shigella flexneri: two-domain structure and proteolytic activity. Microbiology. 1995;144:1815–1822. doi: 10.1099/00221287-144-7-1815. [DOI] [PubMed] [Google Scholar]
- 2.Bhan M K, Raj P, Levine M M, Kaper J B, Bhandari N, Srivastava R, Kumar R, Sazawal S. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J Infect Dis. 1989;159:1061–1064. doi: 10.1093/infdis/159.6.1061. [DOI] [PubMed] [Google Scholar]
- 3.Brunder W, Schmidt H, Karch H. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol Microbiol. 1997;24:767–78. doi: 10.1046/j.1365-2958.1997.3871751.x. [DOI] [PubMed] [Google Scholar]
- 4.Cravioto A, Tello A, Navarro A, Ruiz J, Villafan H, Uribe F, Eslava C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet. 1991;337:262–264. doi: 10.1016/0140-6736(91)90868-p. [DOI] [PubMed] [Google Scholar]
- 5.de Bernard M, Papini E, de Filippis V, Gottardi E, Telford J, Manetti R, Fontana A, Rappuoli R, Montecucco C. Low pH activates the vacuolating toxin of Helicobacter pylori, which becomes acid and pepsin resistant. J Biol Chem. 1995;270:23937–23940. doi: 10.1074/jbc.270.41.23937. [DOI] [PubMed] [Google Scholar]
- 6.Djafari S, Ebel F, Deibel C, Kramer S, Hudel M, Chakraborty T. Characterization of an exported protease from Shiga toxin-producing Escherichia coli. Mol Microbiol. 1997;25:771–784. doi: 10.1046/j.1365-2958.1997.5141874.x. [DOI] [PubMed] [Google Scholar]
- 7.Donelli G, Fabbri A, Fiorentine C. Bacteroides fragilis enterotoxin induces cytoskeletal changes and surface blebbing in HT-29 cells. Infect Immun. 1996;64:113–119. doi: 10.1128/iai.64.1.113-119.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dota S T, Beristain S, Tomicic T K. Inhibition of heat-labile cholera and Escherichia coli enterotoxins by brefeldin A. Infect Immun. 1993;61:3282–3286. doi: 10.1128/iai.61.8.3282-3286.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eslava C, Villaseca J, Morales R, Navarro A, Cravioto A. Abstracts of the 93rd General Meeting of the American Society for Microbiology 1993. Washington, D.C: American Society for Microbiology; 1993. Identification of a protein with toxigenic activity produced by enteroaggregative Escherichia coli, abstr. B-105. [Google Scholar]
- 10.Eslava C, Navarro-Garcia F, Czeczulin J R, Henderson I, Cravioto A, Nataro J P. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect Immun. 1998;66:3155–3163. doi: 10.1128/iai.66.7.3155-3163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fasano A, Fiorentini C, Donelli G, Uzzau S, Kaper J B, Margaretten K, Ding X, Gualdini S, Comstock L, Goldblum S E. Zonula occludens toxin modulates tight junctions througt protein kinase C-dependent actin reorganization, in vitro. J Clin Investig. 1995;96:710–720. doi: 10.1172/JCI118114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hecht G, Koutsouris A, Pothoulaskis C, LaMont J T, Madara J L. Clostridium difficile toxin B disrupts the barrier fuction of T84 monolayers. Gastroenterology. 1992;102:416–423. doi: 10.1016/0016-5085(92)90085-d. [DOI] [PubMed] [Google Scholar]
- 13.Hecht G, Pothoulaskis C, LaMont J T, Madara J L. Clostridium difficile toxin A perturbs cytoskeleton structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Investig. 1988;82:1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Henderson, I., F. Navarro-García, S. Hicks, and J. Nataro. Unpublished data.
- 14.Hicks S, Candy D C A, Phillips A D. Adhesion of enteroaggregative Escherichia coli to pediatric intestinal mucosa in vitro. Infect Immun. 1996;64:4751–4760. doi: 10.1128/iai.64.11.4751-4760.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keusch G T, Jacewicz M. Primary amines and chloroquine inhibit cytotoxic responses to Shigella toxin and permit late antibody rescue of toxin treated cells. Biochem Biophys Res Commun. 1984;121:69–76. doi: 10.1016/0006-291x(84)90689-2. [DOI] [PubMed] [Google Scholar]
- 16.Knutton S, Phillips A D, Smith H R, Gross R J, Shaw R, Watson P, Price E. Screening for enteropathogenic Escherichia coli in infants with diarrhea by the fluorescent-actin staining test. Infect Immun. 1991;59:365–371. doi: 10.1128/iai.59.1.365-371.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koshy S S, Montrose M H, Sears C L. Human intestinal epithelial cells swell and demonstrate actin arrangement in response to the metalloprotease toxin of Bacteroides fragilis. Infect Immun. 1996;64:5022–5028. doi: 10.1128/iai.64.12.5022-5028.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lencer W I, Strohmeier G, Moe S, Carlson S L, Constable C T, Madara J L. Signal transduction by cholera toxin: processing in vesicular compartments does not require acidification. Am J Physiol. 1995;269:G548–G557. doi: 10.1152/ajpgi.1995.269.4.G548. [DOI] [PubMed] [Google Scholar]
- 19.Lencer W I, de Almeida J B, Moe S, Stow J L, Ausiello D A, Madara J L. Entry of cholera toxin into polarized human intestinal epithelial cells. Identification of an early brefeldin A sensitive event required for A1-peptide generation. J Clin Investig. 1993;92:2941–2951. doi: 10.1172/JCI116917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lencer W I, Constable C, Moe S, Rufo P A, Wolf A, Jobling M G, Ruston S P, Madara J L, Holmes R K, Hirst T R. Proteolytic activation of cholera toxin and Escherichia coli labile toxin by entry into host epithelial cells. Signal transduction by a protease-resistant toxin variant. J Biol Chem. 1997;272:15562–15568. doi: 10.1074/jbc.272.24.15562. [DOI] [PubMed] [Google Scholar]
- 21.Lima A A, Lyerly D M, Wilkins T D, Innes D J, Guerrant R L. Effects of Clostridium difficile toxins A and B in rabbit small and large intestine in vivo and on cultured cells in vitro. Infect Immun. 1988;56:582–588. doi: 10.1128/iai.56.3.582-588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews J B, Awtrey C S, Thompson R, Hung T, Tally K J, Madara J L. Na(+)-K(+)-2Cl-cotransport and Cl- secretion evoked by heat-stable enterotoxin is microfilament dependent in T84 cells. Am J Physiol. 1993;265:G370–G378. doi: 10.1152/ajpgi.1993.265.2.G370. [DOI] [PubMed] [Google Scholar]
- 23.Matthews J B, Smith J A, Tally K J, Awtrey C S, Nguyen H, Rich J, Madara J L. Na+-K+-2Cl- cotransport in intestinal epithelial cells: influence of chloride efflux and F-actin on regulation of contransporter activity and bumetanide binding. J Biol Chem. 1994;269:15703–15709. [PubMed] [Google Scholar]
- 24.McClane B A. Clostridium perfringens enterotoxin acts by producing small molecule permeability alterations in plasma membranes. Toxicology. 1994;87:43–67. doi: 10.1016/0300-483x(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell T J, Ketley J M, Burdon D W, Candy D C, Stephen J. Biological mode of action of Clostridium difficile toxin A: a novel enterotoxin. J Med Microbiol. 1987;23:211–219. doi: 10.1099/00222615-23-3-211. [DOI] [PubMed] [Google Scholar]
- 26.Moncrief J S, Obiso R, Barroso L A, Kling J J, Wright R L, Van Tassell R L, Lyerly D M, Wilkins T D. The enterotoxin of Bacteroides fragilis is a metalloprotease. Infect Immun. 1995;63:175–181. doi: 10.1128/iai.63.1.175-181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nataro J P, Yikang D, Cookson S, Cravioto A, Savarino S J, Guers L D, Levine M M, Tacket C O. Heterogeneity of enteroaggregative Escherichia coli virulence demonstrated in volunteers. J Infect Dis. 1995;171:465–468. doi: 10.1093/infdis/171.2.465. [DOI] [PubMed] [Google Scholar]
- 28.Nataro J P, Kaper J B, Robins-Browne R, Prado V, Vial P A, Levine M M. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. J Pediatr Infect Dis. 1987;16:829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Nataro J P, Hicks S, Phillips A D, Vial P A, Sears C L. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect Immun. 1996;64:4761–4768. doi: 10.1128/iai.64.11.4761-4768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navarro-García F, Eslava C, Villaseca J M, Lopez-Revilla R, Czeczulin J R, Srinivas S, Nataro J P, Cravioto A. In vitro effects of a high-molecular-weight heat-labile enterotoxin from enteroaggregative Escherichia coli. Infect Immun. 1998;66:3149–3154. doi: 10.1128/iai.66.7.3149-3154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orlandi P A, Cerran P K, Fishman P H. Brefeldin A blocks the response of cultured cells to cholera toxin. J Biol Chem. 1993;268:12010–12016. [PubMed] [Google Scholar]
- 32.Pai M, Kang G, Ramakrishna B S, Venkataraman A, Muliyil J. An epidemic of diarrhoea in south India caused by enteroaggregative Escherichia coli. Indian J Med Res. 1997;106:7–12. [PubMed] [Google Scholar]
- 33.Provence D L, Curtiss R., III Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect Immun. 1994;62:1369–1380. doi: 10.1128/iai.62.4.1369-1380.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajakumar K, Sasakawa C, Adler B. Use of a novel approach, termed island probing, identifies the Shigella flexneri she pathogenicity island which encodes a homolog of the immunoglobulin A protease-like family of proteins. Infect Immun. 1997;65:4606–4614. doi: 10.1128/iai.65.11.4606-4614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reigler M, Sedivy R, Pothoulakis C, Hamilton G, Zacheri J, Bischof G, Consentini E, Feil W, Schiessel R, LaMont J T, Wenzl E. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Investig. 1995;95:2004–2011. doi: 10.1172/JCI117885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saidi R F, Sears C L. Bacteroides fragilis toxin rapidly intoxicates human intestinal epithelial cells (HT29/C1) in vitro. Infect Immun. 1996;64:5029–5034. doi: 10.1128/iai.64.12.5029-5034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saidi R F, Jaeger K, Montrose M H, Wu S, Sears C L. Bacteroides fragilis toxin rearranges the actin cytoskeleton of HT29/C1 cells without direct proteolysis of actin or decrease in F-actin content. Cell Motil Cytoskel. 1997;37:159–165. doi: 10.1002/(SICI)1097-0169(1997)37:2<159::AID-CM8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 38.Smith H R, Cheasty T, Rowe B. Enteroaggregative Escherichia coli and outbreaks of gastroenteritis in UK. Lancet. 1997;350:814–815. doi: 10.1016/s0140-6736(05)62611-6. [DOI] [PubMed] [Google Scholar]
- 39.Stein M, Kenny B, Stein M A, Finlay B B. Characterization of EspC, a 110-kilodalton protein secreted by enteropathogenic Escherichia coli which is homologous to members of the immunoglobulin A protease-like family of secreted proteins. J Bacteriol. 1996;178:6546–6554. doi: 10.1128/jb.178.22.6546-6554.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tousson H, Fuller C M, Benos D J. Apical recruitment of CFTR in T-84 cells is dependent on cAMP and microtubules but not Ca+2 or microfilaments. J Cell Sci. 1996;109:1325–1334. doi: 10.1242/jcs.109.6.1325. [DOI] [PubMed] [Google Scholar]
- 41.Tzipori S, Montanaro J, Robins-Browne R M, Vial P, Gibson R, Levine M M. Studies with enteroaggregative Escherichia coli in the gnotobiotic piglet gastroenteritis model. Infect Immun. 1992;60:5302–5306. doi: 10.1128/iai.60.12.5302-5306.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vial P A, Robins-Browne R, Lior H, Prado V, Kaper J B, Nataro J P, Maneval D, Elsayed A, Levine M M. Characterization of enteroadherent-aggregative Escherichia coli, a putative agent of diarrheal disease. J Infect Dis. 1988;158:70–79. doi: 10.1093/infdis/158.1.70. [DOI] [PubMed] [Google Scholar]
- 43.Wanke C A, Schorling J B, Barret L J, De Souza M A, Guerrant R L. Potential role of adherence traits of Escherichia coli in persistent diarrhea in an urban Brazilian slum. Pediatr Infect Dis J. 1991;10:746–751. doi: 10.1097/00006454-199110000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Weikel C S, Grieco F D, Reuben J, Myers L L, Sack R B. Human colonic epithelial cells, HT29/C1, treated with crude Bacteroides fragilis enterotoxin dramatically alter their morphology. Infect Immun. 1992;60:321–327. doi: 10.1128/iai.60.2.321-327.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]