Abstract
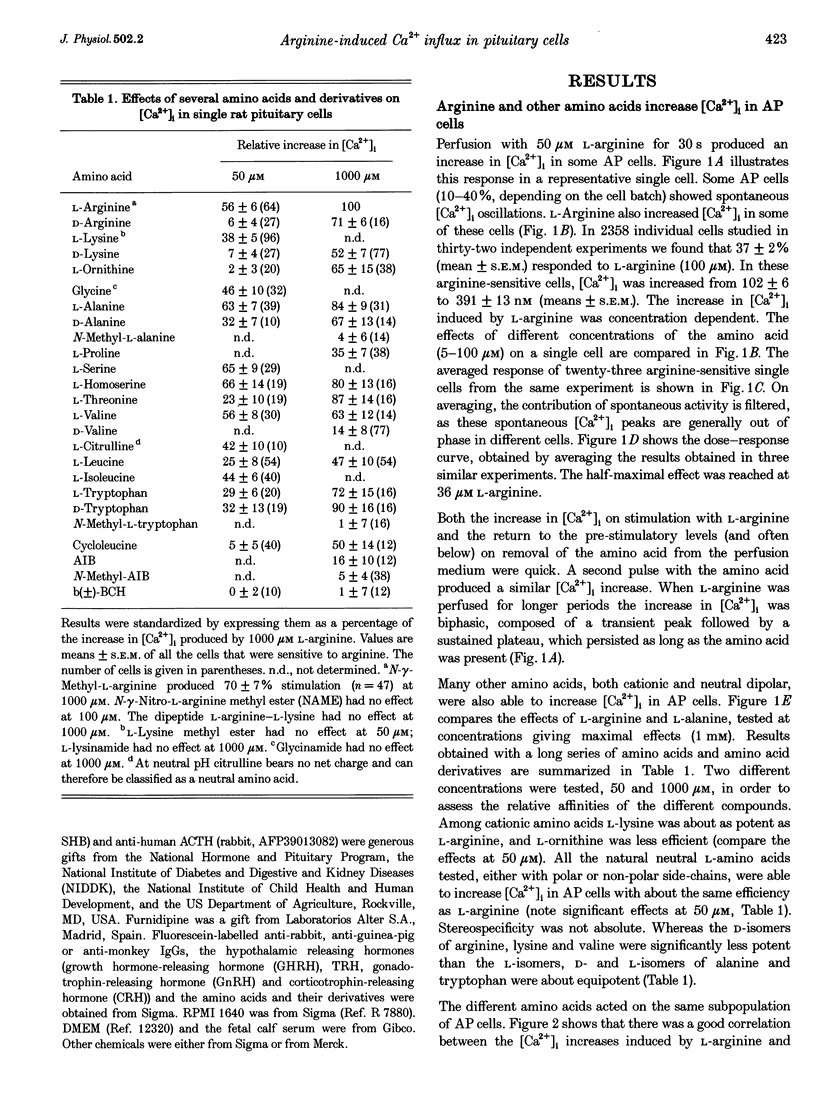
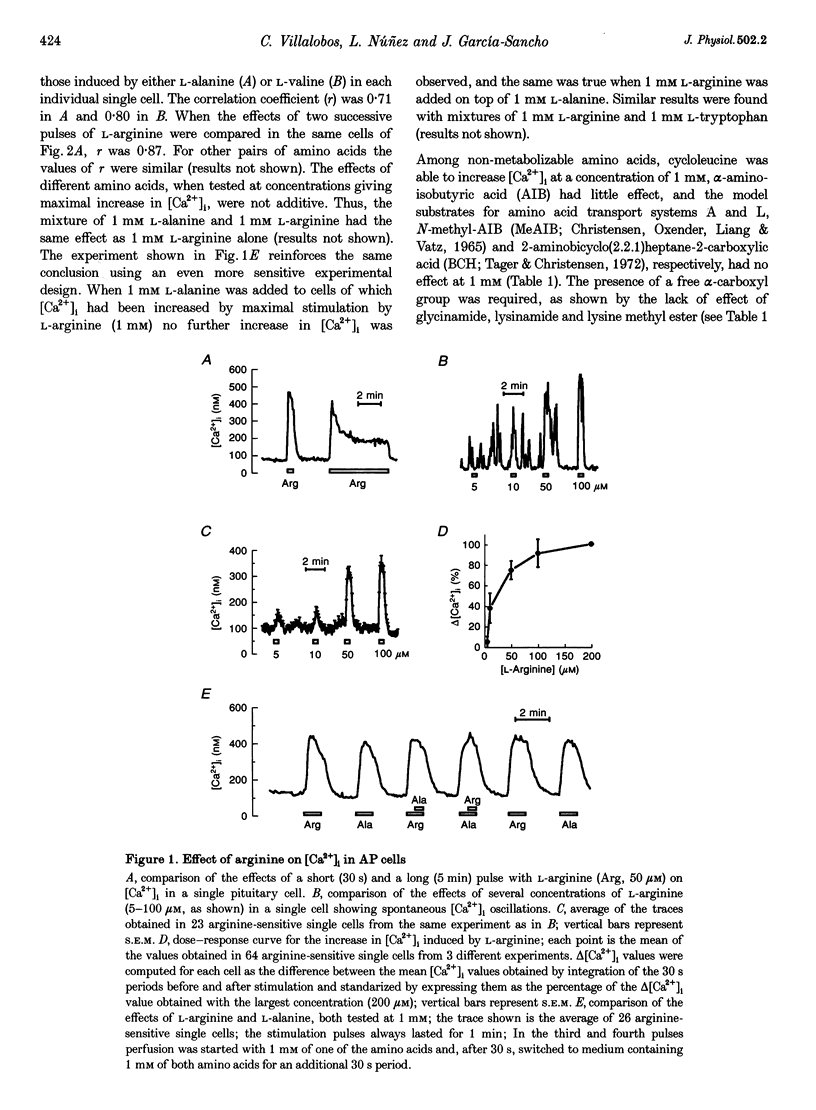
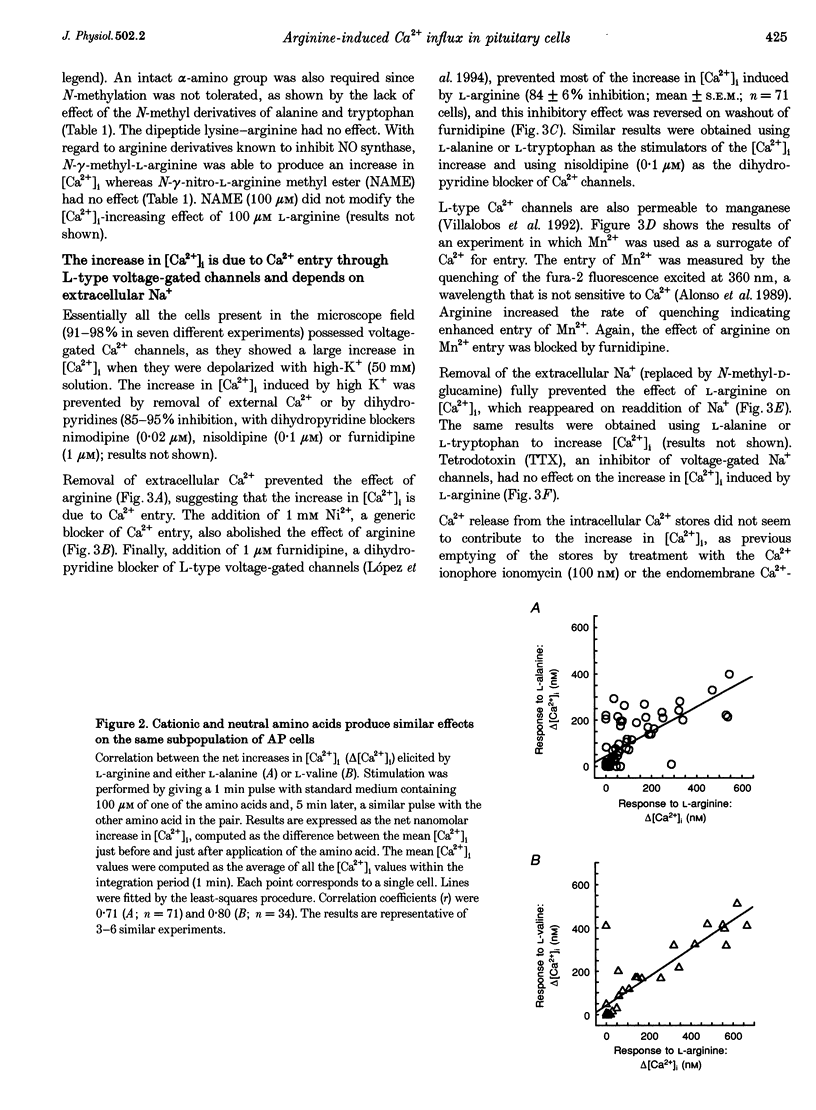
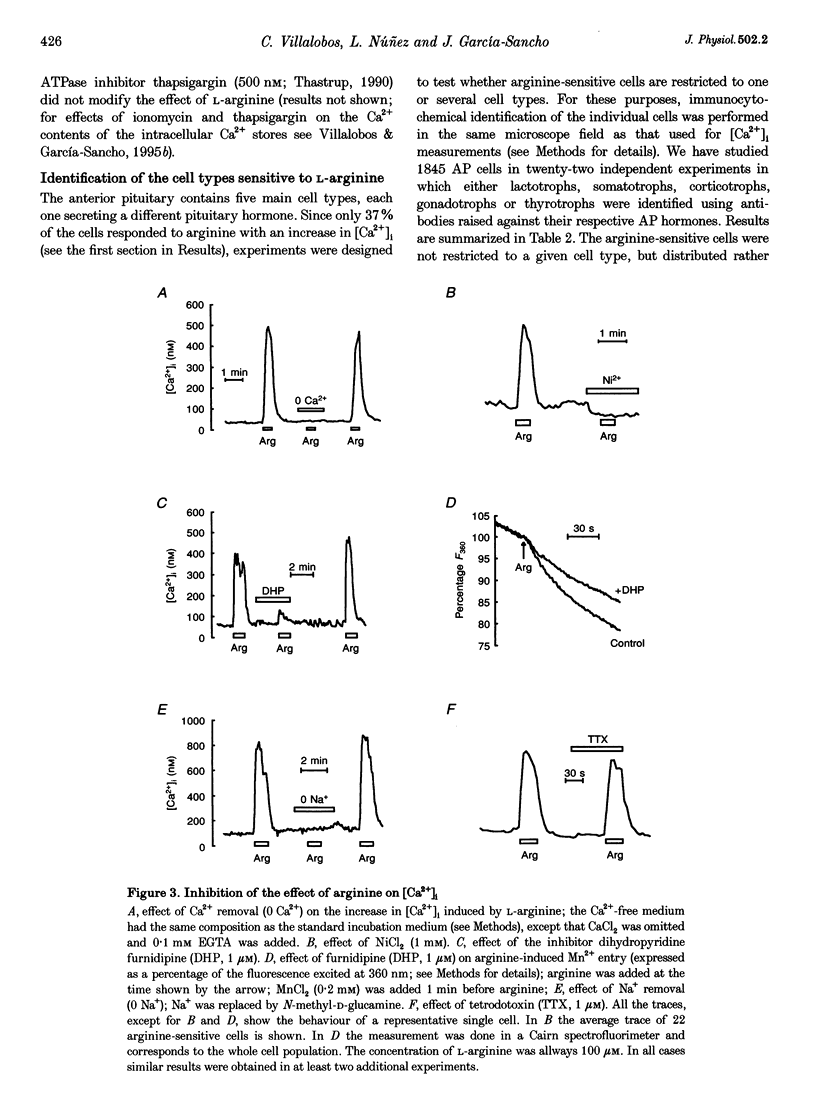
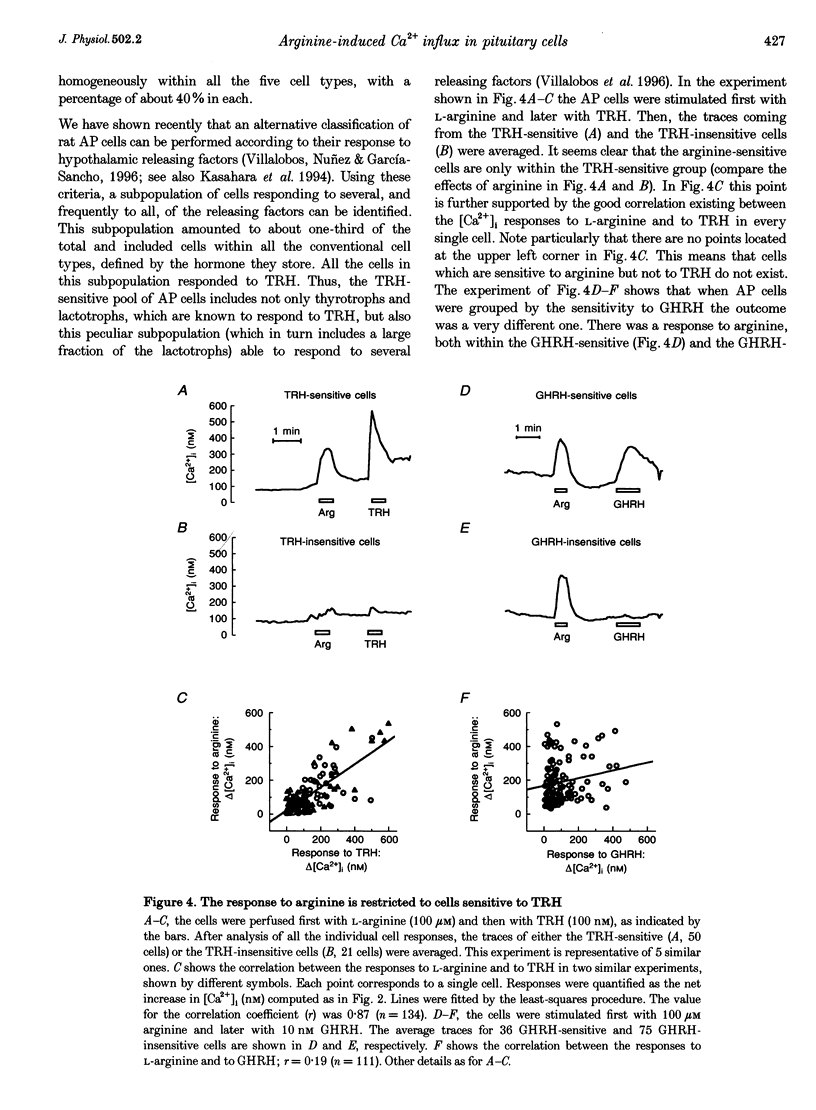
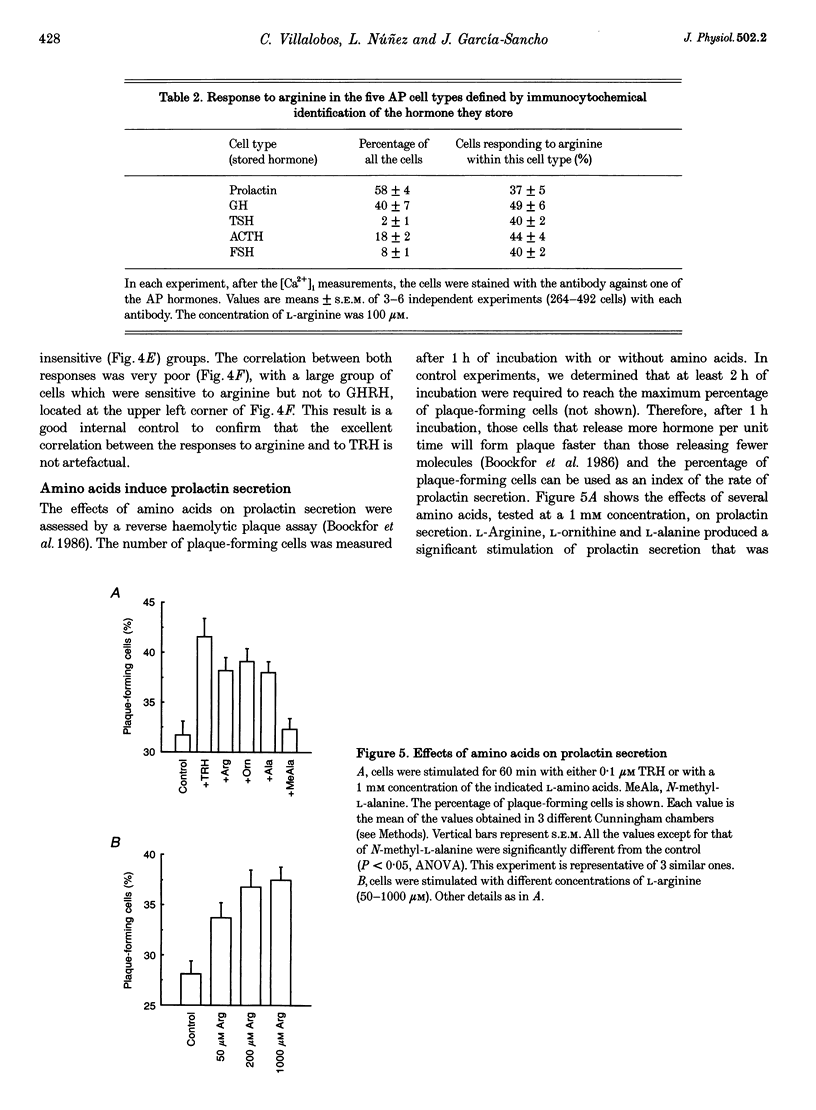
1. Arginine and other amino acids are secretagogues for growth hormone and prolactin in the intact animal, but the mechanism of action is unclear. We have studied the effects of amino acids on cytosolic free calcium concentration ([Ca2+]i) in single rat anterior pituitary (AP) cells. Arginine elicited a large increase of [Ca2+]i) in about 40% of all the AP cells, suggesting that amino acids may modulate hormone secretion by acting directly on the pituitary. 2. Cell typing by immunofluorescence of the hormone the cells store showed that the arginine-sensitive cells are distributed uniformly within all the five AP cell types. The arginine-sensitive cells overlapped closely with the subpopulation of cells sensitive to thyrotrophin-releasing hormone. 3. Other cationic as well as several neutral (dipolar) amino acids had the same effect as arginine. The increase of [Ca2+]i was dependent on extracellular Ca2+ and blocked by dihydropyridine, suggesting that it is due to Ca2+ influx through L-type voltage-gated Ca2+ channels. The [Ca2+]i increase was also blocked by removal of extracellular Na+ but not by tetrodotoxin. The substrate specificity for stimulation of AP cells resembled closely that of the amino acid transport system B0+. We propose that electrogenic amino acid influx through this pathway depolarizes the plasma membrane with the subsequent activation of voltage-gated Ca2+ channels and Ca2+ entry. 4. Amino acids also stimulated prolactin secretion in vitro with a similar substrate specificity to that found for the [Ca2+]i increase. Existing data on the stimulation of secretion of other hormones by amino acids suggest that a similar mechanism could apply to other endocrine glands.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso M. T., Sanchez A., García-Sancho J. Monitoring of the activation of receptor-operated calcium channels in human platelets. Biochem Biophys Res Commun. 1989 Jul 14;162(1):24–29. doi: 10.1016/0006-291x(89)91956-6. [DOI] [PubMed] [Google Scholar]
- Barros F., Villalobos C., García-Sancho J., del Camino D., de la Peña P. The role of the inwardly rectifying K+ current in resting potential and thyrotropin-releasing-hormone-induced changes in cell excitability of GH3 rat anterior pituitary cells. Pflugers Arch. 1994 Feb;426(3-4):221–230. doi: 10.1007/BF00374775. [DOI] [PubMed] [Google Scholar]
- Blachier F., Leclercq-Meyer V., Marchand J., Woussen-Colle M. C., Mathias P. C., Sener A., Malaisse W. J. Stimulus-secretion coupling of arginine-induced insulin release. Functional response of islets to L-arginine and L-ornithine. Biochim Biophys Acta. 1989 Sep 19;1013(2):144–151. doi: 10.1016/0167-4889(89)90042-6. [DOI] [PubMed] [Google Scholar]
- Boockfor F. R., Hoeffler J. P., Frawley L. S. Analysis by plaque assays of GH and prolactin release from individual cells in cultures of male pituitaries. Evidence for functional heterogeneity within rat mammotrope and somatotrope populations. Neuroendocrinology. 1986;42(1):64–70. doi: 10.1159/000124250. [DOI] [PubMed] [Google Scholar]
- Christensen H. N., Handlogten M. E., Thomas E. L. Na plus-facilitated reactions of neutral amino acids with a cationic amino acid transport system. Proc Natl Acad Sci U S A. 1969 Jul;63(3):948–955. doi: 10.1073/pnas.63.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H. N., Liang M., Archer E. G. A distinct Na+-requiring transport system for alanine, serine, cysteine, and similar amino acids. J Biol Chem. 1967 Nov 25;242(22):5237–5246. [PubMed] [Google Scholar]
- Christensen H. N., Oxender D. L., Liang M., Vatz K. A. The use of N-methylation to direct route of mediated transport of amino acids. J Biol Chem. 1965 Sep;240(9):3609–3616. [PubMed] [Google Scholar]
- Dobson P. R., Brown B. L. The preparation, culture, and incubation of rat anterior pituitary cells for static and dynamic studies of secretion. Methods Enzymol. 1985;109:293–298. doi: 10.1016/0076-6879(85)09094-2. [DOI] [PubMed] [Google Scholar]
- Drews G., Krippeit-Drews P. NO synthase activity does not influence electrical activity of mouse pancreatic B-cells. Biochem Biophys Res Commun. 1995 May 25;210(3):914–920. doi: 10.1006/bbrc.1995.1744. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hallam T. J., Rink T. J. Agonists stimulate divalent cation channels in the plasma membrane of human platelets. FEBS Lett. 1985 Jul 8;186(2):175–179. doi: 10.1016/0014-5793(85)80703-1. [DOI] [PubMed] [Google Scholar]
- Kasahara K., Tasaka K., Masumoto N., Mizuki J., Tahara M., Miyake A., Tanizawa O. Characterization of rat pituitary cells by their responses to hypothalamic releasing hormones. Biochem Biophys Res Commun. 1994 Mar 30;199(3):1436–1441. doi: 10.1006/bbrc.1994.1391. [DOI] [PubMed] [Google Scholar]
- Lamberts S. W., Macleod R. M. Regulation of prolactin secretion at the level of the lactotroph. Physiol Rev. 1990 Apr;70(2):279–318. doi: 10.1152/physrev.1990.70.2.279. [DOI] [PubMed] [Google Scholar]
- López M. G., Artalejo A. R., García A. G., Neher E., García-Sancho J. Veratridine-induced oscillations of cytosolic calcium and membrane potential in bovine chromaffin cells. J Physiol. 1995 Jan 1;482(Pt 1):15–27. doi: 10.1113/jphysiol.1995.sp020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López M. G., Villarroya M., Lara B., Martínez Sierra R., Albillos A., García A. G., Gandía L. Q- and L-type Ca2+ channels dominate the control of secretion in bovine chromaffin cells. FEBS Lett. 1994 Aug 8;349(3):331–337. doi: 10.1016/0014-5793(94)00696-2. [DOI] [PubMed] [Google Scholar]
- O'Leary R., O'Connor B. Thyrotropin-releasing hormone. J Neurochem. 1995 Sep;65(3):953–963. doi: 10.1046/j.1471-4159.1995.65030953.x. [DOI] [PubMed] [Google Scholar]
- OXENDER D. L., CHRISTENSEN H. N. DISTINCT MEDIATING SYSTEMS FOR THE TRANSPORT OF NEUTRAL AMINO ACIDS BY THE EHRLICH CELL. J Biol Chem. 1963 Nov;238:3686–3699. [PubMed] [Google Scholar]
- Prevarskaya N., Skryma R., Vacher P., Bresson-Bepoldin L., Odessa M. F., Rivel J., San Galli F., Guerin J., Dufy-Barbe L. Gonadotropin-releasing hormone induced Ca2+ influx in nonsecreting pituitary adenoma cells: role of voltage-dependent Ca2+ channels and protein kinase C. Mol Cell Neurosci. 1994 Dec;5(6):699–708. doi: 10.1006/mcne.1994.1084. [DOI] [PubMed] [Google Scholar]
- Schally A. V., Coy D. H., Meyers C. A. Hypothalamic regulatory hormones. Annu Rev Biochem. 1978;47:89–128. doi: 10.1146/annurev.bi.47.070178.000513. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Warner T. D., Ishii K., Sheng H., Murad F. Insulin secretion from pancreatic B cells caused by L-arginine-derived nitrogen oxides. Science. 1992 Feb 7;255(5045):721–723. doi: 10.1126/science.1371193. [DOI] [PubMed] [Google Scholar]
- Thastrup O. Role of Ca2(+)-ATPases in regulation of cellular Ca2+ signalling, as studied with the selective microsomal Ca2(+)-ATPase inhibitor, thapsigargin. Agents Actions. 1990 Jan;29(1-2):8–15. doi: 10.1007/BF01964706. [DOI] [PubMed] [Google Scholar]
- Van Winkle L. J., Campione A. L., Gorman J. M. Na+-independent transport of basic and zwitterionic amino acids in mouse blastocysts by a shared system and by processes which distinguish between these substrates. J Biol Chem. 1988 Mar 5;263(7):3150–3163. [PubMed] [Google Scholar]
- Van Winkle L. J., Christensen H. N., Campione A. L. Na+-dependent transport of basic, zwitterionic, and bicyclic amino acids by a broad-scope system in mouse blastocysts. J Biol Chem. 1985 Oct 5;260(22):12118–12123. [PubMed] [Google Scholar]
- Van Winkle L. J., Haghighat N., Campione A. L., Gorman J. M. Glycine transport in mouse eggs and preimplantation conceptuses. Biochim Biophys Acta. 1988 Jun 22;941(2):241–256. doi: 10.1016/0005-2736(88)90185-x. [DOI] [PubMed] [Google Scholar]
- Villalobos C., Fonteriz R., López M. G., García A. G., García-Sancho J. Inhibition of voltage-gated Ca2+ entry into GH3 and chromaffin cells by imidazole antimycotics and other cytochrome P450 blockers. FASEB J. 1992 Jun;6(9):2742–2747. doi: 10.1096/fasebj.6.9.1319362. [DOI] [PubMed] [Google Scholar]
- Villalobos C., García-Sancho J. Capacitative Ca2+ entry contributes to the Ca2+ influx induced by thyrotropin-releasing hormone (TRH) in GH3 pituitary cells. Pflugers Arch. 1995 Oct;430(6):923–935. doi: 10.1007/BF01837406. [DOI] [PubMed] [Google Scholar]
- Villalobos C., García-Sancho J. Glutamate increases cytosolic calcium in GH3 pituitary cells acting via a high-affinity glutamate transporter. FASEB J. 1995 Jun;9(9):815–819. doi: 10.1096/fasebj.9.9.7601345. [DOI] [PubMed] [Google Scholar]
- Villalobos C., Núez L., Garcia-Sancho J. Functional glutamate receptors in a subpopulation of anterior pituitary cells. FASEB J. 1996 Apr;10(5):654–660. doi: 10.1096/fasebj.10.5.8621065. [DOI] [PubMed] [Google Scholar]
- White M. F. The transport of cationic amino acids across the plasma membrane of mammalian cells. Biochim Biophys Acta. 1985 Dec 9;822(3-4):355–374. doi: 10.1016/0304-4157(85)90015-2. [DOI] [PubMed] [Google Scholar]


