Summary:
Sacral reconstruction post tumor resection has historically been executed with the placement of hardware or free tissue transfer. Reconstruction using a vascularized bone graft is an alternative that avoids the complications associated with hardware placement or free tissue transfer. This article describes the first documented case of spinoplastic reconstruction using an iliac crest vascularized bone graft (IC-VBG) after the resection of a sacral ependymoma. This is a case of a 17-year-old boy with a history of a sacral myxopapillary ependymoma. He presented to a local emergency department complaining of 6 months of urinary incontinence and progressive paresthesias affecting the left lower extremity. Magnetic resonance imaging was significant for a lesion located in the extradural spinal canal. Biopsy confirmed a myxopapillary ependymoma, World Health Organization grade II. The initial intervention involved tumor resection with titanium hardware placement. He subsequently required replacement of the titanium hardware with carbon fiber, secondary to the need for surveillance imaging. He underwent harvesting and inset of the IC-VBG at the time of hardware replacement. IC-VBG is a safe and effective modality for spinoplastic reconstruction. It enhances the potential for solid bony union and offers a practical alternative to free bone transfer. This approach provides an asset to add to a reconstructive surgeon’s armamentarium, making it an essential tool for reconstructive surgeons working in conjunction with spinal surgery colleagues.
Ependymomas are a rare subtype of primary central nervous system tumors, making up only 1.9% of primary central nervous system tumors among adults, but are the most common spinal tumors in patients younger than 19 years of age.1 There is currently a paucity of documented cases providing insights into the complexities of reconstruction after resection of sacral ependymomas. The aim of this study was to present the first documented case of spinoplastic reconstruction using an iliac crest vascularized bone graft (IC-VBG) after the resection of a sacral ependymoma. This report highlights the surgical technique, outcome, and follow-up of this procedure. We aimed to offer insights that can guide future surgical decisions and contribute to the expanding body of knowledge in the field of oncological spinoplastic reconstruction.
CLINICAL PRESENTATION
This study was deemed exempt by the institutional review board. The patient and parents consented to the procedure and to the publication of his images.
A 17-year-old boy presented to the emergency department with a 6-month history of urinary incontinence and progressive paresthesia of the left lower extremity. A physical examination revealed decreased reflexes and sensation in the left lower extremity and signs of early saddle abnormalities. Magnetic resonance imaging demonstrated severe cauda equina syndrome with a likely neuroblastic tumor in the spinal cord. Biopsy of the lesion was obtained via interventional radiology, and pathology confirmed a myxopapillary ependymoma (MPE), World Health Organization grade II.
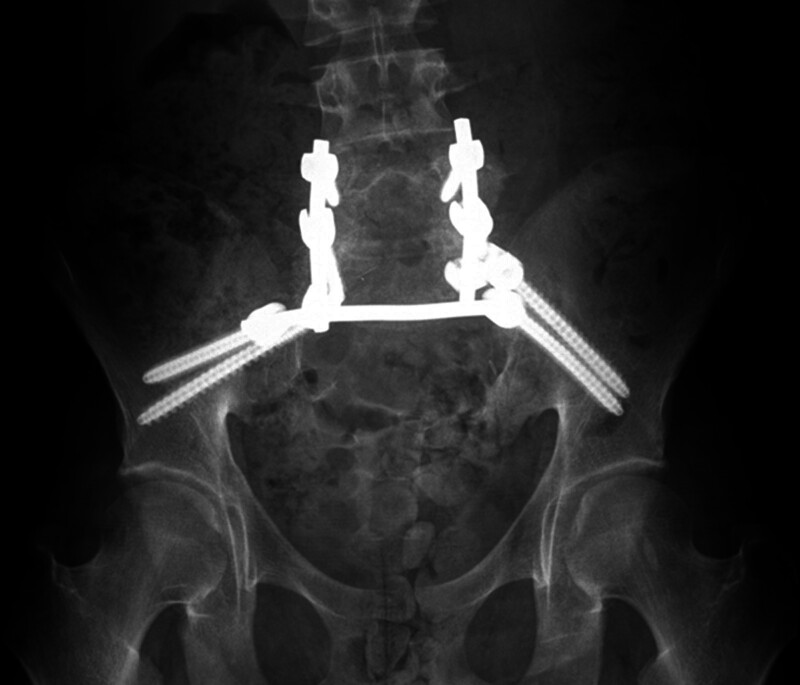
The patient initially underwent an anterior resection of the tumor with posterior spinal arthrodesis and fusion using titanium hardware (Fig. 1). Postoperatively, the patient continued to endorse bowel and bladder incontinence and paresthesias of the lower back, buttocks, and along the posterior aspect of the lower extremities, bilaterally.
Fig. 1.
Preoperative imaging demonstrating existing titanium hardware that was subsequently removed and replaced with carbon fiber hardware.
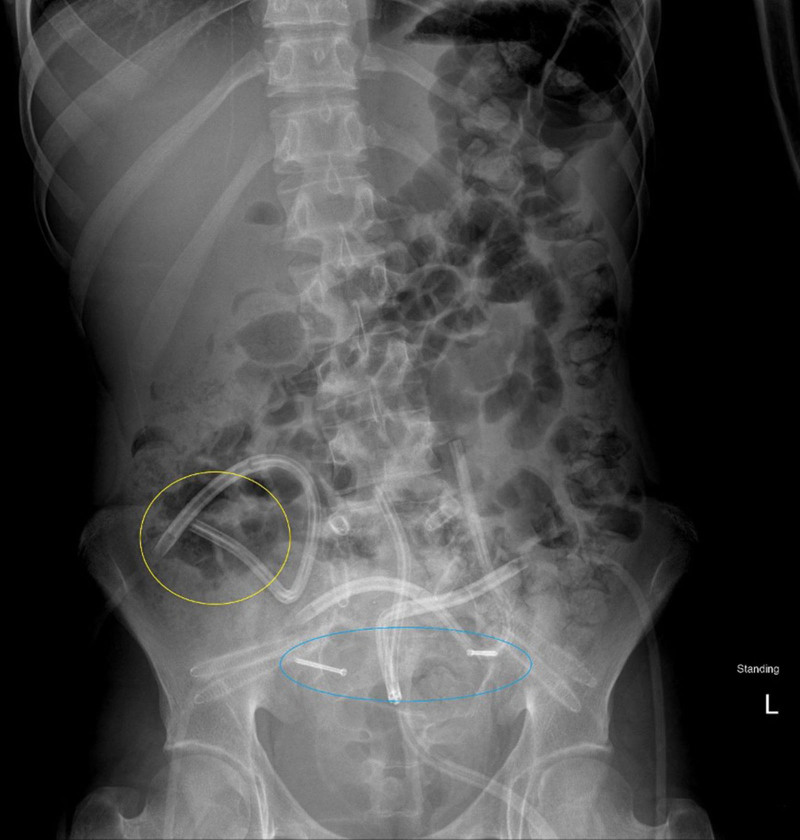
After the procedure, radiation oncology recommended postoperative proton beam therapy for local control and improved long-term survival. Current literature indicates that patients with titanium hardware have reduced local control rates due to scatter that results in poor imaging resolution and poor modeling of the proton dose. Nonmetallic hardware reduces hardware scatter and improves modeling of proton dose, which improves treatment efficacy.1 As such, the patient and his parents consented to the removal of prior titanium hardware, replacement with carbon fiber instrumentation, and IC-VBG to the spine to reinforce the sacral defect and introduce healthy, vascularized tissue into the region of the tumor resection (Figs. 2, 3).
Fig. 2.
Postoperative imaging that shows the carbon fiber hardware in place, with drains, and the vascularized iliac bone graft taken from the right hip (yellow) and secured transversely across the sacrum with 2 screws (blue).
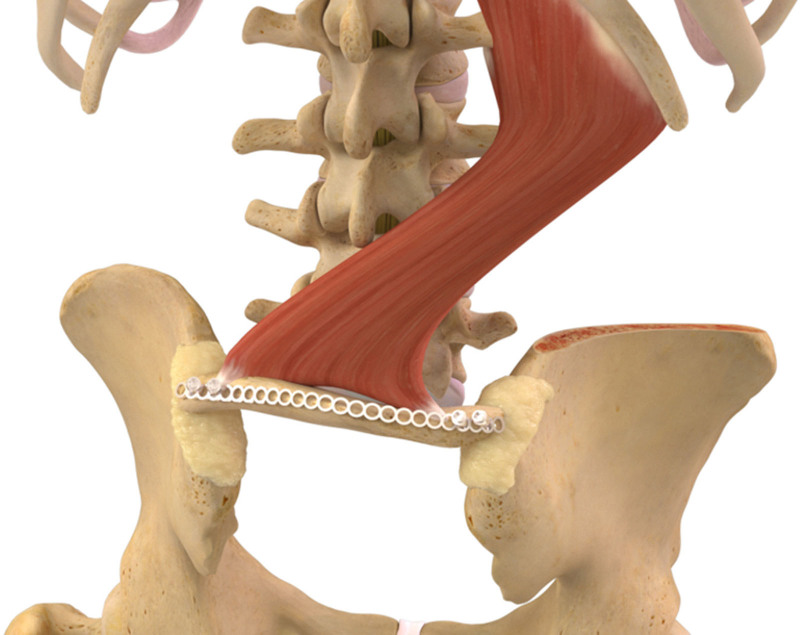
Fig. 3.
Illustration of the vascularized iliac crest bone graft in place and secured over the sacral deficiency.
SURGICAL TECHNIQUE
The previous hardware was removed using a posterior approach followed by new pedicle carbon screws placed at L3, L4, and L5, along with 2 iliac screws at S1 and S2. The fusion was completed using 2 carbon rods and a bridging carbon rod between the iliac screws. Subsequently, dissection of the right iliac crest was performed. The dorsal attachments of the iliac crest were dissected, preserving the quadratus lumborum muscle, which served as the vascular pedicle. Subperiosteal dissection was taken to minimize the risk of damaging the cluneal nerve. A 2 × 6 cm bicortical segment of posterior iliac crest was harvested, 1 cm from the posterior superior iliac crest, with careful attention to preserve the quadratus lumborum muscle attachments. A transmuscular tunnel was dissected under the multifidus and longissimus muscles, extending from the transverse processes of the lumbar spine to the lateral aspect of the quadratus lumborum. The graft was detached from the iliac crest and rotated beneath the lumbar paraspinal muscles. Indocyanine green dye was intravenously administered, and the vascularity of the graft was visualized using the SPY Elite machine (Stryker, Kalamazoo, MI). The medullary surface of the bone graft was placed in direct contact with the decorticated surfaces of the spine to address bony deficiency in the sacrum and secured with 2 titanium screws (Fig. 3). Additional allograft bone was placed around the graft. Paraspinous muscle flaps provided durable soft-tissue coverage over the construct, and the skin was closed using 2 surgical drains, 1 deep and 1 in the subcutaneous plane. Postoperatively, the patient progressed well and mobilized using a thoracolumbosacral orthosis brace, and no significant complications were reported.
DISCUSSION
The role of plastic surgery in the field of spinal surgery is evolving.2 Historically, plastic surgery played a limited role in spinal wound management.3 However, due to the expanding indications for spinal surgery, the plastic surgeon can play a large role in addressing complications of spinal surgery.3 Postoperative wound complications can occur in up to 40% of complicated patients.4 Patients such as ours who undergo resection of portions of the osseous spine may have a compromised recipient bed due to radiation therapy, resulting in the need for complex spinal reconstruction.5 The field of spinoplastic surgery is characterized by the use of vascularized bone grafts (VBGs).2 VBGs have superior outcomes compared to their nonvascularized and allogenic counterparts due to their excellent blood supply, as they supply fully vascularized bone to the recipient site without microsurgical techniques or pedicle dissection.6 Various techniques have been described using VGBs from the vertebral spinous processes, scapula, occiput, ribs, and the iliac crest.2 There is an increasing body of evidence suggesting that the inclusion of VBGs enhances bony union secondary to osteogenic, osteoinductive, and osteoconductive properties.7
Ependymomas are neuroepithelial tumors that may occur in the central canal of the spinal cord.1 Extradural MPEs are rare and can involve the sacral bone in various locations.8 Clinically, 73% of these patients present with back pain but can also present with more localized pain due to sacral erosion.9 Patients can also present with limb weakness, radiculopathy, or sensory impairment.9 Our patient’s main complaints were urinary incontinence, paresthesia, decreased reflexes, and decreased sensation in the left lower extremity. The imaging modality of choice for MPE is magnetic resonance imaging with and without contrast.10 Adjuvant radiotherapy may not significantly decrease recurrence rates but has shown significantly longer 10-year progression-free survival.1 As such, our patient was advised to pursue postoperative proton therapy. Surgical resection is the mainstay of treatment for MPEs.1 The extent of resection is the most important prognostic factor,1 with gross total resection demonstrating lower recurrence rates.6 Achieving gross total resection is difficult due to close adhesion to nerve roots, and production of myxoid matrix and can leave large bony defects requiring stabilization.1
Potential complications of this procedure must be discussed. Common donor site harvest pain stems from superior cluneal nerve injury. Submuscular dissection decreases the risk of injury to this nerve. Dissection of the ventral iliac wing and quadratus muscle also poses a potential risk to retroperitoneal structures such as the iliac vessels and the ureters. By using blunt dissection in a strictly subperiosteal plane on the crest and under the superior cluneal nerve branches, this complication can be avoided.
CONCLUSIONS
IC-VBG has proven effective in spinoplastic reconstruction. It enhances the potential for solid bony union and offers a practical alternative to free bone transfer. This technique follows consistent anatomical principles, facilitating a relatively straightforward harvest within the operative field while minimizing donor site complications. Furthermore, it allows for the use of a living autograft in spinoplastic reconstruction, eliminating the need for the microsurgical anastomosis typically associated with free bone transfers. This approach provides a valuable option and a potential salvage strategy, making it an essential tool for reconstructive surgeons working with spine surgery colleagues in this region.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
ACKNOWLEDGMENTS
The authors express their deepest gratitude to all contributing authors and Dr. Marco Innocenti from the Rizzoli Orthopaedics Institute in Bologna, Italy, and Dr. Elisabetta Pataia from the Department of Orthopedics and Traumatology at the Università Cattolica del Sacro Cuore in Rome, Italy, for their invaluable input and dedication to this project. Their expertise, insights, and collaboration were essential in shaping the depth and breadth of this work.
Footnotes
Published online 25 November 2024.
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.Rudà R, Bruno F, Pellerino A, et al. Ependymoma: evaluation and management updates. Curr Oncol Rep. 2022;24:985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeger JL, Simoni A, Shvedova M, et al. Spino-plastic surgery: a literature review of vascularized bone grafts and their uses in spine reconstruction. Orthoplast Surg. 2023;14:15–22. [Google Scholar]
- 3.Do A, Davis MJ, Abu-Ghname A, et al. The historical role of the plastic surgeon in spine reconstruction. Semin Plast Surg. 2021;35:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen LE, Fullerton N, Mundy LR, et al. Optimizing successful outcomes in complex spine reconstruction using local muscle flaps. Plast Reconstr Surg. 2016;137:295–301. [DOI] [PubMed] [Google Scholar]
- 5.Horn SR, Dhillon ES, Poorman GW, et al. Epidemiology and national trends in prevalence and surgical management of metastatic spinal disease. J Clin Neurosci. 2018;53:183–187. [DOI] [PubMed] [Google Scholar]
- 6.Bohl MA, Mooney MA, Catapano JS, et al. Pedicled vascularized bone grafts for posterior lumbosacral fusion: a cadaveric feasibility study and case report. Spine Deform. 2018;6:498–506. [DOI] [PubMed] [Google Scholar]
- 7.Reece EM, Raghuram AC, Bartlett EL, et al. Vascularized iliac bone graft for complex closure during spinal deformity surgery. Plast Reconstr Surg Glob Open. 2019;7:e2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández-de Thomas RJ, Amaral-Nieves N, De Jesus O, et al. Rare sacral extradural grade II ependymoma: a comprehensive review of literature. BMJ Case Rep. 2021;14:e246540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh MC, Kim JM, Kaur G, et al. Prognosis by tumor location in adults with spinal ependymomas. J Neurosurg Spine. 2013;18:226–235. [DOI] [PubMed] [Google Scholar]
- 10.Chiappa KH. Evoked Potentials in Clinical Medicine. 3rd ed. Raven Press; 1990. [Google Scholar]