Abstract
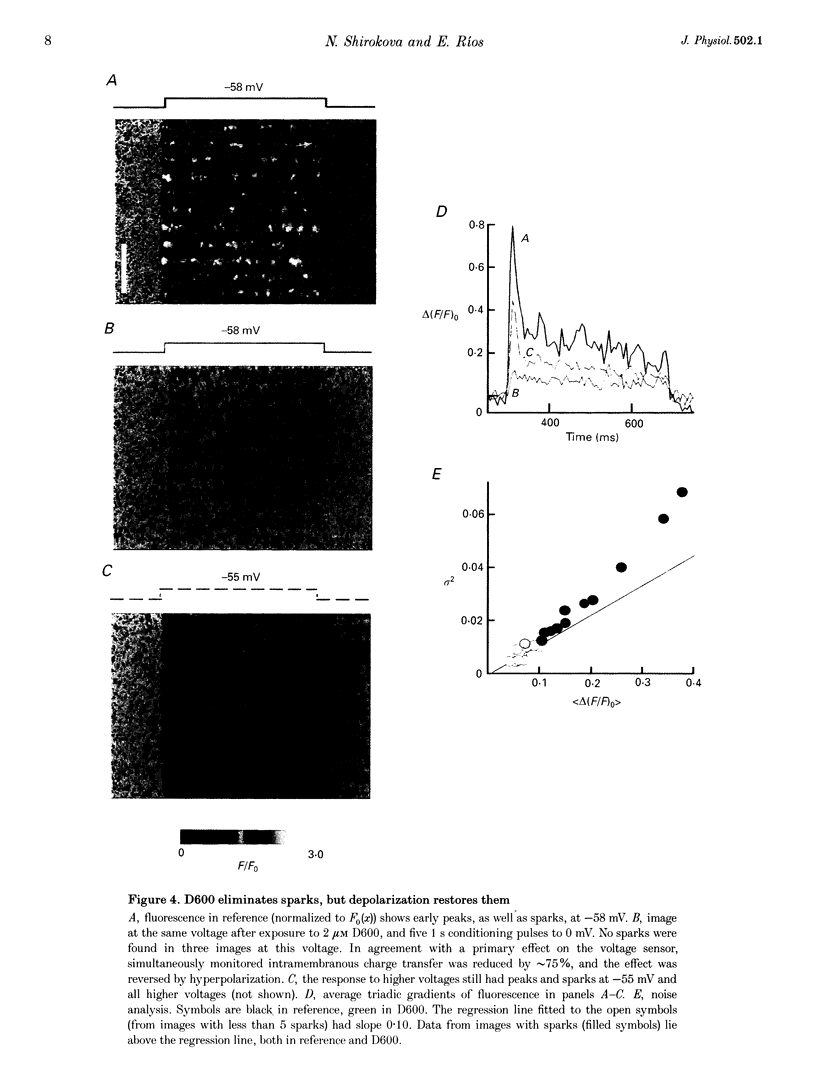
1. Fluo-3 fluorescence associated with Ca2+ release was recorded with confocal microscopy in single muscle fibres. Clamp depolarization to -65 or -60 mV elicited Ca2+ sparks with amplitudes and spatial widths distributed approximately normally, with mean values of 0.79 of resting fluorescence and 0.8 micron (S.D., 0.17 and 0.2 micron; n = 193), respectively. Given these distributions, events of amplitude less than 0.45 or width less than 0.4 micron are unlikely to be sparks. 2. Low voltage depolarization (-72 mV) elicited only one spark per triad every 6 s, but generated a relative increase in fluorescence at triads of 0.05. This increase must therefore have been due to events smaller than sparks. 3. The variance/mean ratio of triadic fluorescence gradients averaged 0.11 at low voltages and increased severalfold at the higher voltages at which sparks appeared, indicating the existence of at least two event amplitudes. 4. Tetracaine (200 microM) reversibly abolished sparks and the early peak of Ca2+ release at all voltages. In its presence, discrete events were smaller than the spark criterion, and triadic gradients had a variance/mean ratio of 0.11. 5. The phenylalkylamine D600 (2 microM) reduced release at all voltages, abolishing sparks and the peak of Ca2+ release at low but not at high voltages. 6. The parallel abolition of all sparks and the peak of Ca2+ release indicates that both phenomena are activated by Ca2+. The restoration of sparks by voltage in D600 suggests that release in small events provides the trigger Ca2+ for activation of sparks.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Art J. J., Goodman M. B. Rapid scanning confocal microscopy. Methods Cell Biol. 1993;38:47–77. doi: 10.1016/s0091-679x(08)60999-1. [DOI] [PubMed] [Google Scholar]
- Blatter L. A., Hüser J., Ríos E. Sarcoplasmic reticulum Ca2+ release flux underlying Ca2+ sparks in cardiac muscle. Proc Natl Acad Sci U S A. 1997 Apr 15;94(8):4176–4181. doi: 10.1073/pnas.94.8.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block B. A., Imagawa T., Campbell K. P., Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988 Dec;107(6 Pt 2):2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemond V., Csernoch L., Klein M. G., Schneider M. F. Voltage-gated and calcium-gated calcium release during depolarization of skeletal muscle fibers. Biophys J. 1991 Oct;60(4):867–873. doi: 10.1016/S0006-3495(91)82120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M. G., Cheng H., Santana L. F., Jiang Y. H., Lederer W. J., Schneider M. F. Two mechanisms of quantized calcium release in skeletal muscle. Nature. 1996 Feb 1;379(6564):455–458. doi: 10.1038/379455a0. [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Submicroscopic calcium signals as fundamental events of excitation--contraction coupling in guinea-pig cardiac myocytes. J Physiol. 1996 Apr 1;492(Pt 1):31–38. doi: 10.1113/jphysiol.1996.sp021286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Zang W. J., Wier W. G. Ca2+ sparks involving multiple Ca2+ release sites along Z-lines in rat heart cells. J Physiol. 1996 Nov 15;497(Pt 1):31–38. doi: 10.1113/jphysiol.1996.sp021747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro G., Csernoch L., Uribe I., Ríos E. Differential effects of tetracaine on two kinetic components of calcium release in frog skeletal muscle fibres. J Physiol. 1992 Nov;457:525–538. doi: 10.1113/jphysiol.1992.sp019392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratusevich V. R., Balke C. W. Factors shaping the confocal image of the calcium spark in cardiac muscle cells. Biophys J. 1996 Dec;71(6):2942–2957. doi: 10.1016/S0006-3495(96)79525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E., Stern M. D. Calcium in close quarters: microdomain feedback in excitation-contraction coupling and other cell biological phenomena. Annu Rev Biophys Biomol Struct. 1997;26:47–82. doi: 10.1146/annurev.biophys.26.1.47. [DOI] [PubMed] [Google Scholar]
- Shirokova N., Pizarro G., Ríos E. A damped oscillation in the intramembranous charge movement and calcium release flux of frog skeletal muscle fibers. J Gen Physiol. 1994 Sep;104(3):449–476. doi: 10.1085/jgp.104.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. D. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992 Aug;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. F. Inferences about membrane properties from electrical noise measurements. Biophys J. 1972 Aug;12(8):1028–1047. doi: 10.1016/S0006-3495(72)86141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sárközi S., Szentesi P., Cseri J., Kovács L., Csernoch L. Concentration-dependent effects of tetracaine on excitation-contraction coupling in frog skeletal muscle fibres. J Muscle Res Cell Motil. 1996 Dec;17(6):647–656. doi: 10.1007/BF00154059. [DOI] [PubMed] [Google Scholar]
- Tsugorka A., Ríos E., Blatter L. A. Imaging elementary events of calcium release in skeletal muscle cells. Science. 1995 Sep 22;269(5231):1723–1726. doi: 10.1126/science.7569901. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Egan T. M., López-López J. R., Balke C. W. Local control of excitation-contraction coupling in rat heart cells. J Physiol. 1994 Feb 1;474(3):463–471. doi: 10.1113/jphysiol.1994.sp020037. [DOI] [PMC free article] [PubMed] [Google Scholar]