Abstract
Merozoite surface protein 4 (MSP4) of Plasmodium falciparum is a glycosylphosphatidylinositol-anchored integral membrane protein of 272 residues that possesses a single epidermal growth factor (EGF)-like domain near the carboxyl terminus. We have expressed both full-length MSP4 and a number of fragments in Escherichia coli and have used these recombinant proteins to raise experimental antisera. All recombinant proteins elicited specific antibodies that reacted with parasite-derived MSP4 by immunoblotting. Antibody reactivity was highly dependent on the protein conformation. For example, reduction and alkylation of MSP4 almost completely abolished the reactivity of several antibody preparations, including specificities directed to regions of the protein that do not contain cysteine residues and are far removed from the cysteine-containing EGF-like domain. This indicated the presence of conformation-dependent epitopes in MSP4 and demonstrated that proper folding of the EGF-like domain influenced the antigenicity of the entire molecule. The recombinant proteins were used to map epitopes recognized by individuals living in areas where malaria is endemic, and at least four distinct regions are naturally antigenic during infection. Binding of human antibodies to the EGF-like domain was essentially abrogated after reduction of the recombinant protein, indicating the recognition of conformational epitopes by the human immune responses. This observation led us to examine the importance of conformation dependence in responses to other integral membrane proteins of asexual stages. We analyzed the natural immune responses to a subset of these antigens and demonstrated that there is diminished reactivity to several antigens after reduction. These studies demonstrate the importance of reduction-sensitive structures in the maintenance of the antigenicity of several asexual-stage antigens and in particular the importance of the EGF-like domain in the antigenicity of MSP4.
Malaria infection of humans, particularly that due to Plasmodium falciparum, is one of the most widespread infectious diseases in the tropics and exacts an enormous public health burden of both deaths and economic loss due to illness. The incidence of clinical cases and deaths is increasing because of the decreasing effectiveness of specific chemotherapy and vector control programs. Although alternative control measures such as impregnated bed nets show some promise, it is generally agreed that an effective subunit vaccine would be an important advance in combating this disease (20). Current evidence suggests that such a vaccine will contain multiple proteins from all stages of parasite development, including the asexual blood stage, and considerable effort is being devoted to identification of asexual-stage proteins that would induce host-protective responses (2, 20). Since a major component of natural immunity to the asexual stage in humans is antibody, the appropriate vaccine components are likely to be the exposed proteins of the parasite, such as merozoite surface proteins (MSPs), rhoptry proteins, and proteins on the infected-erythrocyte surface (2, 20).
Integral membrane proteins of the merozoite surface that appear to be targets of protective immune responses include MSP1 (21), MSP2 (33), apical membrane antigen 1 (AMA1) (28), and the 175-kDa erythrocyte binding antigen (EBA175) (13). Immune responses to these antigens have been shown to interfere with merozoite invasion in vitro and in some cases to offer protection from infection in animal models (2). One of the best-studied MSPs is MSP1, a large protein that undergoes a series of processing events to yield a number of fragments that associate with the merozoite surface (6, 9). Of these, the carboxyl-terminal 19-kDa fragment, which contains two epidermal growth factor (EGF)-like domains, remains on the surface of the invading merozoite and is carried into the newly invaded erythrocytes (5, 7). Antibodies directed against this region are capable of interfering with invasion (5, 8, 14), animals actively immunized with this region are protected against subsequent challenge (12, 23, 24), and naturally acquired antibodies to this region are associated with clinical immunity to P. falciparum malaria (19).
Several members of this group of MSPs contain highly conserved cysteine residues that are found in all allelic variants of these antigens identified in field isolates. These cysteines are apparently involved in maintaining the tertiary structure of these proteins, and protective antibodies are preferably induced by correctly conformed protein. This has been well demonstrated with MSP1 and AMA1, where denatured protein does not induce the same level of protective immunity as nondenatured protein (17, 18, 24). This is also likely to be the case with EBA175, which is extremely rich in cysteine residues and intramolecular disulfide bonds (31). This question has not been studied in the case of MSP2, although it should be noted that the mature protein contains a pair of cysteine residues in a completely conserved region of the carboxyl terminus (33).
MSP4 is a newly identified MSP, with an observed molecular mass of 40 kDa, present in all isolates of P. falciparum so far examined (25). Nucleotide sequencing studies revealed that the predicted protein contains both a hydrophobic signal sequence and a signal for glycosylphosphatidylinositol (GPI) attachment. GPI attachment was confirmed by biosynthetic labeling studies which revealed that myristic acid is incorporated into MSP4. Phase separation experiments showed that the mature protein is partitioned into the Triton X-114-soluble fraction, a membrane fraction in which AMA1 and MSP2 are also found (16, 33), and immunofluorescence localization studies revealed a staining pattern typical of MSPs. Of particular interest is the presence of a single EGF-like domain in the carboxyl terminus of the protein which shows the typical spacing of cysteine residues observed in MSP1 but in which the intervening residues are quite dissimilar (25). We set out to examine the structural and antigenic properties of MSP4, particularly with respect to the EGF-like domain. We determined that this region is crucial for the proper conformation of the entire protein and that antibody reactivity of some sera to the protein is greatly reduced when the EGF-like domain is disrupted, even in regions of the protein that do not participate in intramolecular disulfide bonds. The protein is immunogenic in laboratory animals, and several regions of the protein are naturally antigenic during malaria infection of humans. The reactivity of human antisera is also strongly influenced by the correct folding of the EGF-like domain. We examined the conformational dependence of the human antibody response to other membrane-associated proteins of the parasite and found that antibody reactivity to several of these antigens is markedly reduced under conditions that disrupt disulfide bonds.
MATERIALS AND METHODS
Parasites.
P. falciparum parasites were cultured in vitro by standard procedures (37). Infected erythrocytes were harvested from asynchronous cultures, and the parasites were isolated by lysis with 0.15% saponin, washed with phosphate-buffered saline (30), and stored at −70°C until required. The sequence of MSP4 in AA01 is identical to that in D10 with the exception of an Asp→Gly substitution in fragment D.
Construction of recombinant plasmids to express different parts of MSP4.
Fragments of the MSP4 sequence were either amplified by PCR with P. falciparum D10 cDNA as template (fragments A, C, D, and E) or generated from a w2mef cDNA clone (fragment B) (25). The sequence of MSP4B in w2mef is identical to that in D10 except for a single glycine residue deletion (25). Primers contained restriction sites, and the inserts were digested with restriction endonucleases and ligated into appropriately cut pGEX vectors (AMRAD Pharmacia Biotech, Melbourne, Victoria, Australia) or pTrcHis vector (Invitrogen, Carlsbad, Calif.). The recombinant plasmids were transformed into Escherichia coli BL21 (Novagen, Milwaukee, Wis.) for protein expression and entirely sequenced to confirm cloning in the correct reading frame and the absence of mutations. The expression constructs are designated A, B, C, D, and E, and their relative positions are shown in Fig. 1.
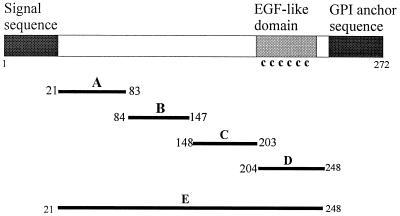
FIG. 1.
(Top) Structure of MSP4 and positions of the expression constructs. Black boxes at the left and right represent the signal sequence and the GPI anchor sequence, respectively; the shaded box indicates the EGF-like domain which contains the six cysteine residues. A, B, C, and D are four MSP4 fragments cloned in pGEX vectors, and E is the full-length MSP4 lacking signal and anchor sequences cloned in both pGEX and pTrcHis vectors. The first and last amino acid residues of MSP4 included in each recombinant protein are indicated. (Bottom) Coomassie blue-stained SDS-PAGE gel showing the five recombinant MSP4 GST fusion proteins GST-MSP4A (lane A), GST-MSP4B (lane B), GST-MSP4C (lane C), GST-MSP4D (lane D), and GST-MSP4E (lane E) and the hexahistidine fusion of full-length MSP4 (lane E-His). As a control, GST is included. Positions of molecular mass standards (kilodaltons) are shown at the left.
Expression and purification of fusion proteins.
Expression of fusion proteins was induced with 2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (Progen Industries Limited, Darra, Queensland, Australia). The glutathione S-transferase (GST) fusion proteins were purified by affinity chromatography on glutathione agarose (Sigma Chemical Company, St. Louis, Mo.) and eluted with 10 mM reduced glutathione in 50 mM Tris-HCl, pH 9.6 (32). The hexahistidine fusion was purified with TALON metal affinity resin under native conditions by using a batch-gravity flow column purification procedure according to the manufacturer’s instructions (Clontech Laboratories, Palo Alto, Calif.). The purity and integrity of the fusion proteins were assessed by Coomassie blue staining of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, and the concentration was measured by the Bio-Rad (Hercules, Calif.) protein assay according to the manufacturer’s instructions. A control GST was purified from E. coli BL21 transformed with the pGEX vector alone.
Human and animal antisera.
For production of antisera to recombinant MSP4 fragments, female New Zealand White rabbits were used for immunization. For each recombinant protein, two rabbits were injected subcutaneously with 100 μg of GST fusion protein in complete Freund’s adjuvant (Difco Laboratories, Detroit, Mich.), followed by monthly boosting with 50 μg of GST fusion protein in incomplete Freund’s adjuvant (Difco Laboratories). The antibody reactivity was tested by immunoblotting with parasite-derived MSP4. Rabbit antiserum S508 was prepared by multiple immunization with full-length MSP4 expressed in VR1020 (Vical Industries, San Diego, Calif.) followed by a single boost of 50 μg of the hexahistidine fusion of full-length MSP4 (38). Human immune sera were obtained with informed consent from residents of the Madang region of Papua New Guinea, and a pool of sera was made from 22 individuals with documented exposure to P. falciparum infection (25). The antibodies to MSP2 and MSP5 (26) were raised in our laboratory, and the antibody to MSP119 (monoclonal antibody [MAb] 4H9/19) (15) was kindly provided by Juan Cooper (Queensland Institute of Medical Research, Brisbane, Australia).
SDS-PAGE and immunoblotting.
Protein preparations were either untreated (nonreduced), reduced, or reduced and alkylated. Under nonreducing conditions, proteins were solubilized in SDS sample buffer (125 mM Tris-HCl [pH 6.8], 4% [wt/vol] SDS) before being loaded for SDS-PAGE. To obtain reducing conditions, 50 mM dithiothreitol (DTT) was included in the sample buffer to disrupt the disulfide bonds (14). Proteins were reduced and alkylated by first being treated with DTT, followed by the addition of iodoacetic acid to a final concentration of 50 mM (17). Proteins were fractionated by SDS-PAGE, and the gel was either stained with Coomassie blue or electrophoretically transferred to nitrocellulose for immunoblotting as previously described (25). Primary antibody binding was detected with either anti-rabbit or anti-human immunoglobulin conjugated with horseradish peroxidase (Silenus Laboratories, Melbourne, Victoria, Australia), as appropriate, followed by development with Renaissance chemiluminescence reagent (NEN Life Science Products, Boston, Mass.).
Preabsorption of antisera and thrombin cleavage of GST carrier.
For depletion of the antibody reactivity to GST, the rabbit antisera raised with recombinant MSP4 fragments were diluted in 5% bovine serum albumin in 0.05 M Tris-HCl (pH 7.4)–0.15 M NaCl–0.05% (vol/vol) Tween 20 and incubated with 50 μg of purified GST per ml at 4°C overnight. For depletion of the human antibody reactivity to fragments of MSP4, diluted human sera were incubated with 50 μg of the appropriate recombinant MSP4 fragment per ml at 4°C overnight. To isolate the recombinant MSP4 fragments A and B from the GST carrier, 50 μg of the GST fusion proteins was incubated with thrombin in 100 μl of cleavage buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 2.5 mM CaCl2) at 25°C for 2 h.
Triton X-114 partitioning.
To enrich for membrane-associated proteins, a Triton X-114 partitioning experiment was performed as described previously (34). Briefly, P. falciparum AA01 parasites were lysed in the presence of 0.5% Triton X-114 (Sigma Chemical Company), the parasite lysate was centrifuged to remove insoluble material, and the supernatant was loaded onto a cushion of 6% sucrose in 0.06% Triton X-114. Phase separation was conducted by incubation at 37°C for 5 min followed by centrifugation at 500 × g for 5 min. The Triton X-114-enriched layer was washed three times with PBS prior to processing.
ELISA.
The reactivity of human immune sera with recombinant MSP4D was tested by indirect enzyme-linked immunosorbent assay (ELISA) as described by others (19) with some modifications. Briefly, microtiter plates (Immulon 2; Dynatech Laboratories, Chantilly, Va.) were coated with either MSP4D-GST fusion protein or a GST control overnight at 4°C. Serum was diluted to 1/500 and tested in duplicate on both antigens. After being washed, the plates were further incubated with alkaline phosphatase-conjugated goat anti-human immunoglobulin (Silenus Laboratories) and developed with p-nitrophenyl phosphate (Sigma Chemical Company). The optical density (OD) was read at 405 nm, and the OD value for the GST control was subtracted from that for the fusion protein to obtain a specific OD for the response to MSP4D. Antibody-positive sera are defined as those giving an OD above the normal range (mean + 3 standard deviations for the ODs of 30 control sera from adults not exposed to malaria).
To assess the importance of conformational epitopes maintained by disulfide bonds, the MSP4D-GST protein was reduced and alkylated by incubation with 50 mM DTT at 37°C for 30 min, followed by addition of 50 mM iodoacetic acid (17). The treated protein was used to coat the microtiter plates and tested in parallel with the nonreduced protein.
RESULTS
Construction of various MSP4 fusion proteins.
In order to study the antigenicities of different regions of MSP4, a number of constructs were designed for expression of MSP4 in E. coli as full-length mature protein or as protein fragments (Fig. 1). A, B, C, and D are fragments each spanning approximately one-quarter of the mature molecule, whereas E encompasses the entire MSP4 sequence lacking only the signal and anchor sequences. There is no sequence homology between various fragments; the EGF-like domain which contains six cysteine residues is located in the D fragment. The margins of this fragment were designed to maintain the same relative spacings that had been used successfully in expression of the EGF-like domains of MSP1 (14). These five sequences were cloned into the fusion vector of pGEX to produce GST fusion proteins, and the resulting proteins were purified from cell extracts by affinity chromatography on glutathione agarose followed by elution with reduced glutathione. The results showed that all four MSP4 fragments were produced as abundant, soluble fusion proteins as judged by SDS-PAGE, whereas the full-length MSP4 was obtained in lower yield with a number of prominent smaller bands (Fig. 1). These GST fusion proteins are designated GST-MSP4A, GST-MSP4B, GST-MSP4C, GST-MSP4D, and GST-MSP4E. The calculated molecular masses of these fusion proteins are ∼34, ∼34, ∼33, ∼32, and ∼53 kDa, respectively; the molecular mass of GST alone is about 27 kDa. MSP4 in parasites has an observed molecular mass of 40 kDa on SDS-PAGE, which is much higher than would be predicted, a phenomenon often noted for malaria antigens (1). Thus, the major band in GST-MSP4E at ∼50 kDa is unlikely to be the full-length product. We interpret it to be one of a number of partially degraded expression products which include the smaller bands. The higher band at about 75 kDa is likely to be a contaminating E. coli protein copurified with the recombinant fusion protein, as it was not recognized by antibodies directed to MSP4 (data not shown).
In order to produce undegraded full-length MSP4, other expression systems were tried, one of which was the pTrcHis vector in E. coli. We designed a 3′ primer containing a hexahistidine sequence and cloned the resultant PCR product into a pTrcHisA vector from which the sequence encoding the N-terminal hexahistidine tag had been removed. The expressed product contained a carboxy-terminal hexahistidine tag which favored purification of full-length product. This fusion is designated MSP4E-His, and it contains two closely migrating products running at about 40 kDa on SDS-PAGE (Fig. 1B). N-terminal sequencing revealed that the higher band is a full-length product and the lower one is a truncated form lacking 21 amino acid residues (data not shown). This fusion protein was used in subsequent experiments.
Immunogenicity of recombinant MSP4 in laboratory animals.
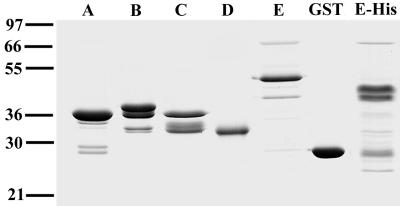
Recombinant GST fusion proteins corresponding to different parts of MSP4 were used to immunize rabbits, and the resultant antisera were tested for specific reactivity with MSP4 by immunoblot analysis. All four fusion proteins elicited antibodies recognizing both recombinant and parasite-derived MSP4. In particular, they all reacted with the expected 40-kDa protein in parasite lysates. These antisera were tested with parasites from several strains, including D10 and AA01, and the reactivities were identical. Prebleed rabbit sera did not react with a 40-kDa band or with other parasite proteins (data not shown). Figure 2 shows the reactivities of antisera to recombinant MSP4 with AA01 parasite lysates that were either nonreduced, reduced, or reduced and alkylated. Treatment of the lysates with reducing agents resulted in a small decrease in the mobility of MSP4 as measured by SDS-PAGE. This shift in mobility of reduced proteins has also been noted in studies on the carboxyl-terminal 19-kDa fragment of MSP1 and presumably reflects changes consequent to disruption of intramolecular disulfide bonds in the EGF-like domain (11, 14). The MSP4A antisera recognized a single band in all three parasite preparations (Fig. 2A), a feature shared by all of the other antisera raised against recombinant fragments, including anti-MSP4D (Fig. 2B) and anti-MSP4B and anti-MSP4C (data not shown). Previous experiments have demonstrated that antibodies to GST do not react with malaria parasites, either by immunoblotting or by immunofluorescence (25).
FIG. 2.
Reactivities of antisera raised to recombinant MSP4 fragments against parasite lysates. AA01 parasite extracts were either untreated (lanes 1), reduced (lanes 2), or reduced and alkylated (lanes 3) prior to SDS-PAGE and transferred to nitrocellulose. The blots were probed with rabbit antiserum raised to recombinant MSP4A (A) and rabbit antiserum raised to recombinant MSP4D (B). Positions of molecular mass standards (kilodaltons) are shown at the left.
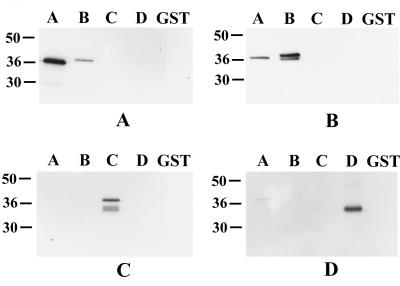
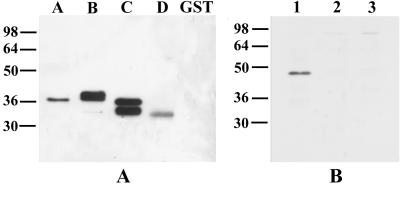
The fine mapping of the specificity of the antibodies raised to the different regions of MSP4 was performed by reacting them against a panel of the various constructs of MSP4. Reactivity against GST was depleted by extensive absorption, and GST was included as a control in the immunoblots. Figure 3 shows that immunization produced specific antibodies that recognized the immunizing fragment. The multiple bands seen in MSP4B and MSP4C correspond to those seen in samples of the purified fusion proteins (Fig. 1) and are probably due to partial degradation of the product. There was clear evidence of cross-reactivity between antisera to MSP4A and MSP4B, which could not be the result of anti-GST reactivity (Fig. 3). It was possible that cross-reactive antibodies were directed to the fusion region, i.e., to an epitope composed of a combination of GST and MSP4 sequences. To test this possibility, recombinant proteins MSP4A and MSP4B were pretreated with thrombin to cleave the proteins at the fusion junctions, and the digestion products were subjected to immunoblot analysis. Both antisera recognized the isolated MSP4A and MSP4B fragments (data not shown), demonstrating that cross-reactive antibodies were directed to authentic MSP4 sequences.
FIG. 3.
Specificities of the antibodies raised to different regions of MSP4. Equivalent amounts (0.01 μg) of recombinant GST-MSP4A (lanes A), GST-MSP4B (lanes B), GST-MSP4C (lanes C), GST-MSP4D (lanes D), and GST alone were separated by SDS-PAGE, transferred to nitrocellulose, and then probed with the antibodies raised to GST-MSP4A (A), GST-MSP4B (B), GST-MSP4C (C), and GST-MSP4D (D). All of the antisera were preabsorbed against GST. Positions of molecular mass standards (kilodaltons) are given at the left in each panel.
Influence of reduction on the antigenicity of MSP4.
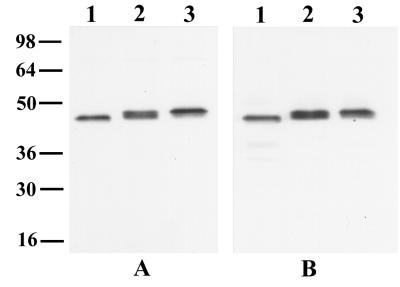
The requirement for correct protein conformation has been shown to be an essential part of protective immunity to MSPs such as MSP1 and AMA1. We set out to determine whether there was evidence for a similar requirement for MSP4 by raising antisera that may recognize conformational epitopes of MSP4. To do this, we made use of the recently described procedure of DNA vaccination and raised antisera in rabbits to full-length MSP4 by a combination of priming with plasmid DNA and boosting with recombinant protein. The resulting antisera were used to examine the importance of the redox state for the antigenicity of MSP4 by reaction with parasite lysates as well as various recombinant fragments of MSP4 under nonreducing or reducing conditions (Fig. 4). The antisera clearly recognized recombinant fragments MSP4A, MSP4B, MSP4C, and MSP4D irrespective of whether the proteins were reduced and alkylated (Fig. 4A) or untreated (data not shown). The intensity of the reactivity to fusion proteins was similar regardless of the redox state, and this experiment demonstrated that these sera reacted with at least three distinct regions of the protein. When the antisera were tested with parasite lysates that had been electrophoresed under nonreducing conditions, under reducing conditions, or after reduction and alkylation, it was surprising that although the antibodies reacted with nonreduced MSP4, there was no reactivity to either reduced or reduced and alkylated parasite material (Fig. 4B). Identical results were obtained with sera from mice immunized and boosted with the MSP4 DNA construct (data not shown). The weak higher-molecular-mass band at ∼80 kDa in lanes 2 and 3 of Fig. 4B is not likely to be a dimer of MSP4, as it is not recognized by other antisera to MSP4, such as the rabbit antisera to MSP4B and MSP4C (data not shown) and MSP4D (Fig. 2B). Further, it is not present in lane 1, which contained nonreduced parasite material. We therefore interpret it to be a cross-reactive epitope which was exposed by the reducing agent. These results demonstrate that reduction-sensitive structures present in the native MSP4 molecule are the predominant structures of the protein recognized by antibodies to presumably native MSP4. As MSP4A, MSP4B, and MSP4C do not contain any cysteine residues, this experiment further demonstrates that the correct conformational arrangement of the EGF-like domain is crucial for the antigenicity of the entire protein, including regions that are not immediately adjacent in the primary structure.
FIG. 4.
Reactivity of an experimental antiserum to recombinant and parasite-derived MSP4. (A) Equivalent amounts (0.1 μg) of recombinant GST-MSP4A (lane A), GST-MSP4B (lane B), GST-MSP4C (lane C), GST-MSP4D (lane D), and GST alone were reduced and alkylated before being subjected to SDS-PAGE, transferred to nitrocellulose, and then probed with rabbit antibodies raised by a combination of priming with a DNA vaccine construct and boosting with MSP4E-His. (B) AA01 parasite extracts were either untreated (lane 1), reduced (lane 2), or reduced and alkylated (lane 3) before SDS-PAGE and transferred to nitrocellulose, and the blot was probed with the same antiserum. Positions of molecular mass standards (kilodaltons) are shown at the left in each panel.
Reactivity of the recombinant fusion proteins with human immune sera.
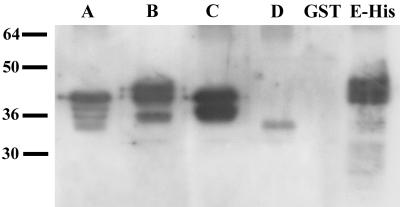
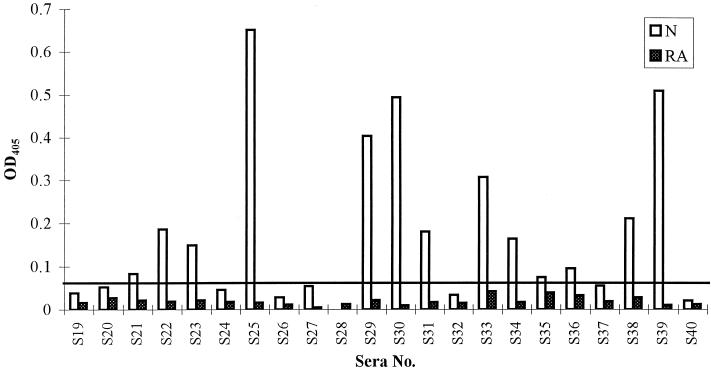
To assess whether these fusion proteins are in a configuration that can be recognized by human immune sera and to evaluate the human antibody responses to MSP4 during malaria infection, an equal amount of each fusion protein was subjected to SDS-PAGE under nonreducing conditions and transferred to nitrocellulose. The blot was probed with a pool of patient sera that had been preabsorbed against GST to deplete reactivity to the fusion partner. Figure 5 shows that all recombinant fragments of MSP4 were recognized to various degrees by the pooled sera, with reactivity against MSP4D being much weaker than the strong reactivity observed with fragments MSP4A, MSP4B, and MSP4C. Treatment of the recombinant proteins with a reducing agent did not affect the reactivity of human antisera with the latter three fragments but removed all detectable binding to MSP4D (data not shown). To confirm the reactivity of patient sera with the EGF-like domain, an ELISA with 22 individual patient sera was performed (Fig. 6). Thirteen of the 22 sera had detectable reactivity to MSP4D, showing ODs higher than the mean + 3 standard deviations for a panel of nonimmune sera. Reduction and alkylation of MSP4D led to the complete abolition of antibody reactivity (Fig. 6). These experiments demonstrated that several regions of MSP4 are immunogenic during natural infection and that the recombinant proteins of most parts of MSP4 can be produced in E. coli with the natural conformation of the antigen.
FIG. 5.
Reactivities of human immune sera to recombinant MSP4 fusion proteins. Equivalent amounts (0.1 μg) of recombinant GST-MSP4A (lane A), GST-MSP4B (lane B), GST-MSP4C (lane C), GST-MSP4D (lane D), GST alone, and MSP4E-His were separated by SDS-PAGE under nonreducing conditions, transferred to nitrocellulose, and then probed with a pool of human immune sera that had been preabsorbed against GST. Positions of molecular mass standards (kilodaltons) are given at the left.
FIG. 6.
ELISA examining the reactivities of 22 patient sera to MSP4D either as a nonreduced (N) or as a reduced and alkylated (RA) antigen. The graph shows the ODs at 405 nm (OD405) of duplicate wells for each serum sample diluted to 1/500. Thirty control sera from areas where malaria is not endemic were tested at the same dilution, and the mean value + 3 standard deviations is defined as the cutoff point (shown as a horizontal line).
The observed cross-reactivity between rabbit antisera to MSP4A and MSP4B complicated the interpretation of the number of distinct epitopes on MSP4 recognized by patient sera. To investigate this, we performed an experiment in which the same pooled human sera were extensively preabsorbed against MSP4B before reaction with MSP4A and vice versa. Strong reactivity to MSP4A was still detectable after the reactivity to MSP4B was removed. Similarly, preabsorption of human sera against MSP4A did not remove reactivity to MSP4B (data not shown). This clearly demonstrated that there are additional unique epitopes in MSP4 in these regions, over and above any possible cross-reactive epitopes. Thus, infection with P. falciparum is capable of inducing antibodies to at least four distinct epitopes in MSP4.
Reactivity of human sera with reduction-sensitive epitopes on membrane-associated antigens of the asexual blood stage parasites.
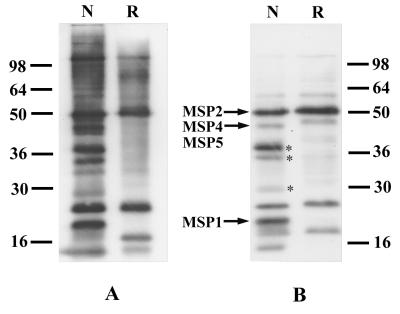
The profound decrease in reactivity of experimental sera to epitopes of MSP4 after reduction of the protein, coupled with previous observations of the importance of properly conformed MSP1 and AMA1 for antigenicity, led us to examine whether this may be an important general feature of the naturally induced immune response. Accordingly, we probed an immunoblot of P. falciparum asexual-stage parasite lysates treated in various ways with a pool of human immune sera. The parasite proteins were electrophoresed under either nonreducing or reducing conditions. There was a clear decrease in antigenic reactivity with proteins that had been reduced; however, the pattern was complex, and it was difficult to assign identities to individual proteins (Fig. 7A). To simplify interpretation, we performed Triton X-114 partitioning to enrich for membrane-associated proteins and surface antigens of asexual-stage parasites prior to treatment with the reducing agent. In the main such enrichment appears to be effective for proteins smaller than approximately 80 kDa (33, 34). Several proteins that showed considerable loss of antigenicity after reduction were identified; these included polypeptides of 38, 35, 30, and 19 kDa. Other polypeptides, such as those at 50 and 21 kDa, remained unchanged in reactivity after reduction (Fig. 7B). In order to assign identities to some of these proteins, an immunoblot was divided into multiple strips and probed individually with monospecific reagents to several proteins known or predicted to occur in the Triton X-114 detergent phase, including antibodies to MSP1, MSP2, MSP4, and MSP5. The 19-kDa band appears to be MSP119, which has been shown to possess reduction-sensitive epitopes (5), and the reduction-resistant band at 50 kDa is probably MSP2. In the range of 30 to 40 kDa, there are several reduction-sensitive bands. Due to the fact that MSP4 and MSP5 comigrated under the conditions used (26), it was difficult to determine whether MSP4 is one of them. Of note are a number of unidentified membrane-associated proteins with reduction-sensitive epitopes; the most prominent of these are the antigens at 38, 35, and 30 kDa.
FIG. 7.
Reduction-sensitive epitopes recognized by human sera on antigens of asexual-blood-stage parasites. (A) Reactivities of human sera to parasite proteins prepared under nonreducing (lane N) and reducing (lane R) conditions. (B) Reactivities of human sera to Triton X-114 phase-enriched parasite proteins under nonreducing (lane N) and reducing (lane R) conditions. The bands corresponding to MSP1, MSP2, MSP4, and MSP5 are indicated by arrows; the unidentified antigens at 38, 35, and 30 kDa with reduction-sensitive epitopes are indicated by asterisks. Positions of molecular mass standards (kilodaltons) are given in each panel.
DISCUSSION
This study provides the first evidence that the antigenicity of MSP4 is conformation dependent and that correct folding of the EGF-like domain is crucial for this. The sequence of MSP4 has been fully determined, and the only cysteine residues in the mature protein are the six residues located in the MSP4D region, which participate in the three disulfide bonds needed to stabilize the EGF-like domain. Therefore, treatments to reduce or to both reduce and alkylate this protein will directly affect only the MSP4D portion of the protein. The possibility that reduction may affect antigenicity through reaction with other residues such as histidine in fragments A, B, and C appears to be remote, as treatment of these isolated fragments does not diminish antigenicity. Correct folding of the EGF-like domains has been shown to be important for their antigenicity in MSP1 (11, 12, 14, 24). In particular, it has been shown that reduction of this domain leads to loss of reactivity of a number of conformation-dependent MAbs to this region (11, 14). Further, separation of the two EGF-like domains results in loss of some immunological specificities. Immunization with the two EGF-like domains as separate recombinant proteins results in an antibody response qualitatively different from that after immunization with both regions expressed in one protein (11, 12, 14). The unusual finding with MSP4 is that disruption of the EGF-like domain affects not only the antigenicity of this domain but also other parts of the molecule which lack disulfide bonds. The mechanism for this is unclear and must await the determination of the three-dimensional structure of the protein. Presumably, it involves transmission of some conformational change throughout the molecule, leading to a marked alteration in structure. The alternative that the disruption of the disulfide bonds leads to masking of other regions of the molecule seems unlikely, as it would need to simultaneously mask three distinct epitopes in MSP4A, MSP4B, and MSP4C. There is no similar phenomenon described for other malaria antigens containing EGF-like domains, but it may be that the appropriate experiments have not been performed, due to the need for epitope mapping of a polyspecific response. To address whether MSP4 plays a role in parasite invasion and the possible function of conformation-dependent epitopes, we are currently investigating the activity of the antibodies raised to recombinant proteins and by DNA vaccination in parasite growth inhibition assays. We are also performing challenge experiments with the Plasmodium yoelii homologue of MSP4/5. Preliminary studies indicate that some of the rabbit sera raised to different regions of MSP4 can inhibit parasite growth in vitro (39).
It is intriguing to note the importance of disulfide bonding to the immunogenicity of a number of asexual-stage proteins associated with the merozoite membrane. It has already been established that the Triton X-114 phase contains some of the known host protective antigens, such as AMA1 and MSP2 (28, 33). This population of antigens is important in host protective immunity, as immunization of mice with the Triton X-114 phase from Plasmodium chabaudi results in significant protection (22a). Previous studies with pooled human sera identified a subset of membrane-associated antigens in a sample of Triton X-114-enriched detergent phase separated under reducing conditions (33). There are several differences between that study and ours in terms of the proteins observed. Some of these differences are undoubtedly due to the different strains of parasites used and to a different percentage of acrylamide in the gel. Nevertheless, there are several proteins absent in that study, which may be explained by the presence of reduction-sensitive epitopes on some of the asexual-stage parasite antigens. Several of these antigens are of unknown identity, and further work will be required to identify them. This experiment also makes the important point that the naturally occurring immune response to several of these membrane-associated proteins is directed almost exclusively to conformational epitopes. This is particularly so for the proteins of 38, 35, and 30 kDa. It may be that the malaria genome project will allow their identification, given that we know that they must possess the general features of hydrophobic sequence, cysteine residues, and asexual-stage expression and be of a certain molecular mass.
Different regions of MSP4 are capable of inducing specific antibodies in laboratory animals which can recognize MSP4 in the parasite. Of note is that despite the difficulties in proper folding of the EGF-like domain, the antiserum raised to MSP4D was still capable of reacting with native MSP4. It is likely that expressed MSP4D exists as a mixture of proteins with different conformations, and at least some of these must be a reasonable approximation of the native protein. These may well be a minority, however, as the pooled human immune sera recognized this fusion protein quite poorly. The observation of cross-reactivity among antisera raised to MSP4A and MSP4B is quite surprising, as there is very little sequence similarity between the two fragments. However, the thrombin cleavage experiment clearly demonstrates that the epitope exists within the primary sequence of MSP4. Perhaps the closest match is the three consecutive residues EKK or EEK found in both fragments, but this is a somewhat weak basis for a shared epitope. It may be that the epitope is also partly conformational, but there is no evidence for this. We can completely exclude the possibility of a mix-up during the course of immunization of the rabbits. The serum to MSP4B was raised some 2 years prior to the construction of fragments MSP4A, MSP4C, and MSP4D, and this serum shows clear evidence of cross-reaction to MSP4A.
Using pooled immune sera from malaria patients, we have clearly demonstrated that MSP4 is immunogenic during natural infection and that its antigenicity is influenced by the correct folding of the EGF-like domain. The fact that the recombinant proteins we have constructed can be recognized by sera taken from malaria patients suggests that at least some of the epitopes are expressed in the correct conformation. There are at least four distinct epitopes in this relatively small protein (the size of the mature protein is approximately 233 residues). The weaker recognition of the MSP4D fragment suggests either that this region is poorly recognized during natural infection or that antibodies to the EGF-like domain may be directed to the conformation-dependent epitopes lacking in the recombinant protein. The latter is more likely to be the case, as studies with MSP1 showed that 12 of 19 MAbs bound to the 19-kDa carboxyl-terminal fragment that is composed of the two EGF-like domains (15). There is relatively little precise epitope mapping data for asexual-stage antigens during natural infection. In general, the reactivity to repetitive antigens is somewhat restricted, being predominately to the repeats, whereas nonrepetitive antigens have a larger number of epitopes. Analysis of the human antibody response to ring-infected erythrocyte surface antigen and S antigens suggests that the majority of the natural antibody response is directed against the tandem repeats (3, 27). Antibody responses to MSP2 appear to be predominantly against the repeat region (29, 35) and to a lesser extent the dimorphic regions (36). Analysis of the response to rhoptry high-molecular-weight polypeptide 3, an antigen that lacks repetitive sequences, identified five distinct B-cell epitopes recognized by immune sera (10). Such studies support the proposition that the net effect of the presence of repeats is to focus immune reactivity to repeat regions and render other regions of the protein immunologically invisible. Epidemiological studies to examine the frequency and magnitude of epitope-specific MSP4 responses in several areas of endemicity and to determine whether these antibody responses correlate with clinical immunity are in progress.
All MSPs tested to date have shown the ability to induce some level of host protective immunity following active immunization (2). It is reasonable to conclude that this may also be true for MSP4. Our studies indicate that scrupulous attention will need to be paid to attaining the correct conformation of this protein in order to induce antibodies capable of reacting with the native protein in the parasite. This will be particularly so for antibodies to the EGF-like domain. Whereas the EGF-like domain of MSP1 has been expressed in a conformationally correct form in E. coli (11), it has been necessary to express other proteins containing EGF-like domains in yeast (4, 22). It would appear from these studies that MSP4 will require the use of DNA immunization approaches or appropriate eukaryotic host-vector combinations. We are currently addressing this issue by using yeast expression systems, to enable the testing of MSP4 as a vaccine in primate challenge experiments.
ACKNOWLEDGMENTS
We thank Sue Cranmer and John Menting for advice and assistance, Emanuela Handman for review of the manuscript, and Juan Cooper for provision of MAb 4H9/19. John Menting provided the data on the N-terminal sequencing of MSP4E-His.
This work was supported by the Australia National Health and Medical Research Council (NH&MRC) and the U.S. Agency for International Development (USAID). Lina Wang is a recipient of a Monash University Graduate Scholarship.
REFERENCES
- 1.Anders R F, Coppel R L, Brown G V, Kemp D J. Antigens with repeated amino acid sequences from the asexual blood stages of Plasmodium falciparum. Prog Allergy. 1988;41:148–172. [PubMed] [Google Scholar]
- 2.Anders R F, Saul A J. Candidate antigens for an asexual blood stage vaccine against falciparum malaria. In: Good M F, Saul A J, editors. Molecular immunological considerations in malaria vaccine development. Boca Raton, Fla: CRC Press; 1993. pp. 169–208. [Google Scholar]
- 3.Anders R F, Shi P T, Scanlon D B, Leach S J, Coppel R L, Brown G V, Stahl H D, Kemp D J. Synthetic peptides as antigens. Ciba Found Symp. 1986;199:164–183. doi: 10.1002/9780470513286.ch10. [DOI] [PubMed] [Google Scholar]
- 4.Barr P J, Green K M, Gibson H L, Bathurst I C, Quakyi I A, Kaslow D C. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med. 1991;174:1203–1208. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackman M J, Heidrich H G, Donachie S, McBride J S, Holder A A. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990;172:379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackman M J, Holder A A. Secondary processing of the Plasmodium falciparum merozoite surface protein-1 (MSP1) by a calcium-dependent membrane-bound serine protease: shedding of MSP133 as a noncovalently associated complex with other fragments of the MSP1. Mol Biochem Parasitol. 1992;50:307–315. doi: 10.1016/0166-6851(92)90228-c. [DOI] [PubMed] [Google Scholar]
- 7.Blackman M J, Ling I T, Nicholls S C, Holder A A. Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol Biochem Parasitol. 1991;49:29–33. doi: 10.1016/0166-6851(91)90127-r. [DOI] [PubMed] [Google Scholar]
- 8.Blackman M J, Scottfinnigan T J, Shai S, Holder A A. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J Exp Med. 1994;180:389–393. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackman M J, Whittle H, Holder A A. Processing of the Plasmodium falciparum major merozoite surface protein-1: identification of a 33-kilodalton secondary processing product which is shed prior to erythrocyte invasion. Mol Biochem Parasitol. 1991;49:35–44. doi: 10.1016/0166-6851(91)90128-s. [DOI] [PubMed] [Google Scholar]
- 10.Brown H J, Coppel R L. Primary structure of a Plasmodium falciparum rhoptry antigen. Mol Biochem Parasitol. 1991;49:99–110. doi: 10.1016/0166-6851(91)90133-q. [DOI] [PubMed] [Google Scholar]
- 11.Burghaus P A, Holder A A. Expression of the 19-kilodalton carboxy-terminal fragment of the Plasmodium falciparum merozoite surface protein-1 in Escherichia coli as a correctly folded protein. Mol Biochem Parasitol. 1994;64:165–169. doi: 10.1016/0166-6851(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 12.Calvo P A, Daly T M, Long C A. Plasmodium yoelii - the role of the individual epidermal growth factor-like domains of the merozoite surface protein-1 in protection from malaria. Exp Parasitol. 1996;82:54–64. doi: 10.1006/expr.1996.0007. [DOI] [PubMed] [Google Scholar]
- 13.Camus D, Hadley T J. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science. 1985;230:553–556. doi: 10.1126/science.3901257. [DOI] [PubMed] [Google Scholar]
- 14.Chappel J A, Holder A A. Monoclonal antibodies that inhibit Plasmodium falciparum invasion in vitro recognise the first growth factor-like domain of merozoite surface protein-1. Mol Biochem Parasitol. 1993;60:303–312. doi: 10.1016/0166-6851(93)90141-j. [DOI] [PubMed] [Google Scholar]
- 15.Cooper J A, Cooper L T, Saul A J. Mapping of the region predominantly recognized by antibodies to the Plasmodium falciparum merozoite surface antigen MSA 1. Mol Biochem Parasitol. 1992;51:301–312. doi: 10.1016/0166-6851(92)90080-4. [DOI] [PubMed] [Google Scholar]
- 16.Crewther P E, Culvenor J G, Silva A, Cooper J A, Anders R F. Plasmodium falciparum: two antigens of similar size are located in different compartments of the rhoptry. Exp Parasitol. 1990;70:193–206. doi: 10.1016/0014-4894(90)90100-q. [DOI] [PubMed] [Google Scholar]
- 17.Crewther P E, Matthew M, Flegg R H, Anders R F. Protective immune responses to apical membrane antigen 1 of Plasmodium chabaudi involve recognition of strain-specific epitopes. Infect Immun. 1996;64:3310–3317. doi: 10.1128/iai.64.8.3310-3317.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deans J A, Jean W C. Structural studies on a putative protective Plasmodium knowlesi merozoite antigen. Mol Biochem Parasitol. 1987;26:155–166. doi: 10.1016/0166-6851(87)90139-3. [DOI] [PubMed] [Google Scholar]
- 19.Egan A F, Morris J, Barnish G, Allen S, Greenwood B M, Kaslow D C, Holder A A, Riley E M. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-Kda C-terminal fragment of the merozoite surface antigen, PfMSP-1. J Infect Dis. 1996;173:765–769. doi: 10.1093/infdis/173.3.765. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman S, Coppel R L, Chulay J. Vaccines. In: Oaks S C, Mitchell V S, Pearson G W, Carpenter C C J, editors. Malaria: obstacles and opportunities. Washington, D.C: National Academy Press; 1991. pp. 169–210. [PubMed] [Google Scholar]
- 21.Holder A A, Freeman R R. The three major antigens on the surface of Plasmodium falciparum merozoites are derived from a single high molecular weight precursor. J Exp Med. 1984;160:624–629. doi: 10.1084/jem.160.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaslow D C, Bathurst I C, Lensen T, Ponnudurai T, Barr P J, Keister D B. Saccharomyces cerevisiae recombinant pfs25 adsorbed to alum elicits antibodies that block transmission of Plasmodium falciparum. Infect Immun. 1994;62:5576–5580. doi: 10.1128/iai.62.12.5576-5580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.Lew, A. Personal communication.
- 23.Ling I T, Ogun S A, Holder A A. The combined epidermal growth factor-like modules of Plasmodium yoelii merozoite surface protein-1 are required for a protective immune response to the parasite. Parasite Immunol. 1995;17:425–433. doi: 10.1111/j.1365-3024.1995.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 24.Ling I T, Ogun S A, Holder A A. Immunization against malaria with a recombinant protein. Parasite Immunol. 1994;16:63–67. doi: 10.1111/j.1365-3024.1994.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 25.Marshall V M, Silva A, Foley M, Cranmer S, Wang L, McColl D J, Kemp D J, Coppel R L. A second merozoite surface protein (MSP-4) of Plasmodium falciparum that contains an epidermal growth factor-like domain. Infect Immun. 1997;65:4460–4467. doi: 10.1128/iai.65.11.4460-4467.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall V M, Wu T, Coppel R L. Close linkage of three merozoite surface protein genes on chromosome 2 of Plasmodium falciparum. Mol Biochem Parasitol. 1998;94:13–25. doi: 10.1016/s0166-6851(98)00045-0. [DOI] [PubMed] [Google Scholar]
- 27.Perlmann H, Perlmann P, Berzins K, Wahlin B, Troye B M, Hagstedt M, Andersson I, Hogh B, Petersen E, Bjorkman A. Dissection of the human antibody response to the malaria antigen Pf155/RESA into epitope specific components. Immunol Rev. 1989;112:115–132. doi: 10.1111/j.1600-065x.1989.tb00555.x. [DOI] [PubMed] [Google Scholar]
- 28.Peterson M G, Marshall V M, Smythe J A, Crewther P E, Lew A, Silva A, Anders R F, Kemp D. Integral membrane protein located in the apical complex of Plasmodium falciparum. Mol Cell Biol. 1989;9:3151–3154. doi: 10.1128/mcb.9.7.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranford-Cartwright L C, Taylor R R, Asgarijirhandeh N, Smith D B, Roberts P E, Robinson V J, Babiker H A, Riley E M, Walliker D, McBride J S. Differential antibody recognition of FC27-like Plasmodium falciparum merozoite surface protein MSP2 antigens which lack 12 amino acid repeats. Parasite Immunol. 1996;18:411–420. doi: 10.1046/j.1365-3024.1996.d01-137.x. [DOI] [PubMed] [Google Scholar]
- 30.Rosenthal P J. Plasmodium falciparum—effects of proteinase inhibitors on globin hydrolysis by cultured malaria parasites. Exp Parasitol. 1995;80:272–281. doi: 10.1006/expr.1995.1033. [DOI] [PubMed] [Google Scholar]
- 31.Sim B, Orlandi P A, Haynes J D, Klotz F W, Carter J M, Camus D, Zegans M E, Chulay J D. Primary structure of the 175K Plasmodium falciparum erythrocyte binding antigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. J Cell Biol. 1990;111:1877–1884. doi: 10.1083/jcb.111.5.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 33.Smythe J A, Coppel R L, Brown G V, Ramasamy R, Kemp D J, Anders R F. Identification of two integral membrane proteins of Plasmodium falciparum. Proc Natl Acad Sci USA. 1988;85:5195–5199. doi: 10.1073/pnas.85.14.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smythe J A, Murray P J, Anders R F. Improved temperature-dependent phase separation using Triton X-114: isolation of integral membrane proteins of pathogenic parasites. J Methods Cell Mol Biol. 1990;2:133–137. [Google Scholar]
- 35.Smythe J A, Peterson M G, Coppel R L, Saul A J, Kemp D J, Anders R F. Structural diversity in the 45-kilodalton merozoite surface antigen of Plasmodium falciparum. Mol Biochem Parasitol. 1990;39:227–234. doi: 10.1016/0166-6851(90)90061-p. [DOI] [PubMed] [Google Scholar]
- 36.Taylor R, Smith D, Robinson V, McBride J, Riley E. Human antibody response to Plasmodium falciparum merozoite surface protein 2 is serogroup specific and predominantly of the immunoglobulin G3 subclass. Infect Immun. 1995;63:4382–4388. doi: 10.1128/iai.63.11.4382-4388.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trager W, Jensen J. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 38.Wang, L., et al. Unpublished data.
- 39.Wu, T. Unpublished data.