Abstract
Background:
The ACTT risk profile, which was developed from ACTT-1 (Adaptive COVID-19 Treatment Trial-1), demonstrated that hospitalized patients with COVID-19 in the high-risk quartile (characterized by low absolute lymphocyte count [ALC], high absolute neutrophil count [ANC], and low platelet count at baseline) benefited most from treatment with the antiviral remdesivir. It is unknown which patient characteristics are associated with benefit from treatment with the immunomodulator baricitinib.
Objective:
To apply the ACTT risk profile to the ACTT-2 cohort to investigate potential baricitinib-related treatment effects by risk quartile.
Design:
Post hoc analysis of ACTT-2, a randomized, double-blind, placebo-controlled trial. (ClinicalTrials.gov: NCT04401579)
Setting:
Sixty-seven trial sites in 8 countries.
Participants:
Adults hospitalized with COVID-19 (n = 999; 85% U.S. participants).
Intervention:
Baricitinib + remdesivir versus placebo + remdesivir.
Measurements:
Mortality, progression to invasive mechanical ventilation (IMV) or death, and recovery, all within 28 days; ALC, ANC, and platelet count trajectories.
Results:
In the high-risk quartile, baricitinib + remdesivir was associated with reduced risk for death (hazard ratio [HR], 0.38 [95% CI, 0.16 to 0.86]; P = 0.020), decreased progression to IMV or death (HR, 0.57 [CI, 0.35 to 0.93]; P = 0.024), and improved recovery rate (HR, 1.53 [CI, 1.16 to 2.02]; P = 0.002) compared with placebo + remdesivir. After 5 days, participants receiving baricitinib + remdesivir had significantly larger increases in ALC and significantly larger decreases in ANC compared with control participants, with the largest effects observed in the high-risk quartile.
Limitation:
Secondary analysis of data collected before circulation of current SARS-CoV-2 variants.
Conclusion:
The ACTT risk profile identifies a subgroup of hospitalized patients who benefit most from baricitinib treatment and captures a patient phenotype of treatment response to an immunomodulator and an antiviral. Changes in ALC and ANC trajectory suggest a mechanism whereby an immunomodulator limits severe COVID-19.
Primary Funding Source:
National Institute of Allergy and Infectious Diseases.
ACTT (Adaptive COVID-19 Treatment Trial) was a series of large, double-blind, randomized, placebo-controlled trials that evaluated treatments for adults hospitalized with COVID-19 and helped define the standard of care. ACTT-1 showed that the antiviral remdesivir reduced time to recovery compared with placebo (1). Given emerging data at the time suggesting that host immune dysregulation was associated with COVID-19 severity, ACTT-2 investigated the immunomodulator baricitinib. Baricitinib, a selective, orally administered Janus kinase (JAK) 1 and 2 inhibitor, inhibits the signaling pathways of several inflammatory cytokines associated with severe COVID-19, such as tumor necrosis factor-a, interleukin-6, and granulocyte colony-stimulating factor (2, 3). ACTT-2 demonstrated that treatment with baricitinib+remdesivir further reduced time to recovery compared with placebo+remdesivir (4). Differential treatment effects were assessed by an 8-point ordinal scale based on supplemental oxygen use (Supplement Table 1, available at Annals.org). The treatment effect of remdesivir in ACTT-1 was greatest in participants receiving low-flow oxygen at baseline (ordinal score [OS] 5) (1), and the treatment effect for baricitinib+remdesivir in ACTT-2 was greatest in participants receiving high-flow oxygen or noninvasive ventilation (OS6) (4). On the basis of these subgroup observations and data from additional clinical trials, society and government guidelines on COVID-19 management make the strongest recommendations for remdesivir use in patients requiring low-flow supplemental oxygen and for baricitinib use in patients requiring high-flow oxygen or noninvasive ventilation (5).
There may, however, be patient-specific characteristics that, when added to oxygen requirement, more precisely define who may benefit from a particular COVID-19 therapeutic, such as baricitinib. For example, ACTT-1 participants with a low absolute lymphocyte count (ALC), a high absolute neutrophil count (ANC), and a low platelet count at baseline were more likely to develop severe disease outcomes (6). These parameters were selected in previous work that applied data mining techniques to develop a risk profile, hereafter referred to as the “ACTT risk profile,” which predicted risk for progression to invasive mechanical ventilation (IMV) or death. Although oxygen requirement of participants at baseline, or the baseline OS, was included in the risk profile by design, its contribution to the risk profile (hazard ratio [HR], 0.91) was substantially lower than the contributions of ANC (HR, 1.93), ALC (HR, 0.58), and platelet count (HR, 0.63) (Supplement Table 2, available at Annals.org), and compared with baseline OS alone, the ACTT risk profile better predicted progression to IMV or death (area under the receiver operating characteristic curve [AUROC], 0.73 vs. 0.53; P < 0.001) (6). When the treatment effect of remdesivir was assessed within risk quartiles, those in the high-risk quartile derived the greatest benefit. This quartile included a range of oxygen requirements, including some patients who required either IMV or extracorporeal membrane oxygenation (ECMO) (OS7), a group for which remdesivir is not generally recommended in treatment guidelines. These findings inspired the hypothesis that respiratory status alone is insufficient for identification of subgroups that benefit most from different COVID-19 therapeutics, as some persons within each oxygen requirement group may benefit more than others.
In this study, we applied the ACTT risk profile to the ACTT-2 data set and explored the treatment effect of baricitinib by risk quartile to ascertain whether, similar to our findings with remdesivir, we could more precisely characterize who will benefit from baricitinib.
Methods
Data Set
ACTT-1 (ClinicalTrials.gov: NCT04280705) enrolled 1062 participants from 21 February to 19 April 2020 (1), and ACTT-2 (ClinicalTrials.gov: NCT04401579) enrolled 1033 participants from 8 May to 1 July 2020 (4). Overall, ACTT-1 and ACTT-2 enrolled patients from 81 hospitals in 11 countries (82% U.S. participants); ACTT-2 included 67 sites in 8 countries (85% U.S. participants). Eligibility criteria and clinical assessments conducted through study day 28 were similar between the trials. Steroids were permitted only for non–COVID-19 indications. A total of 999 ACTT-2 participants who received their assigned treatment and had no missing data for ACTT risk profile contributions were analyzed (Supplement Tables 3 and 4, available at Annals.org). The OS was assigned at enrollment into ACTT (Supplement Table 1); enrolled participants had an OS of 4 to 7 at baseline (OS4: hospitalized, no supplemental oxygen; OS5: low-flow supplemental oxygen; OS6: noninvasive ventilation or high-flow nasal cannula; OS7: IMV or ECMO). The primary outcome was time to recovery, defined as time until hospital discharge or the first day of hospitalization without a need for medical care (OS1 to OS3).
Statistical Analysis
Prognostic Performance of the Risk Profile
Participant characteristics were compared (including ACTT risk profile components) between trial stages and risk quartiles. Baseline upper respiratory viral load (VL) (collected via oropharyngeal or nasopharyngeal swab) and C-reactive protein (CRP) level were compared between the high-risk and least-risk ACTT quartiles using Wilcoxon–Mann–Whitney tests. The prognostic performance of the ACTT risk profile was assessed in the ACTT-2 data set by calculating the AUROC for mortality, progression to IMV or death, and recovery within 28 days. For patients requiring IMV at baseline, the composite outcome of IMV or death was defined as the time to death.
Treatment Effect of Baricitinib
The treatment effect of baricitinib+remdesivir versus placebo+remdesivir was estimated using ACTT-2 data for each quartile of ACTT risk profile for the outcomes of mortality, progression to IMV or death, and recovery within 28 days. Fine–Gray competing-risks analysis (7) was used for consistency with the analyses used to develop the ACTT risk profile (6), and Cox regression was performed as a sensitivity analysis (8). A Fine–Gray competing-risk model with interaction terms between ACTT risk quartile and treatment was used to compare baricitinib response between quartiles. Statistical methods followed the PATH (Predictive Approaches to Treatment effect Heterogeneity) guidelines for assessing treatment effect heterogeneity (9, 10). Hazard ratios were used to determine relative treatment effects, and differences in cumulative incidence were estimated based on Kaplan–Meier survival curves.
Exploratory Analyses Using Individual Laboratory Parameters to Predict Benefit of Baricitinib
Exploratory analyses were performed to assess whether individual laboratory parameters might also predict benefit from baricitinib. Fine–Gray competing-risks models were repeated with quartiles defined by CRP level, ALC, or ANC individually. Additional details are provided in the Supplement (available at Annals.org).
Exploratory Analyses of Changes in Hematologic and Other Laboratory Parameters During Treatment
Temporal trends in laboratory parameters in the ACTT risk profile (ALC, ANC, and platelet count) as well as VL and CRP level were compared between treatment groups with t tests on log-transformed ratio changes from baseline to study day 5. Trends in ALC, ANC, and platelet count within the baricitinib+remdesivir group alone were compared between risk quartiles with t tests on the log-transformed ratio changes.
Role of the Funding Source
The protocols were designed by the ACTT investigators and the study sponsor, with input from the manufacturers of remdesivir (Gilead) and baricitinib (Eli Lilly). ACTT investigators and site staff gathered the data, which were analyzed by statisticians at The Emmes Company and the National Institute of Allergy and Infectious Diseases (NIAID). The funder, NIAID, participated in writing of the manuscript and submission of the manuscript for publication.
Results
Characteristics of the ACTT Risk Profile
Distributions of ALC, ANC, and platelet count were similar between ACTT-1 and ACTT-2 participants overall and within each quartile (Table 1). Risk quartiles were defined with the combined ACTT-1 and ACTT-2 populations, and ACTT-2 participants were evenly distributed by treatment across quartiles (Supplement Table 5, available at Annals.org). In ACTT-2, all risk quartiles included representation from each OS category, although ACTT-2 had fewer participants in the OS7 category (IMV or ECMO) than ACTT-1 (11% vs. 27%) and more in the OS5 category (low-flow supplemental oxygen [55% vs. 41%]) and within each risk quartile (Table 1). For both ACTT-1 and ACTT-2, the OS5 category had the greatest representation in each risk quartile.
Table 1.
Distribution of Variables in the ACTT Risk Profile Among ACTT-1 Participants vs. ACTT-2 Participants
| Covariate | Least Risk | Lower Risk | Moderate Risk | High Risk | Overall | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| ACTT-1 | ACTT-2 | ACTT-1 | ACTT-2 | ACTT-1 | ACTT-2 | ACTT-1 | ACTT-2 | ACTT-1 | ACTT-2 | |
| (n = 242) | (n = 271) | (n = 277) | (n = 236) | (n = 291) | (n = 221) | (n = 241) | (n = 271) | (n = 1051) | (n = 999) | |
|
| ||||||||||
| Median baseline ALC (IQR), × 109 cells/L | 1.4 (1.1–1.8) | 1.5 (1.2–1.9) | 1.1 (0.9–1.4) | 1.1 (0.9–1.3) | 0.9 (0.7–1.1) | 0.9 (0.7–1.2) | 0.6 (0.4–0.8) | 0.7 (0.5–0.9) | 1.0 (0.7–1.3) | 1.0 (0.7–1.4) |
| Median baseline ANC (IQR), × 109 cells/L | 3.4 (2.3–4.7) | 3.3 (2.3–4.4) | 4.6 (3.4–5.9) | 4.3 (3.4–5.7) | 5.7 (4.1–7.9) | 5.9 (4.5–7.4) | 8.1 (5.9–10.7) | 7.8 (5.8–11.0) | 5.1 (3.5–7.5) | 5.1 (3.6–7.2) |
| Median baseline platelet count (IQR), × 109 cells/L | 256 (195–354) | 251 (182–314) | 228 (172–284) | 222 (172–284) | 215 (159–276) | 204 (168–262) | 192 (151–251) | 207 (159–257) | 220 (167–287) | 218 (170–279) |
| Baseline ordinal score, n (%)* 4 (hospitalized, not requiring supplemental oxygen, requiring ongoing medical care) |
38 (16) | 52 (19) | 44 (16) | 39 (17) | 34 (12) | 22 (10) | 22 (9) | 24 (9) | 138 (13) | 137 (14) |
| 5 (hospitalized, requiring supplemental oxygen) | 120 (50) | 172 (63) | 115 (42) | 129 (55) | 105 (36) | 106 (48) | 95 (39) | 138 (51) | 435 (41) | 545 (55) |
| 6 (hospitalized, receiving noninvasive ventilation or high-flow oxygen devices) | 37 (15) | 38 (14) | 49 (18) | 47 (20) | 64 (22) | 62 (28) | 43 (18) | 65 (24) | 193 (18) | 212 (21) |
| 7 (hospitalized, receiving invasive mechanical ventilation or ECMO) | 47 (19) | 39 (3) | 69 (25) | 21 (9) | 88 (30) | 31 (14) | 81 (34) | 44 (16) | 285 (27) | 105 (11) |
ACTT = Adaptive COVID-19 Treatment Trial; ALC = absolute lymphocyte count; ANC = absolute neutrophil count; ECMO = extracorporeal membrane oxygenation.
Values are column percentages with the given baseline ordinal score value among participants in the specified risk quartile and stage.
Table 2 shows baseline characteristics for ACTT-2 participants by risk quartile. There was greater representation of men and older patients in higher risk quartiles. Baseline VL was higher in the higher risk quartiles and was significantly higher in the high-risk quartile than in the least-risk quartile (the difference in the medians of log10 VL was −0.50 [95% CI, −1.1 to −0.000039]). Median CRP level increased with risk quartile and was significantly higher in the high-risk quartile (174.2 mg/L [CI, 163.0 to 190.0 mg/L]) than in the least-risk quartile (67.0 mg/L [CI, 60.0 to 78.9 mg/L]). Relationships between other participant characteristics and risk quartile were similar to those in ACTT-1 (Supplement Table 6, available at Annals.org), although on average the ACTT-1 cohort was older, had less representation of Hispanic participants, and had lower baseline VL compared with the ACTT-2 cohort. Baseline characteristics in the ACTT-2 cohort were balanced between treatment groups (4), as was the risk profile distribution (Supplement Figure 1, available at Annals.org).
Table 2.
Baseline Characteristics of ACTT-2 Participants, by Quartile of ACTT Risk Profile*
| Characteristic | Least Risk (n = 271) | Lower Risk (n = 236) | Moderate Risk (n = 221) | High Risk (n = 271) | Overall (n = 999) |
|---|---|---|---|---|---|
|
| |||||
| Median age (IQR), y | 50 (42–63) | 56 (42–67) | 57 (45–67) | 59 (47–69) | 56 (43–66) |
| Male sex, n (%)† Race, n (%) |
143 (53) | 142 (60) | 154 (70) | 193 (71) | 632 (63) |
| American Indian or Alaska Native | 3 (1.1) | 2 (0.8) | 3 (1.4) | 1 (0.4) | 9 (0.9) |
| Asian | 34 (13) | 26 (11) | 13 (6) | 26 (10) | 99 (10) |
| Black or African American | 56 (21) | 35 (15) | 30 (14) | 32 (12) | 153 (15) |
| White | 119 (44) | 114 (48) | 111 (50) | 132 (49) | 476 (48) |
| Hispanic or Latino ethnicity, n (%) | 128 (47) | 114 (48) | 111 (50) | 156 (58) | 509 (51) |
| Median body mass index (IQR), kg/m2 | 31.8 (27.5–38.2) | 30.8 (26.9–36.9) | 31.1 (26.7–35.7) | 29.4 (25.6–33.8) | 30.7 (26.6–36.0) |
| Median baseline CRP level (IQR), mg/L | 67.0 (34.1–113.2) | 108.0 (64.5–174.7) | 147.3 (93.9–204.6) | 174.2 (108.4–245.3) | 124.0 (64.4–190.0) |
| Median baseline log10 VL (IQR)‡ | 4.0 (0.0–5.1) | 4.2 (0.3–5.2) | 4.2 (2.5–5.3) | 4.5 (2.9–5.8) | 4.2 (1.4–5.4) |
| VL data, n (%)§ | 168 (62) | 162 (69) | 157 (71) | 171 (63) | 658 (66) |
| Median days from symptom onset to enrollment (IQR) | 8.0 (5.0–11.0) | 8.0 (6.0–11.0) | 8.0 (5.0–10.0) | 8.0 (5.0–10.0) | 8.0 (5.0–10.0) |
| Immunodeficiency, n (%) | 8 (3.0) | 8 (3.4) | 8 (3.6) | 6 (2.2) | 30 (3.0) |
ACTT = Adaptive COVID-19 Treatment Trial; CRP = C-reactive protein; VL = viral load.
Risk quartiles were derived from ACTT-1 and ACTT-2 participants combined.
Percentages are among participants in the specified risk quartile.
Collected via oropharyngeal or nasopharyngeal swab.
Collection was planned only for a subset of participants.
Prognostic Performance of the ACTT Risk Profile
In predictions of the outcomes of recovery, death, and IMV or death within 28 days in the ACTT-2 population, AUROC values ranged from 0.56 to 0.73 for the ACTT risk profile (Supplement Figure 2 and Supplement Table 7, available at Annals.org).
Treatment Effect of Baricitinib
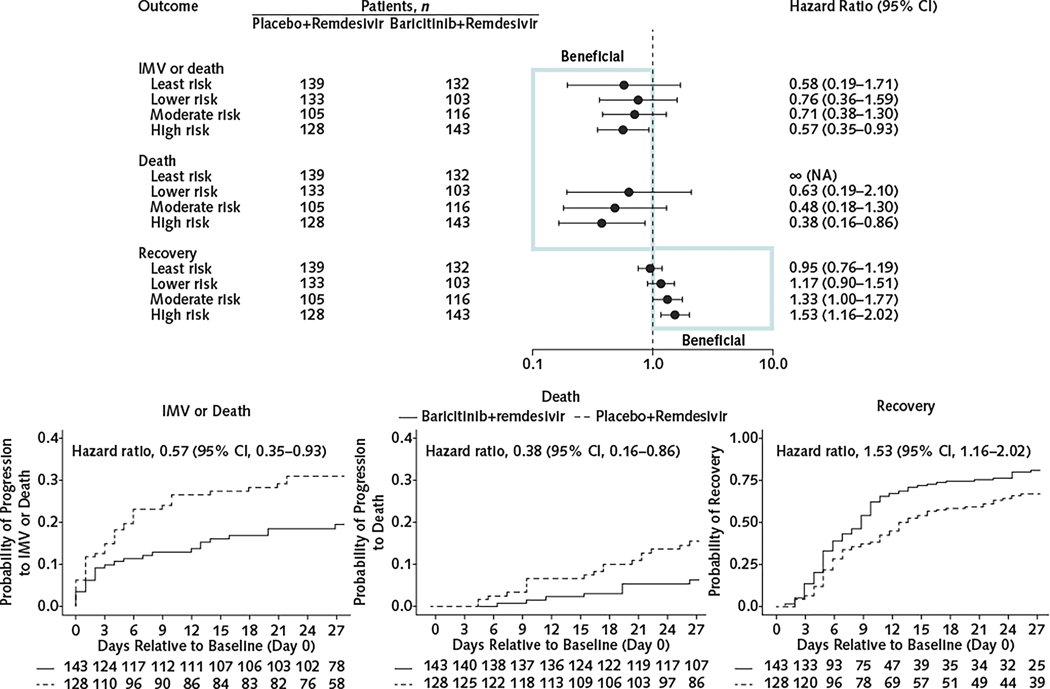
When outcomes within quartiles of ACTT risk profile were assessed, patients in the high-risk quartile receiving baricitinib+remdesivir had significantly improved clinical outcomes within 28 days compared with placebo+remdesivir recipients (Figure 1) for mortality (subdistribution HR, 0.38 [CI, 0.16 to 0.86]; P = 0.020), IMV or death (HR, 0.57 [CI, 0.35 to 0.93]; P = 0.024), and recovery (HR, 1.53 [CI, 1.16 to 2.02]; P = 0.002). Although treatment effect estimates were not significant for the moderate-, lower-, or least-risk categories, the size of the baricitinib treatment effect increased with increasing risk quartiles (Figure 1, top). Results from Cox regression analysis and for treatment effects quantified as differences in cumulative incidence were similar (Supplement Figures 3 and 4, available at Annals.org). Interaction tests showed that the treatment effect of baricitinib for recovery was 1.60 times higher (CI, 1.10 to 2.32) in the high-risk group than in the least-risk group (Supplement Table 10, available at Annals.org). An analysis that adjusted for baseline values of OS, age, sex, log10 CRP level, and number of comorbidities also showed significant benefit from baricitinib in the high-risk group for mortality, recovery, and IMV or death (Supplement Figure 5, available at Annals.org).
Figure 1.
Effect of baricitinib+remdesivir compared with placebo+remdesivir in hospitalized patients with COVID-19 for the outcomes of death, IMV or death, and recovery within 28 days, by ACTT risk quartile.
ACTT = Adaptive COVID-19 Treatment Trial; IMV = invasive mechanical ventilation; NA = not applicable. Top. Effect of baricitinib+remdesivir compared with placebo+remdesivir. Fine–Gray subdistribution hazard ratios and 95% CIs are shown. Bottom. Kaplan–Meier curves for the high-risk quartile.
Exploratory Analyses Using Individual Laboratory Parameters to Predict Benefit of Baricitinib
Sensitivity analyses that estimated treatment effects by quartile of CRP level and ANC suggested that the ACTT risk profile distinguished a subgroup that benefited most from baricitinib better than either CRP level or ANC alone (Supplement Figures 6 and 7, available at Annals.org). When treatment effects were assessed by ALC quartile, participants with an ALC below 0.74 × 109 cells/L experienced a significant benefit from baricitinib+remdesivir compared with placebo+remdesivir for mortality, recovery, and IMV or death within 28 days (Supplement Figure 9, available at Annals.org), which was similar to our result with the ACTT risk profile. In contrast, the size of the treatment effect of baricitinib did not show a clear relationship for the higher quartiles.
Exploratory Analyses of Changes in Hematologic Parameters and Other Laboratory Parameters During Treatment
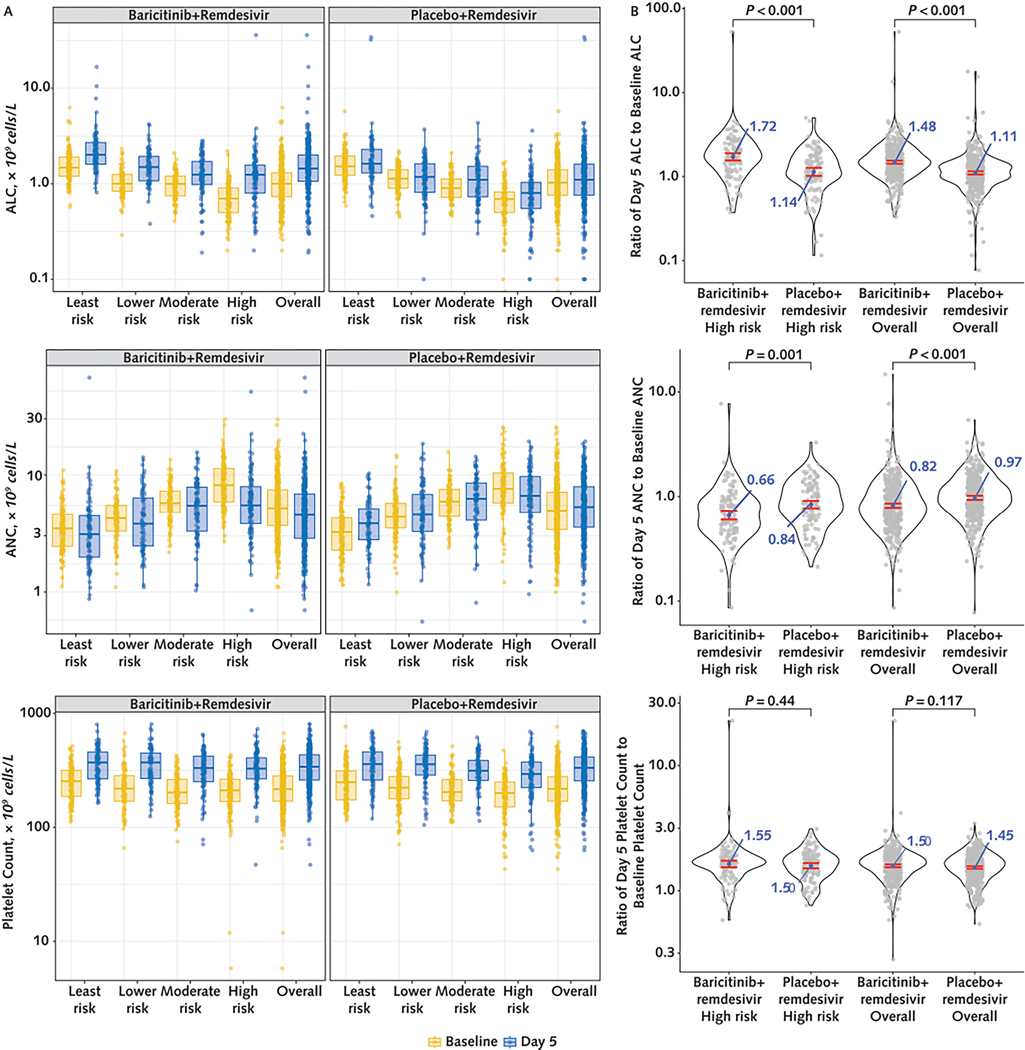
Recipients of baricitinib+remdesivir had higher ALC and lower ANC by day 5 than those who received placebo+remdesivir (Figure 2). Participants who received baricitinib+remdesivir had a significantly greater geometric mean (GM) ratio change from baseline to day 5 in ALC (1.48 [CI, 1.42 to 1.55] vs. 1.11 [CI, 1.06 to 1.16]) and ANC (0.82 [CI, 0.78 to 0.86] vs. 0.97 [CI, 0.93 to 1.02]) than those receiving placebo+remdesivir. When the high-risk quartile was assessed, the GM ratio changes in ALC and ANC from baseline to day 5 also differed significantly between treatment groups. Recipients of baricitinib+remdesivir in the high-risk quartile had a GM ratio change in ALC of 1.72 (CI, 1.56 to 1.90) (that is, values at day 5 were 72% higher on average than values at baseline), which was significantly greater than the change among placebo+remdesivir recipients (1.14 [CI, 1.02 to 1.27]). The GM ratio change in ANC among recipients of baricitinib+remdesivir in the high-risk quartile was 0.66 (CI, 0.60 to 0.73) (34% lower on average at day 5 than at baseline) and 0.84 for those who received placebo+remdesivir (CI, 0.77 to 0.91); these changes also differed significantly. The magnitude of the treatment effect in the high-risk quartile on ALC and ANC trajectory was larger than that in the overall population. An interaction test showed that the treatment effect in the high-risk quartile was significantly larger than the effect in the moderate-risk (P = 0.003) and least-risk (P = 0.039) quartiles for ALC (Supplement Table 11, available at Annals.org). Analogous models were fit for the log-transformed changes in ANC, platelet count, CRP level, and VL, but interaction terms were not significant. Platelet count increased by day 5 in both treatment groups, but the increases were similar between groups and by risk quartile (Figure 2).
Figure 2.
Changes in ALC, ANC, and platelet count after initiation of treatment with baricitinib+remdesivir vs. placebo+remdesivir in hospitalized patients with COVID-19.
Panel A shows distributions of ALC, ANC, and platelet count at baseline (yellow) and at day 5 (blue) by risk quartile and treatment group. Panel B shows the distributions of ratio change in hematologic parameters from baseline to day 5 in the high-risk quartile and in the overall study population by treatment group. The center of the ratio change distribution is quantified by the geometric mean of the ratio change with the 95% CI. P values are from t tests on the log-transformed ratio changes. ALC = absolute lymphocyte count; ANC = absolute neutrophil count.
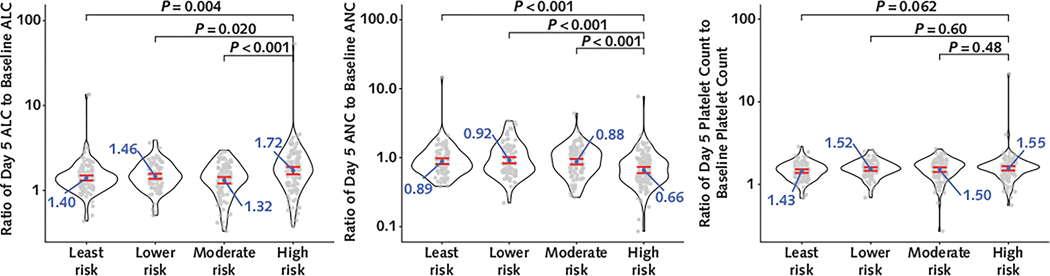
When the baricitinib+remdesivir group was analyzed alone, the GM ratio change in ALC from baseline to day 5 in the high-risk quartile was 1.72 (CI, 1.56 to 1.90), which was significantly larger than the changes in the moderate-risk quartile (1.32 [CI, 1.21 to 1.44]), the lower-risk quartile (1.46 [CI, 1.36 to 1.57]), and the least-risk quartile (1.40 [CI, 1.31 to 1.50]) (Figure 3). Significantly larger decreases in ANC were also seen in the high-risk quartile (GM ratio change, 0.66 [CI, 0.60 to 0.73]) compared with the moderate-risk quartile (0.88 [CI, 0.80 to 0.96]), the lower-risk quartile (0.92 [CI, 0.83 to 1.02]), and the least-risk quartile (0.89 [CI, 0.81 to 0.97]). There were no significant differences in the magnitude of changes in platelet count by quartile in the baricitinib+remdesivir group.
Figure 3.
Differences in the magnitude of change from baseline to day 5 for ALC, ANC, and platelet count in baricitinib+remdesivir recipients in the high-risk quartile.
The figure shows changes in ALC (left), ANC (middle), and platelet count (right), expressed as a ratio of the value at day 5 to the baseline value, by ACTT risk quartile in the baricitinib + remdesivir treatment group. The center of the ratio change distribution is quantified by the geometric mean of the ratio change with the 95% CI. P values are from t tests on the log-transformed ratio changes. ACTT = Adaptive COVID-19 Treatment Trial; ALC = absolute lymphocyte count; ANC = absolute neutrophil count.
Viral load decreased from baseline to day 5 overall and within each risk quartile, but changes were similar between treatment groups (Supplement Figure 11, available at Annals.org). In contrast, although CRP values decreased by day 5 in both treatment groups, they decreased significantly more in the baricitinib+remdesivir group (GM ratio change, 0.43 [CI, 0.40 to 0.47]) than in the placebo+remdesivir group (GM ratio change, 0.59 [CI, 0.54 to 0.64]) (Supplement Figure 11). Significant differences in GM ratio change in CRP level from baseline to day 5 were also seen in the high-risk quartile (0.42 [CI, 0.36 to 0.49] for baricitinib+remdesivir and 0.62 [CI, 0.52 to 0.73] for placebo+remdesivir).
Discussion
Several risk stratification tools can predict progression to severe COVID-19 outcomes; however, a gap exists in knowledge of whether such tools can be applied to guide COVID-19 treatment strategies. Risk scores with high prognostic performance do not necessarily identify subgroups with strong treatment benefit, as parameters related to treatment responsiveness may differ biologically from those related to disease outcome (11). In this study, the ACTT risk profile, in addition to defining a group of participants who were more likely to require IMV or die, identified a subgroup with differential benefit from immunomodulator treatment. Within the high-risk quartile, characterized by participants with higher ANC, lower ALC, and lower platelet count, the baricitinib+remdesivir group had a markedly reduced hazard of death (by a factor of 0.38) and a reduced hazard of IMV or death (by a factor of 0.57). The ACTT risk profile can identify a subgroup of hospitalized patients with COVID-19 who benefit from remdesivir, as shown previously (6), and from baricitinib, as shown here.
Although most COVID-19 therapeutic trials report analyses stratified by baseline oxygen requirements, the ACTT risk profile differentiates risk in a more precise manner (6). Baseline oxygen requirement, defined by the OS, was included in the ACTT risk profile by design, but its contribution to risk stratification was lower than the contributions of the hematologic parameters. Of note, 60% of patients within the high-risk quartile in ACTT-2 required no oxygen or low-flow oxygen at baseline. These data suggest that baseline oxygen requirement is an incomplete proxy for COVID-19 severity. Although respiratory status is used in major treatment guidelines to guide management, the findings presented here suggest that a biomarker-based approach utilizing simple parameters found in a bedside complete blood count provides complementary information on who might benefit from baricitinib treatment.
Baricitinib is a selective and reversible inhibitor of JAK 1/2 that is used in the treatment of autoimmune diseases. In large studies of patients with rheumatoid arthritis who were treated with baricitinib, increases in the ALC, including T and B cells, were observed in the first weeks of treatment, whereas ANC decreased significantly (12, 13). In this study, in the very different clinical setting of hospitalized patients with COVID-19, we observed significant increases in ALC and decreases in ANC after 5 days of baricitinib treatment (Figure 2), and the ALC increases and ANC decreases were larger in magnitude than for control participants. Although studies early in the pandemic showed that low ALC (14–16) and high ANC (17–19) are hallmarks of severe COVID-19, treatment effects on these parameters have not been assessed in large therapeutic trials. When assessing the trajectory of these markers solely within the baricitinib-treated group, we found the largest trajectory changes in ALC and ANC within the high-risk quartile (Figure 3), in which a significant treatment effect on mortality was also observed. Although this study was not designed to evaluate the mechanism of baricitinib, we hypothesize that baricitinib-dependent enhancement of ALC and suppression of ANC may affect lymphocyte and neutrophil function in host defense and may limit deleterious inflammation. This is supported by findings in a small cohort of hospitalized patients with COVID-19 evaluated early in the pandemic, where baricitinib treatment increased ALC, with significant increases specifically in CD4+ and CD8+ T-cell counts—both of which are implicated in host defense against SARS-CoV-2—as well as B cells and SARS-CoV-2–specific antibodies by 1 week (20). Enhancement of ALC with baricitinib treatment may also limit immune dysregulation (21, 22), thus decreasing pulmonary inflammation. In addition, baricitinib-dependent reductions in ANC may limit neutrophil-mediated pathology in COVID-19. Neutrophils are present within pulmonary infiltrates in human COVID-19 postmortem samples (23), and neutrophils are implicated in immunopathology in severe COVID-19 (24, 25). In a nonhuman primate model of COVID-19, baricitinib treatment decreased neutrophil recruitment to the lungs and lung pathology (26). Taken together, changes in ALC and ANC trajectory mediated by baricitinib and most pronounced in the high-risk quartile may affect immune defense and limit inflammation in COVID-19, thereby improving outcomes.
The ACTT risk profile predicts benefit in the high-risk quartile from 2 very different COVID-19 therapeutics: an antiviral, as previously shown (4), and an immunomodulator, as shown in this study. Therefore, we hypothesize that parameters reflected in the profile, including levels of lymphocytes and neutrophils in the blood at baseline, capture a patient phenotype of responsiveness to treatment. This phenotype may comprise persons who are unable to effectively reduce viral burden or limit deleterious inflammation and who derive particular benefit from an antiviral or an immunomodulator.
These data have implications for the design of future COVID-19 treatment trials and interpretation of clinical trial data sets assessing immunomodulators for hospitalized patients with COVID-19. Pivotal trials supporting the use of dexamethasone (27), baricitinib (4, 28), and tocilizumab (29, 30) stratified patients with COVID-19 into subgroups based on baseline oxygen requirement, with society and government COVID-19 treatment guidelines adopting a similar approach (5). Although the COV-BARRIER (28) and RECOVERY trials (27) used CRP or other inflammatory markers as part of their enrollment criteria, no clear evidence exists that persons with elevated levels of inflammatory markers benefit more from an immunomodulator. The REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform for Community-acquired Pneumonia) trial reported that beneficial effects of tocilizumab were seen across all prespecified CRP subgroups (29). To our knowledge, no other clinical trials have assessed clinical benefit from an immunomodulator with relation to dynamics in hematologic parameters, and these data suggest the relevance of these measurements in predicting treatment response.
This analysis has several limitations. First, this is a post hoc analysis. Although recovery and mortality within 28 days were prespecified end points, we also analyzed IMV or death within 28 days for consistency with analyses that were performed to develop the ACTT risk profile. Results for these disease severity outcomes, regardless of prespecification, were consistent with treatment benefit. Low event rates in lower-risk quartiles limit the power to detect treatment response for death and for IMV or death, but event rates were adequate when recovery was analyzed, and an interaction test showed a significantly higher treatment effect on recovery within 28 days in the high-risk quartile compared with the least-risk quartile (P = 0.013). We also opted not to assess whether variables that were unavailable at the time of risk profile development, such as CRP level or VL, should be included. Rather, we describe these variables within the context of the risk profile, and the high-risk quartile had statistically significant differences in baseline CRP level and VL, suggesting that the ACTT risk profile captures some of the variability in risk quantified by these variables. Although the ACTT risk profile predicted benefit of baricitinib better than CRP level or ANC alone, when participants were stratified by ALC into quartiles, the lowest quartile, defined by an upper limit of 0.74 × 109 cells/L, was found to predict baricitinib treatment response against mortality, IMV or death, and recovery. However, unlike the ACTT risk profile, higher ALC quartiles did not show a pattern with baricitinib treatment response. Future studies of ALC, ALC dynamics, and JAK inhibitors during COVID-19 are warranted. Another limitation of this study is that corticosteroid use may affect the variables included in the risk profile and clinical outcomes. When steroid use was assessed in ACTT-2, preenrollment corticosteroid use was low, and overall use was well balanced between treatment groups within quartiles (Supplement Tables 12 and 13, available at Annals.org). Of note, ACTT-2 excluded many patients with immunosuppression or immunodeficiencies for safety reasons; therefore, our results cannot be generalized to this population, which has accounted for 12% to 20% of COVID-19–related hospitalizations recently (31, 32). Finally, the data collected here are from an early stage of the COVID-19 pandemic. Some data suggest that ALC and ANC remain important predictors of severe COVID-19 as Omicron subvariants have become dominant (33), but further studies are needed to determine the importance of these laboratory parameters against current SARS-CoV-2 variants and in patients with prior immunity due to infection or vaccination.
Despite these limitations, ACTT-2 was a large, double-blind, randomized, placebo-controlled trial that assessed baricitinib+remdesivir in the relative absence of competing therapies and had detailed characterization of participants that was seen in few other studies. Patients identified as being at high risk for severe COVID-19 outcomes by the ACTT risk profile benefit significantly from baricitinib treatment, and baricitinib robustly reverses trends in leukocyte parameters, suggesting a potential mechanism through which it enhances host defense and limits pathology. We observed diversity in ALC and ANC parameters within oxygen requirement categories and capture a patient phenotype of responsiveness to baricitinib treatment based on simple complete blood count parameters.
Baricitinib and other immunomodulators are showing increasing importance for treatment of infectious diseases, such as severe pneumonia, often in combination with antimicrobial therapy (4, 27, 28, 34). The data presented here suggest that hematologic parameters related to host immune status may help identify patients at risk for severe disease and also direct therapies to those who are most likely to benefit. Therapeutic trial design for COVID-19 and other infectious diseases characterized by immune dysregulation should incorporate readily available biomarkers, which may help to identify more specific and personalized treatment approaches.
Supplementary Material
Acknowledgment:
The authors thank the patients who enrolled in the ACTT studies and their families as well as the members of the ACTT study group and study team. They also thank the Frederick National Laboratory for Cancer Research for performing the CRP measurements and Shannon Gallagher for her advice on this project.
Financial Support:
Data from ACTT-1 and ACTT-2 were used in this analysis. Both trials were sponsored and primarily funded by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), through NIAID grants UM1AI148684, UM1AI148576, UM1AI148573, UM1AI148575, UM1AI148452, UM1AI148685, UM1AI148450, and UM1AI148689. The trials have been supported in part by federal funds from the Defense Health Program of the U.S. Department of Defense and by the governments of Denmark, Japan, Mexico, and Singapore. The trial site in South Korea received funding from the Seoul National University Hospital. Support for the London International Coordinating Centre was also provided by the U.K. Medical Research Council (grant no. MRC_UU_12023/23). This project has been funded in whole or in part by federal funds from the National Cancer Institute, NIH, under contract no. 75N910D00024. Dr. Rapaka was supported by a Mentored Clinical Scientist Research Career Development Award from NIAID/NIH (K08 AI143923) during this work.
Footnotes
Disclaimer: The content of this article does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M23-2593.
Data Sharing Statement: The authors have indicated they will not yet be sharing data but plan to in the future after the completion of ongoing secondary analyses.
References
- 1.Beigel JH, Tomashek KM, Dodd LE, et al. ; ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stebbing J, Krishnan V, de Bono S, et al. ; Sacco Baricitinib Study Group. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol Med. 2020;12:e12697. doi: 10.15252/emmm.202012697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sims JT, Krishnan V, Chang CY, et al. Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19. J Allergy Clin Immunol. 2021;147:107–111. doi: 10.1016/j.jaci.2020.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalil AC, Patterson TF, Mehta AK, et al. ; ACTT-2 Study Group Members. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Accessed at www.covid19treatmentguidelines.nih.gov on 30 January 2024.
- 6.Paules CI, Gallagher SK, Rapaka RR, et al. Remdesivir for the prevention of invasive mechanical ventilation or death in coronavirus disease 2019 (COVID-19): a post hoc analysis of the Adaptive COVID-19 Treatment Trial-1 cohort data. Clin Infect Dis. 2022;74:1260–1264. doi: 10.1093/cid/ciab695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Austin PC, Fine JP. Practical recommendations for reporting Fine–Gray model analyses for competing risk data. Stat Med. 2017;36:4391–4400. doi: 10.1002/sim.7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kent DM, Paulus JK, van Klaveren D, et al. The Predictive Approaches to Treatment effect Heterogeneity (PATH) statement. Ann Intern Med. 2020;172:35–45. doi: 10.7326/M18-3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kent DM, van Klaveren D, Paulus JK, et al. The Predictive Approaches to Treatment effect Heterogeneity (PATH) statement: explanation and elaboration. Ann Intern Med. 2020;172:W1–W25. doi: 10.7326/M18-3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simms L, Barraclough H, Govindan R. Biostatistics primer: what a clinician ought to know—prognostic and predictive factors. J Thorac Oncol. 2013;8:808–813. doi: 10.1097/JTO.0b013e318292bdcd [DOI] [PubMed] [Google Scholar]
- 12.Tanaka Y, McInnes IB, Taylor PC, et al. Characterization and changes of lymphocyte subsets in baricitinib-treated patients with rheumatoid arthritis: an integrated analysis. Arthritis Rheumatol. 2018;70:1923–1932. doi: 10.1002/art.40680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kay J, Harigai M, Rancourt J, et al. Changes in selected haematological parameters associated with JAK1/JAK2 inhibition observed in patients with rheumatoid arthritis treated with baricitinib. RMD Open. 2020;6. doi: 10.1136/rmdopen-2020-001370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lv Z, Wang W, Qiao B, et al. The prognostic value of general laboratory testing in patients with COVID-19. J Clin Lab Anal. 2021;35:e23668. doi: 10.1002/jcla.23668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang G, Kovalic AJ, Graber CJ. Prognostic value of leukocytosis and lymphopenia for coronavirus disease severity. Emerg Infect Dis. 2020;26:1839–1841. doi: 10.3201/eid2608.201160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797–806. doi: 10.1002/jmv.25783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meizlish ML, Pine AB, Bishai JD, et al. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Adv. 2021;5:1164–1177. doi: 10.1182/bloodadvances.2020003568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bronte V, Ugel S, Tinazzi E, et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest. 2020; 130:6409–6416. doi: 10.1172/JCI141772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta P, McAuley DF, Brown M, et al. ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solimani F, Meier K, Ghoreschi K. Janus kinase signaling as risk factor and therapeutic target for severe SARS-CoV-2 infection. Eur J Immunol. 2021;51:1071–1075. doi: 10.1002/eji.202149173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calabrese F, Pezzuto F, Fortarezza F, et al. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European pulmonary pathologists. Virchows Arch. 2020;477:359–372. doi: 10.1007/s00428-020-02886-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKenna E, Wubben R, Isaza-Correa JM, et al. Neutrophils in COVID-19: not innocent bystanders. Front Immunol. 2022;13:864387. doi: 10.3389/fimmu.2022.864387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veras FP, Pontelli MC, Silva CM, et al. SARS-CoV-2–triggered neutrophil extracellular traps mediate COVID-19 pathology. J Exp Med. 2020;217:e20201129. doi: 10.1084/jem.20201129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoang TN, Pino M, Boddapati AK, et al. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell. 2021; 184:460–475.e21. doi: 10.1016/j.cell.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marconi VC, Ramanan AV, de Bono S, et al. ; COV-BARRIER Study Group. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9:1407–1418. doi: 10.1016/S2213-2600(21)00331-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon AC, Mouncey PR, Al-Beidh F, et al. ; REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans RA, Dube S, Lu Y, et al. Impact of COVID-19 on immunocompromised populations during the Omicron era: insights from the observational population-based INFORM study. Lancet Reg Health Eur. 2023;35:100747. doi: 10.1016/j.lanepe.2023.100747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singson JRC, Kirley PD, Pham H, et al. ; COVID-NET Surveillance Team. Factors associated with severe outcomes among immunocompromised adults hospitalized for COVID-19 - COVID-NET, 10 states, March 2020–February 2022. MMWR Morb Mortal Wkly Rep. 2022;71:878–884. doi: 10.15585/mmwr.mm7127a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu W, Shi Q, Chen F, et al. The derived neutrophil to lymphocyte ratio can be the predictor of prognosis for COVID-19 Omicron BA.2 infected patients. Front Immunol. 2022;13:1065345. doi: 10.3389/fimmu.2022.1065345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dequin P-F, Meziani F, Quenot J-P, et al. ; CRICS-TriGGERSep Network. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388:1931–1941. doi: 10.1056/NEJMoa2215145 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.