Abstract
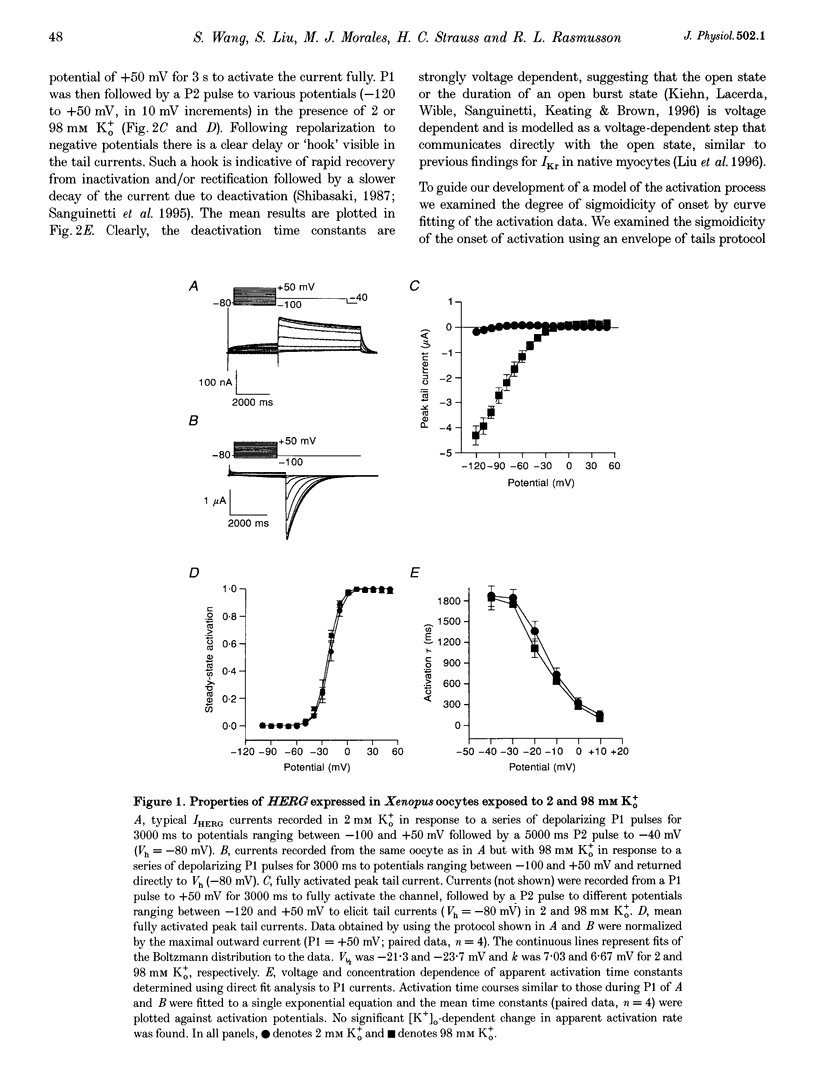
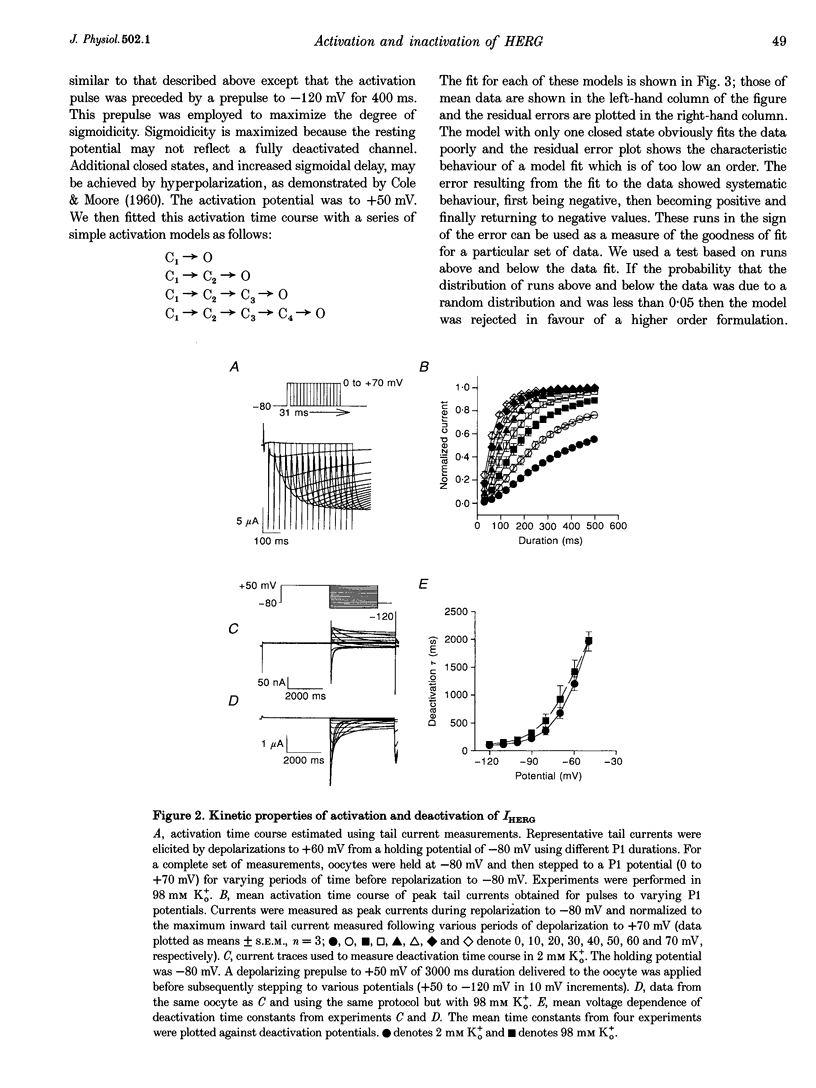
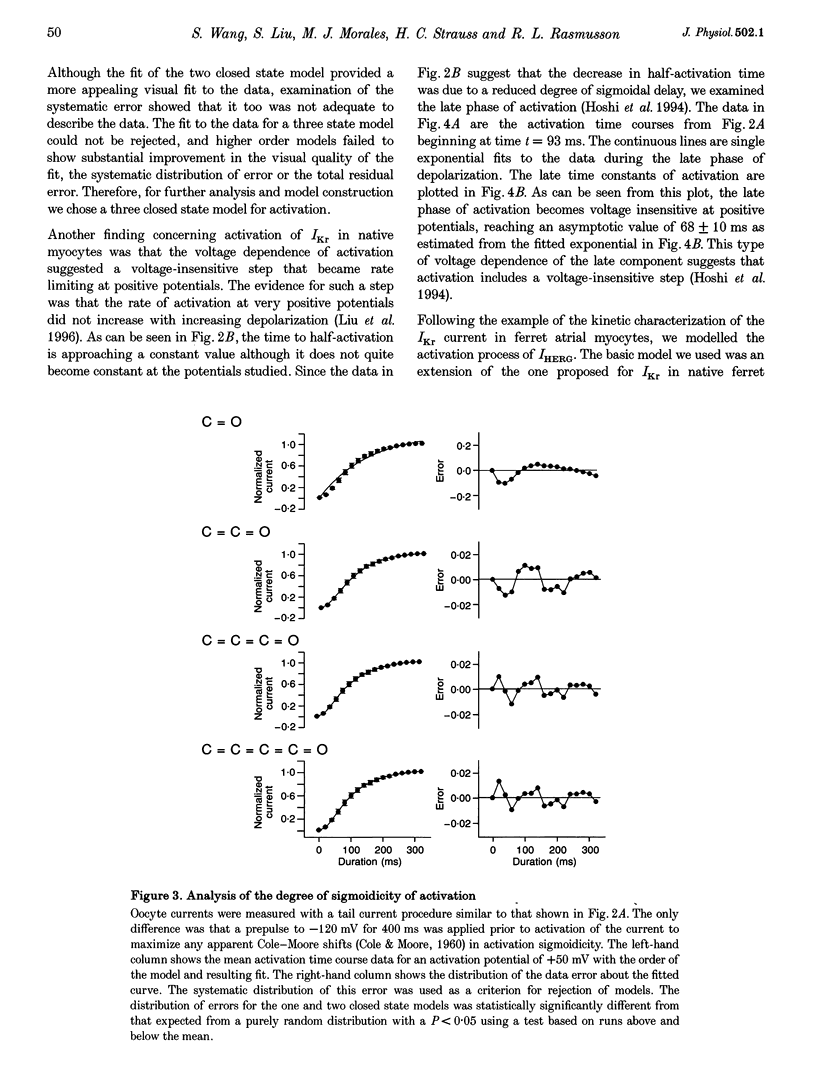
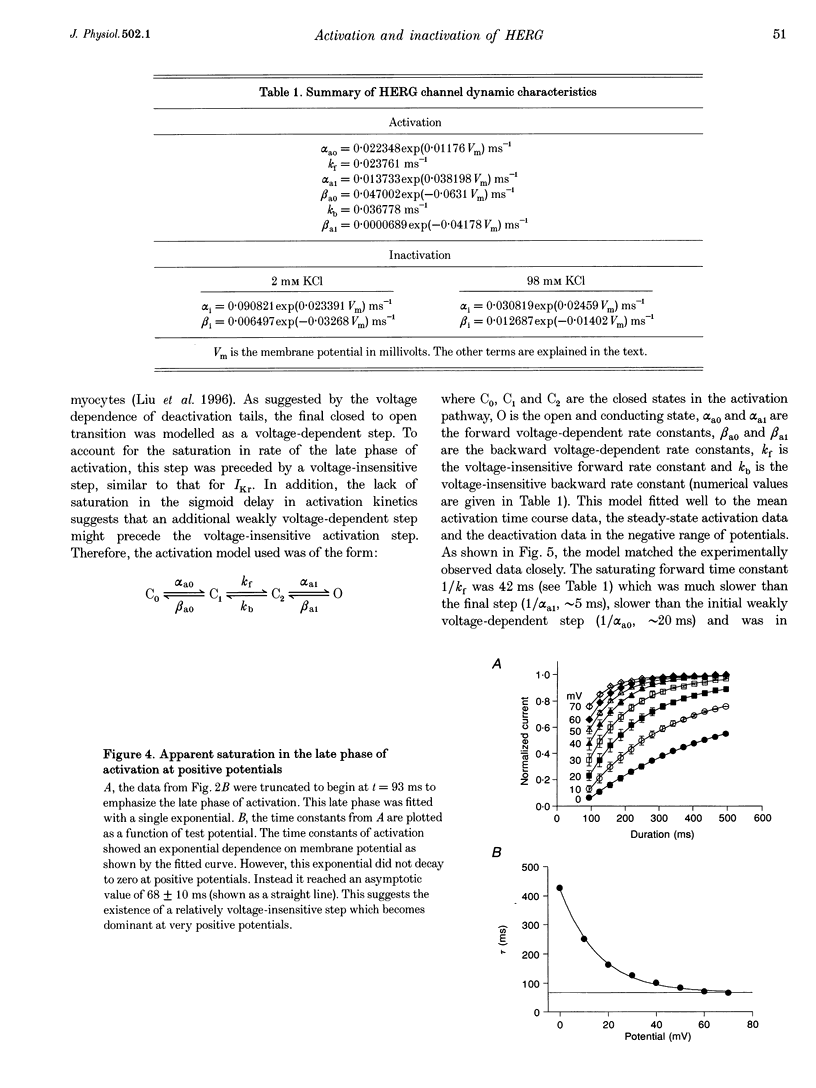
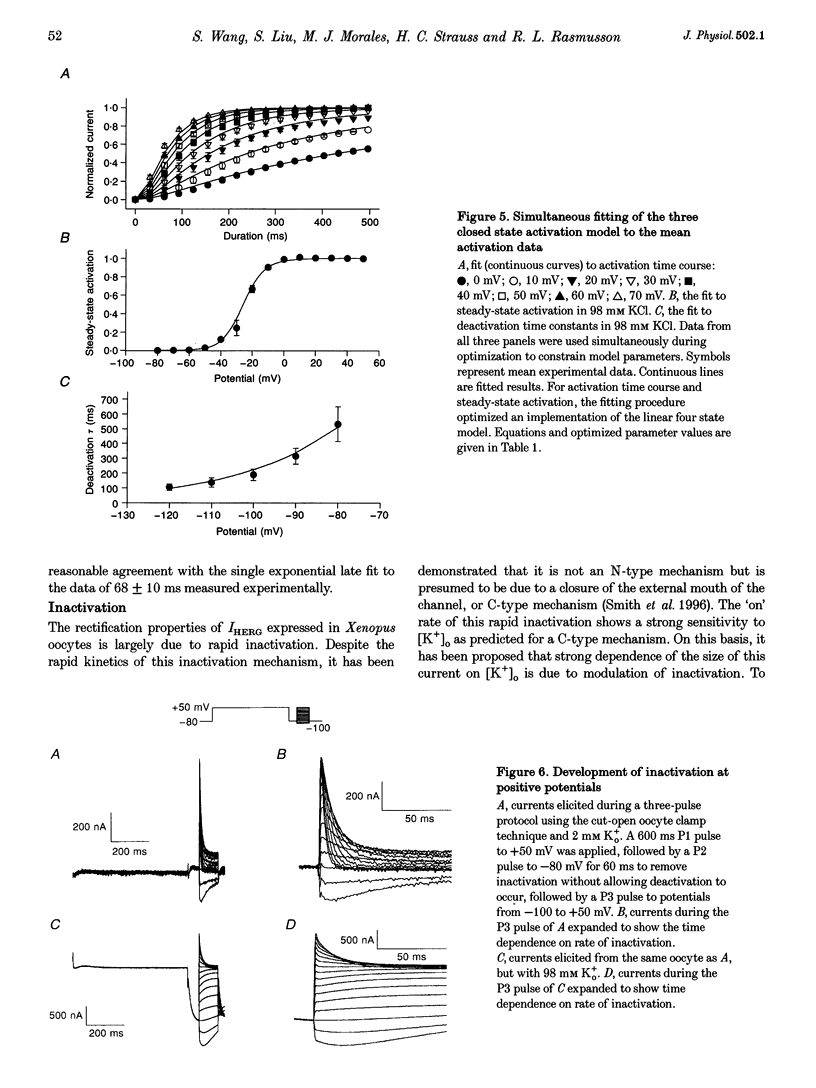
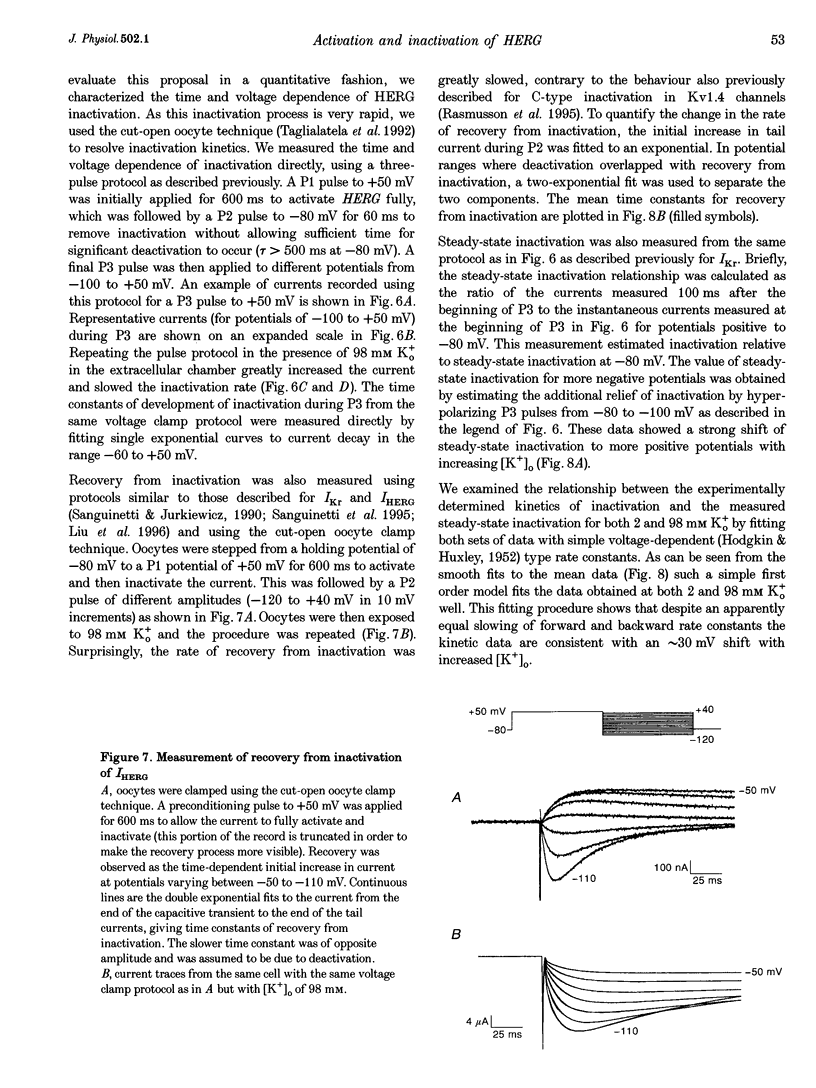
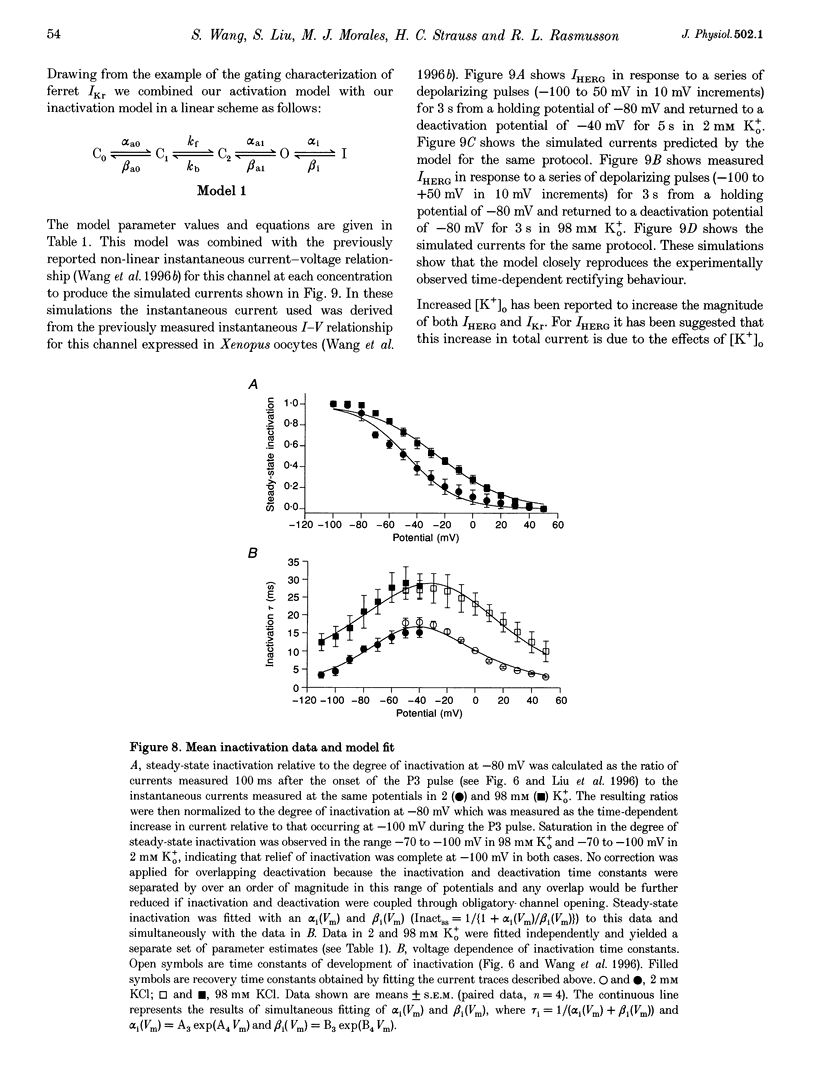
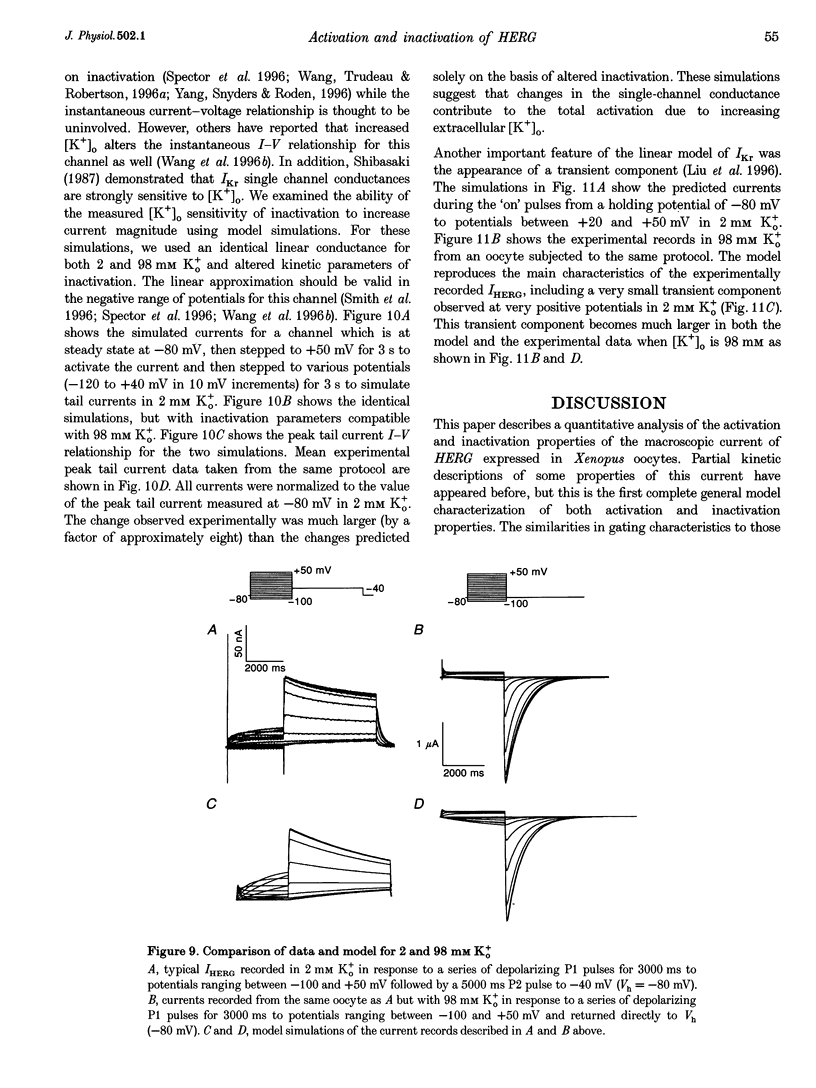
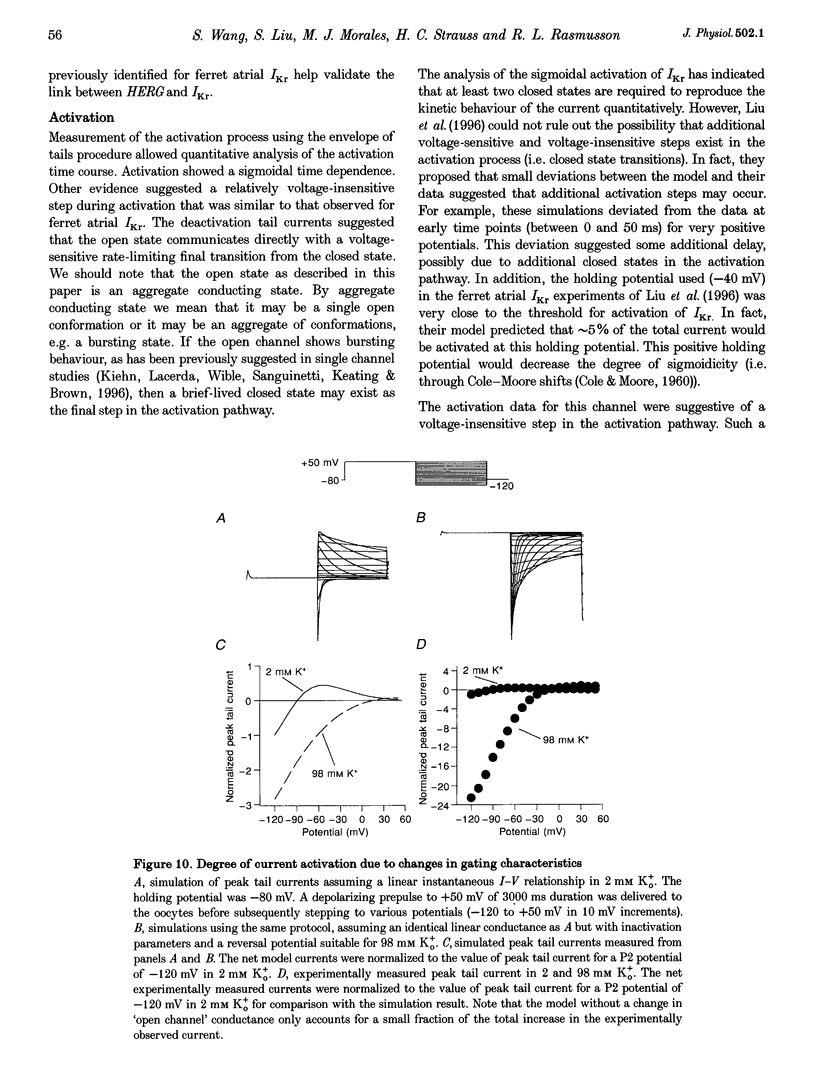
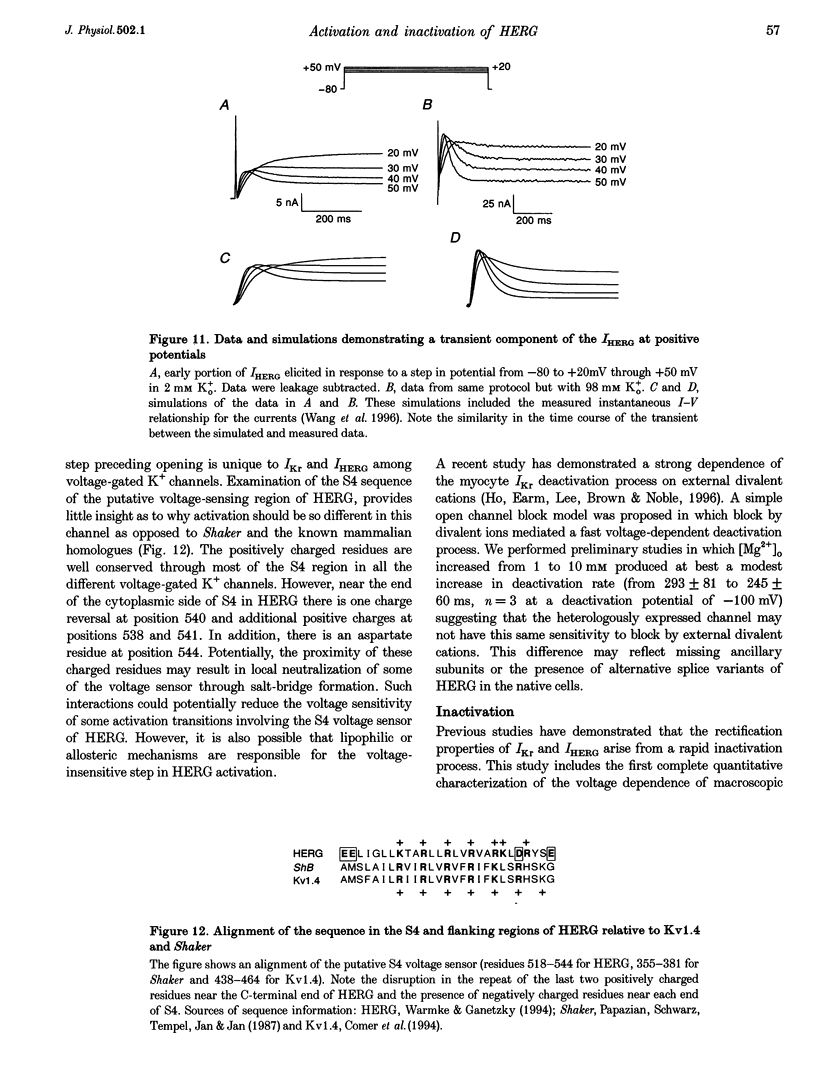
1. The human ether à-go-go-related gene (HERG) encodes a K+ channel that is believed to be the basis of the delayed rectified current, IKr, in cardiac muscle. We studied HERG expressed in Xenopus oocytes using a two-electrode and cut-open oocyte clamp technique with [K+]0 of 2 and 98 mM. 2. The time course of activation of the channel was measured using an envelope of tails protocol and demonstrated that activation of the heterologously expressed HERG current (IHERG) was sigmoidal in onset. At least three closed states were required to reproduce the sigmoid time course. 3. The voltage dependence of the activation process and its saturation at positive voltages suggested the existence of at least one relatively voltage-insensitive step. A three closed state activation model with a single voltage-insensitive intermediate closed state was able to reproduce the time and voltage dependence of activation, deactivation and steady-state activation. Activation was insensitive to changes in [K+]0. 4. Both inactivation and recovery time constants increased with a change of [K+]0 from 2 to 98 mM. Steady-state inactivation shifted by approximately 30 mV in the depolarized direction with a change from 2 to 98 mM K+0. 5. Simulations showed that modulation of inactivation is a minimal component of the increase of this current by [K+]0, and that a large increase in total conductance must also occur.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anumonwo J. M., Freeman L. C., Kwok W. M., Kass R. S. Delayed rectification in single cells isolated from guinea pig sinoatrial node. Am J Physiol. 1992 Mar;262(3 Pt 2):H921–H925. doi: 10.1152/ajpheart.1992.262.3.H921. [DOI] [PubMed] [Google Scholar]
- Balser J. R., Bennett P. B., Roden D. M. Time-dependent outward current in guinea pig ventricular myocytes. Gating kinetics of the delayed rectifier. J Gen Physiol. 1990 Oct;96(4):835–863. doi: 10.1085/jgp.96.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baukrowitz T., Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995 Oct;15(4):951–960. doi: 10.1016/0896-6273(95)90185-x. [DOI] [PubMed] [Google Scholar]
- COLE K. S., MOORE J. W. Potassium ion current in the squid giant axon: dynamic characteristic. Biophys J. 1960 Sep;1:1–14. doi: 10.1016/s0006-3495(60)86871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. L., Rasmusson R. L., Qu Y., Strauss H. C. The calcium-independent transient outward potassium current in isolated ferret right ventricular myocytes. I. Basic characterization and kinetic analysis. J Gen Physiol. 1993 Apr;101(4):571–601. doi: 10.1085/jgp.101.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. L., Rasmusson R. L., Strauss H. C. Ionic current mechanisms generating vertebrate primary cardiac pacemaker activity at the single cell level: an integrative view. Annu Rev Physiol. 1992;54:279–302. doi: 10.1146/annurev.ph.54.030192.001431. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Use-dependent block and use-dependent unblock of the delayed rectifier K+ current by almokalant in rabbit ventricular myocytes. Circ Res. 1993 Nov;73(5):857–868. doi: 10.1161/01.res.73.5.857. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Voltage- and time-dependent block of the delayed K+ current in cardiac myocytes by dofetilide. J Pharmacol Exp Ther. 1992 Aug;262(2):809–817. [PubMed] [Google Scholar]
- Castellino R. C., Morales M. J., Strauss H. C., Rasmusson R. L. Time- and voltage-dependent modulation of a Kv1.4 channel by a beta-subunit (Kv beta 3) cloned from ferret ventricle. Am J Physiol. 1995 Jul;269(1 Pt 2):H385–H391. doi: 10.1152/ajpheart.1995.269.1.H385. [DOI] [PubMed] [Google Scholar]
- Clay J. R., Ogbaghebriel A., Paquette T., Sasyniuk B. I., Shrier A. A quantitative description of the E-4031-sensitive repolarization current in rabbit ventricular myocytes. Biophys J. 1995 Nov;69(5):1830–1837. doi: 10.1016/S0006-3495(95)80053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer M. B., Campbell D. L., Rasmusson R. L., Lamson D. R., Morales M. J., Zhang Y., Strauss H. C. Cloning and characterization of an Ito-like potassium channel from ferret ventricle. Am J Physiol. 1994 Oct;267(4 Pt 2):H1383–H1395. doi: 10.1152/ajpheart.1994.267.4.H1383. [DOI] [PubMed] [Google Scholar]
- Curran M. E., Splawski I., Timothy K. W., Vincent G. M., Green E. D., Keating M. T. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995 Mar 10;80(5):795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- De Biasi M., Hartmann H. A., Drewe J. A., Taglialatela M., Brown A. M., Kirsch G. E. Inactivation determined by a single site in K+ pores. Pflugers Arch. 1993 Jan;422(4):354–363. doi: 10.1007/BF00374291. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. The cardiac hyperpolarizing-activated current, if. Origins and developments. Prog Biophys Mol Biol. 1985;46(3):163–183. doi: 10.1016/0079-6107(85)90008-2. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho W. K., Earm Y. E., Lee S. H., Brown H. F., Noble D. Voltage- and time-dependent block of delayed rectifier K+ current in rabbit sino-atrial node cells by external Ca2+ and Mg2+. J Physiol. 1996 Aug 1;494(Pt 3):727–742. doi: 10.1113/jphysiol.1996.sp021528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990 Oct 26;250(4980):533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Shaker potassium channel gating. I: Transitions near the open state. J Gen Physiol. 1994 Feb;103(2):249–278. doi: 10.1085/jgp.103.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkiewicz N. K., Sanguinetti M. C. Rate-dependent prolongation of cardiac action potentials by a methanesulfonanilide class III antiarrhythmic agent. Specific block of rapidly activating delayed rectifier K+ current by dofetilide. Circ Res. 1993 Jan;72(1):75–83. doi: 10.1161/01.res.72.1.75. [DOI] [PubMed] [Google Scholar]
- Liu S., Rasmusson R. L., Campbell D. L., Wang S., Strauss H. C. Activation and inactivation kinetics of an E-4031-sensitive current from single ferret atrial myocytes. Biophys J. 1996 Jun;70(6):2704–2715. doi: 10.1016/S0006-3495(96)79840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Barneo J., Hoshi T., Heinemann S. H., Aldrich R. W. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1993;1(1):61–71. [PubMed] [Google Scholar]
- McAllister R. E., Noble D., Tsien R. W. Reconstruction of the electrical activity of cardiac Purkinje fibres. J Physiol. 1975 Sep;251(1):1–59. doi: 10.1113/jphysiol.1975.sp011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraki K., Imaizumi Y., Watanabe M., Habuchi Y., Giles W. R. Delayed rectifier K+ current in rabbit atrial myocytes. Am J Physiol. 1995 Aug;269(2 Pt 2):H524–H532. doi: 10.1152/ajpheart.1995.269.2.H524. [DOI] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazian D. M., Schwarz T. L., Tempel B. L., Jan Y. N., Jan L. Y. Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila. Science. 1987 Aug 14;237(4816):749–753. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- Rasmusson R. L., Morales M. J., Castellino R. C., Zhang Y., Campbell D. L., Strauss H. C. C-type inactivation controls recovery in a fast inactivating cardiac K+ channel (Kv1.4) expressed in Xenopus oocytes. J Physiol. 1995 Dec 15;489(Pt 3):709–721. doi: 10.1113/jphysiol.1995.sp021085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M. C., Jiang C., Curran M. E., Keating M. T. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995 Apr 21;81(2):299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Jurkiewicz N. K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990 Jul;96(1):195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönherr R., Heinemann S. H. Molecular determinants for activation and inactivation of HERG, a human inward rectifier potassium channel. J Physiol. 1996 Jun 15;493(Pt 3):635–642. doi: 10.1113/jphysiol.1996.sp021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. J Physiol. 1987 Jun;387:227–250. doi: 10.1113/jphysiol.1987.sp016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata E. F., Giles W. R. Ionic currents that generate the spontaneous diastolic depolarization in individual cardiac pacemaker cells. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7796–7800. doi: 10.1073/pnas.82.22.7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. L., Baukrowitz T., Yellen G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature. 1996 Feb 29;379(6568):833–836. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- Spector P. S., Curran M. E., Zou A., Keating M. T., Sanguinetti M. C. Fast inactivation causes rectification of the IKr channel. J Gen Physiol. 1996 May;107(5):611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialatela M., Toro L., Stefani E. Novel voltage clamp to record small, fast currents from ion channels expressed in Xenopus oocytes. Biophys J. 1992 Jan;61(1):78–82. doi: 10.1016/S0006-3495(92)81817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau M. C., Warmke J. W., Ganetzky B., Robertson G. A. HERG sequence correction. Science. 1996 May 24;272(5265):1087–1087. [PubMed] [Google Scholar]
- Trudeau M. C., Warmke J. W., Ganetzky B., Robertson G. A. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995 Jul 7;269(5220):92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- Verheijck E. E., van Ginneken A. C., Bourier J., Bouman L. N. Effects of delayed rectifier current blockade by E-4031 on impulse generation in single sinoatrial nodal myocytes of the rabbit. Circ Res. 1995 Apr;76(4):607–615. doi: 10.1161/01.res.76.4.607. [DOI] [PubMed] [Google Scholar]
- Wang S., Morales M. J., Liu S., Strauss H. C., Rasmusson R. L. Time, voltage and ionic concentration dependence of rectification of h-erg expressed in Xenopus oocytes. FEBS Lett. 1996 Jul 1;389(2):167–173. doi: 10.1016/0014-5793(96)00570-4. [DOI] [PubMed] [Google Scholar]
- Wang Z., Fermini B., Nattel S. Delayed rectifier outward current and repolarization in human atrial myocytes. Circ Res. 1993 Aug;73(2):276–285. doi: 10.1161/01.res.73.2.276. [DOI] [PubMed] [Google Scholar]
- Wang Z., Fermini B., Nattel S. Rapid and slow components of delayed rectifier current in human atrial myocytes. Cardiovasc Res. 1994 Oct;28(10):1540–1546. doi: 10.1093/cvr/28.10.1540. [DOI] [PubMed] [Google Scholar]
- Warmke J. W., Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]


