Abstract
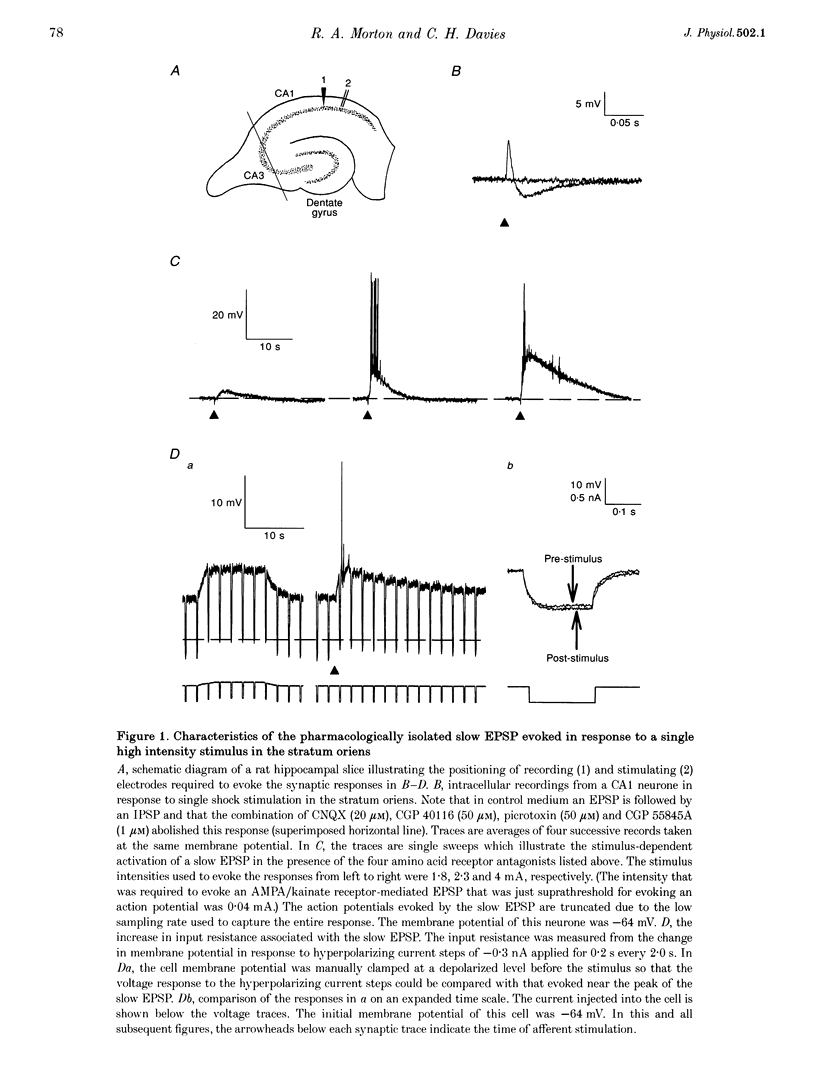
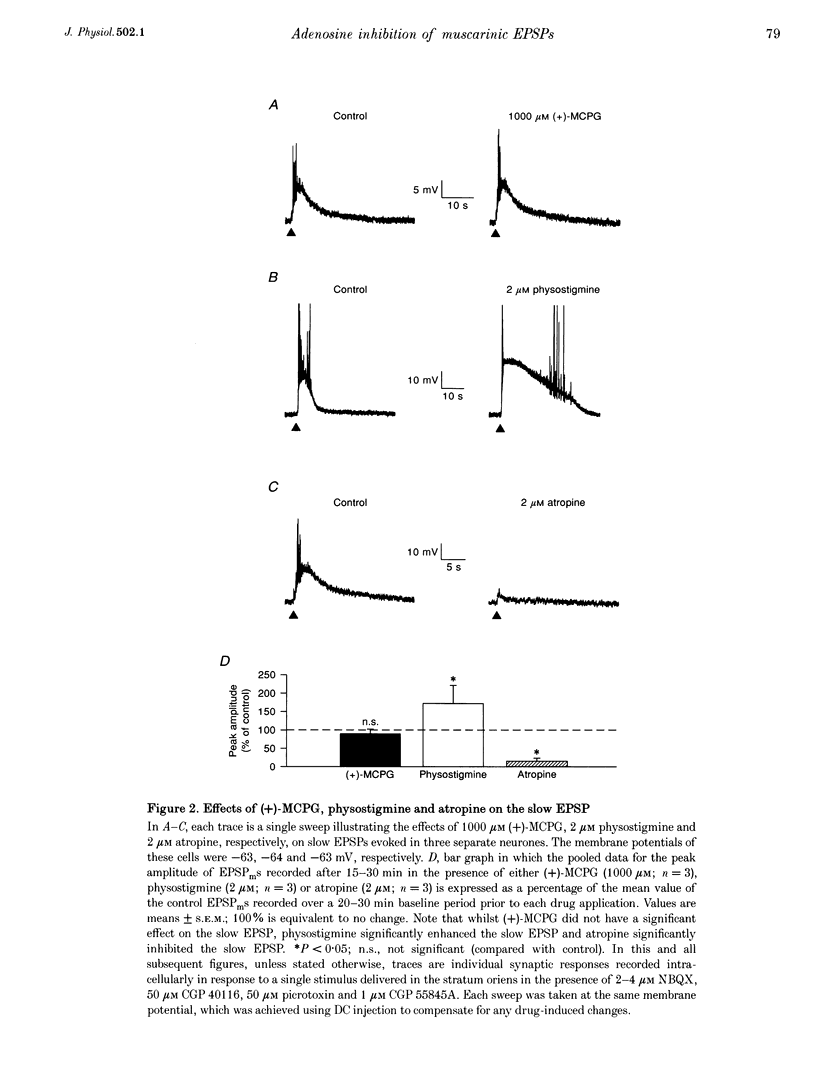
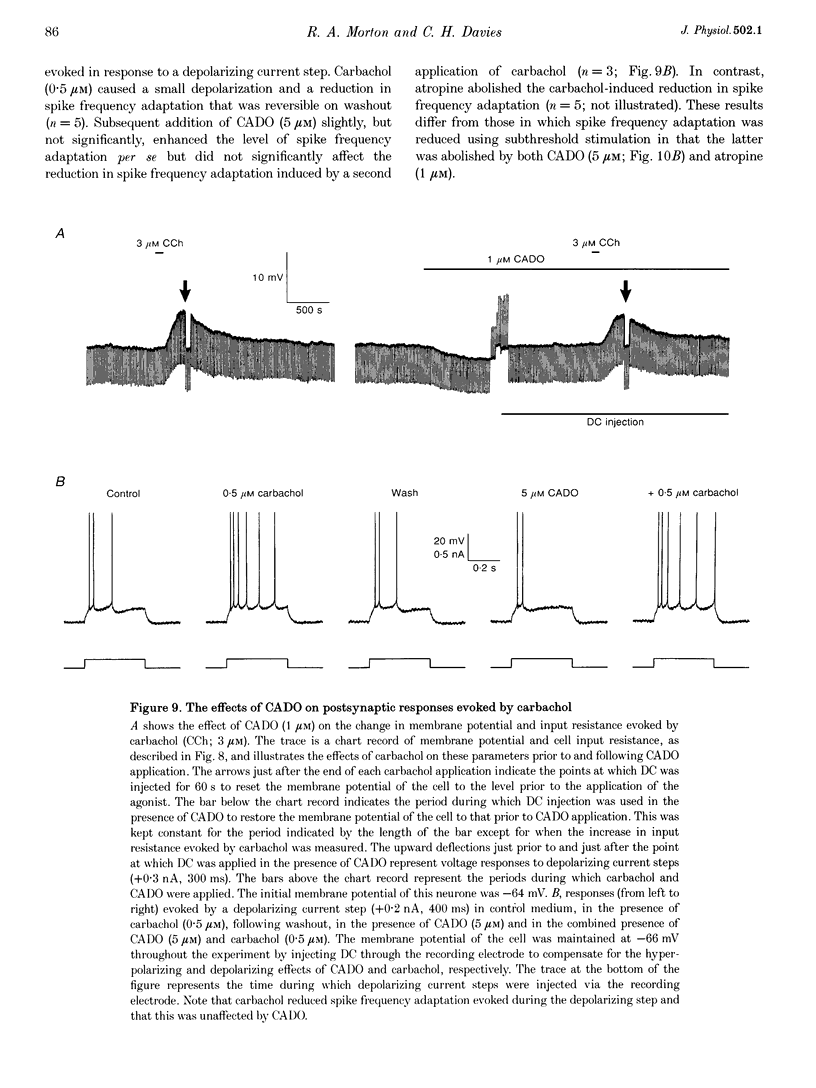
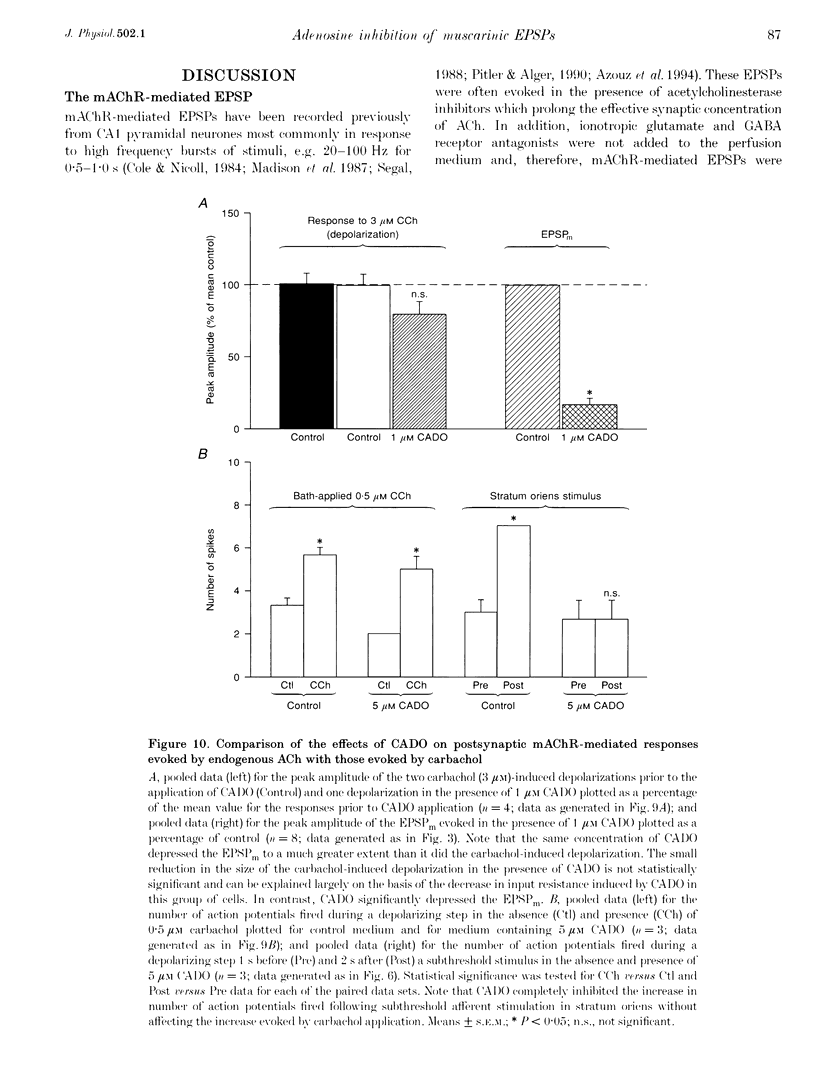
1. Intracellular current clamp recordings were made from CA1 pyramidal neurones in rat hippocampal slices. Experiments were performed in the presence of ionotropic glutamate receptor antagonists and gamma-aminobutyric acid (GABA) receptor antagonists to block all fast excitatory and inhibitory synaptic transmission. A single stimulus, delivered extracellularly in the stratum oriens, caused a reduction in spike frequency adaptation in response to a depolarizing current step delivered 2 s after the stimulus. A 2- to 10-fold increase in stimulus intensity evoked a slow excitatory postsynaptic potential (EPSP) which was associated with a small increase in input resistance. The peak amplitude of the EPSP occurred approximately 2.5 s after the stimulus and its magnitude (up to 30 mV) and duration (10-50 s) increased with increasing stimulus intensity. 2. The slow EPSP was unaffected by the metabotropic glutamate receptor antagonist (+)-alpha-methyl-4-carboxyphenylglycine ((+)-MCPG; 1000 microM) but was greatly enhanced by the acetylcholinesterase inhibitor physostigmine (1-5 microM). Both the slow EPSP and the stimulus-evoked reduction in spike frequency adaptation were inhibited by the muscarinic acetylcholine receptor (mAChR) antagonist atropine (1-5 microM). These results are consistent with these effects being mediated by mAChRs. 3. Both the mAChR-mediated EPSP (EPSPm) and the associated reduction in spike frequency adaptation were reversibly depressed (up to 97%) by either adenosine (100 microM) or its non-hydrolysable analogue 2-chloroadenosine (CADO; 0.1-5.0 microM). These effects were often accompanied by postsynaptic hyperpolarization (up to 8 mV) and a reduction in input resistance (up to 11%). The selective adenosine A1 receptor agonists 2-chloro-N6-cyclopentyladenosine (CCPA; 0.1-0.4 microM) and R(-)N6-(2-phenylisopropyl)-adenosine (R-PIA; 1 microM) both depressed the EPSPm. In contrast, the adenosine A2A receptor agonist 2-p-(2-carboxyethyl)-phenethylamino-5'-N-ethylcarboxamidoadenosine (CGS 21680; 0.5-1.0 microM) did not significantly affect the EPSPm. 4. The selective adenosine A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; 0.2 microM) fully reversed the depressant effects of both adenosine (100 microM) and CADO (1 microM) on the EPSPm and the stimulus-evoked reductions in spike frequency adaptation. 5. DPCPX (0.2 microM) alone caused a small but variable mean increase in the EPSPm of 22 +/- 19% and enabled activation of an EPSPm by a previously subthreshold stimulus. In contrast, the selective adenosine kinase inhibitor 5-iodotubercidin (5-IT; 10 microM) inhibited the EPSPm by 74 +/- 10%, an effect that was reversed by DPCPX. 6. The concentration-response relationship for the depressant action of CADO on the EPSPm more closely paralleled that for its presynaptic depressant action on glutamate-mediated EPSPs than that for postsynaptic hyperpolarization. The respective mean IC50 and EC50 concentrations for these effects were 0.3, 0.8 and 3.0 microM. 7. CADO (1-5 microM) did not have a significant effect on the postsynaptic depolarization, increase in input resistance and reduction in spike frequency adaptation evoked by carbachol (0.5-3.0 microM). All these effects were abolished by atropine (1 microM). 8. These data provide good evidence for an adenosine A1 receptor-mediated inhibition of mAChR-mediated synaptic responses in hippocampal CA1 pyramidal neurones. This inhibition is mediated predominantly presynaptically, is active tonically and can be enhanced when extracellular levels of endogenous adenosine are raised.
Full text
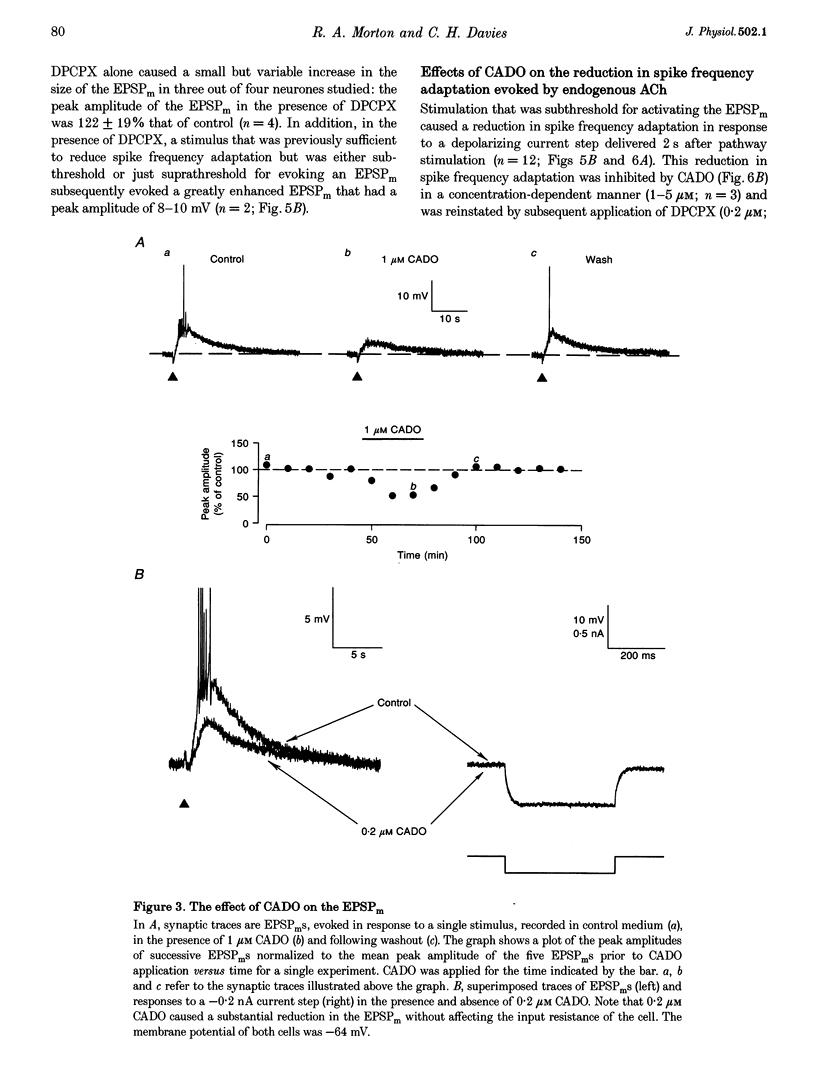
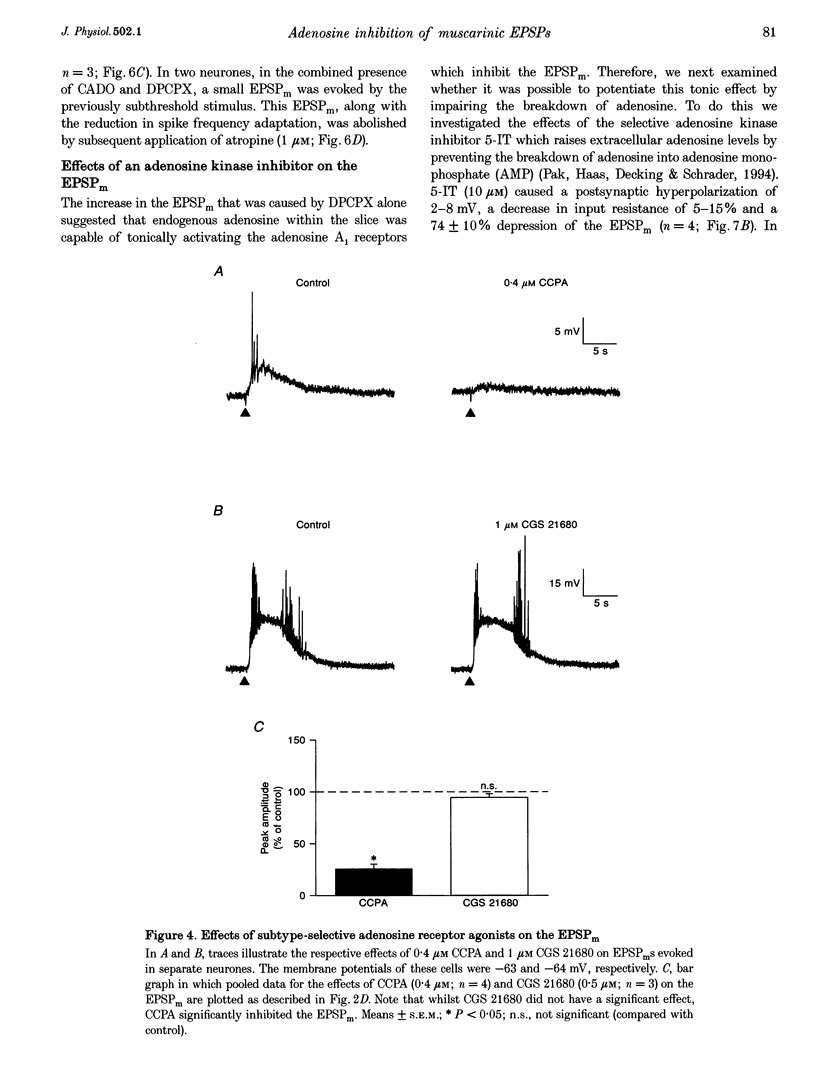
PDF


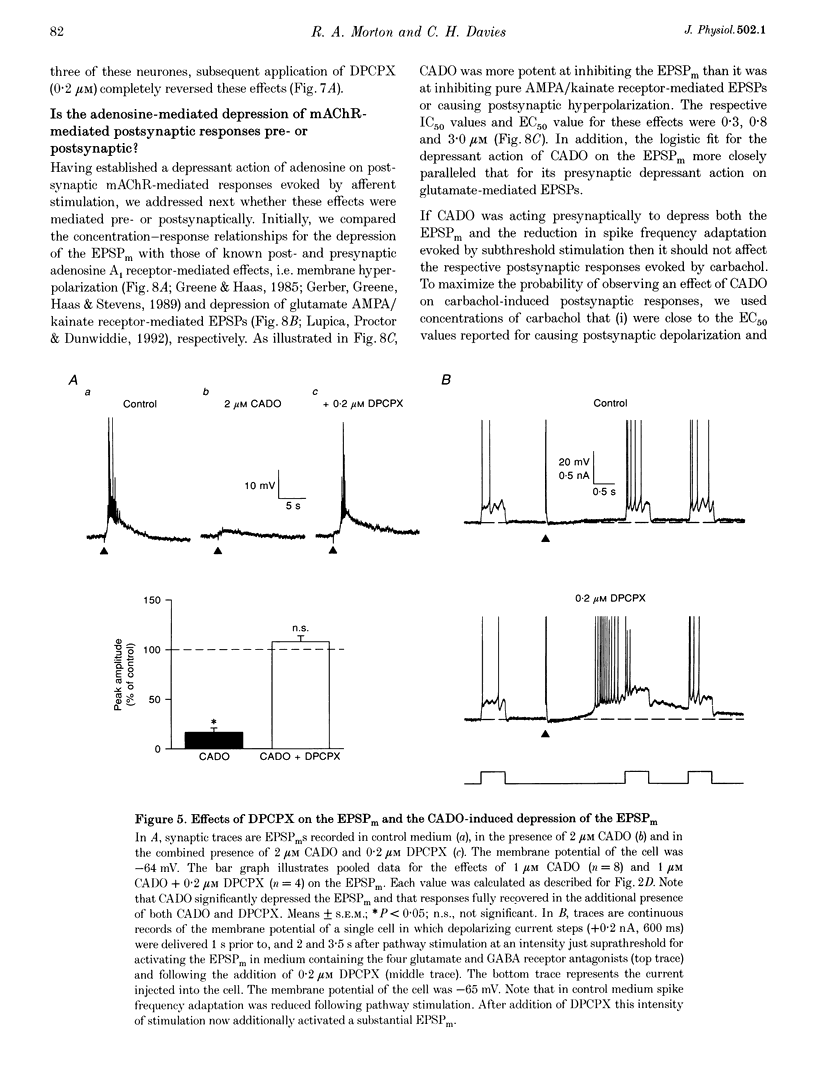
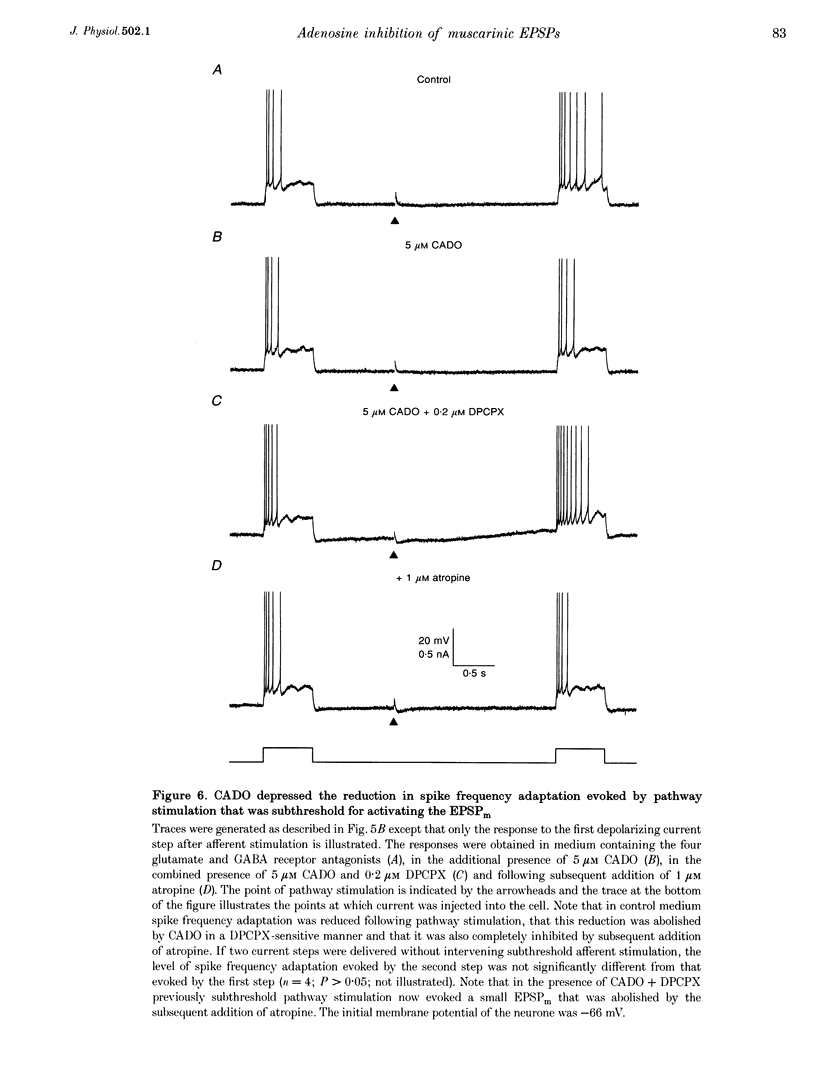



Selected References
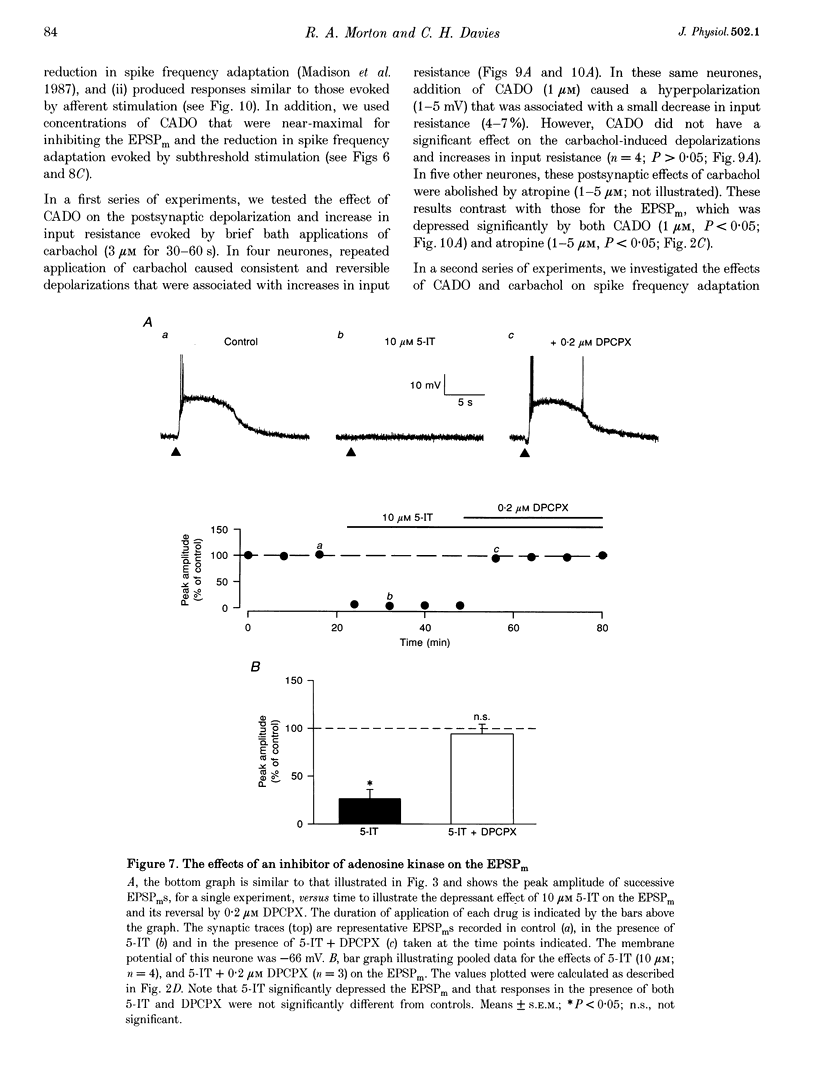
These references are in PubMed. This may not be the complete list of references from this article.
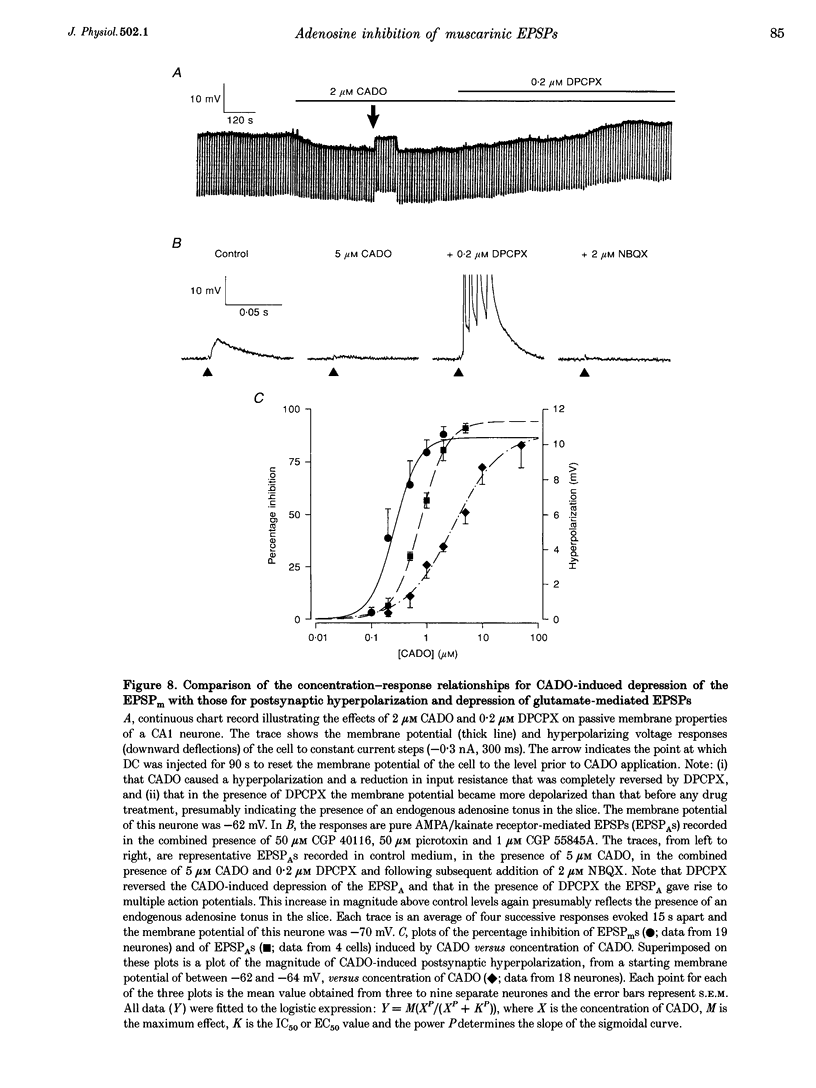
- Azouz R., Jensen M. S., Yaari Y. Muscarinic modulation of intrinsic burst firing in rat hippocampal neurons. Eur J Neurosci. 1994 Jun 1;6(6):961–966. doi: 10.1111/j.1460-9568.1994.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Bianchi R., Wong R. K. Excitatory synaptic potentials dependent on metabotropic glutamate receptor activation in guinea-pig hippocampal pyramidal cells. J Physiol. 1995 Sep 15;487(Pt 3):663–676. doi: 10.1113/jphysiol.1995.sp020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpak S., Gähwiler B. H. Glutamate mediates a slow synaptic response in hippocampal slice cultures. Proc Biol Sci. 1991 Mar 22;243(1308):221–226. doi: 10.1098/rspb.1991.0035. [DOI] [PubMed] [Google Scholar]
- Cole A. E., Nicoll R. A. Acetylcholine mediates a slow synaptic potential in hippocampal pyramidal cells. Science. 1983 Sep 23;221(4617):1299–1301. doi: 10.1126/science.6612345. [DOI] [PubMed] [Google Scholar]
- Cole A. E., Nicoll R. A. Characterization of a slow cholinergic post-synaptic potential recorded in vitro from rat hippocampal pyramidal cells. J Physiol. 1984 Jul;352:173–188. doi: 10.1113/jphysiol.1984.sp015285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colino A., Halliwell J. V. Carbachol potentiates Q current and activates a calcium-dependent non-specific conductance in rat hippocampus in vitro. Eur J Neurosci. 1993 Sep 1;5(9):1198–1209. doi: 10.1111/j.1460-9568.1993.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Connolly G. P., Stone T. W. Adenosine selectively depresses muscarinic compared with non-muscarinic receptor mediated depolarisation of the rat superior cervical ganglion. Gen Pharmacol. 1995 Jul;26(4):865–873. doi: 10.1016/0306-3623(94)00257-n. [DOI] [PubMed] [Google Scholar]
- Cunha R. A., Milusheva E., Vizi E. S., Ribeiro J. A., Sebastião A. M. Excitatory and inhibitory effects of A1 and A2A adenosine receptor activation on the electrically evoked [3H]acetylcholine release from different areas of the rat hippocampus. J Neurochem. 1994 Jul;63(1):207–214. doi: 10.1046/j.1471-4159.1994.63010207.x. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T. V., Diao L. Extracellular adenosine concentrations in hippocampal brain slices and the tonic inhibitory modulation of evoked excitatory responses. J Pharmacol Exp Ther. 1994 Feb;268(2):537–545. [PubMed] [Google Scholar]
- Dutar P., Bassant M. H., Senut M. C., Lamour Y. The septohippocampal pathway: structure and function of a central cholinergic system. Physiol Rev. 1995 Apr;75(2):393–427. doi: 10.1152/physrev.1995.75.2.393. [DOI] [PubMed] [Google Scholar]
- Dutar P., Lamour Y., Nicoll R. A. Galanin blocks the slow cholinergic EPSP in CA1 pyramidal neurons from ventral hippocampus. Eur J Pharmacol. 1989 May 19;164(2):355–360. doi: 10.1016/0014-2999(89)90477-9. [DOI] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Classification of muscarinic responses in hippocampus in terms of receptor subtypes and second-messenger systems: electrophysiological studies in vitro. J Neurosci. 1988 Nov;8(11):4214–4224. doi: 10.1523/JNEUROSCI.08-11-04214.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B., Dunwiddie T. V., Bergman B., Lindström K. Levels of adenosine and adenine nucleotides in slices of rat hippocampus. Brain Res. 1984 Mar 12;295(1):127–136. doi: 10.1016/0006-8993(84)90823-0. [DOI] [PubMed] [Google Scholar]
- Gahwiler B. H., Brown D. A. Functional innervation of cultured hippocampal neurones by cholinergic afferents from co-cultured septal explants. Nature. 1985 Feb 14;313(6003):577–579. doi: 10.1038/313577a0. [DOI] [PubMed] [Google Scholar]
- Gerber U., Greene R. W., Haas H. L., Stevens D. R. Characterization of inhibition mediated by adenosine in the hippocampus of the rat in vitro. J Physiol. 1989 Oct;417:567–578. doi: 10.1113/jphysiol.1989.sp017819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber U., Lüthi A., Gähwiler B. H. Inhibition of a slow synaptic response by a metabotropic glutamate receptor antagonist in hippocampal CA3 pyramidal cells. Proc Biol Sci. 1993 Nov 22;254(1340):169–172. doi: 10.1098/rspb.1993.0142. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L., Hirst G. D. The effect of adenosine on the release of the transmitter from the phrenic nerve of the rat. J Physiol. 1972 Aug;224(3):629–645. doi: 10.1113/jphysiol.1972.sp009916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. W., Haas H. L. Adenosine actions on CA1 pyramidal neurones in rat hippocampal slices. J Physiol. 1985 Sep;366:119–127. doi: 10.1113/jphysiol.1985.sp015788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothman E. W., Bertram E. H., 3rd, Stringer J. L. Functional anatomy of hippocampal seizures. Prog Neurobiol. 1991;37(1):1–82. doi: 10.1016/0301-0082(91)90011-o. [DOI] [PubMed] [Google Scholar]
- Lupica C. R., Proctor W. R., Dunwiddie T. V. Presynaptic inhibition of excitatory synaptic transmission by adenosine in rat hippocampus: analysis of unitary EPSP variance measured by whole-cell recording. J Neurosci. 1992 Oct;12(10):3753–3764. doi: 10.1523/JNEUROSCI.12-10-03753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison D. V., Lancaster B., Nicoll R. A. Voltage clamp analysis of cholinergic action in the hippocampus. J Neurosci. 1987 Mar;7(3):733–741. doi: 10.1523/JNEUROSCI.07-03-00733.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. B., Lupica C. R., Dunwiddie T. V. Activity-dependent release of endogenous adenosine modulates synaptic responses in the rat hippocampus. J Neurosci. 1993 Aug;13(8):3439–3447. doi: 10.1523/JNEUROSCI.13-08-03439.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak M. A., Haas H. L., Decking U. K., Schrader J. Inhibition of adenosine kinase increases endogenous adenosine and depresses neuronal activity in hippocampal slices. Neuropharmacology. 1994 Sep;33(9):1049–1053. doi: 10.1016/0028-3908(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Pedarzani P., Storm J. F. PKA mediates the effects of monoamine transmitters on the K+ current underlying the slow spike frequency adaptation in hippocampal neurons. Neuron. 1993 Dec;11(6):1023–1035. doi: 10.1016/0896-6273(93)90216-e. [DOI] [PubMed] [Google Scholar]
- Pitler T. A., Alger B. E. Activation of the pharmacologically defined M3 muscarinic receptor depolarizes hippocampal pyramidal cells. Brain Res. 1990 Nov 26;534(1-2):257–262. doi: 10.1016/0006-8993(90)90137-z. [DOI] [PubMed] [Google Scholar]
- Segal M., Fisher A. AF102B, a muscarinic M1 receptor agonist, mimics some effects of acetylcholine on neurons of rat hippocampus slices. Eur J Pharmacol. 1992 Sep 10;220(1):103–106. doi: 10.1016/0014-2999(92)90019-z. [DOI] [PubMed] [Google Scholar]
- Segal M. Synaptic activation of a cholinergic receptor in rat hippocampus. Brain Res. 1988 Jun 14;452(1-2):79–86. doi: 10.1016/0006-8993(88)90011-x. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. On the mechanism by which adenosine receptor activation inhibits the release of acetylcholine from motor nerve endings. J Physiol. 1984 Jan;346:243–256. doi: 10.1113/jphysiol.1984.sp015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. M., Capogna M., Scanziani M. Presynaptic inhibition in the hippocampus. Trends Neurosci. 1993 Jun;16(6):222–227. doi: 10.1016/0166-2236(93)90160-n. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Haas H. L., Gähwiler B. H. Comparison of the actions of adenosine at pre- and postsynaptic receptors in the rat hippocampus in vitro. J Physiol. 1992;451:347–363. doi: 10.1113/jphysiol.1992.sp019168. [DOI] [PMC free article] [PubMed] [Google Scholar]


