Abstract
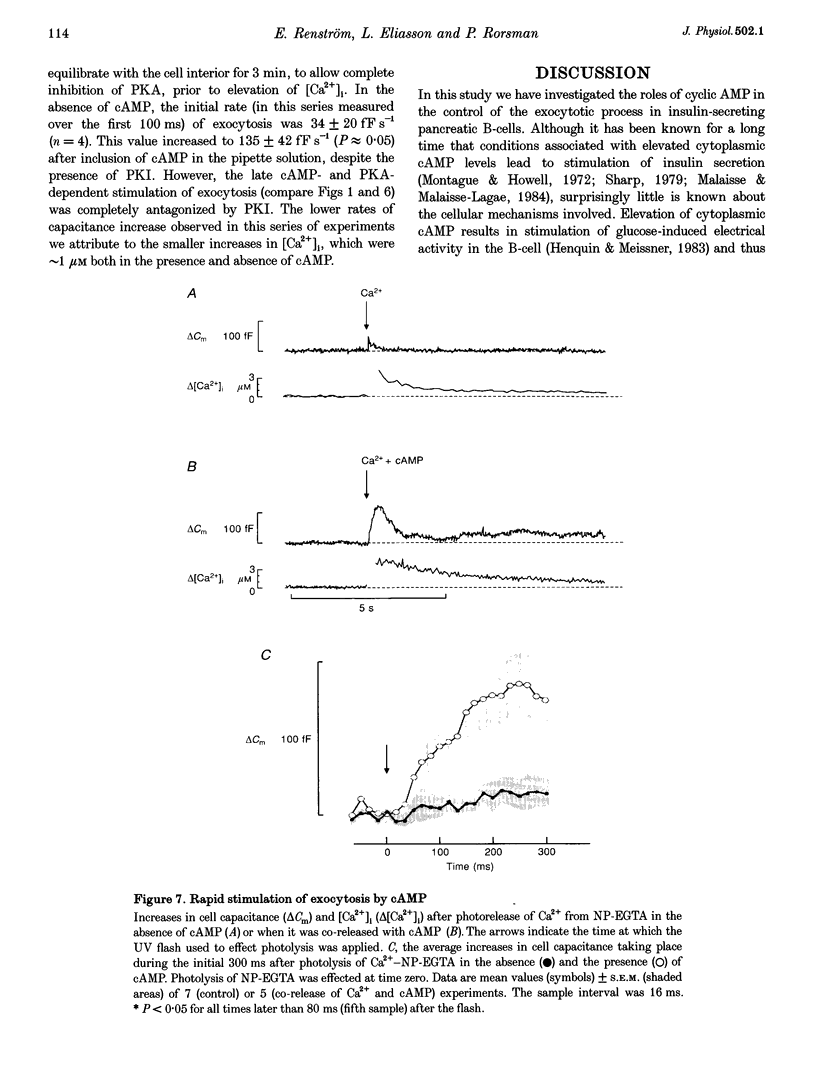
1. The mechanisms by which cAMP stimulates Ca(2+)-dependent insulin secretion were investigated by combining measurements of whole-cell Ca2+ currents, the cytoplasmic free Ca2+ concentration ([Ca2+]i) and membrane capacitance in single mouse B-cells maintained in tissue culture. 2. Cyclic AMP stimulated exocytosis > 4-fold in whole-cell experiments in which secretion was evoked by intracellular dialysis with a Ca(2+)-EGTA buffer with a [Ca2+]i of 1.5 microM. This effect was antagonized by inhibitors of protein kinase A (PKA). 3. Photorelease of cAMP from a caged precursor potentiated exocytosis at Ca2+ concentrations which were themselves stimulatory (> or = 60 nM), but was without effect in the complete absence of Ca2+. 4. Elevation of intracellular cAMP (by exposure to forskolin) evoked a 6-fold PKA-dependent enhancement of the maximal exocytotic response (determined as the maximum increase in cell capacitance that could be elicited by a train of depolarizations) in perforated-patch whole-cell recordings. 5. Exocytosis triggered by single depolarizations in standard whole-cell recordings was strongly potentiated by cAMP, but in this case the effect was unaffected by PKA inhibition. 6. When exocytosis was triggered by Ca2+ released from Ca(2+)-NP-EGTA ('caged Ca2+'), cAMP exerted a dual stimulatory effect on secretion: a rapid (initiated within 80 ms) PKA-independent phase and a late PKA-dependent component. 7. We conclude that cAMP stimulates insulin secretion both by increasing the release probability of secretory granules already in the readily releasable pool and by accelerating the refilling of this pool.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammälä C., Ashcroft F. M., Rorsman P. Calcium-independent potentiation of insulin release by cyclic AMP in single beta-cells. Nature. 1993 May 27;363(6427):356–358. doi: 10.1038/363356a0. [DOI] [PubMed] [Google Scholar]
- Bertuzzi F., Berra C., Socci C., Davalli A. M., Calori G., Freschi M., Piemonti L., De Nittis P., Pozza G., Pontiroli A. E. Glucagon improves insulin secretion from pig islets in vitro. J Endocrinol. 1995 Oct;147(1):87–93. doi: 10.1677/joe.0.1470087. [DOI] [PubMed] [Google Scholar]
- Bokvist K., Eliasson L., Ammälä C., Renström E., Rorsman P. Co-localization of L-type Ca2+ channels and insulin-containing secretory granules and its significance for the initiation of exocytosis in mouse pancreatic B-cells. EMBO J. 1995 Jan 3;14(1):50–57. doi: 10.1002/j.1460-2075.1995.tb06974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J., Li J., Davidson N., Lester H. A., Zinn K. Heteromeric olfactory cyclic nucleotide-gated channels: a subunit that confers increased sensitivity to cAMP. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8890–8894. doi: 10.1073/pnas.91.19.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttler L., Collins B. J., Marone P. A., Szabo M. The effect of isobutylmethylxanthine, forskolin, and cholera toxin on growth hormone release from pituitary cell cultures of perinatal and mature rats. Endocr Res. 1993 Mar;19(1):33–46. doi: 10.3109/07435809309035406. [DOI] [PubMed] [Google Scholar]
- Distler M., Biel M., Flockerzi V., Hofmann F. Expression of cyclic nucleotide-gated cation channels in non-sensory tissues and cells. Neuropharmacology. 1994 Nov;33(11):1275–1282. doi: 10.1016/0028-3908(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Gillis K. D., Misler S. Enhancers of cytosolic cAMP augment depolarization-induced exocytosis from pancreatic B-cells: evidence for effects distal to Ca2+ entry. Pflugers Arch. 1993 Jul;424(2):195–197. doi: 10.1007/BF00374612. [DOI] [PubMed] [Google Scholar]
- Gillis K. D., Mossner R., Neher E. Protein kinase C enhances exocytosis from chromaffin cells by increasing the size of the readily releasable pool of secretory granules. Neuron. 1996 Jun;16(6):1209–1220. doi: 10.1016/s0896-6273(00)80147-6. [DOI] [PubMed] [Google Scholar]
- Gremlich S., Porret A., Hani E. H., Cherif D., Vionnet N., Froguel P., Thorens B. Cloning, functional expression, and chromosomal localization of the human pancreatic islet glucose-dependent insulinotropic polypeptide receptor. Diabetes. 1995 Oct;44(10):1202–1208. doi: 10.2337/diab.44.10.1202. [DOI] [PubMed] [Google Scholar]
- Heinemann C., Chow R. H., Neher E., Zucker R. S. Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+. Biophys J. 1994 Dec;67(6):2546–2557. doi: 10.1016/S0006-3495(94)80744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Dibutyryl cyclic AMP triggers Ca2+ influx and Ca2+-dependent electrical activity in pancreatic B cells. Biochem Biophys Res Commun. 1983 Apr 29;112(2):614–620. doi: 10.1016/0006-291x(83)91508-5. [DOI] [PubMed] [Google Scholar]
- Jones P. M., Fyles J. M., Howell S. L. Regulation of insulin secretion by cAMP in rat islets of Langerhans permeabilised by high-voltage discharge. FEBS Lett. 1986 Sep 15;205(2):205–209. doi: 10.1016/0014-5793(86)80898-5. [DOI] [PubMed] [Google Scholar]
- Joshi C., Fernandez J. M. Capacitance measurements. An analysis of the phase detector technique used to study exocytosis and endocytosis. Biophys J. 1988 Jun;53(6):885–892. doi: 10.1016/S0006-3495(88)83169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague W., Howell S. L. Mode of action of adenosine 3':5'-cyclic monophosphate in insulin secretion. Biochem J. 1972 Feb;126(3):13P–14P. doi: 10.1042/bj1260013p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Zucker R. S. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993 Jan;10(1):21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Zawalich K. C., Ganesan S., Calle R., Zawalich W. S. Physiology and pathophysiology of insulin secretion. Diabetes Care. 1990 Jun;13(6):655–666. doi: 10.2337/diacare.13.6.655. [DOI] [PubMed] [Google Scholar]
- Renström E., Eliasson L., Bokvist K., Rorsman P. Cooling inhibits exocytosis in single mouse pancreatic B-cells by suppression of granule mobilization. J Physiol. 1996 Jul 1;494(Pt 1):41–52. doi: 10.1113/jphysiol.1996.sp021474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P., Ammälä C., Berggren P. O., Bokvist K., Larsson O. Cytoplasmic calcium transients due to single action potentials and voltage-clamp depolarizations in mouse pancreatic B-cells. EMBO J. 1992 Aug;11(8):2877–2884. doi: 10.1002/j.1460-2075.1992.tb05356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. W. The adenylate cyclase-cyclic AMP system in islets of Langerhans and its role in the control of insulin release. Diabetologia. 1979 May;16(5):287–296. doi: 10.1007/BF01223617. [DOI] [PubMed] [Google Scholar]
- Sikdar S. K., Zorec R., Mason W. T. cAMP directly facilitates Ca-induced exocytosis in bovine lactotrophs. FEBS Lett. 1990 Oct 29;273(1-2):150–154. doi: 10.1016/0014-5793(90)81072-v. [DOI] [PubMed] [Google Scholar]
- Smith P. A., Duchen M. R., Ashcroft F. M. A fluorimetric and amperometric study of calcium and secretion in isolated mouse pancreatic beta-cells. Pflugers Arch. 1995 Sep;430(5):808–818. doi: 10.1007/BF00386180. [DOI] [PubMed] [Google Scholar]
- Sutherland E. W. Studies on the mechanism of hormone action. Science. 1972 Aug 4;177(4047):401–408. doi: 10.1126/science.177.4047.401. [DOI] [PubMed] [Google Scholar]
- Thomas P., Wong J. G., Almers W. Millisecond studies of secretion in single rat pituitary cells stimulated by flash photolysis of caged Ca2+. EMBO J. 1993 Jan;12(1):303–306. doi: 10.1002/j.1460-2075.1993.tb05657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Wong J. G., Lee A. K., Almers W. A low affinity Ca2+ receptor controls the final steps in peptide secretion from pituitary melanotrophs. Neuron. 1993 Jul;11(1):93–104. doi: 10.1016/0896-6273(93)90274-u. [DOI] [PubMed] [Google Scholar]
- Thorens B. Glucagon-like peptide-1 and control of insulin secretion. Diabete Metab. 1995 Dec;21(5):311–318. [PubMed] [Google Scholar]
- Wollheim C. B., Ullrich S., Meda P., Vallar L. Regulation of exocytosis in electrically permeabilized insulin-secreting cells. Evidence for Ca2+ dependent and independent secretion. Biosci Rep. 1987 May;7(5):443–454. doi: 10.1007/BF01362507. [DOI] [PubMed] [Google Scholar]
- de Wit R. J., Hekstra D., Jastorff B., Stec W. J., Baraniak J., Van Driel R., Van Haastert P. J. Inhibitory action of certain cyclophosphate derivatives of cAMP on cAMP-dependent protein kinases. Eur J Biochem. 1984 Jul 16;142(2):255–260. doi: 10.1111/j.1432-1033.1984.tb08279.x. [DOI] [PubMed] [Google Scholar]
- von Rüden L., Neher E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science. 1993 Nov 12;262(5136):1061–1065. doi: 10.1126/science.8235626. [DOI] [PubMed] [Google Scholar]