Abstract
The use of combined essential oils (EOs) is a new technique that can improve their preservative effects while minimizing their sensory impact in foods. The aim of this study was to determine the chemical profile of three essential oils (EOs) extracted from Lavandula stoechas L. (Ls), Thymus zygis L. (Tz), and Eucalyptus camaldulensis Dehnh (Ec) and to evaluate their synergistic antibacterial activity for optimal inhibition against Bacillus subtilis, Escherichia coli, and Staphylococcus aureus using an augmented Simplex centroid mixing scheme. The essential oils were extracted by hydrodistillation and analyzed via gas chromatography–mass spectrometry. Anti-bacterial potency was evaluated by disk diffusion. Chemical analysis revealed the main compounds in Lavandula stoechas (Ls) essential oil: camphor (36.15%), followed by fenchone (16.57%) and Z-8-hydroxy linalool (8.28%). The Thymus zygis (Tz) essential oil is dominated by δ-terpineol (27.64%), δ-3-carene (15.7%), and thymol (14.17%). In contrast, the Eucalyptus camaldulensis (Ec) essential oil contains mainly 1,8-cineole (43.61%), γ-terpinene (11.71%), and α-terpineol (10.58%). The optimal mixture is the binary association of 40% E. camaldulensis EO and 60% T. zygis EO, which provides an effective inhibition diameter (ID) of 13.37 mm to inhibit S. aureus. Furthermore, the formulation of 27% and 73% EOs of E. camaldulensis and T. zygis, respectively, corresponds to the mixture required to achieve the optimum inhibition diameter (ID = 11.55 mm) against E. coli. In addition, the mixture of 29% EO of E. camaldulensis and 71% EO of T. zygis is the optimum mixture to inhibit B. subtilis, with an inhibition diameter of 12.31 mm. These findings highlight the potency of antibacterial formulations of these essential oils and suggest that they might be used as substitutes for conventional drugs to prevent the development of bacteria responsible for serious infections and food spoilage.
Keywords: antibacterial activity, essential oil, Lavandula stoechas, Thymus zygis, Eucalyptus camaldulensis, augmented Simplex centroid
1. Introduction
For many years, the food industry has relied on synthetic preservatives to prevent microbial contamination in packaged foods [1]. However, these artificial preservatives pose significant health risks, causing side effects such as headaches, nausea, weakness, cancers, and anorexia [2]. Consequently, the food industry is moving towards using natural food products or extracts, which require protection against spoilage and microbial contamination throughout their shelf life.
For thousands of years, medicinal and aromatic plants have been utilized to combat human-infectious diseases due to their preservative and pharmacological properties. Numerous secondary metabolites from these plants have shown significant biological activities, making them highly valuable [3]. Among these, essential oils (EOs) stand out as concentrated hydrophobic liquids rich in volatile plant-derived compounds. They have been employed for various medical and health purposes for millennia [4]. The biological activities of essential oils, including antioxidant, antifungal, and antibacterial effects, are well documented [5,6]. Additionally, their safe application as natural food preservatives have been noted [7]. However, achieving similar antibacterial and preservative effects observed in laboratory tests often requires high concentrations [8], leading to potential overdoses and alterations in food flavor. As a result, research has shifted to exploring the synergistic effects of different essential oil combinations to enhance their efficacy while reducing the necessary concentration [9].
Lavandula stoechas L., belonging to the Lamiaceae family, is an evergreen shrub characterized by its foliage and dark-purple-to-violet flowers, which are highly aromatic. This plant can reach a height of up to 100 cm and grows naturally in Mediterranean regions. In Morocco, it is found in various areas, particularly in the Rif, Middle Atlas, and High Atlas regions [10,11]. Used for its medicinal properties, its benefits are attributed to its bioactive compounds, such as camphor, terpineol, eucalyptol, fenchone, and linalool [12,13]. Thanks to its phytochemical composition, L. stoechas is widely employed in traditional medicine as well as in the food and cosmetic industries. In Morocco, it is used to treat inflammations, nephrotic syndromes, rheumatic diseases, and as an antispasmodic agent. It is also recognized for its antidiabetic properties and its use in the treatment of hypertension [14]. Research has highlighted its essential oils’ antimicrobial, antioxidant, antileishmanial, insecticidal, anti-inflammatory, and anticancer activities [15,16,17]. These biological properties are attributed to its high fenchone/camphor chemotype content. However, the results of its biological activities vary from study to study, and these differences may be due to variations in the chemical compositions of its essential oils, influenced by environmental conditions and regional differences [18].
Thymus zygis L. is an aromatic plant from the Lamiaceae family, is widely distributed in the Iberian Peninsula, and has a long history of use as a spice. It is characterized by its small, linear, lanceolate leaves and produces clusters of small tubular flowers that range in color from white to pale pink. It grows in Mediterranean regions and is found in Morocco, where it develops spontaneously in forest clearings, rocky pastures of low to medium mountains, primarily in the High Atlas, North Atlantic, Middle Atlas, Essaouira region and the Mediterranean coast, particularly in cold, semi-arid, humid, and sub-humid bioclimatic areas [19]. Although this species is widely used for its therapeutic benefits in the pharmaceutical, cosmetic, and perfumery industries [20], the essential oil of T. zygis mainly contain phenolic compounds, such as thymol, and alcoholic compounds, such as terpineol, which contribute to their various bioactive properties, including anti-inflammatory, antifungal, antimicrobial, and antioxidant effects [21,22]. While the major components are well-known for their biological significance, the minor constituents also play an important role in enhancing the effects of the main components through synergistic and additive mechanisms [23,24].
Eucalyptus camaldulensis Dehnh, native to Australia, is a species that belongs to the Myrtaceae family [25]. In Morocco, it is primarily planted in the northwestern region of the country [26]. Eucalyptus trees are large and can reach heights of over 100 m. Their evergreen, aromatic leaves are entire, leathery, and have a high cutin content [27]. The plant is widely used in traditional therapies for various ailments, particularly as an antiseptic and astringent [28]. Numerous studies have focused on the cosmetic and pharmaceutical applications of E. camaldulensis leaves, especially in the treatment of respiratory diseases [29]. Furthermore, many studies have examined the antioxidant, antimicrobial, and antifungal properties of its essential oils [30]. The main compounds typically found in the essential oil of E. camaldulensis leaves include 1,8-cineole, γ-terpinene, p-cymene, and α-pinene [31,32]. Notably, 1,8-cineole is considered a major bioactive component due to its numerous biological activities.
This study aimed to evaluate the antibacterial effects of the combined essential oils of three plants, L. stoechas, T. zygis, and E. camaldulensis, against three bacterial strains: Bacillus subtilis, Staphylococcus aureus, and Escherichia coli. This combination was chosen with the aim of enhancing efficacy and reducing the required amount of essential oils, thereby minimizing their toxicity and negative impact. To achieve this, an augmented Simplex centroid mixture design was employed to develop polynomial models to highlight the synergy between the essential oils against the bacterial strains.
2. Materials and Methods
2.1. Plant Material
Samples of the aerial parts of T. zygis, L. stoechas, and E. camaldulensis were collected in June 2021 from the regions of El Hoceima (North Morocco: 35°08′09.8″ N; 4°05′10.7″ W), Azrou (Middle Atlas of Morocco: 33°25′48″ North, 5°12′36″ West), and Mamora (Northwest Morocco: 34°16′16.0″ N; 6°25′28.1″ W), respectively. The identification of the species was confirmed at the Scientific Institute of Rabat (Morocco) by Mohammed Sghir Taleb (a research professor at the Scientific Institute. His research focuses on botany, plant ecology, aromatics, and socio-economics).
2.2. Extraction of Essential Oils
The aerial parts of L. stoechas, T. zygis, and E. camaldulensis were subjected to hydrodistillation using a Clevenger-type apparatus [33]. Three distillations were carried out by boiling 200 g of plant material with two liters of distilled water for three hours. The obtained essential oils were placed in hermetically sealed glass vials and stored at a temperature of 4 °C until use. The essential-oil yield was determined relative to the dry matter and evaluated from 3 samples of 20 g dried for 48 h in an oven at 60 °C. The essential oil yields of the samples were determined using the formula specified by [34]. All experiments were performed in triplicate.
| Yield % = (weight of EO obtained by distillation (g)/weight of dry biomass (g)) × 100; |
2.3. Gas Chromatography–Mass Spectrometry (GC/MS) Analysis
The essential oils were subjected to chemical analysis using gas chromatography coupled with mass spectrometry (GC/MS) and flame ionization detection (GC–FID). The GC/MS analysis was employed to quantify the components, while the GC–FID analysis was used for identification purposes. All samples were analyzed by gas chromatography using an HP-5 capillary column (30 m × 0.25 mm, film thickness 0.25 µm), an FID detector, and an injector set at 275 °C. The equipment was sourced from Hewlett–Packard, located in Palo Alto, California, USA. After an initial five-minute interval, the oven temperaturewas gradually increased from 50 °C to 250 °C at a rate of 4 °C/min. Nitrogen was used as the carrier gas at a flow rate of 1.8 mL/min. The samples, diluted 1/50 in methanol, were injected in a volume of 1 µL in split mode at a ratio of 1/50 and a flow rate of 72.1 mL/min. The proportions of the components present in the essential oils were expressed as percentages, determined by peak area normalization. Retention indices (RI) on the HP-5 MS column were calculated using a homologous series of alkanes ranging from C9 to C28.
The gas chromatography–mass spectrometry (GC/MS) analysis was performed using a Hewlett–Packard gas chromatograph (HP 6890) coupled to a mass spectrometer (HP and stationary syringe 5973). An HP-5MS column (30 m × 0.25 mm, 0.25 µm film thickness) was used. The column temperature was initially set at 50 °C, then gradually increased to 250 °C at a rate of 2 °C/min. Helium gas with a purity of 99.995% served as the carrier gas, flowing at a rate of 1.5 mL/min, with a split ratio of 1/74.7, corresponding to a flow rate of 112 mL/min. Components were identified using a NIST 98 spectral library in the mass spectrometer. The ionization voltage was maintained at 70 eV, the ion source temperature was set at 230 °C, and the mass scan range was between 35 and 450 m/z. Component identification was verified by comparing the elution order of the compounds with the relative retention indices reported in the literature.
2.4. Tested Organisms
The bacterial strains Bacillus subtilis (ATCC 6633), Escherichia coli (ATCC 8739), and Staphylococcus aureus (ATCC 6538) belong to the collection of the Microbiology Laboratory at the Center for Innovation, Research, and Training, Rabat, Morocco. The strains were inoculated from a master culture maintained on agar at 4 °C, placed on nutrient agar plates, and incubated at 37 °C for 24 h.
2.5. Antibacterial Activity of Essential Oils
The antibacterial activity of the essential oils (EOs) was evaluated using a disk diffusion method, known for its reliability and reproducibility. This method involves placing a sterile disk soaked in EO on a freshly growing bacterial lawn and measuring the inhibition zone diameter, which reflects the antibacterial activity of the EOs. For this test, 15 mL of Tryptic Soy Agar (TSA) was poured into each Petri dish, and 100 µL of a bacterial suspension with a density equivalent to 0.5 McFarland standard (108 CFU/mL) was added. Sterile filter paper disks (6 mm) were then impregnated with 5 μL of EO and placed on the inoculated Petri dishes. Tetracycline (300 µg) was used as a positive reference standard to determine the sensitivity of the tested strains. The Petri dishes were incubated at 37 °C for 24 h. After incubation, the diameter of the inhibition zone was measured in millimeters. All tests were conducted in triplicate [35].
2.6. Mixture Design and Statistical Analysis
The mixture design is an experiment in which the responses are presumed to be influenced by the relative proportions of the mixture’s constituents, rather than the total quantity of the mixture. This approach was implemented to identify the optimal formulation while simultaneously reducing the number of experiments. Consequently, it enables the identification of the correlation between the variables and the experimental responses that were quantified.
2.6.1. Chosen Design
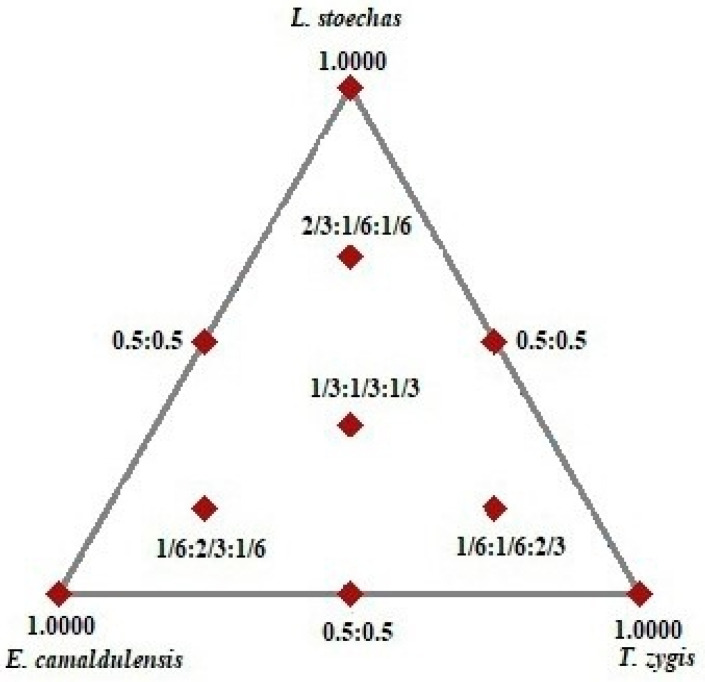
In this work, an augmented centroid design (Figure 1) was employed to determine the optimal formulation components that provide the most effective combination of EOs to achieve increased antibacterial activities (i.e., the highest diameter of inhibition (DI)).
Figure 1.
Positions of experimental points for augmented Simplex-centroid designs.
To establish the experimental setup, a polynomial model explaining the relationship between a response and the factors under consideration was constructed using the experimental data of the selected design. This design includes ten experiments divided as follows: three diluted EOs in the triangle’s vertices (experiments 1, 2, and 3), 0.5/0.5 mixtures (experiments 4, 5, and 6), an equal proportionate mixture of the three constituents (experiments 7), and control points (experiments 8, 9, and 10) [36]. To evaluate pure error and compare it with lack of fit, experiment 7 was repeated three times, resulting in 12 experiments for this design. The sum of the components of the mixture was 100%.
Table 1 shows 12 ternary combinations of the three Eos (L. stoechas, E. camaldulensis, and T. zygis) synthesized using an augmented centroid design.
Table 1.
Experience matrix of augmented centroid design.
| Experiment Number | L. stoechas | E. camaldulensis | T. zygis |
|---|---|---|---|
| 1 | 1 | 0 | 0 |
| 2 | 0 | 1 | 0 |
| 3 | 0 | 0 | 1 |
| 4 | 0.5 | 0.5 | 0 |
| 5 | 0.5 | 0 | 0.5 |
| 6 | 0 | 0.5 | 0.5 |
| 7 | 1/3 | 1/3 | 1/3 |
| 8 | 1/3 | 1/3 | 1/3 |
| 9 | 1/3 | 1/3 | 1/3 |
| 10 | 2/3 | 1/6 | 1/6 |
| 11 | 1/6 | 2/3 | 1/6 |
| 12 | 1/6 | 1/6 | 2/3 |
2.6.2. Chosen Mathematical Model
The chosen model is a special cubic model, which is a linear model with third-order interactions, having the following general form:
With:
-
-
Y represents the response expressed in mm for the diameter of inhibition (DI) and in µg/mL for IC50.
-
-
α1, α2, α3 are the coefficients of the linear terms.
-
-
α12, α23, α13 are the coefficients of the binary interaction terms.
-
-
α123: coefficient of the ternary interaction term.
-
-
ɛ: error term.
2.6.3. Statistical Analysis
To evaluate the significance of the fitted model, an ANOVA test was performed. The Fratio (CMR/CMr), which is the ratio of the mean square due to regression to the mean square due to residuals, was calculated. To assess the quality of the model fit, we also calculated the Fratio (CMR/CMr), which is the ratio of the mean square due to lack of fit to the mean square due to pure error, as described by [37].
The quality of the chosen model was expressed by the coefficient of determination, R2. A value closer to 1 indicates that the variability explained by the model is much greater than the variability explained by the residuals [38].
To determine the significance of factors, a t-test was employed at a significance level of 95%. This test involves calculating the ratio of the coefficient value to its standard error. The resulting statistic is the t-value, from which the probability that the coefficient is zero can be assessed. In the coefficient table, each factor is accompanied by its t-value and p-value. A smaller p-value indicates greater statistical significance of the coefficient [37].
To finalize the optimization step, the mixture design was used to identify a compromise setting that leads to the desired response. Additionally, the “Desirability” function was applied to determine the precise optimal setting with a compromise percentage [37].
3. Result and Discussion
3.1. Essential Oil Yields
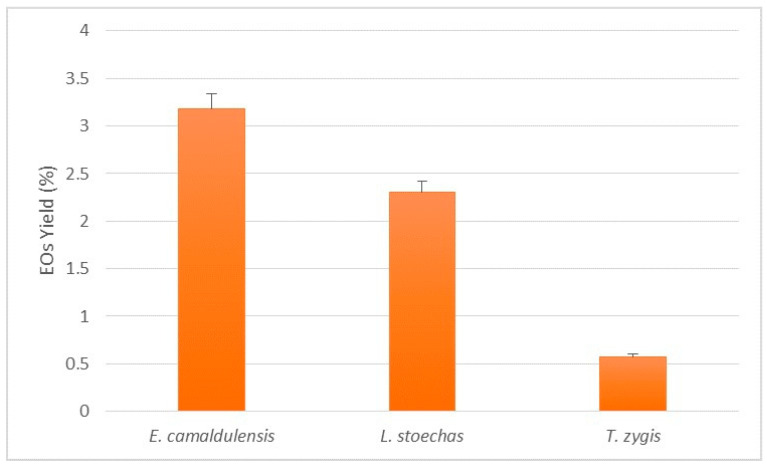
The hydrodistillation extraction of essential oils from E. camaldulensis, L. stoechas, and T. zygis yielded 3.18%, 2.30%, and 0.57%, respectively (Figure 2). Different results have been reported for E. camaldulensis essential oils in previous studies: in Iran, the yield was 2.10% [39], in Malaysia, it ranged from 0.46% to 1.4% [40], while in Nicosia, Cyprus, it was reported to be approximately 2.40% [41]. Similarly, the yield of L. stoechas essential oils varies across different regions: in Sidi Slimane, Northwest Morocco, it was 2.10% [42], in Tunisia, it ranged from 0.72% to 0.95% [43], and in Italy, it was notably lower, at 0.11% [44]. For T. zygis from Taza, Northeast Morocco, a low yield of 0.3% was reported [45], whereas T. zygis subsp. sylvestris from Portugal showed a higher yield of 1.2% [46].
Figure 2.
Average yield of the essential oils as a function the plants studied.
3.2. Chemical Composition of Essential Oils
The main components present in the essential oils obtained by hydrodistillation and the blend of essential oils according to the mixture design plan are summarized in Table 2. Chromatographic analyses by GC and GC/MS identified 60 compounds in L. stoechas essential oil, accounting for 99.49% of the composition. Monoterpenes were the predominant components in L. stoechas essential oil, comprising 84.15%, followed by sesquiterpenes at 15.24% (Table 2). The predominant compounds were camphor (36.15%), fenchone (16.57%), and Z-8-hydroxy linalool (8.28%). Comparisons with the chemical composition of L. stoechas essential oils from other countries revealed qualitative and quantitative differences. For instance, in Algeria, α-fenchone (39%), camphor (18.5%), and bornyl acetate (7.79%) were dominant [47]. In Tunisia, camphor (15.32–50.63%), fenchone (6.57–34.70%), and 1,8-cineole (0.05–13.45%) were found to be major constituents [11]. In Taounate, Northern Morocco, camphor (43.97%), fenchone (30.39%), and camphene (4.09%) dominated [48].
Table 2.
Chemical composition in percentage of the essential oils of L. stoechas (Ls), T. zygis (Tz) and E. camaldulensis (Ec) and their combinations analyzed by GC/MS.
| No | Compounds | RI | Ls | Tz | Ec | Ls/Tz (0.5:0.5) | Ls/Ec (0.5:0.5) | Tz/Ec (0.5:0.5) | Ls/Ec/Tz (1/3:1/3:1/3) |
Ls/Ec/Tz (2/3:1/6:1/6) |
Ls/Ec/Tz (1/6:2/3:1/6) |
Ls/Ec/Tz (1/6:1/6:2/3) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Heptanal | 901 | - | 0.14 | - | - | 0.07 | - | - | - | - | - |
| 2 | Santalina triene | 906 | 0.34 | - | - | - | 0.11 | - | - | 0.17 | 0.05 | 0.19 |
| 3 | Tricyclene | 921 | 1.45 | - | 0.17 | - | 0.18 | - | - | - | - | |
| 4 | α-Thujane | 924 | 4.33 | 0.05 | 3.49 | 2.31 | 4.45 | 2.59 | 3.35 | 3.79 | 2.73 | 2.45 |
| 5 | α-Pinene | 932 | 4.24 | 3.98 | 0.32 | 3.39 | 2.14 | 2.3 | 3.15 | 3.58 | 1.2 | 3.6 |
| 6 | Norbornen-2-ol | 941 | - | 0.11 | - | - | - | - | - | - | - | - |
| 7 | Fenchene | 946 | 0.21 | - | - | - | 0.09 | - | 0.1 | 0.14 | - | - |
| 8 | Verbinene | 961 | 0.12 | 1.65 | - | 0.86 | 0.09 | 1.51 | 1.27 | 0.81 | 1.21 | 1.23 |
| 9 | Sabinene | 969 | 0.28 | 0.13 | 0.25 | - | 1.02 | - | - | - | - | - |
| 10 | β-Pinene | 974 | 0.19 | 0.24 | 0.35 | 0.19 | 0.28 | 0.29 | 0.31 | 0.24 | 0.26 | 0.25 |
| 11 | cis Pinane | 982 | 0.14 | - | - | 0.17 | - | - | 0.12 | 0.12 | - | 0.14 |
| 12 | Myrcene | 988 | - | 0.07 | - | 0.09 | - | - | - | - | - | - |
| 13 | oxide linalool | 991 | 0.07 | - | - | - | - | - | - | - | - | - |
| 14 | 2-Octanol | 994 | - | - | 0.1 | - | 0.23 | 0.27 | 0.22 | 0.18 | 0.3 | - |
| 15 | δ-2-Carene | 1001 | 2.28 | 0.37 | 0.1 | 0.93 | 1.26 | 0.32 | 0.79 | 1.49 | 0.33 | 0.39 |
| 16 | δ-3-Carene | 1008 | - | 15.7 | 0.06 | 8.87 | - | 11.31 | 8.74 | 5.06 | 6.22 | 12.27 |
| 17 | α-Terpinene | 1014 | 1.05 | 0.42 | - | - | 0.08 | - | - | - | - | - |
| 18 | ρ-Cymene | 1020 | 1.26 | 2.44 | 4.93 | 0.79 | 4.74 | - | - | 1.46 | - | - |
| 19 | Limonene | 1024 | 0.89 | - | - | 1.2 | - | - | - | - | - | - |
| 20 | 1,8-cineol | 1026 | - | - | 43.61 | - | 24.53 | 21.93 | 14.32 | 8.39 | 28.77 | 9.76 |
| 21 | E,β-Ocymene | 1044 | 0.19 | 1.85 | - | 2.65 | 0.05 | - | - | - | - | - |
| 22 | γ-Terpinene | 1054 | - | 0.72 | 11.71 | 0.59 | 2.91 | 5.84 | 4.08 | 2.32 | 4.98 | 4.66 |
| 23 | cis hydrate Sabinene | 1065 | 0.4 | - | 0.13 | - | 0.18 | - | - | 0.19 | 0.1 | 0.05 |
| 24 | trans Oxide linalool | 1067 | - | 0.6 | - | 7.67 | - | - | - | - | - | - |
| 25 | Camphelilone | 1078 | - | 1.38 | 0.85 | 1.77 | - | 0.53 | 0.54 | - | - | - |
| 26 | Fenchone | 1084 | 16.57 | - | - | - | 8.16 | - | 5.52 | 9.78 | 3.09 | 3.02 |
| 27 | Terpinolene | 1086 | - | 0.48 | 2.3 | - | - | 1.59 | 1.28 | 1.66 | 1.57 | 1.39 |
| 28 | Linalool | 1095 | 2.86 | 0.16 | - | 0.29 | 0.1 | 1.6 | - | - | - | |
| 29 | Trans-Hydrate Sabinene | 1098 | - | - | 0.27 | - | - | - | - | - | - | - |
| 30 | α-Fenchocamphorone | 1104 | 0.28 | - | - | - | 0.34 | - | 0.22 | 0.38 | 0.23 | 0.14 |
| 31 | 6-Camphenol | 1111 | - | 0.23 | - | - | - | 0.23 | - | - | - | 0.26 |
| 32 | endo-Fenchol | 1114 | 1.34 | - | - | 0.36 | 0.42 | - | 0.33 | 0.4 | 0.34 | - |
| 33 | trans Hydrate Pinene | 1119 | - | - | 0.04 | - | - | - | - | - | - | - |
| 34 | dehydro Linalool | 1131 | - | 4.99 | - | - | - | - | - | - | - | - |
| 35 | trans-β-dihydro Terpineol | 1134 | - | - | 0.39 | - | - | - | - | - | - | - |
| 36 | cis hydrate Pinene | 1139 | - | - | 1.42 | - | 0.57 | - | - | - | - | - |
| 37 | Camphor | 1141 | 36.15 | - | - | 23.77 | 23.6 | - | 15.82 | 28.94 | 9.91 | 10.03 |
| 38 | β-Oxide-Pinene | 1154 | 3.21 | - | - | 0.4 | 1.81 | - | 0.3 | 0.15 | - | - |
| 39 | δ-Terpineol | 1162 | - | 27.64 | 1.16 | 16.8 | 2.39 | 16.15 | 11.29 | 7.12 | 7.05 | 19.48 |
| 40 | Thujanol | 1164 | - | 1.12 | - | - | - | - | - | - | - | - |
| 41 | cis Oxyde Linalool | 1170 | 2.05 | 0.93 | - | 1.68 | - | - | 0.86 | - | 3.02 | 0.86 |
| 42 | Terpinene-4-ol | 1174 | 1.36 | - | 3.91 | 1.06 | 2.55 | 2.45 | 2.12 | 1.75 | 3.32 | 1.88 |
| 43 | iso-Verbanol | 1176 | - | 0.31 | - | - | - | - | - | 0.8 | - | - |
| 44 | ρ-Cymen-8-ol | 1179 | 0.38 | - | - | 0.3 | - | - | 1.13 | 0.6 | - | - |
| 45 | neo-Verbanol | 1182 | - | 0.84 | - | 0.41 | - | - | - | - | - | - |
| 46 | α-Terpineol | 1186 | - | - | 10.58 | - | 2.82 | 5.47 | 1.98 | 1.18 | 4.63 | 1.78 |
| 47 | ϒ-Terpineol | 1199 | 0.6 | - | 0.22 | - | 0.39 | - | - | 0.38 | 0.52 | 0.39 |
| 48 | Verbenone | 1204 | 1.23 | 0.05 | 0.35 | 0.55 | - | 0.39 | 0.7 | 0.24 | 0.11 | |
| 49 | trans piperitol | 1207 | - | - | 0.11 | - | - | 0.06 | - | - | - | - |
| 50 | acetate Octenol | 1208 | 0.34 | - | - | - | 0.13 | - | - | - | - | - |
| 51 | acetate Octanol | 1211 | - | - | - | - | - | - | 0.15 | 0.22 | 0.06 | 0.22 |
| 52 | Formate Linalool | 1214 | 0.12 | - | - | - | 0.05 | - | - | 0.1 | - | - |
| 53 | trans Carveol | 1215 | - | 2.14 | - | 1 | - | 1.11 | 0.79 | 0.44 | 0.44 | - |
| 54 | acetate Endo-Fenchyl | 1218 | 0.11 | - | - | - | - | - | - | - | - | - |
| 55 | cis acetate hydrate Sabinene | 1219 | - | - | 0.37 | - | - | - | - | - | - | - |
| 56 | cis-Carveol | 1226 | - | 1.77 | - | 0.6 | - | 1.19 | 0.76 | 0.36 | - | 0.95 |
| 57 | Tetra hydro-acetate Linalool | 1231 | 0.22 | - | - | 0.25 | - | - | - | - | - | - |
| 58 | Pulegone | 1233 | - | - | 1.02 | - | 0.57 | - | - | 0.16 | 0.98 | - |
| 59 | Carvone | 1239 | 0.14 | - | 0.23 | - | 0.07 | 0.15 | 0.02 | 0.07 | 0.12 | - |
| 60 | trans acetate hydrate Sabinene | 1253 | - | - | 0.13 | - | 0.05 | - | 0.02 | - | 0.06 | - |
| 61 | Carvenone | 1255 | 0.05 | - | - | - | - | - | - | - | - | - |
| 62 | cis oxide Carvone | 1259 | 0.07 | - | - | - | 0.08 | - | - | - | - | - |
| 63 | iso-3-acetate Thujanol | 1267 | 0.12 | - | 0.33 | 0.07 | 0.44 | 0.42 | 0.32 | 0.14 | 0.62 | 0.16 |
| 64 | trans-Oxide Carvone | 1273 | 0.35 | 4.13 | 0.6 | 3.95 | - | 4 | 2.64 | 1.67 | 1.75 | 4.56 |
| 65 | neoiso-3-acetate Thujanol | 1281 | - | - | 0.2 | - | 0.23 | - | - | - | 0.35 | - |
| 67 | trans-acetate Oxide Linalool | 1287 | 0.05 | - | - | - | - | - | - | - | - | - |
| 68 | Thymol | 1289 | - | 14.17 | - | 5.91 | - | 6.81 | 4.43 | 2.28 | 2.8 | 7.84 |
| 69 | ρ-Cymen-7-ol | 1290 | - | - | 1.35 | - | 0.27 | - | - | - | - | - |
| 70 | trans acetate Verbenyl | 1291 | - | 0.06 | - | - | - | - | - | - | - | - |
| 71 | acetate dehydro Carveol | 1306 | 0.3 | - | - | 0.12 | 0.14 | - | 0.15 | 0.18 | - | - |
| 72 | Iso acetate Verbanol | 1308 | 0.26 | 0.31 | - | 0.18 | 0.08 | 0.16 | 0.14 | 0.1 | 0.05 | 0.21 |
| 73 | δ-Acetate Terpinyl | 1316 | - | - | 0.08 | - | - | - | - | - | - | - |
| 74 | neo-iso acetate Verbanol | 1328 | 0.02 | 0.02 | - | - | - | - | - | - | 0.16 | - |
| 75 | δ-Elemene | 1335 | - | - | 0.95 | - | 1.06 | 1.03 | 0.7 | 0.38 | 1.63 | 0.36 |
| 76 | acetate Verbanol | 1340 | 0.02 | 0.03 | - | 0.06 | - | - | - | - | - | - |
| 77 | α-acetate Terpinyl | 1346 | - | - | 0.51 | - | - | 0.03 | 0.03 | 0.07 | - | 0.04 |
| 78 | cis-acetate Carvyl | 1365 | - | - | 0.04 | - | - | 0.03 | 0.04 | - | - | |
| 79 | α-Copaene | 1374 | - | 0.08 | - | - | - | - | 0.04 | - | - | 0.04 |
| 80 | β-Elemen | 1389 | 0.15 | - | - | 0.05 | 0.05 | - | 0.05 | 0.07 | - | - |
| 81 | β-Longipinene | 1400 | - | 0.07 | - | - | - | - | 0.04 | - | - | - |
| 82 | Longifolene | 1407 | - | - | 0.05 | - | - | 0.05 | - | - | - | - |
| 83 | E-Caryophyllene | 1417 | - | 2.75 | 0.35 | 1.42 | - | 1.55 | 1.07 | 0.51 | 0.59 | 1.88 |
| 84 | Carvone hydrate | 1422 | - | - | 0.26 | - | - | - | - | - | - | - |
| 85 | 4,8-β-epoxy-Caryophyllane | 1423 | 0.1 | - | - | - | - | - | - | - | - | - |
| 86 | γ-Elemene | 1434 | - | 0.07 | - | - | - | - | - | - | - | - |
| 87 | Aromadendrene | 1439 | - | - | 0.24 | - | 0.06 | 0.05 | 0.07 | - | 0.09 | - |
| 88 | α-Humulene | 1452 | - | 0.12 | 0.23 | 0.05 | - | 0.1 | 0.07 | - | - | 0.08 |
| 89 | Sesquisabinene | 1457 | - | 0.17 | - | 0.14 | - | 0.12 | 0.13 | 0.12 | - | 0.13 |
| 90 | 9-epi-E-Caryophyllene | 1464 | 0.13 | - | 0.09 | - | 0.1 | - | - | - | 0.11 | - |
| 91 | 10-epi-β-Acoradiene | 1474 | 0.05 | - | - | - | - | - | - | - | - | - |
| 92 | β-Thujaplicin | 1475 | - | 0.16 | - | - | - | - | - | - | - | 0.09 |
| 93 | γ-Muurolene | 1478 | - | - | 0.25 | - | - | - | 0.1 | - | 0.09 | - |
| 94 | Germacrene D | 1484 | 0.4 | - | - | 0.1 | 0.12 | - | 0.16 | 0.17 | 0.16 | 0.07 |
| 95 | β-Selinene | 1489 | - | 0.39 | - | 0.11 | - | 0.09 | 0.13 | - | - | 0.1 |
| 96 | δ-Selinene | 1492 | 0.23 | - | 0.2 | - | - | - | - | 0.11 | 0.11 | - |
| 97 | α-Selinene | 1498 | 0.07 | - | - | - | - | - | - | - | - | - |
| 98 | α-Muurolene | 1500 | - | - | 0.38 | - | - | - | - | - | - | - |
| 99 | β-Bisaboline | 1505 | - | 0.13 | - | - | - | 0.04 | 0.04 | - | - | 0.05 |
| 100 | γ-Cadinene | 1513 | - | 0.27 | 0.07 | - | - | 0.09 | - | - | - | 0.19 |
| 101 | 7-epi-α-Selinene | 1520 | 0.68 | - | - | 0.41 | 0.25 | - | 0.25 | 0.45 | 0.13 | - |
| 102 | δ-Cadinene | 1522 | - | - | 0.09 | - | - | - | - | - | - | - |
| 103 | α-Cadinene | 1537 | 0.39 | 0.03 | - | - | 0.15 | 0.15 | - | 0.2 | 0.07 | 0.05 |
| 104 | α-Calacorene | 1544 | 1.18 | - | - | 0.51 | 0.52 | - | 0.35 | 0.78 | 0.2 | 0.16 |
| 105 | Elemol | 1548 | - | 0.03 | 2.11 | - | 0.41 | 0.38 | 0.23 | 0.68 | 0.11 | |
| 106 | β-Calacorene | 1564 | 0.42 | - | 0.22 | 0.08 | 0.2 | - | 0.1 | 0.14 | 0.17 | 0.05 |
| 107 | Davanone B | 1564 | - | - | - | - | - | 0.07 | - | - | - | - |
| 108 | Caryophyllenyl Alcohol | 1570 | - | 0.21 | - | 0.2 | - | - | - | - | - | - |
| 109 | Germacrene D-4-ol | 1574 | - | - | 0.07 | - | 0.05 | - | - | - | - | - |
| 110 | trans hydrate sesquisabinene | 1577 | 0.05 | 0.22 | - | - | - | - | 1.14 | 0.57 | - | 0.79 |
| 111 | Oxide Caryophellene | 1582 | - | 2.24 | 0.3 | 1.51 | 1.4 | 1.77 | 1.35 | 0.74 | 2.43 | 2.04 |
| 112 | Davanone | 1587 | - | - | 1.1 | - | 0.57 | 1.87 | - | - | 1.34 | - |
| 113 | cis-β-Elemenone | 1589 | - | 0.12 | - | - | - | - | - | - | - | - |
| 114 | Viridiflorol | 1592 | 0.22 | - | - | - | - | - | - | - | - | - |
| 115 | Widdrol | 1599 | - | - | 0.23 | - | 0.18 | 0.26 | 0.15 | - | 0.23 | 0.13 |
| 116 | trans-β-Elemenone | 1601 | - | 0.09 | - | - | - | - | - | - | - | - |
| 117 | trans Isolongifolanone | 1612 | - | - | 0.08 | - | - | 0.1 | - | - | - | - |
| 118 | Z-8-hydroxy Linalool | 1619 | 8.28 | - | - | 3.83 | 4.02 | - | 2.76 | 5.1 | 1.86 | 1.38 |
| 119 | trans Isolongifolanone | 1625 | - | - | 0.28 | - | 0.17 | - | 0.04 | - | 0.1 | 0.02 |
| 120 | E-Sesquilavandulol | 1631 | 0.21 | - | - | 0.15 | - | - | - | 0.23 | - | - |
| 121 | α-Acorenol | 1632 | - | - | 0.15 | - | - | - | - | - | - | - |
| 122 | cis-Cadin-4-en-7-ol | 1635 | - | 0.69 | - | 0.62 | - | 0.68 | 0.53 | 0.61 | 0.52 | 0.55 |
| 123 | epi-α-Cadinol | 1638 | 0.59 | - | - | - | 0.46 | - | - | - | - | - |
| 124 | epi-α-Muurolol | 1644 | - | - | 0.18 | - | - | - | - | - | - | - |
| 125 | β-Eudesmol | 1649 | - | 0.35 | - | - | - | - | - | 0.73 | - | 0.13 |
| 126 | α-Eudesmol | 1652 | - | 0.28 | - | - | - | 0.28 | - | - | - | - |
| 127 | 1,2-dihydro-8-hydroxy-2E-Linalool | 1654 | 0.63 | - | - | 0.33 | 0.53 | - | 0.55 | - | - | - |
| 128 | dehydro-Eudesmol | 1661 | - | - | 0.54 | - | - | 0.26 | - | - | 0.57 | - |
| 129 | 14-hydroxy-Z-Caryophyllene | 1666 | - | 0.39 | - | 0.36 | - | 0.29 | 0.31 | - | 0.28 | 0.31 |
| 130 | epi-β-Bisabolol | 1670 | 0.39 | - | - | - | 0.07 | - | - | 0.4 | - | - |
| 131 | acetate Davanol | 1689 | - | 0.09 | - | - | - | - | - | - | - | - |
| 132 | acetate caryophyllene | 1701 | 0.7 | - | - | 0.32 | 0.34 | - | 0.23 | 0.44 | 0.14 | 0.15 |
| 133 | 2E,6Z-Farnesol | 1714 | 0.23 | - | 0.1 | - | 0.8 | 0.06 | 0.05 | 0.1 | - | - |
| 134 | 2E,6Z Farnesol | 1715 | - | - | - | - | - | - | - | - | - | |
| 135 | Z-acetate Sesquilavandyl | 1732 | 0.06 | - | - | - | 0.06 | - | - | - | - | - |
| 136 | 2E,6E-Farnesol | 1742 | 0.02 | - | - | - | - | - | 0.05 | - | - | - |
| 137 | β-Acoradienol | 1762 | 0.08 | - | - | - | - | - | 0.03 | 0.05 | - | - |
| 138 | γ-acetate Eudesmol | 1783 | 0.04 | - | - | - | - | - | - | - | - | - |
| Total | 99.49 | 99.63 | 99.68 | 99.4 | 99.51 | 96.31 | 100.04 | 99.36 | 98.96 | 94.34 | ||
| Monoterpenes | 84.19 | 94.1 | 90.61 | 89.15 | 87.94 | 86.96 | 89.25 | 87.5 | 87.46 | 88.27 | ||
| Sesquiterpenes | 15.3 | 5.53 | 9.07 | 10.25 | 11.57 | 9.35 | 10.79 | 11.86 | 11.5 | 6.07 | ||
| Hydrocarbons | 23.68 | 36.59 | 29.29 | 27.67 | 24.08 | 31.26 | 29.84 | 25.75 | 24.91 | 30.24 | ||
| Ketones | 54.84 | 5.77 | 4.42 | 29.84 | 34.11 | 6.72 | 25.19 | 41.7 | 17.76 | 17.88 | ||
| Aldehydes | - | 0.14 | - | - | 0.07 | - | - | - | - | - | ||
| Esters | 0.17 | 0.06 | 0.63 | - | 0.06 | 0.06 | 0.07 | 0.07 | - | 0.04 | ||
| Ethers | - | - | 43.61 | - | 24.53 | 21.93 | 14.32 | 8.39 | 28.77 | 9.76 | ||
| Alcohols | 20.8 | 37.45 | 21.62 | 33.97 | 16.66 | 26.94 | 24.64 | 19.57 | 24.28 | 27.28 | ||
| Phenols | - | 19.73 | 0.11 | 7.92 | - | 9.4 | 5.98 | 3.88 | 3.24 | 9.14 |
No: In order of elution on HP-5ms; Components: Components identified based on retention indices and mass spectra; RI: Retention indices calculated experimentally using homologous series of C9–C28 alkanes; -: Not detected.
T. zygis essential oils revealed the presence of 54 compounds constituting 99.63% of the total composition. The major components were δ-terpineol (27.64%), δ-3-carene (15.7%), thymol (14.17%), dehydro-linalool (4.99%), trans-carvone oxide (4.13%), and α-pinene (3.98%) (Table 2). Monoterpenes represented the dominant class in T. zygis essential oil (94.18%), while sesquiterpenes were detected in smaller quantities (5.47%). These results differ from previous studies; for instance, T. zygis from Ifrane-Boulemane province, Middle Atlas, Morocco, showed dominance of thymol (36.4%), carvacrol (24.1%), and p-cymene (23.5%) [49]. In Serbia, T. zygis essential oil was rich in thymol (35%) and p-cymene (24.1%) [50]. Different distillation techniques revealed that T. zygis essential oil from Portugal was dominated by ρ-cymene (10.5–77%), thymol (1.42–41%), linalool (2.43–11.7%), γ-terpinene (1.11–10%) and carvacrol (0.48–8.67%) [51].
Furthermore, E. camaldulensis essential oil contained 55 identified constituents, comprising 99.68% of the total compounds. Monoterpenes dominated (90.57%), while sesquiterpenes were present in lower amounts (9.03%). The major components were 1,8-cineole (43.61%), γ-terpinene (11.71%), α-terpineol (10.58%), p-cymene (4.93%), terpinene-4-ol (3.91%), and α-thujene (3.49%) (Table 2). Our findings are consistent with previous reports; for instance, E. camaldulensis from Burkina Faso was dominated by 1,8-cineole (44.17%), followed by α-pinene (14.70%) and o-cymene (14.11%) [52]. In Pakistan, E. camaldulensis essential oil was rich in eucalyptol (30.43%), α-pinene (10.35%), and spathulenol (10.15%) [53], while in Iran, ρ-cymene (18.86%), α-pinene (16.56%), alloaromadendrene (12.26%), and 1,8-cineole (11.79%) were major components [54].
These variations can be attributed to various factors including environmental conditions such as soil type, precipitation, climate, seasonal conditions, harvest timing, extraction and processing methods, duration of action, plant origin, phenological stage of the plant, and genetic influences [55,56].
Regarding the composition of binary blends of essential oils, the Ls/Tz (0.5:0.5) sample contains a significant proportion of monoterpenes (89.15%) and a low proportion of sesquiterpenes (10.25%). The main components are camphor (23.77%), δ-terpineol (16.8%), δ-3-carene (8.87%), trans-oxide linalool (7.67%), thymol (5.91%), trans-oxide carvone (3.95%), and Z-8-hydroxy linalool (3.83%), while fenchone is present in a lower quantity compared to the individual Ls essential oil.
The percentages of 1,8-cineole are similar in both combinations of EO blends Ls/Ec and Tz/Ec (24.53–21.93%, respectively). However, the percentage of δ-3-carene and δ-terpineol in the Ls/Ec sample is less than 10%, reaching 11.31% and 16.15%, respectively, in the Tz/Ec sample. In contrast, camphor is present at 23.6% and absent in both combinations Ls/Ec and Tz/Ec, respectively. The binary EO blends Ls/Ec and Tz/Ec are characterized by a substantial amount of monoterpenes (87.94%, 86.96%), while there is a low amount of sesquiterpenes (11.57%, 9.35%), respectively.
The tertiary combination Ls/Tz/Ec (1/3:1/3:1/3) primarily contains camphor (15.82%), 1,8-cineole (14.32%), and δ-terpineol (11.29%). Other constituents such as δ-3-carene (8.74%), γ-terpinene (4.08%), fenchone (1.28%), α-terpineol (1.98%), and thymol (4.43%) were detected in smaller quantities, although they are present in the individual oils at relatively higher concentrations (over 10%). The percentages of δ-3-carene, fenchone, and thymol conform to the theoretical binary values of the combined EOs Ls, Tz, and Ec in the ratio (1/3:1/3:1/3). Monoterpenes (89.25%) were found at higher levels than sesquiterpenes (10.79%) in the tertiary combination.
The main components detected in the samples, Ls/Tz/Ec (2/3:1/6:1/6), (1/6:2/3:1/6), (1/6:1/6:2/3), are similar to those present in the individual essential oils. However, the results showed that the estimated component percentages in these samples are higher compared to the theoretical binary values, where each sample should contain 66.67% of the plant component with a higher ratio in the blend (2/3 ratio). They are dominated by monoterpenes (87.5%, 87.46%, 88.27%), while the quantities of sesquiterpenes are lower (11.86%, 11.5%, 6.07%), respectively.
3.3. Simple Antibacterial Activity
The results of the antibacterial activity of the EOs from L. stoechas, T. zygis, and E. camaldulensis, assessed using the disk diffusion method against Staphylococcus aureus, Bacillus subtilis, and Escherichia coli, are summarized in Table 3.
Table 3.
Antimicrobial activities of the essential oils against different strains of bacteria by disk diffusion method.
| Microorganisms | DI (mm) | |||
|---|---|---|---|---|
| Plants | Escherichia coli (ATCC 8739) | Bacillus subtilis (ATCC 6633) | Staphylococcus aureus (ATCC 6538) | |
|
T. zygis (5 µL/disk) |
16.11 ± 0.96 | 17.11 ± 0.30 | 18.33 ± 0.89 | |
|
E. camaldulensis (5 µL/disk) |
19.11 ± 0.30 | 22.56 ± 0.59 | 25.00 ± 0.22 | |
|
L. stoechas (5 µL/disk) |
15.78 ± 0.59 | 20.33 ± 0.44 | 20.78 ± 0.74 | |
| Tetracycline | 24 ± 0.33 | 21.67 ± 0.44 | 25.5 ± 0.33 | |
DI: diameter of inhibition; Tetracycline: antibiotic.
The inhibition zone diameters vary based on the EOs’ nature and the tested species’ sensitivity. L. stoechas, T. zygis, and E. camaldulensis exhibited significant antibacterial effects against S. aureus, with inhibition zone diameters of 20.78 ± 0.74 mm, 18.33 ± 0.89 mm, and 25.00 ± 0.22 mm, respectively. This notable antibacterial effect was also observed against B. subtilis, with inhibition zones of 20.33 ± 0.44 mm, 17.11 ± 0.30 mm, and 22.56 ± 0.59 mm for L. stoechas, T. zygis, and E. camaldulensis, respectively. Additionally, E. coli showed significant sensitivity to the EOs of L. stoechas, T. zygis, and E. camaldulensis, with inhibition zone diameters of 15.78 ± 0.59 mm, 16.11 ± 0.96 mm, and 19.11 ± 0.30 mm, respectively. All tested strains exhibited very strong sensitivity to the antibiotic tetracycline.
Previous studies have reported the antibacterial effects of the three EOs studied. Our results corroborate those reported by [57], which show that the EO of L. stoechas causes significant inhibition against the Gram-positive bacterium Staphylococcus aureus (18 ± 0.1 mm), while a weak antibacterial effect was noted against the Gram-negative bacterium Escherichia coli (12 ± 0.2 mm). Conversely, Ez Zoubi et al. [58] reported a strong antibacterial effect of L. stoechas EO against Staphylococcus aureus (17.5 ± 3.1 mm) and a significant effect against Escherichia coli (14.3 ± 0.55 mm). Additionally, the study reported by [42] showed high antibacterial activity of this species collected from Sidi Slimane (northwest Morocco) against Bacillus subtilis (24 mm) and Escherichia coli (28.5 mm).
However, it has previously been demonstrated that the EO of T. zygis from Serbia exhibits weak antibacterial effects against Staphylococcus aureus (5.67 ± 0.58 mm) and Listeria monocytogenes (9.00 ± 1.00 mm) and against Escherichia coli (7.33 ± 0.58 mm), Micrococcus luteus (6.67 ± 0.58 mm), Pseudomonas putida (4.67 ± 0.58 mm), and Enterobacter aerogenes (12.33 ± 0.33 mm) [59]. However, Coimbra et al. [23] reported that the EO of T. zygis from Portugal has a strong antibacterial effect against Staphylococcus aureus (20.67–35.10 mm).
The inhibition zone of E. camaldulensis EO from Pakistan against E. coli ranged from 14.46 ± 0.03 mm to 19.34 ± 0.05 mm, while against B. subtilis, it ranged from 10.77 ± 0.05 mm to 15.81 ± 0.04 mm [60].
The antibacterial activity of essential oils generally depends on their chemical composition and the interaction between their functional groups and the bacterial cell wall, as well as the presence of inactive components and their synergistic interactions. The hydrophilic functional groups in essential oils are crucial for their antimicrobial properties. Phenolic compounds are the most effective, followed by aldehydes, ketones, alcohols, ethers, and hydrocarbons [61].
Gram-negative bacteria exhibit increased resistance due to their cell wall structure, which limits the penetration of hydrophobic compounds like essential oils and their bioactive components through the lipopolysaccharide layer. In contrast, Gram-positive bacteria lack this outer membrane and have a cell wall primarily composed of a thick peptidoglycan layer, which facilitates the diffusion of essential oils through their cell membrane [62].
3.4. Mixture Design Formulation: Antibacterial Activities
3.4.1. Experimental Design
The different combinations of the three essential oils (EOs) studied and the observed responses for each experiment are detailed in Table 4. The experiments were conducted following randomization, and each response is the average of three repetitions.
Table 4.
Different combinations generated by the chosen mixture design and experimental responses.
| Experiment Number | L. stoechas | E. camaldulensis | T. zygis | DI S. aureus (mm) | DI E. coli (mm) | DI B. subtilis (mm) |
|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 0 | 11.00 ± 0.74 | 10.44 ± 0.37 | 11.45 ± 0.59 |
| 2 | 0 | 1 | 0 | 10.39 ± 0.67 | 9.00 ± 0.74 | 9.22 ± 0.59 |
| 3 | 0 | 0 | 1 | 12.11 ± 0.19 | 11.22 ± 0.44 | 11.75 ± 0.81 |
| 4 | 0.5 | 0.5 | 0 | 11.64 ± 0.44 | 9.33 ± 0.22 | 11.67 ± 0.81 |
| 5 | 0.5 | 0 | 0.5 | 11.62 ± 0.37 | 11.33 ± 0.44 | 11.56 ± 0.44 |
| 6 | 0 | 0.5 | 0.5 | 13.30 ± 0.89 | 11.23 ± 0.59 | 11.89 ± 0.96 |
| 7 | 1/3 | 1/3 | 1/3 | 11.44 ± 0.59 | 10.67 ± 0.89 | 10.50 ± 0.44 |
| 8 | 1/3 | 1/3 | 1/3 | 11.33 ± 0.22 | 10.46 ± 0.52 | 10.30 ± 0.59 |
| 9 | 1/3 | 1/3 | 1/3 | 11.42 ± 0.15 | 10.56 ± 0.15 | 10.12 ± 0.30 |
| 10 | 2/3 | 1/6 | 1/6 | 10.89 ± 0.81 | 10.18 ± 0.37 | 11.34 ± 0.15 |
| 11 | 1/6 | 2/3 | 1/6 | 11.46 ± 0.22 | 9.98 ± 0.30 | 10.46 ± 0.96 |
| 12 | 1/6 | 1/6 | 2/3 | 11.97 ± 0.81 | 11.40 ± 0.44 | 11.95 ± 0.52 |
According to Table 4, we can clearly observe that the binary mixture of E. camaldulensis/T. zygis essential oils (tests containing 0.5:0.5) and the ternary mixture of L. stoechas/E. camaldulensis/T. zygis essential oils (tests containing 1/6:1/6:2/3) showed higher inhibition diameters than those of each of the two essential oils separately.
3.4.2. Statistical Validation of the Postulated Model
Based on the analysis of variance table (Table 5), we can conclude that the main effect of the regression is significant, as the p-value is less than 0.05. Furthermore, the models do not exhibit a lack of fit since the p-value for the lack of fit is greater than 0.05.
Table 5.
Analysis of variance for the fitted model.
| Source | Degrees of Freedom | Sum of Squares | Squares Medium | F Report | p-Value | |
|---|---|---|---|---|---|---|
| DI S. aureus | Regression | 6 | 5.64 | 0.9394 | 47.86 | 0.0003 |
| Residual | 5 | 0.0981 | 0.0196 | |||
| Total | 11 | 5.73 | ||||
| Lack of fit | 3 | 0.0913 | 0.0304 | 8.86 | 0.1031 | |
| Pure error | 2 | 0.0069 | 0.0034 | |||
| R 2 | 0.9829 | |||||
| Radj 2 | 0.9624 | |||||
| DI E. coli | Regression | 6 | 6.50 | 1.08 | 69.30 | 0.0001 |
| Residual | 5 | 0.0781 | 0.0156 | |||
| Total | 11 | 6.58 | ||||
| Lack of fit | 3 | 0.0561 | 0.0187 | 1.69 | 0.3921 | |
| Pure error | 2 | 0.0221 | 0.0110 | |||
| R 2 | 0.9881 | |||||
| Radj 2 | 0.9739 | |||||
| DI B. subtilis | Regression | 6 | 7.70 | 1.28 | 10.72 | 0.0099 |
| Residual | 5 | 0.5984 | 0.1197 | |||
| Total | 11 | 8.29 | ||||
| Lack of fit | 3 | 0.5261 | 0.1754 | 4.85 | 0.1756 | |
| Pure error | 2 | 0.0723 | 0.0361 | |||
| R 2 | 0.9278 | |||||
| Radj 2 | 0.8413 | |||||
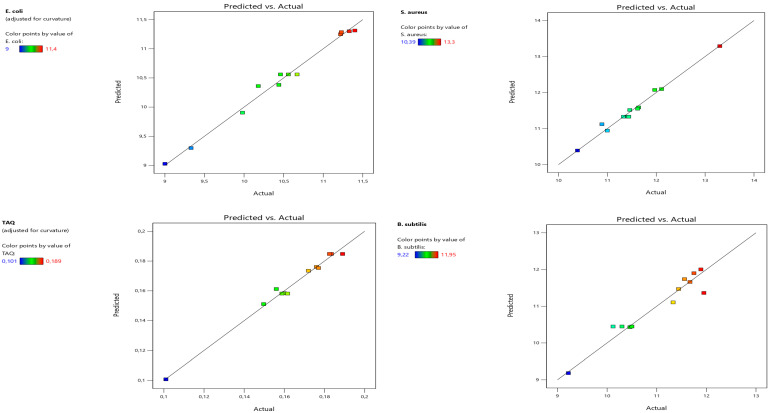
The coefficients of determination range from 0.9278 to 0.9881, indicating a strong agreement between the experimental and predicted values of the fitted model. These results, confirmed by the graph (Figure 3), demonstrate that the curves of the observed values versus the predicted values closely resemble a straight line.
Figure 3.
Scatter plot of observed values vs. predicted values.
3.4.3. Effects of Factors and Models
The effects of all studied factors, along with the statistical t-student values and observed probability (p-value), are summarized in Table 6.
Table 6.
Estimated regression coefficients for the fitted model.
| Terme | Coefficient | Estimation | Std Error | t-Student | p-Value | |
|---|---|---|---|---|---|---|
| DI S. aureus | L. stoechas | b1 | 10.94 | 0.1353 | 80.88 | <0.0001 * |
| E. camaldulensis | b2 | 10.39 | 0.1353 | 76.81 | <0.0001 * | |
| T. zygis | b3 | 12.10 | 0.1353 | 89.40 | <0.0001 * | |
| L. stoechas * E. camaldulensis | b12 | 3.67 | 0.6813 | 5.39 | 0.0030 * | |
| L. stoechas * T. zygis | b13 | 0.1237 | 0.6813 | 0.18 | 0.8631 | |
| E. camaldulensis * T. zygis | b23 | 8.18 | 0.6813 | 12.01 | <0.0001 * | |
| L. stoechas *E. camaldulensis * T. zygis | b123 | −30.90 | 3.71 | −8.34 | 0.0004 * | |
| DI E. coli | L. stoechas | b1 | 10.38 | 0.1207 | 85.98 | <0.0001 * |
| E. camaldulensis | b2 | 9.03 | 0.1207 | 74.76 | <0.0001 * | |
| T. zygis | b3 | 11.25 | 0.1207 | 93.18 | <0.0001 * | |
| L. stoechas * E. camaldulensis | b12 | −1.63 | 0.6080 | −2.67 | 0.0441 * | |
| L. stoechas * T. zygis | b13 | 1.94 | 0.6080 | 3.19 | 0.0242 * | |
| E. camaldulensis * T. zygis | b23 | 4.59 | 0.6080 | 7.55 | 0.0006 * | |
| L. stoechas * E. camaldulensis * T. zygis | b123 | −5.51 | 3.31 | −1.67 | 0.1567 | |
| DI B. subtilis | L. stoechas | b1 | 11.47 | 0.3341 | 34.33 | <0.0001 * |
| E. camaldulensis | b2 | 9.18 | 0.3341 | 27.49 | <0.0001 * | |
| T. zygis | b3 | 11.90 | 0.3341 | 35.62 | <0.0001 * | |
| L. stoechas * E. camaldulensis | b12 | 5.34 | 1.68 | 3.18 | 0.0247 * | |
| L. stoechas * T. zygis | b13 | 0.2153 | 1.68 | 0.13 | 0.9031 | |
| E. camaldulensis * T. zygis | b23 | 5.84 | 1.68 | 3.47 | 0.0178 * | |
| L. stoechas * E. camaldulensis * T. zygis | b123 | −45.09 | 9.15 | −4.93 | 0.0044 * | |
* Statistically significant.
For S. aureus, the statistically significant coefficients are the linear terms b1, b2, and b3. These results indicate that the antibacterial activity against S. aureus depends on all terms of the adapted mathematical model, except for the coefficient corresponding to the binary term b13. The chosen mathematical model is represented by the following Equation:
| Y = 10.94X1 + 10.39X2 + 12.10X3 + 3.67X1X2 + 8.18X2X3 − 30.90X1X2X3 + ɛ |
For E. coli, the statistically significant coefficients are b1, b2, b3, b12, b13, and b23. These results suggest that the antibacterial effect against E. coli is linked to both individual and binary effects. However, no ternary interaction impacts the observed antibacterial action. The chosen mathematical model for the response against E. coli is represented by the following Equation:
| Y = 10.38X1 + 9.03X2 + 11.25X3 − 1.63X1X2 + 1.94X1X3 + 4.59X2X3 + ɛ |
For B. subtilis, the coefficients are statistically significant, with p-values less than 0.05, except for the coefficient corresponding to the binary term b13, which should be excluded from the proposed model. The most significant terms are those representing the effects of the individual components (b1, b2, b3). The chosen mathematical model is represented by the following Equation:
| Y = 11.47X1 + 9.18X2 + 11.90X3 + 5.34X1X2 + 5.84X2X3 − 45.09X1X2X3 + ɛ |
3.4.4. Optimization of Formulation: Inhibition Zone Response
Mixing Profile
The objective of this Section is to identify the optimal formulation of the three EOs that result in an inhibition zone diameter, indicating a sensitivity classified as ‘extremely sensitive’ [63]. Therefore, we will search for the proportions of the components that provide a maximum value of inhibition zone diameter.
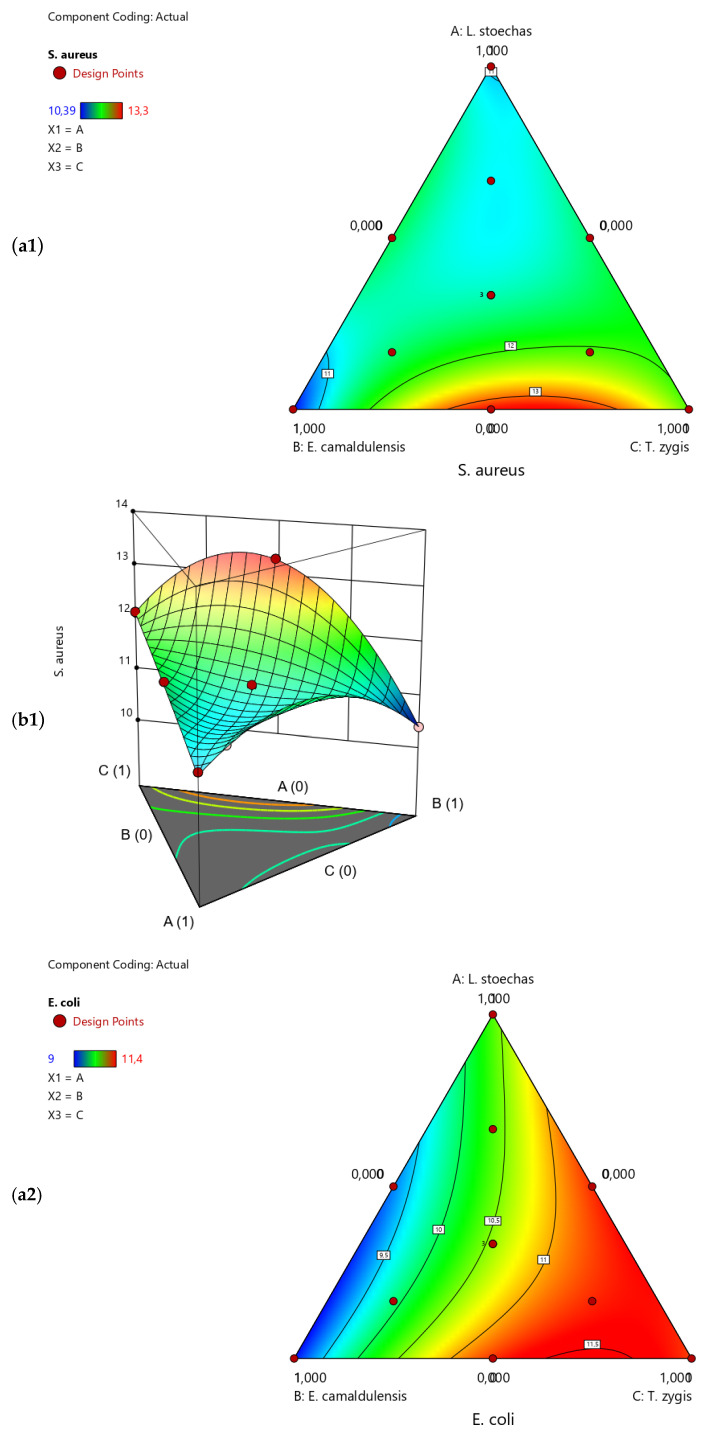
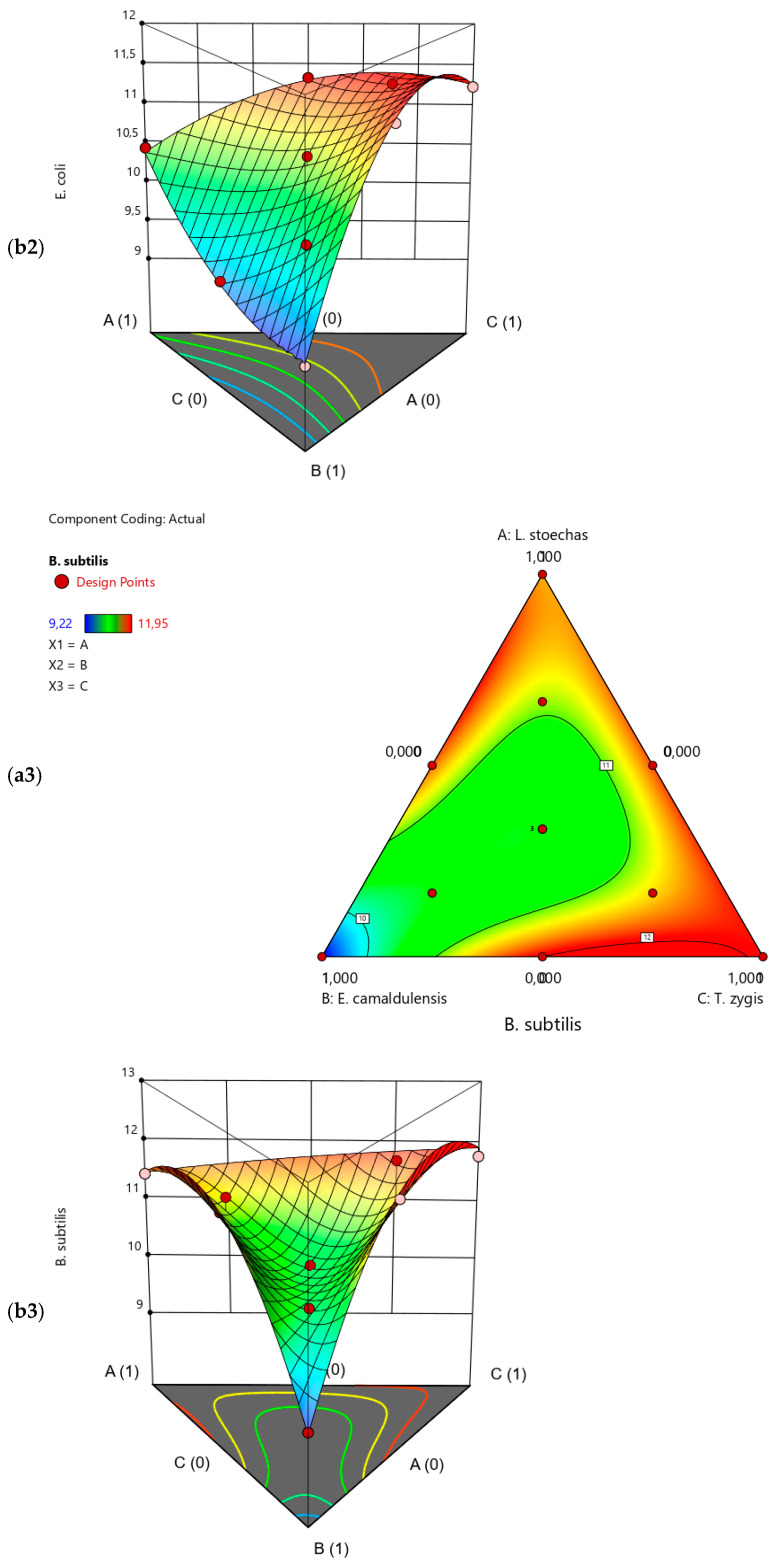
The mixture plot (Figure 4) indicates that the desired diameter (maximum value) can be achieved in two different compromise zones (red areas). The first zone lies along the axis of the triangle formed by the two essential oils, E. camaldulensis and T. zygis, with a minimization of the third essential oil, L. stoechas. The second compromise zone is along the axis of the triangle formed by L. stoechas and T. zygis, with a minimization of E. camaldulensis. These results are more apparent in the mixture and 3D traces, showing that the desired compromise zone is present in two mixing areas.
Figure 4.
(a) Two-dimensional mixing profile and (b) three-dimensional profile showing the zone of maximum di response based on the three constituents.
Study of Desirability
To identify the proportions of the three oils that yield the desired response, we utilized the desirability graph tool. This tool offers the optimal proportions with a level of compromise. The aim is to achieve the maximum inhibition zone values.
The formulations below clearly indicate the proportions of individual EOs necessary to achieve the highest inhibition against the studied strains.
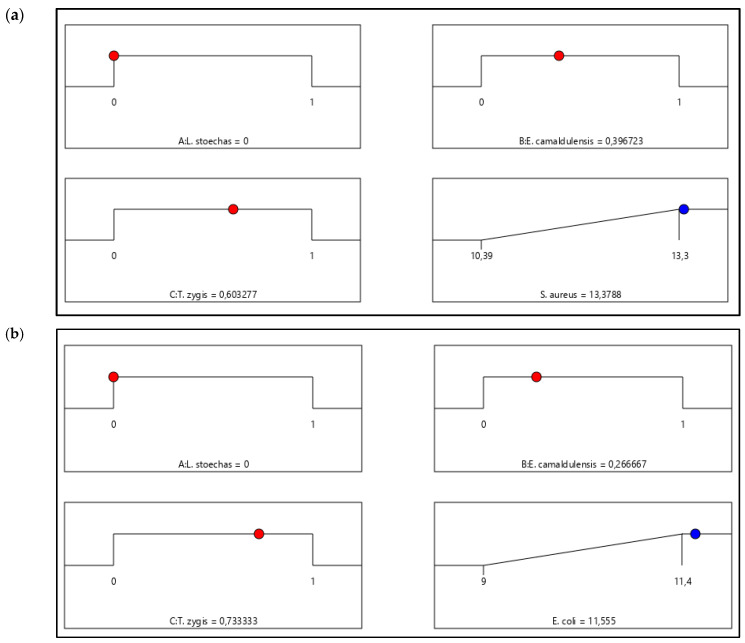
i. Effect of the Formulation against S. aureus ATCC: Figure 5a demonstrates that the maximum inhibition diameter achievable is 13.37 mm, with a desirability of 99.9%. According to the 2D and 3D mixture graph in Figure 4(a1), we can conclude that a mixture of E. camaldulensis EO and T. zygis EO is necessary to reach this inhibition zone value. Furthermore, this value can be achieved by making a mixture composed of 40% E. camaldulensis EO and 60% T. zygis EO.
Figure 5.
Desirability graph revealing the precise proportions of T. zygis, L. stoechas and E. camaldulensis EOs leading to the best antibacterial against S. aureus (a), E. coli (b) and B. subtilis (c).
ii. Effect of the Formulation against E. coli ATCC: Figure 5b illustrates that the maximum inhibition diameter attainable is 11.55 mm. additionally, the 2D and 3D mixture diagrams in Figure 4(a2) specify the exact proportions of E. camaldulensis EO and T. zygis EO required to achieve this value. The desirability test indicates a 99.9% probability of achieving the desired mixture with 27% E. camaldulensis EO and 73% T. zygis EO.
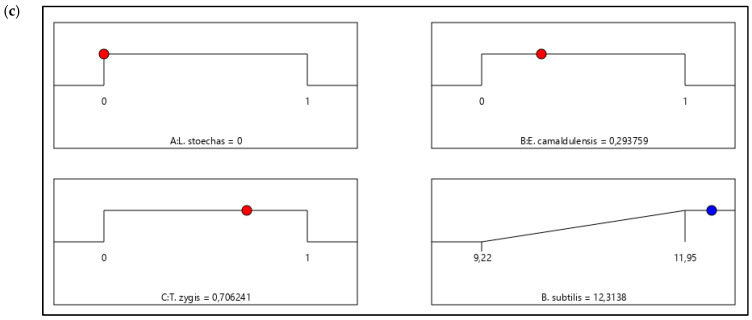
iii. Effect of the Formulation against B. subtilis ATCC: Figure 5c shows that the maximum inhibition diameter achievable is 12.31 mm, with a desirability of 99.9%. This value can be attained by creating a mixture of 29% E. camaldulensis EO and 71% T. zygis EO. Additionally, the 2D and 3D mixture diagrams in Figure 4(a3) indicate the precise proportions of E. camaldulensis EO and T. zygis EO needed to achieve this inhibition against B. subtilis.
Currently, many researchers employ mixture design methodology to analyze potential interactions between different components to identify optimal formulations [37,64,65]. To our knowledge, several studies have reported on the individual effects of selected essential oils, but no research has yet investigated the antibacterial capacity of combinations of L. stoechas, T. zygis, and E. camaldulensis. In this work, the augmented Simplex centroid model was used to optimize the antibacterial activity of essential oil mixtures against B. subtilis, E. coli, and S. aureus. This model reduces variability by examining multiple concentrations, ensuring a more comprehensive assessment of the antibacterial action of these EOs mixtures against these bacteria.
The results demonstrated that the binary mixture of 40% E. camaldulensis EO and 60% T. zygis EO is the necessary mixture to inhibit S. aureus. Also, the mixture of 27% E. camaldulensis EO and 73% T. zygis EO is the necessary mixture to achieve the optimal inhibitory concentration against E. coli. Additionally, the mixture of 29% E. camaldulensis EO and 71% T. zygis EO is the necessary mixture to inhibit B. subtilis. These findings suggest that combinations of thymus and eucalyptus essential oils in ratios of 0.40/0.60, 0.27/0.73, and 0.29/0.71 could be viable alternatives for food safety control against S. aureus, E. coli, and B. subtilis, respectively. In this context, Benkhaira et al. [66] concluded that the mixture of 36% Ruta montana and 64% Clinopodium nepeta is the optimal combination to limit the variability of Staphylococcus aureus. Recently, Jeddi et al. [67] showed that 32% Eucalyptus polybractea cryptonifera, 28% Ormenis mixta, and 40% Lavandula burnatii briquet comprise the optimal mixture against Escherichia coli. Meanwhile, 35% Eucalyptus polybractea cryptonifera, 30% Ormenis mixta and 35% Lavandula burnatii briquet make up the optimal mixture against Staphylococcus aureus. On the other hand, Chraibi et al. [9] reported that 60% and 40% for Thymus satureioides and Myrtus communis and 72% and 28% for Thymus satureioides and Artemisia herba alba predicted the highest antimicrobial effect against Escherichia coli and Staphylococcus aureus, respectively. However, the optimal mixture against E. coli and S. aureus corresponds to 54%/46% and 56%/44% of Mentha pulegium and Mentha piperita EOs, respectively [61].
The synergistic action of combined EOs could be due to the activity of their chemical compounds, particularly δ-terpineol, δ-3-carene, thymol, camphor, fenchone, 1,8-cineole, γ-terpinene, and α-terpineol, which are the main components of the studied essential oils. To our knowledge, the interactions between these molecules have not been previously documented. However, prior research has shown that combinations of 1,8-cineole/thymol, 1,8-cineole/limonene, α-pinene/linalool, and 1,8-cineole/ρ-cymene exhibit synergistic antibacterial activity [68,69,70]. Additionally, another study found that combinations of Lavandula latifolia and camphor were relatively more effective against the pathogens Listeria monocytogenes and Staphylococcus aureus [71].
In general, the antibacterial activity of essential oils is linked to the functional groups present in their active components, which can bind to the cell surface and penetrate the phospholipid bilayer of the cell membrane. This accumulation disrupts the membrane’s structural integrity, altering cellular metabolism and leading to cell death [9]. For instance, in bacteria, it has been shown that phenolic groups like thymol interact with the outer membrane’s constituents, causing degradation and the release of lipopolysaccharides (LPS), which increases membrane permeability and results in significant ATP loss [67]. Similarly, compounds such as α-terpineol, terpinol-4-ol, and δ-terpineol destroy the membrane and cell wall integrity, altering permeability and releasing intracellular substances like nucleic acids and proteins [5]. A study by [72] also demonstrated that 1,8-cineole induces significant outer membrane degradation, cytoplasm reduction, and alters the cell’s physical characteristics. Likewise, camphor disrupts the bacterial membrane’s integrity, leading to bacterial death [63,73].
The combination of 1,8-cineole, camphor, and thymol shows significant antibacterial effects, particularly by disrupting bacterial cell membranes, such as those of S. aureus and E. coli. When used together, these compounds increase membrane permeability, leading to the leakage of intracellular substances and compromising cell integrity. Previous studies have shown that the synergistic interactions between thymol and 1,8-cineole promote structural changes within the membranes, facilitating the influx of antimicrobial compounds and the release of essential ions, thereby disrupting bacterial metabolic functions [74,75]. However, the specific role of camphor in this combination remains to be explored. Research indicates that camphor, due to its terpenoid structure and lipophilic properties, could enhance the membranotropic effects of the other compounds, disrupting bacterial membrane integrity, which may increase membrane fluidity and permeability [76]. This potential synergy could involve cooperative action by modifying bacterial membrane fluidity, increasing permeability, and causing the leakage of vital components such as ions. This disruption leads to potential membrane collapse and bacterial death. Exploring this possibility could provide a deeper understanding of antibacterial mechanisms and contribute to the development of more effective therapeutic strategies.
Overall, the findings of this study provide scientific evidence supporting the potential applications of combined oils to develop new effective antimicrobial agents against resistant bacterial strains. These agents could be beneficial in food packaging and preservation, minimizing the loss of nutritional and organoleptic properties of various food products, as well as in the development of biopharmaceutical products [37,64]. Indeed, essential oils and their components offer solutions to combat bacteria that are safer and more environmentally friendly. Many bioactive molecules are selective and less toxic to humans, animals, and the environment.
4. Conclusions
The present study demonstrated that essential oils extracted from L. stoechas, T. zygis, and E. camaldulensis exhibit significant antimicrobial potential. Whether used alone or in combination, these volatile oils are highly effective against bacterial strains S. aureus, E. coli, and B. subtilis. The antibacterial capacity of the selected essential oils depends on their proportions in the formulation. According to the mixture design, the sensitivity of the bacterial strains can be attributed to the synergistic effect between the active constituents of the combined oils. The combination of Tz/Ec and Ls/Ec/Tz in the ratios 0.5:0.5 and 1/6:1/6:2/3, respectively, showed higher inhibition diameters against Gram-negative strains than against Gram-positive ones. This could be related to higher levels of δ-3-Carene, 1,8-cineole, camphor, and δ-terpineol and the synergistic effect between them.
The binary mixture of 40% E. camaldulensis EO and 60% T. zygis EO constitutes the optimal mixture for inhibiting S. aureus. Similarly, the mixture of 27% E. camaldulensis EO and 73% T. zygis EO is necessary to achieve the optimal inhibitory concentration against E. coli. Additionally, the mixture of 29% E. camaldulensis EO and 71% T. zygis EO is necessary to inhibit B. subtilis. These combinations can serve as alternatives to conventional antibiotics, whose effectiveness is diminishing against certain resistant strains that cause serious pathologies in medicine and quality degradation in the food industry. These results should be considered for the successful application of these natural preservatives in the food industry.
Acknowledgments
Authors are thankful to the Researchers supporting project number (RSPD2024R1057), King Saud University, Riyadh, Saudi Arabia.
Author Contributions
F.A., B.S., S.A., M.K. and J.D.: methodology, formal analysis; F.A., B.S., M.K., M.G. and A.F.: writing—review and editing; F.A., B.S., S.A., R.C. and A.A.S.: software, project administration, resources, F.A., B.S., I.E. and A.A.S.: writing—original; F.A., J.D. and M.O.: supervision, review and editing, F.A., J.D., R.C., A.A.S. and M.K.: conceptualization. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All related data are contained within the manuscript.
Conflicts of Interest
The authors have declared no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sharma K., Guleria S., Razdan V.K., Babu V. Synergistic antioxidant and antimicrobial activities of essential oils of some selected medicinal plants in combination and with synthetic compounds. Ind. Crops Prod. 2020;154:112569. doi: 10.1016/j.indcrop.2020.112569. [DOI] [Google Scholar]
- 2.Bag A., Chattopadhyay R.R. Evaluation of synergistic antibacterial and antioxidant efficacy of essential oils of spices and herbs in combination. PLoS ONE. 2015;10:e0131321. doi: 10.1371/journal.pone.0131321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nafis A., Kasrati A., Jamali C.A., Mezrioui N., Setzer W., Abbad A., Hassani L. Antioxidant activity and evidence for synergism of Cannabis sativa (L.) essential oil with antimicrobial standards. Ind. Crops Prod. 2019;137:396–400. doi: 10.1016/j.indcrop.2019.05.032. [DOI] [Google Scholar]
- 4.El-Gebaly A.S., Sofy A.R., Hmed A.A., Youssef A.M. Combination of nanoparticles (NPs) and essential oils (EOs) as promising alternatives to non-effective antibacterial, antifungal and antiviral agents: A review. Biocatal. Agric. Biotechnol. 2024;57:103067. doi: 10.1016/j.bcab.2024.103067. [DOI] [Google Scholar]
- 5.Huang J., Yang L., Zou Y., Luo S., Wang X., Liang Y., Wei Q. Antibacterial activity and mechanism of three isomeric terpineols of Cinnamomum longepaniculatum leaf oil. Folia Microbiol. 2021;66:59–67. doi: 10.1007/s12223-020-00818-0. [DOI] [PubMed] [Google Scholar]
- 6.Cutillas A.B., Carrasco A., Martinez-Gutierrez R., Tomas V., Tudela J. Thyme essential oils from Spain: Aromatic profile ascertained by GC–MS, and their antioxidant, anti-lipoxygenase and antimicrobial activities. J. Food Drug Anal. 2018;26:529–544. doi: 10.1016/j.jfda.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bencheqroun H.K., Ghanmi M., Satrani B., Aafi A., Chaouch A. Antimicrobial activity of the essential oil of an endemic plant in Morocco, Artemisia mesatlantica. Bull. Soc. R. Sci. Liège. 2021;81:4–21. [Google Scholar]
- 8.Burt S. Essential oils: Their antibacterial properties and potential applications in foods a review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Chraibi M., Fadil M., Farah A., Benkhaira N., Lebrazi S., Fikri-Benbrahim K. Simplex-centroid design as innovative approach in the optimization of antimicrobial effect of Thymus satureioides, Myrtus communis and Artemisia herba alba essential oils against Escherichia coli, Staphylococcus aureus and Candida tropicalis. Exp. Parasitol. 2023;247:108472. doi: 10.1016/j.exppara.2023.108472. [DOI] [PubMed] [Google Scholar]
- 10.Jaouani M., Maouni S., Ettakifi H., Mars N., Taheri F.Z., El Abboudi J., Maouni A. Molecular, biomedical and phytosanitary biodiversity of Lavandula stoechas: A vulnerable and underexploited medicinal plant in Morocco. Sci. Afr. 2024;25:e02296. doi: 10.1016/j.sciaf.2024.e02296. [DOI] [Google Scholar]
- 11.Chograni H., Riahi L., Messaoud C. Variability of qualitative and quantitative secondary metabolites traits among wild genetic resources of Lavandula stoechas L. Biochem. Syst. Ecol. 2021;98:104327. doi: 10.1016/j.bse.2021.104327. [DOI] [Google Scholar]
- 12.Carrasco A., Ortiz-Ruiz V., Martinez-Gutierrez R., Tomas V., Tudela J. Lavandula stoechas essential oil from Spain: Aromatic profile determined by gas chromatography–mass spectrometry, antioxidant and lipoxygenase inhibitory bioactivities. Ind. Crops Prod. 2015;73:16–27. doi: 10.1016/j.indcrop.2015.03.088. [DOI] [Google Scholar]
- 13.Özcan M.M., Starovic M., Aleksic G., Figueredo G., Juhaimi F.A., Chalchat J.C. Chemical composition and antifungal activity of lavender (Lavandula stoechas) oil. Nat. Prod. Commun. 2018;13:895–898. doi: 10.1177/1934578X1801300728. [DOI] [Google Scholar]
- 14.Giray E.S., Kırıcı S., Kaya D.A., Türk M., Sönmez Ö., Inan M. Comparing the Effect of Sub-Critical Water Extraction with Conventional Extraction Methods on the Chemical Composition of Lavandula Stoechas. Talanta. 2008;74:930–935. doi: 10.1016/j.talanta.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 15.Javed M.A., Khan M.A., Arshad H., Aslam N., Iqbal A.A., Ali A., Bukhari M.H. Pharmacological Evaluation of Lavandula stoechas L. for Ethanol-Induced Gastric Mucosal Ulcer. RADS J. Pharm. Pharm. Sci. 2020;8:47–57. doi: 10.37962/jpps.v8i1.361. [DOI] [Google Scholar]
- 16.Karan T. Metabolic profile and biological activities of Lavandula stoechas L. Cell. Mol. Biol. 2018;64:1–7. doi: 10.14715/cmb/2018.64.14.1. [DOI] [PubMed] [Google Scholar]
- 17.Bouyahya A., Et-Touys A., Abrini J., Talbaoui A., Fellah H., Bakri Y., Dakka N. Lavandula stoechas essential oil from Morocco as novel source of antileishmanial, Journal Pre-proof 15 antibacterial and antioxidant activities. Biocatal. Agric. Biotechnol. 2017;12:179–184. doi: 10.1016/j.bcab.2017.10.003. [DOI] [Google Scholar]
- 18.Benali T., Lemhadri A., Harboul K., Chtibi H., Khabbach A., Jadouali S.M., Quesada-Romero L., Louahlia S., Hammani K., Ghaleb A. Chemical Profiling and Biological Properties of Essential Oils of Lavandula stoechas L. Collected from Three Moroccan Sites: In Vitro and In Silico Investigations. Plants. 2023;12:1413. doi: 10.3390/plants12061413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fennane M., Ibn Tattou M., Ouyahya A., El Oualidi J. Flore Pratique du Maroc, 2. Trav. Inst. Sci., Sér. Bot. 2007;38:636. [Google Scholar]
- 20.Bouymajane A., Filali F.R., Ed-Dra A., Aazza M., Nalbone L., Giarratana F., Cacciola F. Chemical profile, antibacterial, antioxidant, and anisakicidal activities of Thymus zygis subsp. gracilis essential oil and its effect against Listeria monocytogenes. Int. J. Food Microbiol. 2022;383:109960. doi: 10.1016/j.ijfoodmicro.2022.109960. [DOI] [PubMed] [Google Scholar]
- 21.Silva A.M., Martins-Gomes C., Souto E.B., Schäfer J., Santos J.A., Bunzel M., Nunes F.M. Thymus zygis subsp. zygis an endemic portuguese plant: Phytochemical profiling, antioxidant, anti-proliferative and anti-inflammatory activities. Antioxidants. 2020;9:482. doi: 10.3390/antiox9060482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drioiche A., Radi F., Zair T. Correlation between the chemical composition and the antimicrobial properties of seven samples of essential oils of endemic Thymes in Morocco against multi-resistant bacteria and pathogenic fungi. Saudi Pharm. J. 2022;30:1200–1214. doi: 10.1016/j.jsps.2022.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coimbra A., Miguel S., Ribeiro M., Coutinho P., Silva L., Duarte A.P., Ferreira S. Thymus zygis essential oil: Phytochemical characterization, bioactivity evaluation and synergistic effect with antibiotics against Staphylococcus aureus. Antibiotics. 2022;11:146. doi: 10.3390/antibiotics11020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moukhles A., Ellaghdach A., Driss A.B., El Amrani M.A., Aghmiz A., Mansour A.I. Chemical profile and in vitro antibacterial potential of essential oils and hydrolat extracts from aerial parts of three wild species of Moroccan Thymus. Sci. Afr. 2022;18:e01434. doi: 10.1016/j.sciaf.2022.e01434. [DOI] [Google Scholar]
- 25.Chiasson H., Bostanian N.J., Vincent C. Acaricidal properties of a Chenopodium-based botanical. J. Econ. Entomol. 2004;97:1373–1377. doi: 10.1093/jee/97.4.1373. [DOI] [PubMed] [Google Scholar]
- 26.Hmiri S., Rahouti M., Habib Z., Satrani B., Ghanmi M., El Ajjouri M. Évaluation du potentiel antifongique des huiles essentielles de Mentha pulegium et d’Eucalyptus Camaldulensis dans la lutte biologique contre les champignons responsables de la détérioration des pommes en conservation. Bull. De La Société R. Des Sci. De Liège. 2011;80:824–836. [Google Scholar]
- 27.Kumar R., Mishra A., Dubey N., Tripathi Y. Evaluation of Chenopodium ambrosioides oil as a potential source of antifungal, antiaflatoxigenic and antioxidant activity. Int. J. Food Microbiol. 2007;115:159–164. doi: 10.1016/j.ijfoodmicro.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Ashraf A., Sarfraz R.A., Mahmood A., ud Din M. Chemical composition and in vitro antioxidant and antitumor activities of Eucalyptus camaldulensis Dehn. leaves. Ind. Crops Prod. 2015;74:241–248. doi: 10.1016/j.indcrop.2015.04.059. [DOI] [Google Scholar]
- 29.Ez-Zriouli R., ElYacoubi H., Imtara H., Mesfioui A., ElHessni A., Al Kamaly O., Rochdi A. Chemical composition, antioxidant and antibacterial activities and acute toxicity of Cedrus atlantica, Chenopodium ambrosioides and Eucalyptus camaldulensis essential oils. Molecules. 2023;28:2974. doi: 10.3390/molecules28072974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehani M., Salhi N., Dahou F., Kasmi S., Mehani I., Segni L., Akram M. Antifungal, Antibacterial and Phytotoxic Activity of Essential Oil from Leaves of Eucalyptus camaldulensis. Phytothérapie. 2022;20:48–62. doi: 10.3166/phyto-2021-0299. [DOI] [Google Scholar]
- 31.Chahomchuen T., Insuan O., Insuan W. Chemical profile of leaf essential oils from four Eucalyptus species from Thailand and their biological activities. Microchem. J. 2020;158:105248. doi: 10.1016/j.microc.2020.105248. [DOI] [Google Scholar]
- 32.Dogan G., Kara N., Bagci E., Gur S. Chemical composition and biological activities of leaf and fruit essential oils from Eucalyptus camaldulensis. Z. Für Naturforschung C. 2017;72:483–489. doi: 10.1515/znc-2016-0033. [DOI] [PubMed] [Google Scholar]
- 33.Clevenger J.F. Apparatus for the determination of volatile oil. J. Am. Pharm. Assoc. 1928;17:346–351. doi: 10.1002/jps.3080170407. [DOI] [Google Scholar]
- 34.Marion C., Pelissier Y., Sabatier R., Andary C., Bessiere J.M. Calculation of Essential Oil Yield without Prior Extraction—Application to the Genus Forsythia Vahl.(Oleaceae) J. Essent. Oil Res. 1994;6:379–387. doi: 10.1080/10412905.1994.9698403. [DOI] [Google Scholar]
- 35.Jaber H., Oubihi A., Ouryemchi I., Boulamtat R., Oubayoucef A., Bourkhiss B., Ouhssine M. Chemical composition and antibacterial activities of eight plant essential oils from Morocco against Escherichia coli strains isolated from different Turkey organs. Biochem. Res. Int. 2021;2021:6685800. doi: 10.1155/2021/6685800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goupy J., Creighton L. Introduction Aux Plans D’exp’eriences. Volume 2 Dunod; Paris, France: 2006. [Google Scholar]
- 37.Fadil M., Fikri-Benbrahim K., Rachiq S., Ihssane B., Lebrazi S., Chraibi M., Farah A. Combined treatment of Thymus vulgaris L., Rosmarinus officinalis L. and Myrtus communis L. essential oils against Salmonella typhimurium: Optimization of antibacterial activity by mixture design methodology. Eur. J. Pharm. Biopharm. 2018;126:211–220. doi: 10.1016/j.ejpb.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Draper N.R., Smith H. Analyse de Régression Appliquée. John Wiley et fils; Hoboken, NJ, USA: 1998. p. 326. [Google Scholar]
- 39.Ebadollahi A., Setzer W.N. Analysis of the Essential Oils of Eucalyptus camaldulensis Dehnh. and E. viminalisLabill. as a Contribution to Fortify Their Insecticidal Application. Nat. Prod. Commun. 2020;15:1–10. doi: 10.1177/1934578X20946248. [DOI] [Google Scholar]
- 40.Mubarak E.E., Ali L.Z., Ahmed I.F.A., Ahmed A.B.A., Taha R.M. Essential oil compositions and cytotoxicity from various organs of Eucalyptus camaldulensis. Int. J. Agric. Biol. 2015;17:320–326. [Google Scholar]
- 41.Yiğit Hanoğlu D., Hanoğlu A., Adediran S.B., Baser K.H.C., Özkum Yavuz D. The essential oil compositions of two Eucalyptus sp.(E. camaldulensis D ehnh. and E. torquata L. uehm.) naturalized to Cyprus. J. Essent. Oil Res. 2023;35:136–142. doi: 10.1080/10412905.2022.2147592. [DOI] [Google Scholar]
- 42.El Hachlafi N., Benkhaira N., Al-Mijalli S.H., Mrabti H.N., Abdnim R., Abdallah E.M., Fikri-Benbrahim K. Phytochemical analysis and evaluation of antimicrobial, antioxidant, and antidiabetic activities of essential oils from Moroccan medicinal plants: Mentha suaveolens, Lavandula stoechas, and Ammi visnaga. Biomed. Pharmacother. 2023;164:114937. doi: 10.1016/j.biopha.2023.114937. [DOI] [PubMed] [Google Scholar]
- 43.Souihi M., Bousnina A., Touati B., Hassen I., Rouissi M., Brahim N.B. Caractérisation morphologique et chimique de deux espèces de Lavande: Lavandula stoechas L. et L. dentata L. en Tunisie. Annales de l’INRAT. 2017;389:1–14. [Google Scholar]
- 44.Sadani S., Shakeri A. Antimicrobial activity of the essential oils of Lavandula stoechas flowers extracted by microwave. J. Med. Plants Stud. 2016;4:224–228. [Google Scholar]
- 45.Zayyad N., Farah A., Bahhou J. Chemical analysis and antibacterial activity of essential oils from three species of Thymus: Thymus zygis, T. algeriensis, and T. bleicherianus. Bull. De La Société R. Des Sci. De Liège. 2014;83:118–132. [Google Scholar]
- 46.Rodrigues V., Cabral C., Evora L., Ferreira I., Cavaleiro C., Cruz M.T., Salgueiro L. Chemical composition, anti-inflammatory activity and cytotoxicity of Thymus zygis L. subsp. sylvestris (Hoffmanns. & Link) Cout. essential oil and its main compounds. Arab. J. Chem. 2019;12:3236–3243. doi: 10.1016/j.arabjc.2015.08.026. [DOI] [Google Scholar]
- 47.Noureddine A., Gherib A., Bakchiche B., Carbonell-Barrachina Á.A., Cano-Lamadrid M. Noguera-Artiaga, Chemical composition, mineral content and antioxidant capacity of phenolic extracts and essential oils of Lavandula stoechas L. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food. 2019;20:423–437. [Google Scholar]
- 48.Ramzi A., Farah A., Ez Zoubi Y., Annemer S., El Ouali Lalami A. Aroma profile and fumigant toxicity of two Moroccan Lavandula species essential oils against Culex pipiens (Diptera: Culicidae) Int. J. Trop. Insect Sci. 2022;42:2663–2672. doi: 10.1007/s42690-022-00795-6. [DOI] [Google Scholar]
- 49.Gourich A.A., Bencheikh N., Bouhrim M., Regragui M., Rhafouri R., Drioiche A., Zair T. Comparative analysis of the chemical composition and antimicrobial activity of four moroccan north middle atlas medicinal plants’ essential oils: Rosmarinus officinalis L., Mentha pulegium L., Salvia officinalis L., and Thymus zygis subsp. gracilis (Boiss.) R. Morales. Chemistry. 2022;4:1775–1788. doi: 10.3390/chemistry4040115. [DOI] [Google Scholar]
- 50.Marinković J., Ćulafić D.M., Nikolić B., Đukanović S., Marković T., Tasić G., Marković D. Antimicrobial potential of irrigants based on essential oils of Cymbopogon martinii and Thymus zygis towards in vitro multispecies biofilm cultured in ex vivo root canals. Arch. Oral Biol. 2020;117:104842. doi: 10.1016/j.archoralbio.2020.104842. [DOI] [PubMed] [Google Scholar]
- 51.Teixeira M.A., Rodrigues A.E. Coupled extraction and dynamic headspace techniques for the characterization of essential oil and aroma fingerprint of Thymus species. Ind. Eng. Chem. Res. 2014;53:9875–9882. doi: 10.1021/ie501301u. [DOI] [Google Scholar]
- 52.Nebié B., Dabiré C.M., Bonzi S., Bationo R.K., Sosso S., Nebié R.C., Duez P. Chemical composition and antifungal activity of the essential oil obtained by co-distillation of Cymbopogon citratus and Eucalyptus camaldulensis from Burkina Faso. J. Pharmacogn. Phytochem. 2023;12:43–48. [Google Scholar]
- 53.Abbas A., Anwar F., Alqahtani S.M., Ahmad N., Al-Mijalli S.H., Shahid M., Iqbal M. Hydro-distilled and supercritical fluid extraction of Eucalyptus camaldulensis essential oil: Characterization of bioactives along with antioxidant, antimicrobial and antibiofilm activities. Dose-Response. 2022;20:1–12. doi: 10.1177/15593258221125477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamzavi F., Moharramipour S. Chemical composition and antifeedant activity of essential oils from Eucalyptus camaldulensis and Callistemon viminalis on Tribolium confusum. Int. J. Agric. Technol. 2017;13:413–424. [Google Scholar]
- 55.Aabouch F., Satrani B., Ameggouz M., Ettaleb I., Assouguem A., Kara M., Ullah R., Bari A., Kaur S., Ghanmi M., et al. Wild Thymus zygis L. ssp. gracilis and Eucalyptus camaldulensis Dehnh.: Chemical composition, antioxidant and antibacterial activities of essential oils. Open Chem. 2024;22:20240050. doi: 10.1515/chem-2024-0050. [DOI] [Google Scholar]
- 56.Yakoubi R., Megateli S., Sadok T.H., Bensouici C., Bağci E. A synergistic interactions of Algerian essential oils of Laurus nobilis L., Lavandula stoechas L. and Mentha pulegium L. on anticholinesterase and antioxidant activities. Biocatal. Agric. Biotechnol. 2021;31:101891. doi: 10.1016/j.bcab.2020.101891. [DOI] [Google Scholar]
- 57.Ghanimi R., Ouhammou A., El Atki Y., Cherkaoui M. Antioxidant and antibacterial activities of essential oils from three Moroccan species (Lavandula mairei Humbert, Lavandula dentata L. and, Lavandula stoechas L.) Lazaroa. 2021;33:64–71. doi: 10.9734/jpri/2021/v33i45B32779. [DOI] [Google Scholar]
- 58.Ez Zoubi Y., El Ouali Lalami A., Bousta D., Polissiou M., Daferera D., Lachkar M., El Khanchoufi A., Farah A. Chemical Composition, Antioxidant and Antimicrobial Activities of the Essential Oil and its Fractions of Lavandula stoechas L. From Morocco. Int. J. Curr. Pharm. Rev. Res. 2017;8:60–67. [Google Scholar]
- 59.Vukić M.D., Čmiková N., Hsouna A.B., Saad R.B., Garzoli S., Schwarzová M., Kačániová M. Thymus zygis, Valuable Antimicrobial (In Vitro and In Situ) and Antibiofilm Agent with Potential Antiproliferative Effects. Plants. 2023;12:3920. doi: 10.3390/plants12233920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saleh M.T., Ayub M.A., Shahid M., Raza M.H., Hussain A., Javed T. Comparison of Essential Oil Yield, Chemical Composition and Biological Activities of Eucalyptus camaldulensis Leaf: Conventional Distillation versus Emerging Superheated Steam Distillation. Iran. J. Pharm. Sci. 2023;19:139–155. doi: 10.22037/ijps.v19i2.43808. [DOI] [Google Scholar]
- 61.Chraibi M., Fadil M., Farah A., Lebrazi S., Fikri-Benbrahim K. Antimicrobial combined action of Mentha pulegium, Ormenis mixta and Mentha piperita essential oils against S. aureus, E. coli and C. tropicalis: Application of mixture design methodology. Food Sci. Technol. 2021;145:111352. doi: 10.1016/j.lwt.2021.111352. [DOI] [Google Scholar]
- 62.Yap P.S.X., Yusoff K., Lim S.-H.E., Chong C.-M., Lai K.-S. Membrane Disruption Properties of Essential Oils—A Double-Edged Sword? Processes. 2021;9:595. doi: 10.3390/pr9040595. [DOI] [Google Scholar]
- 63.Falleh H., Ben Jemaa M., Djebali K., Abid S., Saada M., Ksouri R. Application of the mixture design for optimum antimicrobial activity: Combined treatment of Syzygium aromaticum, Cinnamomum zeylanicum, Myrtus communis, and Lavandula stoechas essential oils against Escherichia coli. J. Food Process. Preserv. 2019;43:e14257. doi: 10.1111/jfpp.14257. [DOI] [Google Scholar]
- 64.Kachkoul R., Benjelloun Touimi G., Bennani B., El Habbani R., El Mouhri G., Mohim M., Lahrichi A. The synergistic effect of three essential oils against bacteria responsible for the development of Lithiasis infection: An optimization by the mixture design. Evid. -Based Complement. Altern. Med. 2021;1:1305264. doi: 10.1155/2021/1305264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Souiy Z., Elaissi A., Jlassi I., Sghair W., Allouch N., Mastouri M., Krifia B. Application of simplex-centroid design methodologies to optimize the anti-bacterial and anti-candidal activity of the mixture of Menthapulegium, Pituranthos Chloranthus and Thymus Algeriensis essential oils. Med. Aromat. Plants. 2021;10:365. [Google Scholar]
- 66.Benkhaira N., Zouine N., Fadil M., Koraichi S.I., El Hachlafi N., Jeddi M., Fikri-Benbrahim K. Application of mixture design for the optimum antibacterial action of chemically-analyzed essential oils and investigation of the antiadhesion ability of their optimal mixtures on 3D printing material. Bioprinting. 2023;34:e00299. doi: 10.1016/j.bprint.2023.e00299. [DOI] [Google Scholar]
- 67.Jeddi M., El Hachlafi N., Fadil M., Benkhaira N., Jeddi S., Benziane Ouaritini Z., Fikri-Benbrahim K. Combination of Chemically-Characterized Essential Oils from Eucalyptus polybractea, Ormenis mixta, and Lavandula burnatii: Optimization of a New Complete Antibacterial Formulation Using Simplex-Centroid Mixture Design. Adv. Pharmacol. Pharm. Sci. 2023;2023:5593350. doi: 10.1155/2023/5593350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tserennadmid R., Takó M., Galgóczy L., Papp T., Pesti M., Vágvölgyi C., Krisch J. Anti yeast activities of some essential oils in growth medium, fruit juices and milk. Int. J. Food Microbiol. 2011;144:480–486. doi: 10.1016/j.ijfoodmicro.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Vuuren S.V., Viljoen A.M. Antimicrobial activity of limonene enantiomers and 1, 8-cineole alone and in combination. Flavour Fragr. J. 2007;22:540–544. doi: 10.1002/ffj.1843. [DOI] [Google Scholar]
- 70.Pina-Vaz C., Gonçalves Rodrigues A., Pinto E. Antifungal activity of Tymus oils and their major compounds. J. Eur. Acad. Dermatol. Venereol. 2004;18:73–78. doi: 10.1111/j.1468-3083.2004.00886.x. [DOI] [PubMed] [Google Scholar]
- 71.Karaca N., Demirci B., Demirci F. Evaluation of Lavandula stoechas L. subsp. stoechas L., Mentha spicata L. subsp. spicata L. essential oils and their main components against sinusitis pathogens. Zeitschrift für Naturforschung C. 2018;73:353–360. doi: 10.1515/znc-2017-0150. [DOI] [PubMed] [Google Scholar]
- 72.Li L., Li Z.W., Yin Z.Q., Wei Q., Jia R.Y., Zhou L.J., Yu W. Antibacterial activity of leaf essential oil and its constituents from Cinnamomum longepaniculatum. Int. J. Clin. Exp. Med. 2014;7:1721–1727. [PMC free article] [PubMed] [Google Scholar]
- 73.Farhanghi A., Aliakbarlu J., Tajik H., Mortazavi N., Manafi L., Jalilzadeh-Amin G. Antibacterial interactions of pulegone and 1,8-cineole with monolaurin ornisin against Staphylococcus aureus. Food Sci. Nutr. 2022;10:2659–2666. doi: 10.1002/fsn3.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mutlu-Ingok A., Devecioglu D., Dikmetas D.N., Karbancioglu-Guler F., Capanoglu E. Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: An updated review. Molecules. 2020;25:4711. doi: 10.3390/molecules25204711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kifer D., Mužinić V., Klarić M.Š. Antimicrobial potency of single and combined mupirocin and monoterpenes, thymol, menthol and 1, 8-cineole against Staphylococcus aureus planktonic and biofilm growth. J. Antibiot. 2016;69:689–696. doi: 10.1038/ja.2016.10. [DOI] [PubMed] [Google Scholar]
- 76.Duda-Madej A., Viscardi S., Grabarczyk M., Topola E., Kozłowska J., Mączka W., Wińska K. Is Camphor the Future in Supporting Therapy for Skin Infections? Pharmaceuticals. 2024;17:715. doi: 10.3390/ph17060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All related data are contained within the manuscript.