ABSTRACT
Background and hypothesis
The MENTOR trial (MEmbranous Nephropathy Trial Of Rituximab) showed that rituximab was noninferior to cyclosporine in inducing complete or partial remission of proteinuria and was superior in maintaining proteinuria remission. However, the cost of rituximab may prohibit first-line use for some patients and health-care payers.
Methods
A Markov model was used to determine the incremental cost-effectiveness ratio (ICER) of rituximab compared with cyclosporine for the treatment membranous nephropathy from the perspective of a health-care payer with a lifetime time horizon. The model was informed by data from the MENTOR trial where possible; additional parameters including cost and utility inputs were obtained from the literature. Sensitivity analyses were performed to evaluate the impact of reduced-cost biosimilar rituximab.
Results
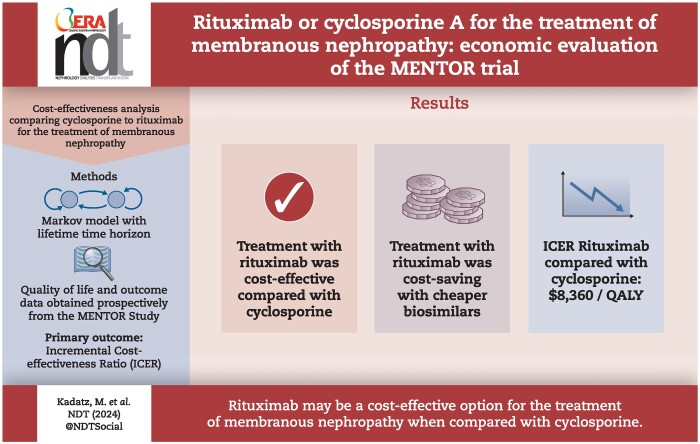
Rituximab for the treatment of membranous nephropathy was cost effective (assuming a willingness-to-pay threshold of $50 000 per quality-adjusted life year (QALY) gained; in $US 2021) compared with cyclosporine, with an ICER of $8373/QALY over a lifetime time horizon. The incremental cost of rituximab therapy was $28 007 with an additional 3.34 QALYs compared with cyclosporine. Lower cost of rituximab biosimilars resulted in a more favorable ICER, and in some cases resulted in rituximab being dominant (lower cost and great benefit) compared to cyclosporine.
Conclusions
Despite the greater cost of rituximab, it may be a cost-effective option for the treatment of membranous nephropathy when compared with cyclosporine. The cost-effectiveness of rituximab is further improved with the use of less expensive biosimilars.
Keywords: cost-effectiveness, cyclosporine, economic evaluation, membranous nephropathy, rituximab
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
Treatment of membranous nephropathy with rituximab has been shown to result in favorable outcomes compared with cyclosporine and is recommended as a first-line treatment; however, rituximab is more expensive than cyclosporine. This study aimed to determine whether using rituximab compared with cyclosporine to treat patients with membranous nephropathy would be cost effective in the long term given its potential benefits.
This study adds:
This study found that using rituximab instead of cyclosporine is cost effective compared to cyclosporine for the treatment of membranous nephropathy. In jurisdictions where the cost of rituximab is lower (such as Canada, UK, Europe), the use of rituximab may be cost-saving to the health-care system.
Potential impact:
This is important information for health-care decision-makers when determining whether rituximab would be funded for patients with membranous nephropathy.
INTRODUCTION
Membranous nephropathy is a leading cause of nephrotic syndrome in adults. [1] While 30% of affected patients will experience spontaneous remission, those who do not achieve remission are at high risk of progression to kidney failure. [2] Furthermore, these patients are at risk for other adverse health outcomes related to the nephrotic syndrome or to immunosuppression therapies used to treat the disease, and accrue significant costs to health-care payers. [3, 4] First-line therapies to induce remission in primary membranous nephropathy have historically included alkylating agents or calcineurin inhibitors. However, alkylating agents are limited by significant drug toxicities and calcineurin inhibitors have a high risk of relapse after completion of therapy.
In 2019 the MEmbranous Nephropathy Trial Of Rituximab (MENTOR) trial demonstrated that rituximab was noninferior to cyclosporine in inducing complete or partial remission of proteinuria after 12 months and was superior in maintaining proteinuria remission after 24 months. [5] Furthermore, patients who received rituximab were observed to have a higher creatinine clearance at the end of 2 years of therapy compared to those treated with cyclosporine, even among the subset of patients who experienced complete or partial remission. With a more favorable side effect profile compared to other alternatives and a higher probability of inducing remission, rituximab is now considered a first-line option therapy in the 2021 KDIGO guidelines for patients with moderate- or high-risk primary membranous nephropathy. [6] However, the greater cost of rituximab may be prohibitive to health-care payers or to patients who must pay out-of-pocket [7].
In this economic evaluation we use prospectively obtained data from the MENTOR trial cohort to determine the cost-effectiveness of rituximab to cyclosporine for the treatment of adults with membranous nephropathy using a Markov model [5]. We hypothesized that despite the higher cost of the drug, that rituximab would be a cost-effective treatment option compared with cyclosporine. The primary objective was to inform policy and health-care decision-makers for treatment guidelines of primary membranous nephropathy.
MATERIALS AND METHODS
Study design
We used Markov models to simulate a fixed cohort of adult patients with primary membranous nephropathy requiring immunosuppressive therapy based on the MENTOR study participants and outcomes. The mean age of this cohort was 52 years; a summary of the characteristics of these patients is presented in Table 1. The model compared the treatment options of rituximab with cyclosporine, as outlined in the MENTOR study. [5] A deterministic method using fixed model inputs was used for the base model. The primary study outcome is the incremental cost-effectiveness ratio (ICER) in US dollars (2021) per quality-adjusted life year (QALY). The base-case model used a health-care payer perspective with a lifetime time horizon and 6-month cycle length. A secondary model with a 24-month time horizon was also performed to align with the duration of follow-up in the MENTOR study. An annual discounting rate of 1.5% was applied to both cost and utility as per current guidelines. [8] A breakdown of the contribution of each health state to cost and utility in the model was calculated. The models were created using TreeAge Pro© 2021 (TreeAge Software, Williamstown, MA, USA). [9] This study was exempt from the requirement of research ethics board review as no patient level data were collected as part of this analysis.
Table 1:
Summary of characteristics of patients included in the MENTOR study.a
| Rituximab | Cyclosporine | |
|---|---|---|
| Age, years (SD) | 51.9 (12.6) | 52.2 (12.4) |
| Sex, male: % (no.) | 72% (47) | 82% (53) |
| Creatinine clearance, ml/min/1.73 m2 (SD) | 84.9 (29.8) | 87.4 (34.4) |
| Median serum albumin, mg/dl (interquartile range) | 2.5 (2.1–2.9) | 2.5 (2.1–2.9) |
| Median urinary protein, g/24 h (interquartile range) | 8.9 (6.8–12.3) | 8.9 (6.7–12.9) |
| Prior treatment with immunosuppressive therapy, % (no.) | 29% (19) | 31% (20) |
| Anti-phospholipase A2 antibody positive, % (no.) | 77% (50) | 71% (46) |
aData adapted from Fervenza et al. NEJM (2019) [5].
Health states and outcomes
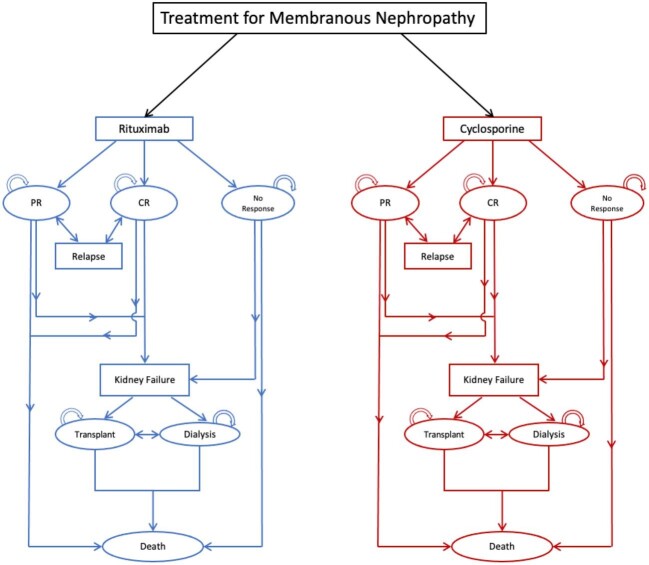
A graphical representation of model structure is shown in Fig. 1. All patients started in a treatment health state (Rituximab or Cyclosporine), and could transition to partial remission (PR), complete remission (CR), or no response (NR) after 6 months of treatment. Patients in the CR and NR state discontinued treatment, while patients in the PR state continued treatment for another 6-month cycle for a total of 12 months of treatment, which aligns with the treatment approach used in the MENTOR trial. Patients in CR or PR state who experienced a relapse would have retreatment for 6 months if they initially had CR or another 12 months if they initially had PR. The duration of retreatment was dependent on remission status after 6 months of retreatment. Patients in any remission state could progress to kidney failure over their lifetime, at which point they could be treated with kidney transplantation or dialysis. Patients could transition to death from any other health state.
Figure 1:
Diagram of base-case Markov model.
Cost, utility, and probability estimates
The model inputs are summarized in Table 2. The cost of rituximab was based on the price of 1 g of Rituxan® therapy in the USA, [10] while the cost of cyclosporine was calculated based on the mean dose of cyclosporine of patients included in the MENTOR study, multiplied by the cost of cyclosporine per milligram. Costs including kidney biopsies, blood work monitoring, and physician assessments were not included in the model given these would be similar in both treatment groups. Outcomes in the first 24 months of the model were informed by data from the MENTOR study and outcomes beyond 24 months were derived using evidence from a focused literature search (see Supplementary material for further discussion). Utility estimates for the health states of treatment phase, PR, CR, and NR were calculated from results of SF-12 data acquired prospectively during the MENTOR study that were mapped to the EQ-5D index to inform quality of life. [11]
Table 2:
Estimates used in the Markov model with their primary assigned values and probability distributions.
| Variable description | Base estimate | Probability distribution | Reference |
|---|---|---|---|
| Cost | |||
| Rituximab (per 1 g) | $9395 | Normal | [10] |
| Cyclosporine (per 1 mg) | $0.058 | Normal | [10] |
| 1 year of dialysis treatment | $83 797 | Normal | [21] |
| Organ acquisition for transplant | $70 747 | Normal | [22] |
| First year of transplant | $77 883 | Normal | [23] |
| Subsequent years of transplant | $23 555 | Normal | [23] |
| Utility | |||
| Treatment | 0.702 | Normal | MENTOR study data |
| CR (varied by time since treatment initiation) | 0.875–0.930 | Normal | |
| PR (varied by time since treatment initiation) | 0.735–0.767 | Normal | |
| No remission | 0.702 | Normal | |
| Kidney failure on dialysis | 0.71 | Normal | [13] |
| Kidney failure with a functioning transplant | 0.82 | Normal | [13] |
| Probability estimates beyond 24 months in modela | |||
| Probability of reaching kidney failure | varied by time after treatment initiation | N/A | [2] |
| Probability of death on dialysis | varied by age | NA | [21] |
| Probability of transplantation after progression to kidney failure | varied by age | NA | [21] |
| Probability of transplant failure | varied by age | NA | [21] |
| Relative risk of death in patients with NR to treatment | 1.69 | Normal | [12] |
| Risk of death from natural causes | varied by age | NA | [24] |
aTransition probabilities for the first 24 months obtained directly from MENTOR study data are detailed in Supplementary Table S2.
Limited mortality data are available for patients with membranous nephropathy who have not progressed to kidney failure. There were no deaths in the 24-month follow-up of the MENTOR study, and so there was no risk of death in the first 24 months in the model. Beyond 24 months, patients with CR and PR who do not develop kidney failure were assigned a risk of death based on the general population risk from 2020 US life table data. Patients with NR have a known increased risk of death and therefore, a hazard ratio for death of 1.69 was applied. [12] No patients in the 24-month follow-up of the MENTOR study experienced progression to kidney failure; beyond 24 months, the probability of progression of kidney disease to kidney failure varied depending on remission status. [2] Utility values of transplant and dialysis and cost data were derived from the literature. [13] All costs were adjusted to 2021 US dollars using the consumer price index.
Assumptions and limitations
Follow-up in the MENTOR study continued for up to 2 years; therefore, outcome data beyond 2 years was derived from existing literature. Furthermore, there were minimal adverse events reported in the MENTOR study and therefore adverse events were not modeled in this evaluation. Since treatment with alkylating agents was not evaluated in the MENTOR study, treatment with alkylating agents was not included in the model. For patients who achieved CR and PR but subsequently relapsed, it was assumed that patients would have a similar probability of achieving CR and PR after repeat treatment.
In the base-case model, patients who did not achieve CR or PR were assumed to undergo no further therapy. Risk of relapse of membranous nephropathy after kidney transplantation was not included in this model. Utility data from the MENTOR study were only available after mapping EQ-5D data from SF-36 surveys, which may have limitations [14].
Sensitivity analyses
To ensure the model findings were robust, we performed a variety of sensitivity analyses. A one-way sensitivity analysis was performed on the model inputs. When available, 95% confidence intervals reported in the literature were used as plausible ranges for these inputs. Otherwise, a range of ±10% was used to generate plausible ranges, except for cyclosporine where a range ±20% was used given potential variation in drug costs across jurisdictions. An independent sensitivity analysis was conducted to examine the impact of the cost of rituximab, which can vary by jurisdiction, as described next.
A probabilistic sensitivity analysis was also performed where uncertainty of parameters was represented by probability distributions for each input parameter (Table 2). The model was then analyzed repeatedly with various combinations of estimates drawn randomly from each distribution. In this case, we repeated the analysis 10 000 times. The distribution of results from the probabilistic analysis are presented in cost-effectiveness acceptability curves with varying thresholds of willingness-to-pay per additional QALY.
It was observed in the MENTOR study that patients treated with cyclosporine had a creatinine clearance that was 18 mL/min/1.73 m2 (95% CI: 5–31 ml/min/1.73 m2) lower than the those treated with rituximab after 24 months, regardless of remission status. We therefore modeled scenarios in which patients treated with cyclosporine had an increased risk of kidney failure based on an expected creatinine clearance of 18 ml/min/1.73 m2 lower than patients treated with rituximab, with a lower limit of 5 ml/min/1.73 m2 and upper limit of 31 ml/min based on the observed confidence intervals in the MENTOR study. In this sensitivity analysis we ascribed a relative hazard for the risk of progression to kidney failure of 1.03 for each additional decrease of creatinine clearance by 1 ml/min/1.73 m2 [2].
Additionally, the base model assumed there would be no subsequent treatment with alternative agents as this was not part of the protocol in the MENTOR study. To evaluate the real-world scenario in which patients might be treated with an alternative agent, we modeled a scenario in which patients who had NR to the initial therapy would be treated with the alternative (i.e. those who had NR to cyclosporine would subsequently be treated with rituximab and vice versa). Treatment with alkylating agents was not evaluated in this scenario.
Finally, there are multiple biosimilars of rituximab currently available, each with varying costs based on jurisdiction or country. We acquired cost estimates for rituximab biosimilars in various countries (Supplementary Table S1) and evaluated the impact of the lower costs on the overall cost-effectiveness of rituximab therapy. It was assumed in this sensitivity analysis that the efficacy of these biosimilars would be equivalent to Rituxan®.
RESULTS
Primary model results
In the base-case deterministic model, treatment with rituximab was associated with an additional 3.35 QALYs, and an increased cost of $28 007 compared with cyclosporine, resulting in a calculated ICER of $8360/QALY (Table 3, Supplementary Fig. 1). In the secondary model with a 24-month time horizon, rituximab treatment was associated with slightly greater QALYs (0.062) with an increased cost of $14 636 compared with cyclosporine, resulting in a calculated ICER of $238 589/QALY (Table 3).
Table 3:
Results of deterministic base-case Markov model of rituximab compared to cyclosporine for the treatment of primary membranous nephropathy with a lifetime and 24-month time horizon.
| Intervention | Cumulative costs ($) | Cumulative QALYs | Incremental cost ($) (rituximab vs. cyclosporine) | Incremental QALYs (rituximab vs. cyclosporine) | ICER (rituximab vs. cyclosporine) |
|---|---|---|---|---|---|
| Lifetime time horizon | |||||
| Rituximab | 76 525 | 13.94 | 28 007 | 3.35 | 8360 |
| Cyclosporine | 48 518 | 10.59 | |||
| 24-month time horizon | |||||
| Rituximab | 17 258 | 1.52 | 14 636 | 0.062 | 238 589 |
| Cyclosporine | 2622 | 1.45 | |||
We found that most of the costs in patients treated with rituximab in the model were derived from drug costs (60.8%), whereas most of the costs in patients treated with cyclosporine were due to progression to kidney failure (80.5%) (Table 4). Furthermore, more patients treated with rituximab achieved complete or PR and had a lower risk of kidney failure compared to cyclosporine, resulting in higher cumulative QALYs over a lifetime time horizon (Table 4).
Table 4:
Summary of categories contributing to the cumulative cost and utility from deterministic analysis of the base model comparing rituximab to cyclosporine for the treatment of primary membranous nephropathy.
| Value | ||
|---|---|---|
| Category | Rituximab | Cyclosporine |
| Cost categories ($) | ||
| Drug cost (%) | 46 543 (60.8) | 8257 (17.0) |
| Dialysis cost (%) | 29 046 (38.0) | 39 049 (80.5) |
| Transplant cost (%) | 936 (0.2) | 1212 (2.5) |
| Total | 76 525 | 48 518 |
| Utility categories (QALYs) | ||
| On treatment (%) | 0.90 (6.5) | 2.59 (12.2) |
| CR (%) | 6.29 (45.2) | 0.091 (0.4) |
| PR (%) | 3.55 (25.5) | 7.73 (36.5) |
| NR (%) | 2.94 (21.1) | 10.09 (47.6) |
| Dialysis (%) | 0.25 (1.8) | 0.66 (3.1) |
| Transplant (%) | 0.007 (<0.1) | 0.018 (<0.1) |
| Total | 13.94 | 21.18 |
Sensitivity analyses
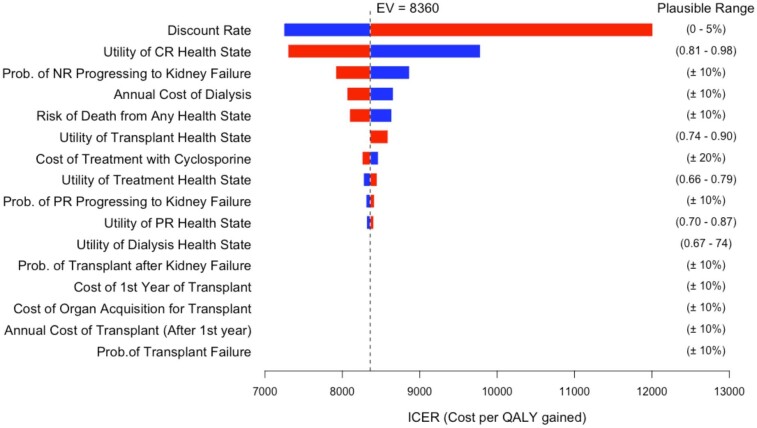
The Markov model results were robust in all one-way sensitivity analyses presented in a tornado diagram (Fig. 2). If the discount rate was increased to 5%, the calculated ICER increased to $12 012/QALY, while is the discount rate was 0%, the ICER decreased to $7245/QALY. If utility of the CR health state increased to 0.98, the calculated ICER decreased to $7245/QALY; whereas, if the utility decreased to 0.81, the ICER increased to $9784/QALY. There were minimal changes in the calculated ICER in the remaining one-way sensitivity analyses, including when the cost of cyclosporine varied by ±20%.
Figure 2:
Tornado diagram of results of one-way sensitivity analyses from Markov model. This graph demonstrates the range of ICERs given the plausible ranges for each input variable. The estimated value (EV) of the ICER from the overall deterministic Markov model is shown on the graph with a dashed line. Prob., probability.
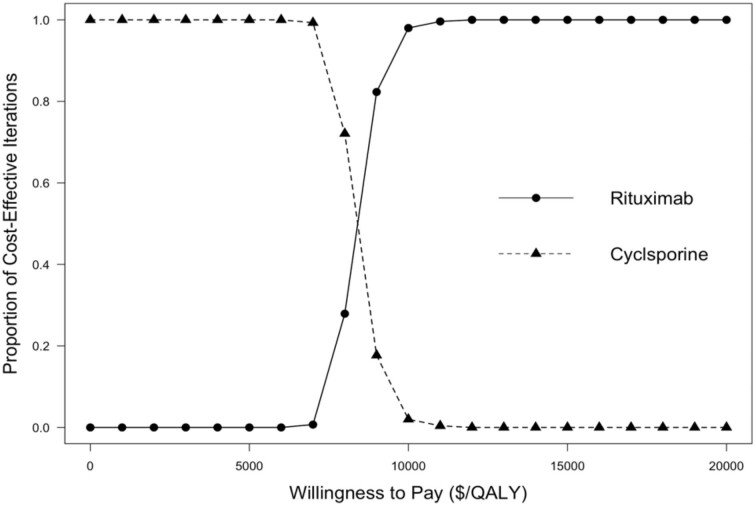
At a willingness-to-pay threshold of $8500/QALY and above, treatment with rituximab was favored in probabilistic analysis (Fig. 3). Rituximab therapy was the favored strategy in 100% of iterations at a willingness-to-pay threshold of $20 000/QALY.
Figure 3:
Cost-effectiveness acceptability curve of the results of the probabilistic sensitivity analysis for the incremental cost-effectiveness of rituximab (RTX) compared with cyclosporine (CsA) for the treatment of primary membranous nephropathy. This graph shows the proportion of iterations where rituximab (circles, solid line) and cyclosporine (triangles, dashed line) were cost effective according to varying willingness-to-pay thresholds. See the Supplementary Figure for these results presented as a scatter plot on the cost-effectiveness plane.
In scenarios where the creatinine clearance of patients treated with cyclosporine was modeled to be 18 ml/min/1.73 m2 lower than those treated with rituximab, as observed in the MENTOR study, the ICER improved modestly from $8373/QALY to $6995/QALY (Table 5). At the extremes of the confidence interval for the observed change in creatinine clearance (5–31 ml/min/1.73 m2), the ICER was estimated to be $8012–6007/QALY. In the scenario where the alternative therapy would be offered to patients who failed initial therapy with either cyclosporine or rituximab, the strategy of initial treatment with rituximab was dominant (less costly and greater benefit) compared with initial cyclosporine strategy.
Table 5:
Model the results of scenario analyses including incremental cost, incremental QALY's and ICER of rituximab compared to cyclosporine therapy for the treatment of membranous nephropathy.
| Scenario | Incremental Cost ($) | Incremental QALYs | ICER ($/QALY) |
|---|---|---|---|
| Base-case results | 28 007 | 3.34 | 8373 |
| Lower creatinine clearance (CrCl) in cyclosporine arm | |||
| CrCl ↓18 ml/min/1.73 m2 | 25 906 | 3.70 | 6995 |
| CrCl ↓5 ml/min/1.73 m2 | 27 477 | 3.43 | 8012 |
| CrCl ↓31 ml/min/1.73 m2 | 24 037 | 4.00 | 6007 |
| Alternative treatment offered | −102 | 3.21 | dominant |
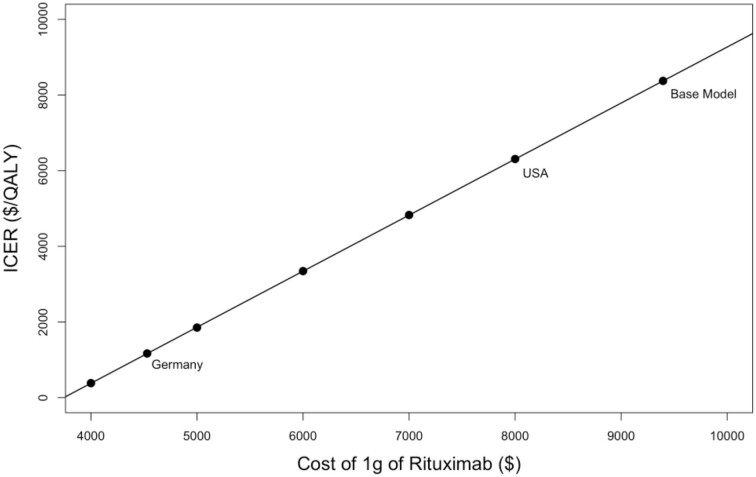
When the price of rituximab in the model was lowered to account for the reduced cost of rituximab biosimilars, the ICER of rituximab compared with cyclosporine improved substantially (Fig. 4) with the largest improvement observed with the current price of rituximab in Canada, and the UK where the treatment with rituximab dominated treatment with cyclosporine (i.e. rituximab was less expensive and more effective). The threshold for cost neutrality was reached if the 1 g cost of rituximab decreased to ∼$3800.
Figure 4:
ICER as a function of the cost of 1 g of rituximab. For reference, the ICER assuming costs of generic biosimilars in Germany and the USA are labeled. Costs of generic biosimilars in Canada and the UK are not shown as these resulted in a dominant ICER where treatment with rituximab was less expensive than treatment with cyclosporine and more effective.
DISCUSSION
We had hypothesized that rituximab would be a cost-effective treatment option compared to cyclosporine for the treatment of primary membranous nephropathy in adults. In this analysis, we found that treatment with rituximab would result in improved quality-adjusted life years with an acceptable cost-effectiveness ratio (ICER of $8373/QALY) when referenced to commonly cited willingness-to-pay thresholds [15, 16, 17]. In scenarios of reduced drug acquisition costs that may occur with biosimilars, rituximab was even more attractive and in some scenarios was dominant (less costly and more effective) compared with cyclosporine. Therefore, rituximab is preferred from the health-care payer perspective compared to cyclosporine.
This is the first economic evaluation to our knowledge that compares rituximab to cyclosporine for the treatment of membranous nephropathy. This analysis is based on the results directly obtained from a randomized controlled trial, which allowed for accurate model inputs of cost and outcomes for the first 24 months of the model. Further, quality of life data for health states of CR, PR, and NR were captured prospectively in the MENTOR study allowing for robust utility estimates for these health states. We were also able to incorporate the observation from the MENTOR study that patients treated with cyclosporine had a lower CrCl at the end of the study follow-up in a sensitivity analysis. Despite a lower CrCl potentially leading to an increased risk of kidney failure in patients treated with cyclosporine, incorporating this observation into our model only marginally improved the ICER in favor of rituximab to $6007/QALY, suggesting that the cost-effectiveness of rituximab is not substantially affected by the assumptions regarding kidney function following treatment, but rather the ability to achieve and maintain remission. Additionally, in a more realistic scenario that likely better approximates usual clinical care in which the alternative therapy would be offered to patients who failed initial therapy with either cyclosporine or rituximab, a strategy of starting treatment with rituximab was cost-saving compared to a strategy of starting treatment with cyclosporine. This further supports the use of rituximab as a potential first-line therapy for patients with primary membranous nephropathy who require immunosuppression.
There are several limitations to our analysis. First, the MENTOR study only captured 24 months of follow-up data, and therefore previously published analyses of retrospective data was used to inform model inputs beyond 24 months. When we limited the time horizon of the model to 24 months, we did not find that rituximab was cost effective with a calculated ICER of $238 589/QALY. This is because none of the patients in the 24 months of the MENTOR study progressed to kidney failure or died, and therefore the benefit of rituximab compared to cyclosporine on health outcomes are not observed over this shorter time horizon. However, our model results indicate the cost-effectiveness of rituximab therapy over a lifetime time horizon is primarily derived from a reduction in the risk of progression to kidney failure that results from better induction and maintenance of both overall remission and CR compared to cyclosporine. While a lifetime horizon is most appropriate, [8, 18] there is uncertainty in extrapolating trial outcomes; if the association of remission and progression to kidney failure differs from existing literature, the result may be altered. Furthermore, the patient population represented in the MENTOR trial were particularly high risk, with median baseline proteinuria of 8.9 g/day, serum albumin of 2.5 g/dl, 74% with positive serum PLA2R antibody (medial level on EURIMMUN assay of 165 RU/ml), and 29–31% having received previous immunosuppression treatment. [5, 19] Therefore, the results of this study may not be applicable to lower risk populations. Additionally, in the MENTOR trial serious adverse events occurred in 31% of patients treated with cyclosporine compared with 17% in those treated with rituximab. [5] This finding was not statistically significant and therefore not included in our model, however, if the rate of adverse events is truly lower in patients treated with rituximab, then the cost-effectiveness of rituximab would be underestimated in our current model. Finally, the MENTOR study only included the treatment options of cyclosporine or rituximab. As such, we were not able to compare the cost-effectiveness of rituximab to alkylating agents, such as cyclophosphamide to rituximab. However, a recent economic evaluation that compared rituximab to cyclophosphamide for the treatment of primary membranous nephropathy and also found that rituximab was a cost-effective treatment option despite the high initial treatment cost with a ICER of £10 246/QALY (∼$12 214 in 2022) [20].
While the upfront cost of rituximab could be regarded as a limitation for routine use, in recent years biosimilar rituximab has become widely available. It is generally thought that these agents have equal efficacy for the treatment of primary membranous nephropathy; our analysis suggests that the lower costs of these agents would dramatically improve the cost-effectiveness of rituximab, and might even result in cost savings in settings where the cost of biosimilars is much lower (i.e. Canada and the UK).
In summary, this trial based economic evaluation found that rituximab was cost effective compared to cyclosporine for the treatment of primary membranous nephropathy in adults, despite the high cost of rituximab therapy. The use of biosimilars may result in cost savings in some countries given the lower drug acquisition costs. These findings can be used by health-care payers to support policy decisions to implement the 2021 KIDOG guideline recommendations of rituximab as a cost-effective first-line therapy for the treatment of patients with primary membranous nephropathy.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the contributions of the MENTOR Trial investigators as follows: Executive Committee: F.C. Fervenza (Principal Investigator), D.C. Cattran (Co-Principal Investigator), G. Appel, D. Gipson, M. Kretzler, and B. Rovin; Study Investigators and Collaborators: USA—Mayo Clinic, Rochester, MN: F.C. Fervenza, J.C. Lieske, N. Leung, S.B. Erickson; Columbia University, New York, NY: J. Radhakrishnan, A. Bomback, J. Hogan, P. Canetta, W. Ahn; Stanford University, Stanford, CA: R. Lafayette, N. Arora, P. Nargund; Ohio State University, Columbus, OH: B. Rovin, A. Alvarado, S. Parikh, L. A. Hebert; Mayo Clinic, Jacksonville, FL: N. Aslam, I. Porter; University of Michigan Medical Center, Ann Arbor, MI: P. Gipson, M. Kretzler, B. Plattner, D. Gipson, L. Mariani, P. Garg, P. Rao; Case Western Reserve University, Cleveland, OH: J. Sedor, J. O'Toole; University of Washington Medical Center, Seattle, WA: J.A. Jefferson, P.J. Nelson; Kansas University Medical Center, Kansas City, KS: E. McCarthy, S. Yarlagadda, N. Jain; University of Alabama at Birmingham, Birmingham, AL: D. Rizk; Cleveland Clinic, Cleveland, OH: J. Simon, S. Gebreselassie; Medical College of Wisconsin, Froedtert Hospital, Milwaukee, WI: S. Blumenthal; New York University Medical Center, New York, NY: L. Beara-Lasic, O. Zhdanova; Mayo Clinic, Scottsdale, AZ: L. Thomas, I. Cohen, M. Keddis; University of Arizona, Tucson, AZ: A. Sussman, B. Thajudeen; University of Mississippi Medical Center, Jackson, MS: L. Juncos, T. Fulop, I. Craici, S. Wagner, A. Dreisbach, D. Monga; University of Miami, Miami, FL: D. Green, A. Mattiazzi, A. Nayer, D. Thomas, L. Barisoni; Washington University School of Medicine, St. Louis, MO: T. Li, and A. Vijayan; Canada—University Health Network, Toronto General Hospital, Toronto, ON: D.C. Cattran, H. Reich, M. Hladunewich; Providence Health Care, St. Paul's Hospital, Vancouver, BC: S. Barbour, A. Levin; Centre Hospitalier Universitaire de Québec, Québec City, QC: D. Philibert, F. Mac-Way, and S. Desmeules; Saudi Arabia—King Abdulaziz University, Jeddah: G. Ankawi; Pathology Adjudication: S. Sethi and C. Avila-Casado; and Quality of Life Measures: H. Beanlands
Contributor Information
Matthew Kadatz, Division of Nephrology, Department of Medicine, Faculty of Medicine, University of British Columbia, Vancouver, Canada; Vancouver Coastal Health Research Institute, Vancouver Coastal Health, Vancouver, Canada.
Scott Klarenbach, Division of Nephrology, Department of Medicine, Faculty of Medicine, University of Alberta, Edmonton, Canada.
Helen So, Division of Nephrology, Department of Medicine, Faculty of Medicine, University of Alberta, Edmonton, Canada.
Fernando C Fervenza, Division of Nephrology & Hypertension, Department of Internal Medicine, Mayo Clinic, Rochester, MN, United States.
Daniel C Cattran, Division of Nephrology, Faculty of Medicine, University of Toronto, Toronto, Canada.
Sean J Barbour, Division of Nephrology, Department of Medicine, Faculty of Medicine, University of British Columbia, Vancouver, Canada; BC Renal, Vancouver, Canada.
MENTOR Study Investigators:
F C Fervenza, D C Cattran, G Appel, D Gipson, M Kretzler, B Rovin, F C Fervenza, J C Lieske, N Leung, S B Erickson, J Radhakrishnan, A Bomback, J Hogan, P Canetta, W Ahn, R Lafayette, N Arora, P Nargund, B Rovin, A Alvarado, S Parikh, L A Hebert, N Aslam, I Porter, P Gipson, M Kretzler, B Plattner, D Gipson, L Mariani, P Garg, P Rao, J Sedor, J O'Toole, J A Jefferson, P J Nelson, E McCarthy, S Yarlagadda, N Jain, D Rizk, J Simon, S Gebreselassie, S Blumenthal, L Beara-Lasic, O Zhdanova, L Thomas, I Cohen, M Keddis, A Sussman, B Thajudeen, L Juncos, T Fulop, I Craici, S Wagner, A Dreisbach, D Monga, D Green, A Mattiazzi, A Nayer, D Thomas, L Barisoni, T Li, A Vijayan, D C Cattran, H Reich, M Hladunewich, S Barbour, A Levin, D Philibert, F Mac-Way, S Desmeules, G Ankawi, S Sethi, C Avila-Casado, and H Beanlands
FUNDING
Genentech and the Fulk Family Foundation provided funding for the MENTOR trial. Genentech provided rituximab for the trial. None of these funding sources had a role in the conduct of the MENTOR trial or in the current analysis.
AUTHORS’ CONTRIBUTIONS
M.K., S.K., and H.S. were involved in the conceptualization, study design, analysis, and writing of this paper. S.J.B. and D.C.C. were involved in the conceptualization, study design, and writing of this paper. F.C.F. was involved in the conceptualization and writing of this paper.
DATA AVAILABILITY STATEMENT
Much of the data presented in this paper are derived from the MENTOR study data, which are not publicly available. Summary data have been included in the paper's tables and Supplementary Materials.
CONFLICT OF INTEREST STATEMENT
Sources of support: M.K. receives financial support from the Michael Smith health Research BC Health Professional Investigator Award. S.K. is supported by the Kidney Health Research Chair and the Division of Nephrology at the University of Alberta. S.K. is Director of the Real World Evidence Consortium, and Alberta Drug and Therapeutic Evaluation Consortium (Universities of Alberta, Calgary, and Institute of Health Economics); these entities receive funding from decision-makers and industry to conduct research. All research funding is made to the academic institution; investigator retains full rights of academic freedom and right to publish. This relationship is not related to the current work.
REFERENCES
- 1. Haas M, Meehan SM, Karrison TG et al. Changing etiologies of unexplained adult nephrotic syndrome: a comparison of renal biopsy findings from 1976-1979 and 1995-1997. Am J Kidney Dis 1997;30:621–31. 10.1016/S0272-6386(97)90485-6 [DOI] [PubMed] [Google Scholar]
- 2. Troyanov S, Wall CA, Miller JA et al. Idiopathic membranous nephropathy: definition and relevance of a partial remission. Kidney Int 2004;66:1199–205. 10.1111/j.1523-1755.2004.00873.x [DOI] [PubMed] [Google Scholar]
- 3. Lee T, Derebail VK, Kshirsagar AV et al. Patients with primary membranous nephropathy are at high risk of cardiovascular events. Kidney Int 2016;89:1111–8. 10.1016/j.kint.2015.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nazareth TA, Kariburyo F, Kirkemo A et al. Patients with idiopathic membranous nephropathy: a real-world clinical and economic analysis of U.S. claims data. JMCP 2019;25:1011–20. 10.18553/jmcp.2019.18456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fervenza FC, Appel GB, Barbour SJ et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med 2019;381:36–46. 10.1056/NEJMoa1814427 [DOI] [PubMed] [Google Scholar]
- 6. Kidney disease: improving global outcomes glomerular diseases work G. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int 2021;100:S1–S276. 10.1016/j.kint.2021.05.021 [DOI] [PubMed] [Google Scholar]
- 7. Cravedi P, Remuzzi G, Ruggenenti P. Rituximab in primary membranous nephropathy: first-line therapy, why not? Nephron Clin Pract 2014;128:261–9. 10.1159/000368589 [DOI] [PubMed] [Google Scholar]
- 8. Guidelines for the Economic Evaluation of Health Technologies. 4th edn, Ottawa, Canada: CADTH; 2017 March. Accessed 2017 January. [Google Scholar]
- 9. TreeAge Pro 2022, R1.2. Williamstown, Massachusetts: TreeAge Software; software available at http://www.treeage.com [Google Scholar]
- 10. Pharmaceutical Prices, Office of Procurement, Acquisition and Logistics. U.S. Department of Veterans Affairs (January 2024, data last accessed), 2022; https://www.va.gov/opal/nac/fss/pharmPrices.asp
- 11. Gray AM, Rivero-Arias O, Clarke PM. Estimating the association between SF-12 responses and EQ-5D utility values by response mapping. Med Decis Making 2006;26:18–29. 10.1177/0272989X05284108 [DOI] [PubMed] [Google Scholar]
- 12. Chou YH, Lien YC, Hu FC et al. Clinical outcomes and predictors for ESRD and mortality in primary GN. Clin J Am Soc Nephrol 2012;7:1401–8. 10.2215/CJN.04500511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wyld M, Morton RL, Hayen A et al. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med 2012;9:e1001307. 10.1371/journal.pmed.1001307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rowen D, Brazier J, Roberts J. Mapping SF-36 onto the EQ-5D index: how reliable is the relationship? Health Qual Life Outcomes 2009;7:27. 10.1186/1477-7525-7-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clement FM, Harris A, Li JJ et al. Using effectiveness and cost-effectiveness to make drug coverage decisions: a comparison of Britain, Australia, and Canada. JAMA 2009;302:1437–43. 10.1001/jama.2009.1409 [DOI] [PubMed] [Google Scholar]
- 16. Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014;371:796–7. 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 17. Chambers JD, Neumann PJ, Buxton MJ. Does medicare have an implicit cost-effectiveness threshold? Med Decis Making 2010;30:E14–27. 10.1177/0272989X10371134 [DOI] [PubMed] [Google Scholar]
- 18. Sanders GD, Neumann PJ, Basu A et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016;316:1093–103. 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 19. Barbour SJ, Fervenza FC, Induruwage D et al. Anti-PLA2R antibody levels and clinical risk factors for treatment nonresponse in membranous nephropathy. Clin J Am Soc Nephrol 2023;8:1283–93. 10.2215/CJN.0000000000000237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamilton P, Kanigicherla D, Venning M et al. Rituximab versus the modified Ponticelli regimen in the treatment of primary membranous nephropathy: a health economic model. Nephrol Dial Transplant 2018;33:2145–55. 10.1093/ndt/gfy049 [DOI] [PubMed] [Google Scholar]
- 21. USRDS . 2019 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases. 2019. [Google Scholar]
- 22. Maximizing medicare cost report reimbursement (6 June 2019, date last accessed). http://organdonationalliance.org/wp-content/uploads/2015/08/ATC_BSullivan_CostReport_062016_S5N0001.pdf
- 23. Schnitzler MA, Skeans MA, Axelrod DA et al. OPTN/SRTR 2016 annual data report: economics. Am J Transplant 2018;18:464–503. 10.1111/ajt.14564 [DOI] [PubMed] [Google Scholar]
- 24. Arias E, Xu JQ. United States Life Tables, 2020 . Vol. 71. Hyattsville, MD: National Vital Statistics Reports. 2022. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Much of the data presented in this paper are derived from the MENTOR study data, which are not publicly available. Summary data have been included in the paper's tables and Supplementary Materials.