ABSTRACT
Background
Persons with chronic kidney disease (CKD) are at increased risk of adverse events, early mortality and multimorbidity. A detailed overview of adverse event types and rates from a large CKD cohort under regular nephrological care is missing. We generated an interactive tool to enable exploration of adverse events and their combinations in the prospective, observational German CKD (GCKD) study.
Methods
The GCKD study enrolled 5217 participants under regular nephrological care with an estimated glomerular filtration rate of 30–60 or >60 mL/min/1.73 m2 and an overt proteinuria. Cardiovascular, cerebrovascular and peripheral vascular, kidney, infection, and cancer events, as well as deaths were adjudicated following a standard operation procedure. We summarized these time-to-event data points for exploration in interactive graphs within an R shiny app. Multivariable adjusted Cox models for time to first event were fitted. Cumulative incidence functions, Kaplan–Meier curves and intersection plots were used to display main adverse events and their combinations by sex and CKD etiology.
Results
Over a median of 6.5 years, 10 271 events occurred in 2947 participants (56.5%), of which 680 participants (13.0%) died. The new publicly available interactive platform enables readers to scrutinize adverse events and their combinations as well as mortality trends as a gateway to better understand multimorbidity in CKD: incident rates per 1000 patient-years varied by event type, CKD etiology and baseline characteristics. Incidence rates for the most frequent events and their recurrence were 113.6 (cardiovascular), 75.0 (kidney) and 66.0 (infection). Participants with presumed diabetic kidney disease and men were more prone to experiencing events.
Conclusion
This comprehensive explorative tool to visualize adverse events (https://www.gckd.org/studienhintergrund/previous-study-results/event-analysis/), their combination, mortality and multimorbidity among persons with CKD may serve as a valuable resourec for patient care, identification of high-risk groups, health services and public health policy planning.
Keywords: adverse events, chronic kidney disease, epidemiology, interactive visualization, multimorbidity
KEY LEARNING POINTS.
What was known:
Individuals diagnosed with chronic kidney disease (CKD) are at increased risk of developing a plethora of adverse outcomes and early mortality.
Interactive data visualization can play a critical role for a better understanding of large datasets by allowing users to explore specific research questions.
Interactive tools to explore adverse events and their combinations as well as mortality and multimorbidity in CKD are missing.
This study adds:
Extensive comorbidity analysis in a large cohort of 5217 participants of whom 56.5% experienced at least one event after 6.5 years of follow-up.
A publicly available resource to interactively explore 10 271 adverse events by baseline characteristics as a gateway to understanding multimorbidity in CKD.
The highest rates per 1000 patient-years were 113.6 (cardiovascular), 75.0 (kidney) and 66.0 (infection), with event distributions showing differential patterns stratified by underlying CKD etiology and sex.
Potential impact:
High risks for cardiovascular, kidney and infection events in persons with CKD require better strategies for prevention.
The interactive tool can be used for hypothesis generation, to identify knowledge gaps that need to be addressed in future projects and to highlight potential intersections for collaborative efforts with other studies.
Differences in event patterns by underlying primary cause of CKD and sex, motivate efforts to apply a more personalized screening and management.
INTRODUCTION
Chronic kidney disease (CKD) is a global health problem with an increasing prevalence in both low- and high-income countries [1–5]. Demographic changes with rising proportions of elderly people, prone to CKD, are posing a challenge for healthcare systems. The annual number of deaths from kidney failure (KF) is 1.2 million, with an additional 1.4 million deaths due to cardiovascular disease (CVD) attributed to CKD. Thus, 4.6% of all deaths are due to CKD, making it the 12th leading cause of death [1].
Etiologies, comorbidities and progression patterns differ markedly among persons with CKD, resulting in a differential risk to develop comorbid conditions such as CVD [6] and subsequent mortality [7]. Cardiovascular (CV) mortality has been reported to be up to 60% higher in persons with CKD compared with the general population [8]. Other well-known adverse events for persons with CKD are initiation of kidney replacement therapy (KRT) [9], acute kidney injury (AKI) [10] and an increased risk of some types of cancer [11]. The high prevalence of multimorbidity in persons with CKD [12] leads to poorer outcomes and an increased use of healthcare services [8, 9]. Most healthcare services are not adequately designed to meet the challenges of multimorbidity [10], but are generally focused on individual diseases [13–15]. Monitoring persons with CKD will help to identify those at higher risk of (multi)morbidity and mortality, which is important given the need for combined management approaches.
Despite the awareness of CKD-related (multi)morbidity and mortality [8], the underlying data has often been generated from population-based studies and registries [16]. Evidence from large cohorts of persons with CKD under regular nephrological care is scarce [17, 18]. Thus, the aims of the present study were to (i) collect and evaluate such data over a period of 6.5 years from the German Chronic Kidney Disease (GCKD) study [19], and (ii) develop a publicly available tool to interactively explore all collected adverse events by baseline characteristics. Such data may have important implications for clinicians, health services and public health policy planning. Moreover, the resulting interactive tool provides an opportunity for hypothesis generation about causes of multimorbidity in CKD, and possibly optimal strategies for prevention.
MATERIALS AND METHODS
Study design and population
The GCKD study, a prospective cohort study of 5217 persons with CKD [19], was previously described with regard to study population and design [19, 20]. Briefly, adult persons with CKD under standard nephrological care who provided written informed consent were recruited from 2010 to 2012 from nephrological out-patient centers. Main inclusion criteria comprised an estimated glomerular filtration rate (eGFR) between 30 and 60 mL/min/1.73 m2 or an eGFR >60 mL/min/1.73 m2 in combination with “overt” albuminuria/proteinuria. The GCKD study has been registered in the national registry for clinical studies (DRKS 00003971) and was approved by the local ethics committees of participating centers [19].
At baseline and at in-person visits, serum and urine was collected in a standardized fashion and transported frozen to a central biobank for storage at –80°C [22]. Trained and certified personnel followed standardized measurements of baseline variables (Supplementary Method 1), and used questionnaires to systematically collect information on demographic and clinical data annually. For this project, event data for the first 6.5 years since baseline (data freeze: March 2021) was available.
Event adjudication
All events are continuously adjudicated from hospital discharge letters, nephrologist out-patient letters and death certificates by trained physicians (Supplementary Method 2; Supplementary data, Fig. S1) following a standard operating procedure (Supplementary Method 3) using a standardized adjudication catalog (Supplementary data, Table S1). The comprehensive adjudication catalog focuses on occurrence of events and comprises eight main event groups (event category “A–H”): CV (“A”), cerebrovascular (“B”), peripheral arterial occlusive disease (“C”), microangiopathy in diabetes (“D”), kidney (“E”), death (“F”), cancer (“G”) and hospitalization due to infection (“H”). Within these categories, events are further classified into subcategories with up to five levels, allowing specification of events as detailed as possible (e.g. “E1a” kidney injury with kidney replacement therapy), resulting in a total of 229 event categories and codes. Within the CV event category, three codes technically not classifying as events were excluded from all analyses: (i) hospital admission to exclude either myocardial infarction (“A7”) or (ii) coronary heart disease (CHD, “A8”), and (iii) no CHD or CHD progression detectable by coronary angiography (“A9a”, “A9c”). Additionally, events belonging to the category microangiopathy in diabetes (“D”) were not included in all analyses of this project due to partial unavailability of information for adjudication.
Statistical analyses
Participants without an event were censored at the end of the observation period (6.5 years; Supplementary data, Fig. S2), or earlier in case of early study termination (N = 547), defined as loss to follow-up or drop-out based on a last known alive date. For each participant, this date was set to the latest available date of any follow-up visit, creatinine measurement, event or hospitalization discharge date.
Descriptive analyses
All events were analyzed descriptively. Graphical representations are made accessible via an R shiny app application at https://www.gckd.org/studienhintergrund/previous-study-results/event-analysis/ and can be explored in an interactive manner using any modern web browser (Supplementary Method 4). Regular updates will be made, as the study progresses and prospective data (anonymized) from later follow-up visits becomes available. Incidence rates (IRs) were calculated for main event categories by dividing the sum of events per category by the total duration of observation both for first events only as well as for all events. For the latter, one participant may contribute multiple events of different and/or the same category (recurrent events) to the results.
Cox proportional hazard analyses
For all Cox proportional hazard analyses endpoints of interest were defined. Endpoints can be made up of several events of interest. Endpoints were coded as 0 for censored, 1 for events of interest, and 2 for the competing event.
The proportional hazard analyses were restricted to the following main endpoints defined as: kidney failure (KF: transplantation or dialysis or death due to foregoing of dialysis: E1a, E1b, F7), myocardial infarction (composite of myocardial infarction, fatal myocardial infarction, fatal coronary artery disease or sudden cardiac death: A1, F1a, F1b, F1c) and all-cause mortality (F). Cause-specific Cox proportional hazard analyses for time to first event were carried out with death from other causes as a competing event. All proportional hazard analyses were adjusted for center, age, sex, body mass index, systolic blood pressure, prevalent diabetes, prevalent CVD, eGFR, log urine albumin–creatinine ratio [log(UACR)], smoking, log C-reactive protein [log(CRP)], cholesterol and a composite comorbidity variable (Supplementary Method 1). Additionally, competing event and subdistribution analyses were carried out to evaluate potential indirect effects [21]. As no remarkable differences between the cause-specific and the subdistribution hazard ratios (HRs) were found, only the former are presented throughout this manuscript. Proportional hazard assumptions were checked with Schoenfeld's residuals. No major deviations were identified (data not shown). No formal statistical significance threshold was applied to the descriptive analyses. For illustrative purposes, cumulative incidence functions for KF and myocardial infarction were displayed by sex as well as G and A risk classification according to the Kidney Disease Improving Global Outcomes (KDIGO) organization [1], and Kaplan–Meier survival estimates for all-cause mortality by sex and presumed primary cause of kidney disease (DKD: diabetic kidney disease; PGD: primary glomerular disease, HKD: hypertensive kidney disease; MISC: miscellaneous).
RESULTS
After a median follow-up period of 6.5 years (Q1: 6.5, Q3: 6.5) a total of 10 271 events were observed in 2947 persons (56.5%) and 2270 persons (43.5%) were censored without an event at the end of the study period or earlier (N = 547; Supplementary data, Fig. S2). In general, older persons, men, persons with lower eGFR and persons with higher UACR at baseline were more likely to experience an event (Supplementary data, Table S2). Compared with the three other primary cause of kidney disease groups, incidence rates per 1000 patient-years including recurrent events of any type were 1.6–2.6 times higher in the DKD group and this tendency was mainly the same when restricting to cardiovascular (code: “A”) and kidney (code: “E”) events (Supplementary data, Table S3). Generally, incidence rates were lowest in the PGD group with few exceptions (code: “E”), yet persons in this group were younger on average. One ubiquitous observation was that men were more likely to experience events than women with only a few exceptions (some infections “H1b” or AKI “E3a2”) where they were at comparable risk (Supplementary data, Table S4). Comparing Kaplan–Meier survival estimates by sex and by primary cause of kidney disease confirmed the risk factor male sex and the vulnerability of the DKD group (Fig. 1). The numbers presented so far included microangiopathy in diabetes (code: “D”), but this category was excluded in the remainder of the manuscript. Furthermore, we provide a publicly available detailed interactive platform for exploring all events in the GCKD study: https://www.gckd.org/studienhintergrund/previous-study-results/event-analysis/. A landing page was established giving background details and explanatory hints of how to use the interactive tool. The user will be able to explore all events interactively overall and by subgroups of certain baseline characteristics. Exploration of the data was facilitated by barplots and treemaps (Supplementary data, Fig. S3).
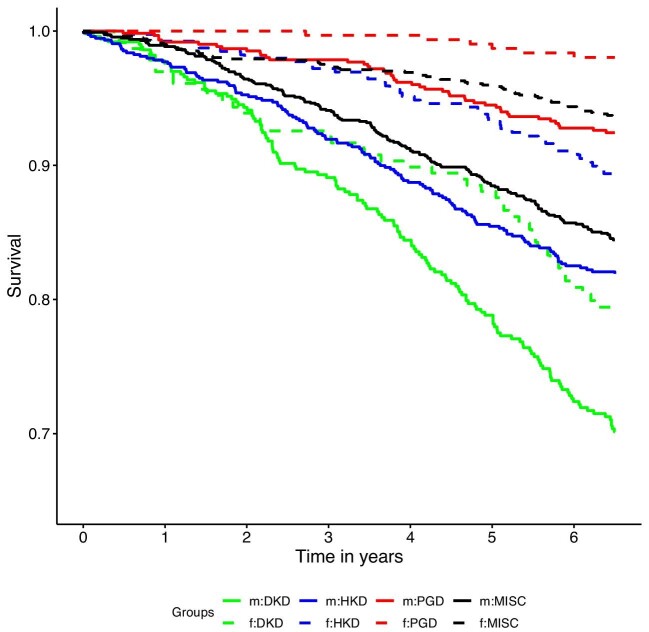
Figure 1:
Kaplan–Meier survival curve for all-cause mortality by sex and primary cause of kidney disease. Solid lines represent male (m) participants, dashed lines female (f) participants. Green: DKD as presumed primary cause of kidney disease; blue: HKD as presumed primary cause of kidney disease; red: PGD as presumed primary kidney disease; black: MISC = any other primary cause of kidney disease. Men diagnosed with DKD had the highest risk of death; women with DKD had a similar risk of death as men with HKD. Men with PGD were at similar risk of death as women with either HKD or MISC. Women with PGD had the lowest risk of death.
Combination of events
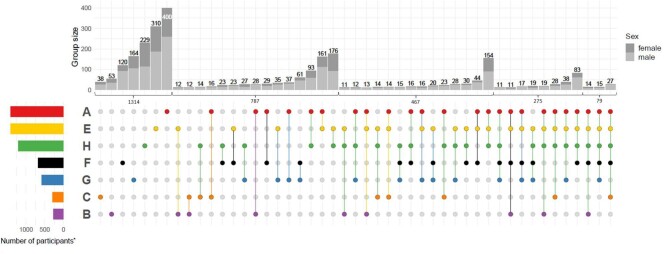
The 2933 persons with events in any main category either experienced events in just one category (N = 1314) or in more than one category (N = 1619, Fig. 2). Persons only experiencing events in one category mainly experienced cardiovascular, kidney or infection events. In total, 787 participants experienced events in two event categories (66% men) and 467 (67% men) in three event categories. Among the 467 persons with events in three event categories, the combination of infection, cardiovascular and kidney events was more than three times as common as any other combination (N = 154 vs 44). Most frequent intersections of two or more event categories included either one of kidney, cardiovascular or infection events.
Figure 2:
Intersection plot of main adverse event categories and their combinations after 6.5 years of follow-up. Main event categories: cardiovascular (“A”), cerebrovascular (“B”), peripheral arterial occlusive disease (PAOD, “C”), kidney (“E”), death (“F”), cancer (“G”), infection (“H”). Plots include recurrent events. Colored intersection plot: indicates intersection of event combinations in main event categories, e.g. 176 participants experienced a combination of adverse kidney events and infection events until the end of the follow-up period, while a total of 787 participants experienced adverse events in two event categories overall. Stacked barplot: number of participants in each intersection by sex. Colored barplot: number of participants with events of a main event category is plotted. *Total number of participants with events in one to five event categories. Intersections with fewer than 11 participants are not shown here. In total, 11 participants had events in six event categories.
Associations of baseline characteristics with kidney failure, myocardial infarction and all-cause mortality
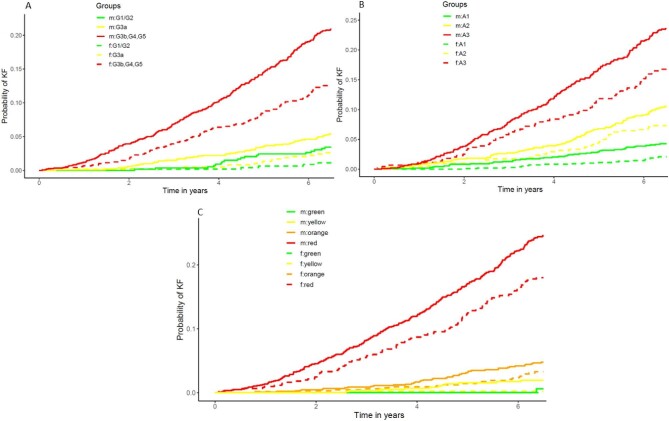
At the end of the follow-up period, 340 persons (6.8%) experienced a myocardial infarction, 478 persons (9.6%) experienced KF and 637 persons (12.8%) died in the complete case cohort of 4989 participants. Overall, adjusted time-to-event analyses with the three main endpoints KF, myocardial infarction and all-cause mortality confirmed most trends observed in the baseline characteristics by event status (Supplementary data, Tables S5–S7). Higher age, male sex, lower eGFR and higher UACR were strongly associated with KF and a 10 mL/min/1.73 m2 decrease in eGFR was associated with a doubling in risk for developing KF after adjusting for other baseline characteristics [HR 2.03, 95% confidence interval (CI) 1.86–2.21, Supplementary data, Table S6]. CKD can be classified by GFR and UACR into G (GFR categories) and A (albuminuria categories) categories. The grid that arises thereby reflects the risk of CKD progression for patients defined by a placing within the grid. The grid has a differential colouring (green to red) to signify low (green, e.g. G1/A1) vs. high (red, e.g. G5/A3) risk for CKD progression. When looking at unadjusted cumulative incidence functions (CIF) for KF by sex and G/A risk classification, where a higher classification in G and/or A corresponds to a higher CIF of KF (Fig. 3A and B), men were also more likely to reach KF. Additionally, the CIF of a man was generally higher than that of a woman where the CIF of a man in CKD stage G1/G2 was, for example, comparable to a woman in G3a. An even greater distinction of persons at high risk for KF compared with the rest of the population was observed when participants were classified into G/A risk categories of CKD progression (Fig. 3C). Here, men had higher CIFs in any of these four categories and women categorized into medium-high risk (“orange”) were at similar risk for KF as men categorized into medium increased risk (“yellow”).
Figure 3:
Cumulative incidence function for kidney failure by sex for KDIGO eGFR and/or UACR categories. Panel (A) by eGFR categories (G1–5), panel (B) by UACR categories (A1–3), (C) combined eGFR and UACR categories. Green: low-risk categories (G1/2A1), yellow: medium-risk categories (G3aA1, G1/2A2, orange: medium-high risk (G3bA1, G3aA2, G1/2A3) and red: high risk categories (G3aA3, G3bA2/3, G4A1–3, G5A1–3). m = men, solid lines; f = women, dashed lines.
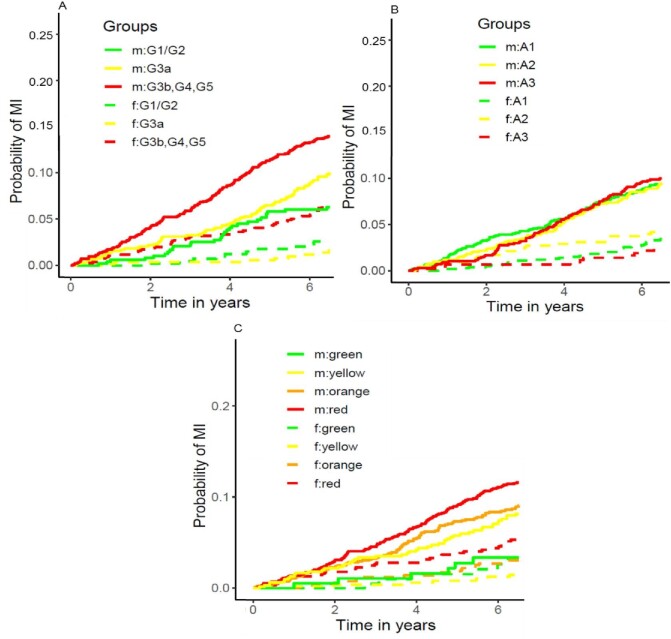
Similar to KF, men were at considerably higher risk than women for the endpoint myocardial infarction after adjusting for baseline characteristics (HR 2.43, 95% CI 1.83–3.22, Supplementary data, Table S5). This effect was only exceeded by prevalent CVD, a known important risk factor for adverse CV events (HR 2.86, 95% CI 2.26–3.62, Supplementary data, Table S5). Confirming the remarkably higher P-value for the association of higher UACR with myocardial infarction than for the association with KF (Supplementary data, Table S6), classification of persons into A categories does not correspond with higher CIFs for myocardial infarction, whereas men have a higher CIF for myocardial infarction regardless of A category (Fig. 4A–C).
Figure 4:
Cumulative incidence function for myocardial infarction by sex for KDIGO eGFR and/or UACR categories. Panel (A) by eGFR categories (G1–5), (B) by UACR categories (A1–3), (C) combined eGFR and UACR categories. Green: low-risk categories, yellow: medium-risk categories, orange: medium-high risk and red: high risk categories. m = men, solid lines; f = women, dashed lines.
For all-cause mortality, important risk factors were higher age, prevalent comorbidities including prevalent diabetes and male sex as well as lower eGFR and higher UACR as expected (Supplementary data, Table S7).
In summary, persons with CKD are at a high risk for cardiovascular, kidney and infection events, as well as combinations of those. Our study reveals men to be more vulnerable than women and persons with DKD to be the most vulnerable compared with the three other primary causes of kidney disease.
DISCUSSION
In this study of 5217 persons with CKD we characterized adverse events (N = 10 271), incidence rates and event combinations over a 6.5-year follow-up period. When considering the event statistics and the individuals lost during the study's course, a loss of approximately 10% of GCKD study participants is a positive result, especially compared with the normally expected percentage of persons with complete follow-up of 60–80% in comparable cohort settings [23, 24]. CKD patients are known to be prone to a plethora of adverse events including KRT, AKI, CV events and some types of cancer [6], but a comprehensive almanac of IRs for a great abundance of adverse events in CKD is missing. These data do not only help in understanding multimorbidity in CKD. For example the economic usefulness of reporting incidence rates for adverse events in CKD lies in its ability to inform cost-effective decision-making, resource allocation and policy development [25]. Possible cost-analyses include direct costs associated with increased healthcare utilization due to adverse events [26] as well as indirect costs like lost productivity due to extended hospital stays or CKD complications [27]. Adjusting policies based on the economic impact of adverse events can lead to more sustainable and cost-effective healthcare practices as well as providing frameworks to achieve a population coverage with kidney specialists (International Society of Nephrology, Global Kidney Disease Health Atlas, 2019; available from: https://www.theisn.org/focus/ckd#health-atlas). Insurance providers or government health agencies, can use this data to negotiate drug prices and coverage policies. In order to fully grasp and explore all events recorded, the development of interactive plots is warranted. Interactive visualization of data can play a critical role in better understanding big data by allowing users to engage with the data in person [28]. Users can interact with multiple facets of the data [29]. Until now, many visualization options have relied on simple, static graphs such as bar charts, which cannot reveal particular facets of the underlying data being presented and thus limit the user's understanding of the demonstrated data [28]. Visualization methods that can show multiple data elements at the same time allow the reader to better identify patterns or to focus on their own areas of interest. Interactive plots have become widely popular during the COVID-19 pandemic (e.g. https://www.euromomo.eu/graphs-and-maps) for tracking and preventing kidney disease in the USA (https://nccd.cdc.gov/ckd/) motivating the implementation of such plots for the GCKD study. Due to its interactive nature with many options, this tool may be additionally valuable in the future for comparing and validating new findings, including from other countries and populations with different demographic structures. The use of a standard programming language as well as software packages in the interactive tool can serve as a basis for other projects in this research field. Researchers and healthcare professionals outside Germany may not only find the data from the GCKD study valuable for further research and collaboration, but the availability of a comprehensive and well-documented dataset may facilitate secondary analyses, validation studies and the development of predictive models, leading to advancements in the understanding and management of CKD globally.
In the GCKD study, older individuals, men and those with lower eGFR and/or higher UACR were more likely to experience an event, aligning with findings from similar studies in CKD patients like the German branch of the Chronic Kidney Disease Outcomes and Practice Patterns (CKDopps) Study [30] and the Chronic Renal Insufficiency Cohort (CRIC) study [31]. CKDopps recruited patients between 2013 and 2016 with data available until 2018, differing notably from the GCKD study in terms of older age, lower eGFR and higher comorbidity load. In CRIC, patients were recruited between 2003 and 2008, sharing some criteria with the GCKD study but representing a multi-ethnic cohort and excluding certain kidney diseases, as well as analyzing only participants with an eGFR of ≤30 mL/min per 1.73 m2 over a median follow-up of 5.5 years. Nonetheless, cumulative incidence for KF, one of CKDopps main outcomes, was higher for participants with higher albuminuria, lower eGFR, male sex and higher age [30]. In the CRIC study proteinuria was highest for participants developing KF as a first event, participants developing a CV event were older and men were also more prone to develop events developing a CVD event and men were also more prone to develop events [31].
In the GCKD study, participants diagnosed with DKD had significantly higher incidence rates (1.6–2.6 times) per 1000 patient-years compared with the other primary causes of kidney disease. This is in line with trends observed in the literature [30]. Additionally, all-cause mortality was highest for DKD in the GCKD study, particularly among men, while women had a lower mortality rate. These findings align with the fact that diabetes-related CKD is among the top four leading causes of death in Germany [4]. It is a well-known fact that men in the general population have a higher risk of mortality than women [32, 33]. However, data from dialysis patients are inconsistent, with some authors reporting attenuation of this fact [34–36] and others observing similar mortality rates in the general population [37]. A recent meta-analysis of cardiovascular mortality in CKD patients came to the conclusion that estimated risks were higher for men than women [38], which is in line with our findings.
When examining IRs, the GCKD study revealed a lower death rate (unadjusted IR 22.4 per 1000 patient-years) among CKD patients than a Korean study (unadjusted IR 134 per 1000 patient-years [39]). However, direct comparability is constrained by significant differences in study populations and because IRs are unadjusted. Notably, the Korean study focused on incident CKD using ICD codes. This is similar to potential discrepancies in the higher mortality rates in CKD patients in the USA (source: https://nccd.cdc.gov/CKD/). Future studies should focus on IRs adjusted for age and population characteristics. This may help mitigate the impact of confounding factors, making the comparison more robust. Furthermore, adjusting for population density could help to account for the differences in the underlying population structures. Making use of the international Network of Chronic Kidney Disease cohort studies a meta-analysis of IRs between studies could shed further light on incidence rates also for other adverse events [40].
Comparative literature on IRs in CKD cohorts categorized by etiology is limited, hindering meaningful study comparisons. A recent study by Ryu et al. examined CKD outcomes based on etiology in 2070 patients from the KoreaN cohort study for Outcome in patients With Chronic Kidney Disease (KNOW-CKD) study over a 6-year follow-up [41]. The study found variations in eGFR decline, with the highest decline in cystic kidney disease, followed by DKD and HKD, while the lowest decline was observed in the PGD group. These results contrast with the GCKD study, where the HKD group had the lowest number reaching KF, attributed to differences in sample sizes, and higher age, male ratio and CVD burden in the PGD group. The Study of Heart and Renal Protection (SHARP) analyzed data on CKD progression to dialysis and all-cause mortality over 6 years in a mixed-ethnicity cohort of 6245 CKD patients [42], observing similar patterns with the highest progression rates in cystic kidney diseases, followed by DKD and PGD, but did not analyze HKD separately.
Evaluation of CIFs by G/A risk classification for KF by sex in the GCKD study also showed that higher G and/or A categories corresponded with a higher CIF for KF for men. In population-based cohorts, women are more likely to be diagnosed with CKD, while the incidence of kidney replacement therapy (KRT; either dialysis or transplantation) is higher in men [43]. Two reasons for this paradigm exist. Either the diagnosis and severity of CKD is overestimated using common estimation equations in women or CKD progression is slower in women compared with men. A new GFR estimation equation [44] might be able to address the seemingly misclassification of women into CKD categories associated with higher progression risk. Even though UACR is a high-risk factor for myocardial infarction in Cox regression analyses this was not reflected in CIFs for myocardial infarction when looking at A categories. Nonetheless, KDIGO reported on a differential relative risk of CV mortality by higher UACR [45], but looking solely at myocardial infarction might not be comparable to a hard endpoint like CV mortality.
Strengths of our study include the large number of participants with CKD under regular nephrological care, the standardized procedures for biomaterial collection and processing, the broad spectrum of events surveyed, the rigorous event adjudication including specific event subcategories based on hard criteria, as well as extensive quality control of the event adjudication by a committee of trained physicians. Our study also has potential limitations. (i) The GCKD study design restricted the cohort to participants with a limited eGFR range and to participants of European ancestry. Therefore, our results may not be generalizable to a wider range of eGFR values, or other ethnicities. (ii) Nephrologist care in Germany may not reflect the situation in CKD cohorts situated in other countries. However, our cohort is comparable to many other Western European countries and can be used for information on persons with CKD of European ancestry worldwide. Because the occurrence of genetic polymorphisms that influence kidney disease progression or drug metabolism differs by ancestry, studies including participants with a more diverse genetic background are an interesting line of future research.
CONCLUSION
Our data on adverse events emphasize the most frequent events in CKD, namely cardiovascular, kidney and infection events, and highlights the importance of managing CKD in an ageing population, with a focus on high-risk individual identification like men and persons with DKD. The interactive tool developed for customized visualization of adverse events, mortality and multimorbidity in patients with CKD may prove to be a valuable resource for patient care, evaluation of surrogate kidney disease outcomes and for guiding public health policy planning.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful for the willingness of the patients to participate in the GCKD study. The enormous effort of the study personnel of the various regional centres is highly appreciated. We thank the large number of nephrologists who provide routine care for the patients and collaborate with the GCKD study. A list of nephrologists currently collaborating with the GCKD study is available at http://www.gckd.org.
Contributor Information
Inga Steinbrenner, Institute of Genetic Epidemiology, Faculty of Medicine and Medical Center – University of Freiburg, Freiburg, Germany.
Fruzsina Kotsis, Institute of Genetic Epidemiology, Faculty of Medicine and Medical Center – University of Freiburg, Freiburg, Germany; Department of Medicine IV, Nephrology and Primary Care, Faculty of Medicine and Medical Center – University of Freiburg, Freiburg, Germany.
Robin Kosch, Peter L. Reichertz Institute for Medical Informatics of TU Braunschweig and Hannover Medical School, Hannover Medical School, Hannover, Germany; Department of Medical Bioinformatics, University Medical Center Göttingen, Göttingen, Germany.
Heike Meiselbach, Department of Nephrology and Hypertension, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany.
Barbara Bärthlein, Medical Centre for Information and Communication Technology, University Hospital Erlangen, Erlangen, Germany.
Helena Stockmann, Department of Nephrology and Medical Intensive Care, Charité-Universitätsmedizin Berlin, Berlin, Germany; Department of Nephrology, University Medical Center Regensburg, Regensburg, Germany.
Jan Lipovsek, Institute of Genetic Epidemiology, Faculty of Medicine and Medical Center – University of Freiburg, Freiburg, Germany.
Helena U Zacharias, Peter L. Reichertz Institute for Medical Informatics of TU Braunschweig and Hannover Medical School, Hannover Medical School, Hannover, Germany.
Michael Altenbuchinger, Department of Medical Bioinformatics, University Medical Center Göttingen, Göttingen, Germany.
Thomas Dienemann, Department of Operative Intensive Care, University Hospital Regensburg, Regensburg, Germany.
Monika Wytopil, Institute of Clinical and Molecular Virology, Universitätsklinikum Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany.
Helena Bächle, Institute of Genetic Epidemiology, Faculty of Medicine and Medical Center – University of Freiburg, Freiburg, Germany.
Claudia Sommerer, Department of Nephrology, University Hospital Heidelberg, Renal Center, Heidelberg, Germany.
Stephanie Titze, Department of Nephrology and Hypertension, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany.
Anke Weigel, Department of Nephrology and Hypertension, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany.
Hansi Weissensteiner, Institute of Genetic Epidemiology, Medical University of Innsbruck, Innsbruck, Austria.
Sebastian Schönherr, Institute of Genetic Epidemiology, Medical University of Innsbruck, Innsbruck, Austria.
Lukas Forer, Institute of Genetic Epidemiology, Medical University of Innsbruck, Innsbruck, Austria.
Nadine S Kurz, Department of Medical Bioinformatics, University Medical Center Göttingen, Göttingen, Germany.
Jan Menne, Department of Nephrology, Rheumatology and Vascular Medicine, KRH Klinikum Siloah, Hannover, Germany.
Georg Schlieper, Zentrum für Nieren-, Hochdruck- und Stoffwechselerkrankungen, Hannover, Germany; Division of Nephrology and Clinical Immunology, University Hospital RWTH Aachen, Aachen, Germany.
Markus P Schneider, Department of Nephrology and Hypertension, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany.
Elke Schaeffner, Institute of Public Health, Charité – Universitätsmedizin Berlin, Berlin, Germany.
Jan T Kielstein, Medical Clinic V Nephrology, Rheumatology, Blood Purification – Academic Teaching Hospital Braunschweig, Braunschweig, Germany.
Thomas Sitter, Department of Nephrology and Hypertension, Ludwig-Maximilians University, Munich, Germany.
Jürgen Floege, Division of Nephrology and Clinical Immunology, University Hospital RWTH Aachen, Aachen, Germany.
Christoph Wanner, Department of Clinical Research and Epidemiology, German Heart Failure Center, University Hospital Würzburg, Würzburg, Germany.
Florian Kronenberg, Institute of Genetic Epidemiology, Medical University of Innsbruck, Innsbruck, Austria.
Anna Köttgen, Institute of Genetic Epidemiology, Faculty of Medicine and Medical Center – University of Freiburg, Freiburg, Germany.
Martin Busch, Department of Internal Medicine III, Nephrology, University Hospital Jena – Friedrich Schiller University Jena, Jena, Germany.
Vera Krane, Department of Clinical Research and Epidemiology, German Heart Failure Center, University Hospital Würzburg, Würzburg, Germany; Department of Medicine I, Division of Nephrology, University Hospital Würzburg, Würzburg, Germany.
Matthias Schmid, Department of Medical Biometry, Informatics, and Epidemiology, University Hospital Bonn, Bonn, Germany.
Kai-Uwe Eckardt, Department of Nephrology and Hypertension, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany; Department of Nephrology and Medical Intensive Care, Charité-Universitätsmedizin Berlin, Berlin, Germany.
Ulla T Schultheiss, Institute of Genetic Epidemiology, Faculty of Medicine and Medical Center – University of Freiburg, Freiburg, Germany; Department of Medicine IV, Nephrology and Primary Care, Faculty of Medicine and Medical Center – University of Freiburg, Freiburg, Germany.
the GCKD Investigators:
Kai-Uwe Eckardt, Heike Meiselbach, Markus P Schneider, Mario Schiffer, Hans-Ulrich Prokosch, Barbara Bärthlein, Andreas Beck, André Reis, Arif B Ekici, Susanne Becker, Ulrike Alberth-Schmidt, Anke Weigel, Sabine Marschall, Eugenia Schefler, Gerd Walz, Anna Köttgen, Ulla T Schultheiß, Fruzsina Kotsis, Simone Meder, Erna Mitsch, Ursula Reinhard, Jürgen Floege, Turgay Saritas, Alice Groß, Elke Schaeffner, Seema Baid-Agrawal, Kerstin Theisen, Kai Schmidt-Ott, Martin Zeier, Claudia Sommerer, Mehtap Aykac, Gunter Wolf, Martin Busch, Andy Steiner, Thomas Sitter, Christoph Wanner, Vera Krane, Antje Börner-Klein, Britta Bauer, Florian Kronenberg, Julia Raschenberger, Barbara Kollerits, Lukas Forer, Sebastian Schönherr, Hansi Weissensteiner, Peter Oefner, Wolfram Gronwald, Matthias Schmid, and Jennifer Nadal
FUNDING
The work of U.T.S., F.K., R.K., H.U.Z. and M.A. was supported within the e:Med junior consortium CKDNapp (https://ckdn.app/), which is funded by grants from the German Ministry of Education and Research (BMBF, grant number 01ZX1912A, 01ZX1912B and 01ZX1912C). The GCKD study was funded by grants from the BMBF (grant number 01ER0804) and the KfH Foundation for Preventive Medicine and corporate sponsors (www.gckd.org).
AUTHORS’ CONTRIBUTIONS
Research idea and study design: I.S., F.K., R.K., J.L., A.K., K.-U.E., H.U.Z., M.A., U.T.S.; data acquisition: H.M., B.B., H.S., T.D., M.W., H.B., C.S., S.T., A.W., H.W., S.S., L.F., J.M., G.S., M.P.S., E.S., J.T.K., T.S., J.F., C.W., F.K., A.K., M.B., V.K., M.S., K.-U.E., U.T.S.; data analysis/interpretation: I.S., F.K., J.L., R.K., A.K., K.-U.E., U.T.S.; statistical analysis: I.S., F.K., R.K., J.L., N.S.K., U.T.S.; supervision or mentorship: H.U.Z., M.A., A.K., K.-U.E., U.T.S. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual's own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
DATA AVAILABILITY STATEMENT
Public posting of individual level participant data is not covered by the informed patient consent form. As stated in the patient consent form and approved by the Ethics Committees, a dataset containing pseudonyms can be obtained by collaborating scientists upon approval of a scientific project proposal by the steering committee of the GCKD study: www.gckd.org. The R shiny app can be accessed through: https://www.gckd.org/studienhintergrund/previous-study-results/event-analysis/.
CONFLICT OF INTEREST STATEMENT
The following conflict of interest statement is taken from the indication in the conflict of interest forms of each co-author. E.S. received consulting fees from AstraZeneca, honoraria, as a speaker from the National Kidney Foundation, is a board member of German Society of Nephrology and a KDIGO Working group. J.T.K. is stockholder with Synlab, BAYER and Quanterix Inc. J.F. received consulting fees and honoraria as a speaker from AstraZeneca, BAYER, Boehringer, CSL Vifor, Stadapharm, and Travere. C.W. received research grants for the institution from Boehringer Ingelheim, Sanofi and consulting fees from Amgen, Amicus, Astellas, AstraZeneca, BAYER, Boehringer Ingelheim, CSL Vifor, Chiesi, Chugai, Fresenius Medical Care, GSK, Idorsia, Eli Lilly, MSD, Novartis, Novo Nordisk, and Sanofi. A.K. received grant funding by the German Research Foundation (DFG) project ID 431984000 (SFB 1453) for the Institute of Genetic Epidemiology Freiburg. M.B. received consulting fees from Vifor Renal Pharma, Boehringer Ingelheim and honoraria as a speaker from AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis Pharma, support for meeting registration/travel from Alexion Pharma. K.U.E. received grant funding from Evotec, Travere, AstraZeneca, consulting fees from Akebia, AstraZeneca, BAYER, Otsuka, Retrophin. J.M. received honoraria as a speaker from AstraZeneca, Boehringer Ingelheim, BAYER, Alexion, Lilly. The remaining authors disclosed no conflicts of interest.
REFERENCES
- 1. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150. [DOI] [PubMed] [Google Scholar]
- 2. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020;395:709–33. 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stel VS, Brück K, Fraser S et al. International differences in chronic kidney disease prevalence: a key public health and epidemiologic research issue. Nephrol Dial Transplant 2017;32:ii129–35. 10.1093/ndt/gfw420 [DOI] [PubMed] [Google Scholar]
- 4. Bruck K, Stel VS, Gambaro G et al. CKD prevalence varies across the European general population. J Am Soc Nephrol 2016;27:2135–47. 10.1681/ASN.2015050542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Webster AC, Nagler EV, Morton RL et al. Chronic kidney disease. Lancet 2017;389:1238–52. 10.1016/S0140-6736(16)32064-5 [DOI] [PubMed] [Google Scholar]
- 6. Masson P, Webster AC, Hong M et al. Chronic kidney disease and the risk of stroke: a systematic review and meta-analysis. Nephrol Dial Transplant 2015;30:1162–9. 10.1093/ndt/gfv009 [DOI] [PubMed] [Google Scholar]
- 7. Go AS, Chertow GM, Fan D et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–305. 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- 8. Tonelli M, Wiebe N, Culleton B et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol 2006;17:2034–47. 10.1681/ASN.2005101085 [DOI] [PubMed] [Google Scholar]
- 9. Ruiz-Ortega M, Rayego-Mateos S, Lamas S et al. Targeting the progression of chronic kidney disease. Nat Rev Nephrol 2020;16:269–88. 10.1038/s41581-019-0248-y [DOI] [PubMed] [Google Scholar]
- 10. He L, Wei Q, Liu J et al. AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int 2017;92:1071–83. 10.1016/j.kint.2017.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stengel B. Chronic kidney disease and cancer: a troubling connection. J Nephrol 2010;23:253–62. [PMC free article] [PubMed] [Google Scholar]
- 12. Barnett K, Mercer SW, Norbury M et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37–43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 13. Johnston MC, Crilly M, Black C et al. Defining and measuring multimorbidity: a systematic review of systematic reviews. Eur J Public Health 2019;29:182–9. 10.1093/eurpub/cky098 [DOI] [PubMed] [Google Scholar]
- 14. Farmer C, Fenu E, O'Flynn N et al. Clinical assessment and management of multimorbidity: summary of NICE guidance. BMJ (Clin Res Ed) 2016;354:i4843. [DOI] [PubMed] [Google Scholar]
- 15. Wallace E, Salisbury C, Guthrie B et al. Managing patients with multimorbidity in primary care. BMJ (Clin Res Ed) 2015;350:h176. [DOI] [PubMed] [Google Scholar]
- 16. Charytan D, Kuntz RE. The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Int 2006;70:2021–30. 10.1038/sj.ki.5001934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sarnak MJ, Amann K, Bangalore S et al. Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol 2019;74:1823–38. 10.1016/j.jacc.2019.08.1017 [DOI] [PubMed] [Google Scholar]
- 18. Herzog CA, Asinger RW, Berger AK et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2011;80:572–86. 10.1038/ki.2011.223 [DOI] [PubMed] [Google Scholar]
- 19. Eckardt KU, Bärthlein B, Baid-Agrawal S et al. The German Chronic Kidney Disease (GCKD) study: design and methods. Nephrol Dial Transplant 2012;27:1454–60. 10.1093/ndt/gfr456 [DOI] [PubMed] [Google Scholar]
- 20. Titze S, Schmid M, Kottgen A et al. Disease burden and risk profile in referred patients with moderate chronic kidney disease: composition of the German Chronic Kidney Disease (GCKD) cohort. Nephrol Dial Transplant 2015;30:441–51. 10.1093/ndt/gfu294 [DOI] [PubMed] [Google Scholar]
- 21. Hsu JY, Roy JA, Xie D et al. Statistical methods for cohort studies of CKD: survival analysis in the setting of competing risks. Clin J Am Soc Nephrol 2017;12:1181–9. 10.2215/CJN.10301016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prokosch HU, Mate S, Christoph J et al. Designing and implementing a biobanking IT framework for multiple research scenarios. Stud Health Technol Inform 2012;180:559–63. [PubMed] [Google Scholar]
- 23. Kristman V, Manno M, Côté P. Loss to follow-up in cohort studies: how much is too much? Eur J Epidemiol 2004;19:751–60. 10.1023/B:EJEP.0000036568.02655.f8 [DOI] [PubMed] [Google Scholar]
- 24. Altman DG. Statistics in medical journals: some recent trends. Stat Med 2000;19:3275–89. [DOI] [PubMed] [Google Scholar]
- 25. Darlington O, Dickerson C, Evans M et al. Costs and healthcare resource use associated with risk of cardiovascular morbidity in patients with chronic kidney disease: evidence from a systematic literature review. Adv Ther 2021;38:994–1010. 10.1007/s12325-020-01607-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jha V, Al-Ghamdi SMG, Li G et al. Global economic burden associated with chronic kidney disease: a pragmatic review of medical costs for the inside CKD research programme. Adv Ther 2023;40:4405–20. 10.1007/s12325-023-02608-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kirkeskov L, Carlsen RK, Lund T et al. Employment of patients with kidney failure treated with dialysis or kidney transplantation-a systematic review and meta-analysis. BMC Nephrol 2021;22:348. 10.1186/s12882-021-02552-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ola O, Sedig K. Beyond simple charts: design of visualizations for big health data. Online J Public Health Inform 2016;8:e195. 10.5210/ojphi.v8i3.7100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chishtie J, Bielska IA, Barrera A et al. Interactive visualization applications in population health and health services research: systematic scoping review. J Med Internet Res 2022;24:e27534. 10.2196/27534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reichel H, Zee J, Tu C et al. Chronic kidney disease progression and mortality risk profiles in Germany: results from the Chronic Kidney Disease Outcomes and Practice Patterns Study. Nephrol Dial Transplant 2020;35:803–10. 10.1093/ndt/gfz260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grams ME, Yang W, Rebholz CM et al. Risks of adverse events in advanced CKD: the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 2017;70:337–46. 10.1053/j.ajkd.2017.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997;349:1269–76. 10.1016/S0140-6736(96)07493-4 [DOI] [PubMed] [Google Scholar]
- 33. Mikkola TS, Gissler M, Merikukka M et al. Sex differences in age-related cardiovascular mortality. PLoS One 2013;8:e63347. 10.1371/journal.pone.0063347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Villar E, Remontet L, Labeeuw M et al. Effect of age, gender, and diabetes on excess death in end-stage renal failure. J Am Soc Nephrol 2007;18:2125–34. 10.1681/ASN.2006091048 [DOI] [PubMed] [Google Scholar]
- 35. Carrero JJ, de Mutsert R, Axelsson J et al. Sex differences in the impact of diabetes on mortality in chronic dialysis patients. Nephrol Dial Transplant 2011;26:270–6. 10.1093/ndt/gfq386 [DOI] [PubMed] [Google Scholar]
- 36. Hecking M, Bieber BA, Ethier J et al. Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: the Dialysis Outcomes and Practice Patterns Study (DOPPS). PLoS Med 2014;11:e1001750. 10.1371/journal.pmed.1001750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jung HY, Jeon Y, Kim YS et al. Sex disparities in mortality among patients with kidney failure receiving dialysis. Sci Rep 2022;12:18555. 10.1038/s41598-022-16163-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shajahan S, Amin J, Phillips JK et al. Relationship between sex and cardiovascular mortality in chronic kidney disease: a systematic review and meta-analysis. PLoS One 2021;16:e0254554. 10.1371/journal.pone.0254554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim KM, Oh HJ, Choi HY et al. Impact of chronic kidney disease on mortality: a nationwide cohort study. Kidney Res Clin Pract 2019;38:382–90. 10.23876/j.krcp.18.0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dienemann T, Fujii N, Orlandi P et al. International Network of Chronic Kidney Disease cohort studies (iNET-CKD): a global network of chronic kidney disease cohorts. BMC Nephrol 2016;17:121. 10.1186/s12882-016-0335-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ryu H, Hong Y, Kang E et al. Comparison of outcomes of chronic kidney disease based on etiology: a prospective cohort study from KNOW-CKD. Sci Rep 2023;13:3570. 10.1038/s41598-023-29844-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haynes R, Staplin N, Emberson J et al. Evaluating the contribution of the cause of kidney disease to prognosis in CKD: results from the Study of Heart and Renal Protection (SHARP). Am J Kidney Dis 2014;64:40–8. 10.1053/j.ajkd.2013.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carrero JJ, Hecking M, Chesnaye NC et al. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 2018;14:151–64. 10.1038/nrneph.2017.181 [DOI] [PubMed] [Google Scholar]
- 44. Pottel H, Björk J, Rule AD et al. Cystatin C-based equation to estimate GFR without the inclusion of race and sex. N Engl J Med 2023;388:333–43. 10.1056/NEJMoa2203769 [DOI] [PubMed] [Google Scholar]
- 45. Kidney Disease: Improving Global Outcome KDIGO 2012 Clinical Practice Guidelines for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013;3:63–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Public posting of individual level participant data is not covered by the informed patient consent form. As stated in the patient consent form and approved by the Ethics Committees, a dataset containing pseudonyms can be obtained by collaborating scientists upon approval of a scientific project proposal by the steering committee of the GCKD study: www.gckd.org. The R shiny app can be accessed through: https://www.gckd.org/studienhintergrund/previous-study-results/event-analysis/.