Abstract
To study the role of tryptophan degradation by indoleamine 2,3-dioxygenase (INDO) in the control of Trypanosoma cruzi or Toxoplasma gondii replication, we used human fibroblasts and a fibrosarcoma cell line (2C4). The cells were cultured in the presence or absence of recombinant gamma interferon (rIFN-γ) and/or recombinant tumor necrosis factor alpha (rTNF-α) for 24 h and were then infected with either T. cruzi or T. gondii. Intracellular parasite replication was evaluated 24 or 48 h after infection. Treatment with rIFN-γ and/or rTNF-α had no inhibitory effect on T. cruzi replication. In contrast, 54, 73, or 30% inhibition of T. gondii replication was observed in the cells treated with rIFN-γ alone, rIFN-γ plus rTNF-α, or TNF-α alone, respectively. The replication of T. gondii tachyzoites in cytokine-activated cells was restored by the addition of extra tryptophan to the culture medium. Similarly, T. gondii tachyzoites transfected with bacterial tryptophan synthase were not sensitive to the microbiostatic effect of rIFN-γ. We also investigated the basis of the cytokine effect on parasite replication by using the three mutant cell lines B3, B9, and B10 derived from 2C4 and expressing defective STAT1α (signal transducer and activator of transcription), JAK2 (Janus family of cytoplasmic tyrosine kinases), or JAK1, respectively, three important elements of a signaling pathway triggered by rIFN-γ. We found that rTNF-α was able to induce low levels expression of INDO mRNA in the parental cell line, as well as the cell line lacking functional JAK2. In contrast to the parental cell line (2C4), rIFN-γ was not able to induce the expression of INDO mRNA or microbiostatic activity in any of the mutant cell lines. These findings indicate the essential requirement of the JAK/STAT pathway for the induction of high levels of INDO mRNA, tryptophan degradation, and the anti-Toxoplasma activity inside human nonprofessional phagocytic cells.
Several of the gene products and functions induced by gamma interferon (IFN-γ) in either professional or nonprofessional phagocytic cells (PPC and NPPC) have been implicated in resistance to microbial infection. In macrophages or PPC the activation by IFN-γ leads to the production of reactive oxygen intermediates (31) and reactive nitrogen intermediates (1, 16), the generation of leukotrine derivatives (54), and tryptophan degradation (8, 35), all of which are involved in the control of intracellular pathogens. Thus, the growth of different intracellular protozoa, such as Leishmania spp., Toxoplasma gondii, and Trypanosoma cruzi (28, 29, 30, 38, 55), is tightly regulated inside macrophages activated by IFN-γ. Many of these microbiostatic-microbicidal functions induced by IFN-γ have also been shown to be potentiated by tumor necrosis factor alpha (TNF-α) (16, 23).
In human NPPC, indoleamine 2,3-dioxygenase (INDO) appears to be the main enzyme induced by IFN-γ and is implicated in the control of the intracellular replication of T. gondii tachyzoites (35). INDO is the first enzyme in a major pathway responsible for the degradation of tryptophan. More specifically, this enzyme catalyzes the oxidative decyclization of l-tryptophan to N-formylkynurenine (36, 48). In both PPC and NPPC, inhibition of intracellular parasite replication induced by IFN-γ can be reversed by the addition of tryptophan to the tissue culture medium (17, 35). These results indicate that the induction of INDO by IFN-γ leads to tryptophan depletion and to the interruption of parasite replication inside the vertebrate host cells (9, 10, 49).
Since, like T. gondii tachyzoites, the T. cruzi amastigotes can also replicate inside most nucleated cells from their vertebrate hosts, it is tempting to speculate that not only macrophages but also NPPC may display mechanisms that will lead to resistance to infection with this latter parasite. In this study, we proposed to investigate the regulation of T. cruzi replication inside NPPC of human origin activated with rIFN-γ and/or rTNF-α. T. gondii parasites were used as a control since their replication is known to be controlled inside human NPPC after activation with IFN-γ. In addition, mutant cell lines were used to test the effect of different elements from the signaling pathway(s) triggered by recombinant IFN-γ [rIFN-γ] on the induction of INDO and the control of intracellular protozoa inside NPPC. Our results, show that (i) in contrast to T. gondii, T. cruzi parasites can grow even in the presence of low levels of tryptophan inside NPPC cells activated with rIFN-γ alone or in combination with rTNF-α; (ii) rTNF-α triggers low levels of INDO mRNA, which was associated with low microbiostatic activity against T. gondii; (iii) rTNF-α also increased, in an additive manner, the effect of IFN-γ on the induction of INDO mRNA as well as on tachyzoite growth regulation inside cells from the fibroblast lineage; and (iv) JAK1 (Janus family of cytoplasmic tyrosine kinases), JAK2, and STAT1α (signal transducer and activator of transcription) are all required for the maximal induction of INDO mRNA, tryptophan degradation, and the control of parasite replication by rIFN-γ alone or when added with rTNF-α.
MATERIALS AND METHODS
Cell lines.
The human fibrosarcoma cell lines 2C4, B3, B9, and B10 and the human foreskin fibroblast line CRL1634 (a gift from Alan Sher, National Institute of Allergy and Infectious Diseases, National Institutes of Health) were used.
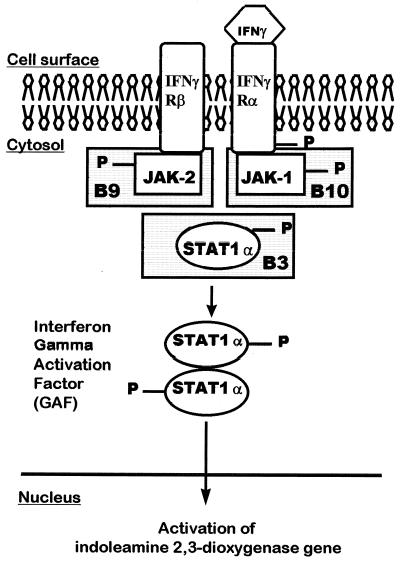
The mutants (B3, B9, and B10) were selected from the 2C4 human cell line, which was derived from a human fibrosarcoma HT1080 (5, 24, 57). The mutant cell lines were mutagenized with ICR 191 (acridine mutagen; Sigma Chemical Co., St. Louis, Mo.). After five rounds of mutagenesis, the cells were fluorescence activator cell sorted and then were grown and clonally selected in the presence of IFN-γ (51). Thus, the mutants termed B3, B9, and B10 were independent isolates derived from 2C4 (5). B3 was defective in STAT1α and B10 and B9 were defective in JAK1 and JAK2, respectively, three important elements of a signaling pathway triggered by IFN-γ (6, 11, 32, 53). The genotypes of these cells (gene deletions) were all confirmed by genetic crosses involving mutants from the same and from different complementation groups, as well as by biochemical analysis (5, 49). Further, by measuring the cytokine-induced expression of membrane surface proteins (e.g., transfected CD2 and major histocompatibility complex class I and class II), the steady-state levels of 9-27,2′,5′-oligoadenylate synthetase, and the guanylate binding protein mRNAs, we confirmed the expected phenotype of the B3, B9, and B10 cell lines. Thus, we showed that B3 and B10 are unresponsive to both IFN type 1 (IFN-α/β) and type 2 (IFN-γ), whereas B9 conserved intact the IFN type 1 signaling pathway (5).
The cells were grown in Dulbecco modified Eagle medium (DMEM; GIBCO Laboratories, Grand Island, N.Y.) or RPMI 1640 medium (GIBCO) and supplemented with 10% heat-inactivated fetal bovine serum, 5 μM l-glutamine, 100 IU of penicillin and 100 μg of streptomycin per ml, and 25 mM of HEPES buffer (pH 7.3). The tryptophan concentrations in the DMEM and RPMI media were 16 mg (78.4 μM) and 5 mg (24.5 μM) per liter, respectively. All cell lines were cultured at 37°C in humidified air containing 5% CO2. The cells were counted in a Neubauer hemocytometer after trypsin treatment, and their viability was assessed by trypan blue exclusion.
Parasites.
The Y strain of T. cruzi (44) and the RH strain of T. gondii were used in the parasite growth assays. An RH strain transfected with the tryptophan synthase (TS) gene from Escherichia coli (43) was used in some experiments. Both parasites were maintained by serial passages in 2C4 human fibroblast monolayer cultures. The T. cruzi trypomastigote and T. gondii tachyzoite forms were obtained on days 5 and 2 of cell culture, respectively.
T. gondii and T. cruzi cell infection.
Confluent cells were trypsinized (0.034% trypsin in 0.1% EDTA; both from Sigma), added to eight-well tissue culture chamber slides (Nunc, Inc.) in duplicates at a final concentration of 3 × 104 cells/well in 430 μl of DMEM or RPMI in the presence or absence of cytokines, and incubated at 37°C in 5% CO2 at 95% humidity for 24 h. The medium was changed after 24 h, and T. gondii or T. cruzi was added at a ratio of 3/1 and 10/1 parasites per cell, respectively, in a volume of 200 μl, with or without the cytokines. After 3 h of infection for T. gondii and 6 h for T. cruzi, the parasites that had not infected the cells were removed by washing, replaced with 430 μl of fresh medium, and further incubated with or without the cytokines for 24 or 48 h at 37°C and 5% CO2. In the case of the T. cruzi, the infected cells were incubated at 33°C in 5% CO2 at 95% humidity (4). After 24 or 48 h of incubation, the chambers were washed with phosphate-buffered saline (PBS), fixed with methanol, stained with May-Grunwald-Giemsa, and mounted on slides. The results evaluated under light microscopy were expressed as an infection index. The infection index is the average number of parasites per 100 cells obtained from three independent experiments performed in duplicate.
In order to verify the effect of exogenous tryptophan in restoring the parasite replication, the 2C4 cells activated with cytokines were cultured as described above, in the presence or absence of exogenous 1 mM l-tryptophan (Sigma), and then infected with either tachyzoite or trypomastigote forms. The intracellular parasites were counted at 24 h postinfection. To further certify a tryptophan requirement for the intracellular growth of T. gondii or T. cruzi, parasite replication was evaluated in a culture medium prepared from a kit supplied by GIBCO that had all the components of a complete medium except tryptophan. The tryptophan-free medium was supplemented with 3% dialyzed heat-inactivated fetal bovine serum and 100 μM indole. Parasite intracellular growth was evaluated at 24 and 48 h postinfection.
Cytophatic assay.
Vaccinia virus strain WR was a gift from C. Jungwirth (University of Würzburg, Würzburg, Germany). It was propagated in Vero cells and purified as previously described by Joklik (19). The 2C4 parental and mutant cell lines were cultured as described above at a density of 1.5 × 104/well on a 24-well plate and, when 90% of confluence was reached, rIFN-γ (500 IU/ml) was added overnight. Fresh medium supplemented with rIFN-γ was added to the cultures, which were then infected with vaccinia virus at a 0.01 multiplicity of infection for 2 h. The infection was stopped by aspirating and replacing the medium with 2 ml of fresh medium. After 30 h, the virus plaques were visualized by adding 1 ml of 0.3% crystal violet in formalin solution (7).
Cytokines.
Human rIFN-γ and rTNF-α, with a specific activity of 3 × 107 U/mg of protein, were provided by Genentech, Inc. (San Francisco, Calif.) and were maintained at 4°C. The concentrations used in the assays were 900 IU/ml for rIFN-γ and 60 IU/ml for rTNF-α. These concentrations were chosen after previous titrations of the cytophatic effect of the vesicular stomatitis virus in 2C4 cells.
INDO mRNA expression assay.
In order to verify the induction of INDO mRNA expression, 75-cm2 culture flasks containing 2 × 106 to 5 × 106 cells were used. Briefly, the cells were incubated in the presence or absence of cytokines for 14 to 16 h. The medium was discarded, and the cells were washed with PBS. Total RNA was extracted with TRIzol (GIBCO) according to the manufacturer’s instructions. The cDNA synthesis was obtained in a final volume of 100 μl containing 200 U of reverse transcriptase (RT) obtained from Moloney murine leukemia virus (Pharmacia), 200 mM concentrations of each deoxynucleoside triphosphate (Promega), 2.5 μl of buffer (0.1 M MgCl2, 0.5 M Tris-HCl, 1 mM DTT, 2 mg of bovine serum albumin per ml; pH 7.2 at 20°C) (Boehringer Mannheim), 240 pmol of oligo-dT10 (Boehringer Mannheim) per μl, 0.1 M dithiothreitol (Bio-Rad), 1 U of the RNase inhibitor RNasin (Promega) per μl, and 0.4 μg of total RNA. The cDNA samples were stored at −20°C.
The INDO mRNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were detected in the host cells by RT-PCR assays. The primers 5′-AGT TGA GAA GTT AAA CAT GC-3′ and 5′-CAT GAT CGT GGA TTT GGT GA-3′ were used to detect INDO mRNA. The expected fragment size was 487 bp. The mRNA expression of the human housekeeping gene GAPDH was used as a reference for the inducible INDO. The primers 5′-GTG GTG AAG CAG GCG TCG-3′ and 5′-GAC TGA GTG TGG CAG GGA-3′ were used to detect GAPDH mRNA expression. The expected fragment size was 311 bp. The RT-PCR program consisted of 30 cycles with an initial denaturation at 95°C for 5 min, annealing at 55°C for 1 min, extension at 72°C for 1 min, and a final annealing of 1 min at 55°C and an extension at 72°C for 5 min. The amplification was carried out in a thermocycler (PTC 100; MJR Research) in a final volume of 10 μl containing 0.5 U of Taq DNA polymerase (CENBIOT, RS, Porto Alegre, Brazil), 200 mM concentrations of each deoxynucleoside triphosphate, 1.5 mM MgCl2, 50 mM KCl, and 10 mM Tris-HCl (pH 8.5), together with 10.0 and 1.0 pmol of the INDO and GAPDH primers, respectively. The reaction mixture was overlaid with 20 μl of mineral oil. After amplification, 3 μl of the products was mixed with 3 μl of a 2× sample buffer (0.25% bromophenol blue, 0.25% xylene cyanol, 30% glycerol) and subjected to electrophoresis through a 6% nondenaturing polyacrylamide gel. Gels were fixed with 10% ethanol–0.5% acetic acid for 10 min, and the bands were revealed by staining with 0.2% silver nitrate for 10 min with 0.75 M NaOH–0.1 M formaldehyde for 10 min as previously described (40).
TS-transfected T. gondii.
The expression of the TS in the transgenic strain of T. gondii (43) was confirmed by PCR. The reaction components were the same as those described for INDO and GAPDH except for the use of 1.0 pmol of primers 5′-CCC CTA TTT TGG TGA GTT TG-3′ and 5′-CCC CTA TTT TGG TGA GTT TG-3′ and either 1.0 or 10.0 ng of template DNA. The expected fragment size was 1,155 bp. The PCR program consisted of 30 cycles, with an initial denaturation at 95°C for 5 min, annealing at 50°C for 1 min, and extension at 72°C for 1 min, followed by annealing at 50°C for 1 min and a final extension of 5 min.
Statistical analysis.
Differences between groups were assessed with the Student t test. P values of <0.05 were considered statistically significant.
RESULTS
Differential regulation of T. cruzi and T. gondii growth in rIFN-γ activated NPPC cells through tryptophan degradation.
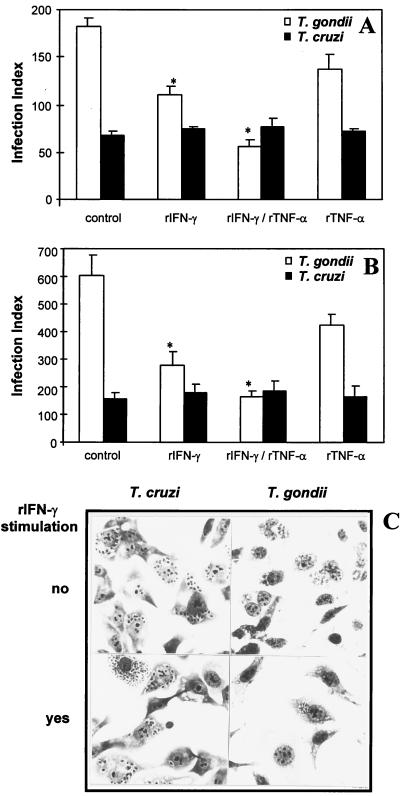
In order to study the regulation of parasite growth inside NPPC, we used the fibrosarcoma cell line (2C4). Initially, we compared the ability of human foreskin fibroblasts (Fig. 1A) and the 2C4 cell line (Fig. 1B), exposed to rIFN-γ and/or rTNF-α, to control T. gondii and T. cruzi replication. The human fibroblasts or 2C4 cells were cultured in the absence or presence of the cytokines rIFN-γ (900 IU/ml) and/or rTNF-α (60 IU/ml) for 24 h and then infected with either T. cruzi trypomastigotes or T. gondii tachyzoites. The cell culture was interrupted at 48 h postinfection, and the intracellular parasites were counted. The results presented on Fig. 1A (human foreskin fibroblasts) and 1B (2C4 cell line) and illustrated in Fig. 1C (2C4 cell line), show that activation of either human fibroblasts or 2C4 cells with rIFN-γ resulted in a strong inhibitory effect of intracellular tachyzoite growth. The rTNF-α alone had a persistent, small inhibitory effect on T. gondii growth, which was observed in different experiments. However, this difference was not statistically significant within a single experiment. The addition of rIFN-γ and rTNF-α resulted in an additive inhibitory effect on the tachyzoite growth. Interestingly, activation of either human foreskin fibroblasts or the 2C4 cell line with rIFN-γ and/or rTNF-α had no effect on the intracellular growth of T. cruzi amastigotes.
FIG. 1.
(A) Effect of rIFN-γ (900 IU/ml) and/or rTNF-α (60 IU/ml) on intracellular parasite replication in human foreskin fibroblasts. The replication was evaluated 48 h postinfection. An asterisk indicates a result statistically different from the control group (P < 0.05). Results indicate the means ± the standard errors of the means from two independent experiments done in duplicate. (B) Effect of rIFN-γ (900 IU/ml) and/or rTNF-α (60 IU/ml) on intracellular parasite replication in 2C4 cells. The replication was evaluated at 48 h postinfection. An asterisk indicates a result statistically different from the control group (P < 0.05). Results indicate the means ± the standard errors of the means from three independent experiments done in duplicate. (C) Illustration of rIFN-γ effect on intracellular replication of T. gondii tachyzoites and T. cruzi amastigotes. Note that, in contrast to T. gondii, T. cruzi growth was observed in the presence or absence of rIFN-γ.
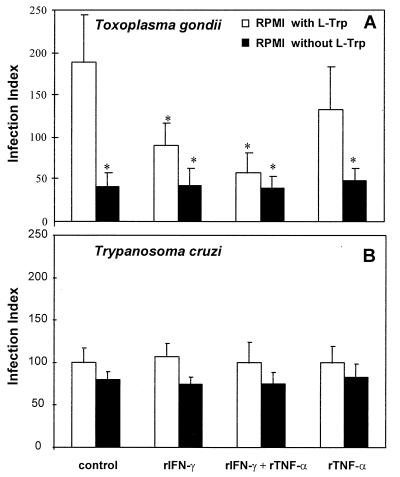
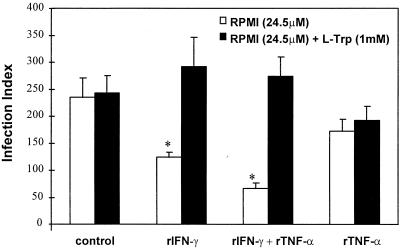
Because previous studies have demonstrated that the activation of human NPPC with IFN-γ results in degradation of the intracellular pool of tryptophan (10, 35, 36), which is responsible for the inhibition of parasite replication, we decided to repeat the above experiment without tryptophan. The 2C4 cell line was cultured in regular medium or in medium lacking tryptophan in the presence or absence of rIFN-γ and/or rTNF-α for 24 h and was then infected with either T. gondii (Fig. 2A) or T. cruzi (Fig. 2B). In agreement with early studies (35), tachyzoite growth was largely inhibited in the tryptophan-free medium even in absence of cytokines. A small but not statistically significant effect on T. cruzi growth was observed in cells cultured in the absence of tryptophan. Thus, our data indicate that T. cruzi amastigotes presented an equal ratio of parasite replication at both low and high levels of tryptophan inside the host cells. Consistent with the hypothesis that tryptophan degradation mediates the inhibition of tachyzoite growth induced by rIFN-γ, we observed that in the presence of an excess amount of tryptophan, the inhibitory effect of intracellular tachyzoite growth induced by either rIFN-γ or rIFN-γ plus rTNF-α was reversed (Fig. 3).
FIG. 2.
Effect of the absence of l-tryptophan (L-Trp) in 2C4 cells stimulated with rIFN-γ (900 IU/ml) and/or rTNF-α (60 IU/ml) and infected with (A) T. gondii or (B) T. cruzi. The cells and parasites were maintained without l-tryptophan as described in Materials and Methods. The replication was evaluated 24 h postinfection. An asterisk indicates a result statistically different from the control group (P < 0.05). Results indicate the means ± the standard errors of the means from three independent experiments done in duplicate.
FIG. 3.
Effect of the addition of l-tryptophan (L-Trp) on the intracellular replication of T. gondii tachyzoites in 2C4 cells activated with rIFN-γ (900 IU/ml) and/or rTNF-α (60 IU/ml). Tryptophan was added to the culture medium at a final concentration of 1 mM immediately after the parasite infection, and the replication was evaluated 24 h later. An asterisk indicates a result statistically different from the control group (P < 0.05). Results indicate the means ± the standard errors of the means from three independent experiments done in duplicate.
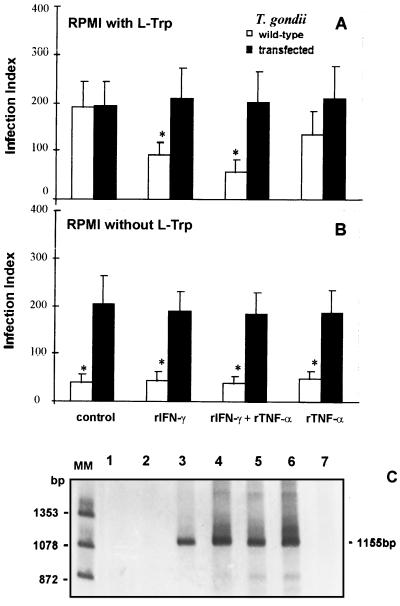
We also tested the ability of T. gondii parasites transfected with the enzyme TS from E. coli to survive inside 2C4 cells under rIFN-γ stimulation. The results presented in Fig. 4 show that, in contrast to the wild type, the replication of transgenic parasites was not sensitive to NPPC exposure to rIFN-γ and/or rTNF-α in the presence (Fig. 4A) or in the absence of tryptophan (Fig. 4B). Figure 4C shows the detection of the TS gene by PCR by using DNA extracted from wild-type tachyzoites (lanes 1 and 2), transfected parasites (lanes 3 and 4), E. coli (lanes 5 and 6), and a negative control of the PCR reaction (lane 7). In contrast to transfected parasites and E. coli, PCR with DNA from wild-type parasites did not yield a PCR product of the predicted size (1,155 bp).
FIG. 4.
Comparison of effects of rIFN-γ (900 IU/ml) and/or rTNF-α (60 IU/ml) on 2C4 human fibroblasts infected with wild-type or transfected strains of T. gondii and maintained in the presence (A) or in the absence (B) of l-tryptophan (L-Trp). Both strains were maintained in tryptophan-free medium. RPMI was supplemented with 3% dialyzed heat-inactivated fetal bovine serum and 100 μM indole. The replication was evaluated 24 h after infection. An asterisk indicates a result statistically different from the control group (P < 0.05). Results indicate the means ± the standard errors of the means from three independent experiments done in duplicate. (C) PCR products of TS from E. coli. PCR was performed as described in Materials and Methods. The PCR product (3 μl) was electrophoresed in 6% polyacrylamide gel and silver stained. DNA of bacteriophage φX digested by endonuclease HaeIII was used as a molecular size marker. Lanes: 1 and 2, DNA from T. gondii RH wild-type strain, 1 and 10 ng, respectively; 3 and 4, T. gondii TS-transfected strain, 1 and 10 ng, respectively; 5 and 6, E. coli, 1 and 10 ng, respectively; 7, negative control (no DNA added). An 1,155-bp fragment was expected.
Study of the involvement of the JAK/STAT pathway in the induction of both INDO mRNA expression and antiparasite effector function by IFN-γ.
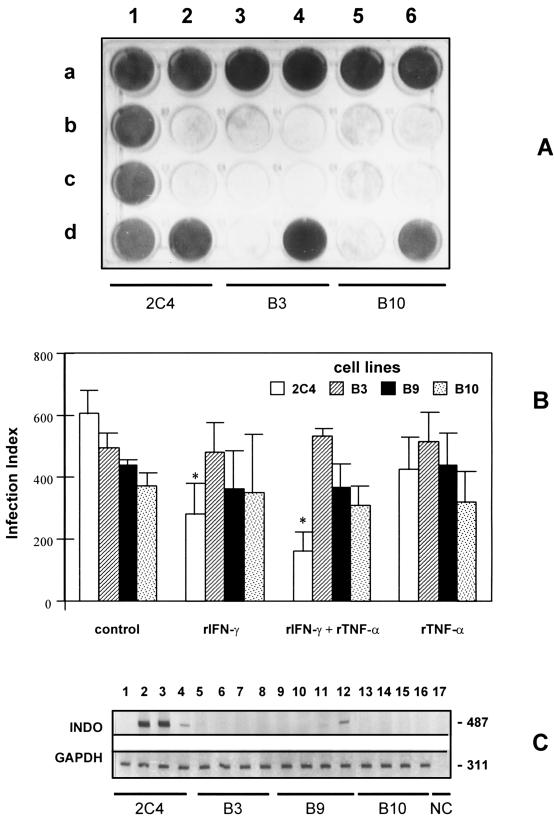
To further investigate the ability of rIFN-γ to induce INDO expression and the regulation of parasite expression in NPPC, we used three mutant cell lines, derived from 2C4 line, that have specific defects on the IFN-γ signaling pathway. As shown in Fig. 5, the mutant cell lines B3, B9, and B10 are defective in STAT1α, JAK2, and JAK1, respectively. Recent studies have demonstrated that cells lacking functional JAK1 can exhibit substantial expression of genes induced by IFN-γ. However, the antiviral activity is only exhibited in cells which have functional JAK1, JAK2, and STAT1α (15, 18, 45). We also tested cells lacking different components from the JAK/STAT pathway (Fig. 5) in their ability to control viral replication (Fig. 6A), T. gondii tachyzoite growth (Fig. 6B), and INDO mRNA expression (Fig. 6C). Interestingly, we found that JAK2-deficient cell line (B9) can express low levels of INDO mRNA in response to rTNF-α but not in response to IFN-γ. As with the antiviral activity, maximal induction of INDO mRNA and control of tachyzoite replication was only observed in cells with functional JAK1 and JAK2 (and STAT1).
FIG. 5.
IFN-γ signaling pathway showing the defects in the human fibroblast mutants B3, B9, and B10.
FIG. 6.
(A) Cytophatic effect of vaccinia virus infectivity assay in parental 2C4 and mutant B3 and B10 cells. Row a shows the controls, in duplicate, with the three cell lines without vaccinia virus and rIFN-γ. b1, c1, and d1 show the protective effect of rIFN-γ (500 IU/ml) against vaccinia virus in 2C4 cells. b2 and c2 show the viral effect on 2C4 cells without rIFN-γ. d2 shows rIFN-γ plus 2C4 cells. b3, c3, and d3 show the viral effect on the B3 mutant cell line plus rIFN-γ. b4 and c4 show the effect of the virus on the mutant B3 cells. d4 shows rIFN-γ plus B3 cells. b5, c5, and d5 show the viral effect on the B10 mutant cell line plus rIFN-γ. b6 and c6 show the viral effect on the B10 cells. d6 shows rIFN-γ plus B10 cells. (B) Effect of rIFN-γ (900 IU/ml) and/or rTNF-α (60 IU/ml) on the parental (2C4) and mutant (B3, B9, and B10) cells infected with T. gondii. The replication was evaluated 48 h after infection. An asterisk indicates a result statistically different from control group (P < 0.05). Results indicate the means ± the standard errors of the means from three independent experiments done in duplicate. (C) Effect of rIFN-γ (900 IU/ml) and/or rTNF-α (60 IU/ml) on the induction of INDO mRNA. The parental (2C4) and mutant (B3, B9, and B10) cells were incubated with rIFN-γ and/or rTNF-α for 14 to 16 h. Lanes 1, 5, 9, and 13 are controls without cytokine; lanes 2, 6, 10, and 14 are rIFN-γ; lanes 3, 7, 11, and 15 are rIFN-γ plus rTNF-α; lanes 4, 8, 12, and 16 are rTNF-α; and lane 17 is the negative control (no cDNA added). Expression of INDO and GAPDH mRNA was detected by RT-PCR. PCR products (3 μl) were electrophoresed in 6% polyacrylamide gel and silver stained. The expected fragment sizes were 487 bp for the INDO and 311 bp for the GAPDH genes.
In addition, we observed a smaller ratio of infectivity when we compared the mutant cells to the parental cell line. However, these differences were observed in some but not all of the experiments performed.
DISCUSSION
Different studies indicate that the activation of human macrophages and NPPC with IFN-γ leads to the induction of INDO, a key enzyme involved in the degradation of the essential amino acid tryptophan. In NPPC and macrophages, tryptophan starvation has been shown to be an important IFN-γ-induced mechanism involved in the regulation of intracellular pathogen replication (3, 10, 35, 55). Thus, the addition of extra tryptophan completely restores replication of the intracellular protozoan T. gondii and the bacteria Chlamydia psittaci and Chlamydia pneumoniae inside human fibroblast and epithelial cells, respectively (8, 35, 46). In contrast, additional tryptophan has only a partial effect in restoring the replication of T. gondii, Leishmania donovani, and C. psittaci inside human macrophages activated with IFN-γ (10, 30). These latter results suggest that mechanisms other than tryptophan degradation may be triggered in PPC by activation with IFN-γ (12, 34, 47).
Because T. cruzi can infect any nucleated cell from its vertebrate host, it is tempting to speculate that cytokine activation of NPPC may also result in the induction of microbiostatic function, which is responsible for the control of parasite replication during the chronic stage of infection. Furthermore, IFN-γ and/or TNF-α have been shown to play an important role in resistance to T. cruzi during the activation of both human and mouse macrophages (13, 26, 27); we therefore decided to study the capacity of these cytokines to induce anti-T. cruzi activity in human fibroblasts. As a control we used T. gondii tachyzoites, whose replication inside human fibroblasts has been shown to be sensitive to activation by IFN-γ. Interestingly, our results showed that in contrast to T. gondii tachyzoites, replication of T. cruzi inside human fibrosarcoma cells or fibroblasts was not affected by cell activation with rIFN-γ. Consistent with these observations, T. cruzi replication was not affected by tryptophan starvation, i.e., when parasites were cultured in tissue culture and medium lacking tryptophan. In contrast, tachyzoite replication was almost abolished in the same culture conditions. In agreement with our findings are the studies showing that T. cruzi replication is not affected in NPPC originating from humans or other mammals activated with human lymphoblastoid IFN and IFN obtained by infecting monolayers of human amniotic cells with inactivated Newcastle disease virus (14, 33). However, different studies suggest that IFN-γ, as well as IFN-α/β, may inhibit T. cruzi replication inside rat or murine NPPC (31, 37). The reasons for these discrepancies are still unclear.
The mechanism by which T. cruzi escapes tryptophan starvation is completely unknown. One possibility would be the expression of an enzyme which is involved in the synthesis of tryptophan. In measuring the amount of indole consumed, we detected only trace amounts of TS activity when we used T. cruzi epimastigote or trypomastigote extracts compared to our findings with E. coli. Moreover, by using primers specific for the conserved E. coli TS sequence, we were unable to amplify a fragment that showed homology with the sequence of E. coli TS (data not shown). Another explanation for this effect would be the generation of free tryptophan in the host due to the parasite protease activity. In fact, early studies demonstrated that parasite treatment with specific protease inhibitors reduced the amount of T. cruzi replication inside host cells (25). It is also noteworthy that all of the parasites sensitive to tryptophan starvation (i.e., T. gondii, L. donovani, and Chlamydia sp.) reside within a parasitophorous vacuole, in contrast to T. cruzi amastigotes, which replicate in the host cell cytoplasm (2). Thus, one could speculate that the residual pool of tryptophan in cells activated with IFN-γ would be less available in the parasitophorous vacuole. It is also possible that the tryptophan starvation only reduces the speed of parasite replication inside NPPC. Since the T. cruzi replication is slower than the T. gondii tachyzoite replication, the proliferation of the former parasite is not affected by the decreased levels of tryptophan inside host cells.
Finally, we also studied the ability of IFN-γ to induce microbiostatic activity in fibroblasts, as well as expression of INDO mRNA in the 2C4 parental cell lines and in derivative cells lacking functional elements (i.e., STAT1α, JAK1, and JAK2) of the IFN-γ signaling pathway. As expected, our results show that 2C4 cells were responsive to IFN-γ, whereas none of mutant cell lines showed antiviral, antiparasitic activity or INDO mRNA expression when stimulated with rIFN-γ. Thus, these results indicate the involvement of the JAK/STAT pathway on the induction of INDO mRNA expression and microbiostatic function in NPPC exposed to IFN-γ.
Interestingly, we found that rTNF-α was able to trigger the expression of low levels of INDO mRNA in the mutant B9 cell line, which has an intact signaling pathway to respond to type 1 IFNs. Previous studies have also shown that TNF-α and IFN-β can trigger the expression of INDO mRNA (41, 42, 52) and that TNF-α is able to induce mRNA synthesis of IFN-β in human fibroblasts (21, 39, 50). In light of our findings, we suggest that TNF-α may be triggering INDO mRNA expression through the induction IFN-β synthesis in human fibroblasts. However, the levels of INDO mRNA induced by TNF-α and IFN-β are apparently not high enough to efficiently control T. gondii replication inside fibroblasts.
These results clearly establish the involvement of the JAK/STAT signaling pathway in the INDO-mediated antimicrobial activity of IFN-γ, as suggested earlier by nucleic acid sequence analysis (22) and functional studies with plasmid constructs containing INDO gene promoter (11, 22). However, in contrast to previously reported studies, our results demonstrate for the first time the involvement of individual components (i.e., JAK1, JAK2, and STAT1) of the IFN-γ signaling cascade in the induction of both INDO mRNA expression and microbiostatic activity in NPPC.
ACKNOWLEDGMENTS
This work was supported in part by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-522.056-95/4), the Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG-CBS 1221/95), and PAPES/FIOCRUZ. R.T.G., A.J.R., and C.A.B. are research fellows from CNPq. I.P.C. and A.C.L.C. are graduate fellows from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and CNPq, respectively.
We are grateful to J. C. Magalhães for technical assistance on the virus infectivity assays.
REFERENCES
- 1.Adams L B, Hibbs J B, Taintor R R, Krahenbuhl J L. Microbiostatic effect of murine activated macrophages for Toxoplasma gondii: role for synthesis of inorganic nitrogen oxides from l-arginine. J Immunol. 1990;144:2725–2729. [PubMed] [Google Scholar]
- 2.Andrews N W. Living dangerously: how Trypanosoma cruzi uses lysosomes to get inside host cells, and then escapes into the cytoplasm. Biol Res. 1993;26:65–67. [PubMed] [Google Scholar]
- 3.Beatty W L, Belanger T A, Desai A A, Morrison R P, Byrne G I. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect Immun. 1994;62:3705–3711. doi: 10.1128/iai.62.9.3705-3711.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertelli M S M, Golgher R R, Brener Z. Intraspecific variation in Trypanosoma cruzi: effect of temperature on the intracellular differentiation in tissue culture. J Parasitol. 1977;63:434–437. [PubMed] [Google Scholar]
- 5.Bonjardim C A. JAK/STAT deficient cell lines. Braz J Med Biol Res. 1998;31:1389–1395. doi: 10.1590/s0100-879x1998001100004. [DOI] [PubMed] [Google Scholar]
- 6.Briscoe J, Rogers N C, Witthuhn B A, Watling D, Harpur A G, Wilks A F, Starck G R, Ihle J N, Kerr I M. Kinase-negative mutants of JAK1 can sustain interferon-γ-inducible gene expression but not an antiviral state. EMBO J. 1996;15:799–809. [PMC free article] [PubMed] [Google Scholar]
- 7.Buller R M L, Chakrabarti S, Moss B, Fredrickson T. Cell proliferative response to Vaccinia virus is mediates by VGF. Virology. 1988;164:182–192. doi: 10.1016/0042-6822(88)90635-6. [DOI] [PubMed] [Google Scholar]
- 8.Byrne G J, Lehmann L K, Landry G J. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun. 1986;53:347–351. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caplen H S, Gupta S L. Differential regulation of a cellular gene by human interferon-γ and interferon-α. J Biol Chem. 1988;263:332–339. [PubMed] [Google Scholar]
- 10.Carlin J M, Borden E C, Byrne G I. Interferon-induced indoleamine 2,3-dioxygenase activity inhibits Chlamydia psittaci replication in human macrophages. J Interferon Res. 1989;9:329–337. doi: 10.1089/jir.1989.9.329. [DOI] [PubMed] [Google Scholar]
- 11.Chon S Y, Hassanain H H, Gupta S L. Cooperative role of interferon regulatory factor-1 and p91 (STAT1) response elements in interferon-γ-inducible expression of human indoleamine 2,3-dioxygenase gene. J Biol Chem. 1996;271:17247–17252. doi: 10.1074/jbc.271.29.17247. [DOI] [PubMed] [Google Scholar]
- 12.de la Maza L M, Peterson E M. Dependence of the in vitro antiproliferative activity of recombinant human γ-interferon on the concentration of tryptophan in culture media. Cancer Res. 1988;48:346–350. [PubMed] [Google Scholar]
- 13.Gazzinelli R T, Oswald I P, Hieny S, James S L, Sher A. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur J Immunol. 1992;22:2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 14.Golgher R R, Bertelli M S M, Petrillo-Peixoto M L, Brener Z. Effect of interferon on the development of Trypanosoma cruzi in tissue culture “Vero cells.”. Mem Inst Oswaldo Cruz. 1980;75:157–160. doi: 10.1590/s0074-02761980000100015. [DOI] [PubMed] [Google Scholar]
- 15.Greelund A C, Farrar M A, Viviano B L, Schreiber R D. Ligand-induced IFN gamma receptor tyrosine phosphorylation couples the receptor to its signal transduction system (p91) EMBO J. 1994;13:1591–1600. doi: 10.1002/j.1460-2075.1994.tb06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green S J, Crawford R M, Hockmeyer J T, Meltzer M S, Nacy C A. Leishmania major amastigotes initiate the l-arginine-dependent killing mechanism in IFN-γ-stimulated macrophages by induction of tumor necrosis factor-α. J Immunol. 1990;145:4290–4297. [PubMed] [Google Scholar]
- 17.Gupta S L, Carlin J M, Pyati P, Daí W, Pfefferkorn E R, Murphy M J., Jr Antiparasitic and antiproliferative effects of indoleamine 2,3-dioxygense enzyme expression in human fibroblasts. Infect Immun. 1994;62:2277–2284. doi: 10.1128/iai.62.6.2277-2284.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heim M H, Kerr I M, Starck G R, Darnell J E. Contribution of STAT SH2 groups to specific interferon signalling by the JAK-STAT pathway. Science. 1995;267:1347–1349. doi: 10.1126/science.7871432. [DOI] [PubMed] [Google Scholar]
- 19.Joklik W R. The purification of four strains of poxvirus. Virology. 1962;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- 20.Kierszenbaum F, Sonnenfeld G. β-Interferon inhibits cell infection by Trypanosoma cruzi. J Immunol. 1984;132:905–908. [PubMed] [Google Scholar]
- 21.Kohase M, Henriksen-DeStefano D, May L T, Vilcek J, Sehgal P B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986;45:659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- 22.Konan K V, Taylor M W. Importance of the two interferon-stimulated response element (ISRE) sequences in the regulation of the human indoleamine 2,3-dioxygenase gene. J Biol Chem. 1996;271:19140–19145. doi: 10.1074/jbc.271.32.19140. [DOI] [PubMed] [Google Scholar]
- 23.Liew F Y, Li Y, Millott S. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990;15:4306–4310. [PubMed] [Google Scholar]
- 24.McKendry R, John J, Flavell D, Muller M, Kerr I M, Stark G R. High-frequency mutagenesis of human cells and characterization of a mutant unresponsive to both α and γ interferons. Proc Natl Acad Sci USA. 1991;88:11455–11459. doi: 10.1073/pnas.88.24.11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meirelles M N L, Juliano L, Carmona E, Silva S G, Costa E M, Murta A C M, Scharfstein J. Inhibitors of the major cysteinyl proteinase (GP57/51) impair host cell invasion and arrest the intracellular development of Trypanosoma cruzi in vitro. Mol Biochem Parasitol. 1992;52:175–184. doi: 10.1016/0166-6851(92)90050-t. [DOI] [PubMed] [Google Scholar]
- 26.Munoz-Fernández M A, Fernández M A, Fresno M. Activation of human macrophages for the killing of intracellular Trypanosoma cruzi by TNF-α and IFN-γ through a nitric oxide-dependent mechanism. Immunol Lett. 1992;33:35–40. doi: 10.1016/0165-2478(92)90090-b. [DOI] [PubMed] [Google Scholar]
- 27.Munoz-Fernández M A, Fernández M A, Fresno M. Synergism between tumor-necrosis factor-alpha and interferon-gamma on macrophage activation for the killing of intracellular Trypanosoma cruzi through a nitric oxide-dependent mechanism. Eur J Immunol. 1992;22:301–307. doi: 10.1002/eji.1830220203. [DOI] [PubMed] [Google Scholar]
- 28.Murray H W, Rubin B Y, Rothermel C D. Killing of intracellular Leishmania donovani by human mononuclear phagocytes: evidence that interferon-γ is the activating lymphokine. J Clin Invest. 1983;72:1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray H W, Rubin B Y, Carriero S M, Denn A T, Harris A M, Jaffee E A. Human mononuclear phagocyte antiprotozoal mechanisms: oxygen-dependent versus oxygen-independent activity against intracellular Toxoplasma gondii. J Immunol. 1985;134:1982–1988. [PubMed] [Google Scholar]
- 30.Murray H W, Szuro-Sudol A, Wellner D, Oca M J, Granger A M, Libby D M, Rothermel C D, Rubin B Y. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infect Immun. 1989;57:845–849. doi: 10.1128/iai.57.3.845-849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nathan C F, Murray H W, Wiebe M E, Rubin B Y. Identification of interferon-γ as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Shea J J. JAKs, STATs, cytokine signal transduction, and immunoregulation: are we there yet? Immunity. 1997;7:1–11. doi: 10.1016/s1074-7613(00)80505-1. [DOI] [PubMed] [Google Scholar]
- 33.Osuna A, Ortega G, Gamarro F, Castanys S, Ruiz-Perez L M. Effect of interferon on the infectivity of Trypanosoma cruzi in cultured HeLa cells. Int J Parasitol. 1985;15:167–170. doi: 10.1016/0020-7519(85)90082-7. [DOI] [PubMed] [Google Scholar]
- 34.Ozaki Y, Edelstein M P, Duch D S. Induction of indoleamine 2,3-dioxygenase: a mechanism of the antitumor activity of interferon-γ. Proc Natl Acad Sci USA. 1988;85:1242–1246. doi: 10.1073/pnas.85.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfefferkorn E R. Interferon-γ blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfefferkorn E R, Eckel M, Rebhun S. Interferon-γ suppresses the growth of Toxoplasma gondii in human fibroblasts through starvation for tryptophan. Mol Biochem Parasitol. 1986;20:215–224. doi: 10.1016/0166-6851(86)90101-5. [DOI] [PubMed] [Google Scholar]
- 37.Plata F, Wietzerbin J, Pons F G, Falcoff E, Eisen H. Synergistic protection by specific antibodies and interferon against infection by Trypanosoma cruzi in vitro. Eur J Immunol. 1984;14:930–935. doi: 10.1002/eji.1830141013. [DOI] [PubMed] [Google Scholar]
- 38.Reed S G. In vivo administration of recombinant IFN-γ induces macrophage activation and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infection. J Immunol. 1988;140:4342–4347. [PubMed] [Google Scholar]
- 39.Reis L F, Ho Lee T, Vilcek J. Tumor necrosis factor acts synergistically with autocrine interferon-beta and increases interferon-beta mRNA levels in human fibroblasts. J Biol Chem. 1989;264:16351–16354. [PubMed] [Google Scholar]
- 40.Santos F R, Pena S D J, Epplen J T. Genetic and population study of a Y-linked tetranucleotide repeat DNA polymorphism. Hum Genet. 1993;90:655–656. doi: 10.1007/BF00202486. [DOI] [PubMed] [Google Scholar]
- 41.Schemer-Avni Y, Wallach D, Sarov I. Inhibition of Chlamydia trachomatis growth by recombinant tumor necrosis factor. Infect Immun. 1988;56:2503–2506. doi: 10.1128/iai.56.9.2503-2506.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitz J L, Carlin J M, Borden E C, Byrne G I. Beta interferon inhibits Toxoplasma gondii growth in human monocyte-derived macrophages. Infect Immun. 1989;57:3254–3256. doi: 10.1128/iai.57.10.3254-3256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sibley L D, Messina M, Niesman I R. Stable DNA transformation in the obligate intracellular parasite Toxoplasma gondii by complementation of tryptophan auxotrophy. Proc Natl Acad Sci USA. 1994;91:5508–5512. doi: 10.1073/pnas.91.12.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva L H P, Nussensweig V. Sobre uma cepa de Trypanosoma cruzi altamente virulenta para o camundongo branco. Fol Clin Biol. 1953;20:191–208. [Google Scholar]
- 45.Stahl N, Farruggella T J, Boulton T G, Zhong Z, Darnell J E, Jr, Yancopoulos G D. Choice of STATs and other substrates specified by modular tyrosine based motifs in cytokine receptors. Science. 1995;267:1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- 46.Summersgill J T, Sahney N N, Charlotte A G, Quinn T C, Ramirez J A. Inhibition of Chlamydia pneumoniae growth in Hep-2 pretreated with gamma interferon and tumor necrosis factor alpha. Infect Immun. 1995;63:2801–2803. doi: 10.1128/iai.63.7.2801-2803.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takikawa O, Kuroiwa T, Yamasaki F, Kido R. Mechanism of interferon-γ action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-γ and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J Biol Chem. 1988;263:2041–2048. [PubMed] [Google Scholar]
- 48.Taylor M W, Grensheng F. Relationship between interferon gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–2522. [PubMed] [Google Scholar]
- 49.Thomas S M, Garrity L F, Brandt C R, Schobert C S, Feng G-S, Taylor M W, Carlin J M, Byrne G J. IFN-γ-mediated antimicrobial response. J Immunol. 1993;150:5529–5534. [PubMed] [Google Scholar]
- 50.Van Damme J, de Ley M, Van Snick J, Dinarello C A, Billiau A. The role of interferon-β1 and the 26-kDa protein (interferon-β2) as mediators of the antiviral effect of interleukin 1 and tumor necrosis factor. J Immunol. 1987;139:1867–1872. [PubMed] [Google Scholar]
- 51.Watling D, Guschin D, Muller M, Silvennoinen O, Witthuhn B A, Quelle F W, Rodgers N C, Starck G R, Ihle J N, Kerr I M. Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon gamma signal transduction pathway. Nature. 1993;366:166–170. doi: 10.1038/366166a0. [DOI] [PubMed] [Google Scholar]
- 52.Werner-Felmayer G, Werner E R, Fuchs D, Hausen A, Reibnegger G, Wachter H. Tumor necrosis factor-alpha and lipopolysaccharide enhance interferon-induced tryptophan degradation and pteridine synthesis in human cells. Biol Chem Hoppe-Seyler. 1989;370:1063–1069. doi: 10.1515/bchm3.1989.370.2.1063. [DOI] [PubMed] [Google Scholar]
- 53.Will A, Hemmann U, Friedmann H, Röllinghoff M, Gessner A. Intracellular murine IFN-γ mediates virus resistance, expression of oligoadenylate synthetase, and activation of STAT transcription factors. J Immunol. 1996;157:4576–4583. [PubMed] [Google Scholar]
- 54.Wirth J J, Kierszembaum F. Effects of leukotriene C4 on macrophage association with and intracellular fate of Trypanosoma cruzi. Mol Biochem Parasitol. 1985;15:1–10. doi: 10.1016/0166-6851(85)90024-6. [DOI] [PubMed] [Google Scholar]
- 55.Wirth J J, Kierszenbaum F, Sonnenfeld G, Zlotnik A. Enhancing effects of gamma interferon on phagocytic cell association with and killing of Trypanosoma cruzi. Infect Immun. 1985;49:61–66. doi: 10.1128/iai.49.1.61-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]