Abstract
T cells bearing the γδ antigen receptor (γδ T cells) can constitute up to 50% of T cells in the peripheral blood and lymphoid organs of young cattle. We present data showing that γδ T cells are involved in immune responses against Theileria parva. γδ T cells isolated from peripheral blood mononuclear cells (PBMC) of T. parva-naive and -immune cattle proliferated in the presence of fixed or unfixed autologous T. parva-infected lymphoblasts (TpL) and heat-stressed concanavalin A (ConA)-induced blasts (ConA blasts) but not untreated ConA blasts. The specificity of response was further evaluated with a panel of γδ T-cell lines and clones. T-cell reactivity was blocked by GB21A, a monoclonal antibody (MAb) specific for the γδ T-cell receptor, but not by MAbs specific for class I and class II major histocompatibility complex (MHC) molecules. In addition, TpL but not ConA blasts from a variety of MHC-mismatched animals induced proliferation of the γδ T-cell lines and clones. These γδ T cells were found to respond to TpL infected with several different parasite stocks and failed to recognize TpL after elimination of the parasite by the theilericidal drug BW 720C. Assays for cytotoxic activity of γδ T cells sorted from bulk cultures of immune PBMC restimulated several times with autologous TpL demonstrated that effector cells whose specificity is similar to that of proliferating cells are generated. These results suggest that bovine γδ T cells are activated by and lyse T. parva-infected cells by recognizing conserved parasite-induced or parasite-derived antigens in an MHC-unrestricted fashion.
Two types of T cells, distinguished by surface expression of either an αβ or a γδ T-cell receptor (TCR), develop independently as separate lineages in vertebrates (27). They constitute the total pool of peripheral T cells and are effectors of both cell-mediated immunity and T-cell help. The majority of mature αβ T cells express either CD8 or CD4 accessory molecules and recognize peptide antigens (Ags) in association with class I or class II major histocompatibility complex (MHC) molecules, respectively. However, the capacity of γδ T cells to recognize diverse Ags and the restriction elements involved remain unclear (27). The effector function of γδ T cells in immune responses in general, and in infectious diseases in particular, is poorly understood, and no consensus has yet emerged about the overall role of these cells in immune systems of different species. γδ T cells in birds, ruminants, humans, and rodents have been studied (9). Some properties of γδ T cells are remarkably conserved, whereas others differ greatly among species. These cells are very scarce in rodents and primates (∼5% of blood lymphocytes) and are distributed preferentially at different mucosal surfaces (27). In contrast, more recent studies with artiodactyls, an order of animals that includes the ruminants and that diverged from the rodent-primate evolutionary stream around 100 million years ago, show that γδ T cells form a much larger proportion of the peripheral T-cell pool (28). Although some γδ T cells become localized at mucosal surfaces in these species, a large pool of cells recirculates among blood, tissue, and lymph and is widely disseminated throughout peripheral body compartments. The prominence of γδ T cells in ruminants provides an opportunity for a detailed analysis of these lymphocytes (29).
Non-TCR lineage-specific markers for bovine and ovine γδ T cells that detect WC1, a 215-kDa Ag that belongs to the scavenger receptor cysteine-rich protein family, have been described. Although the function of the WC1 molecule is not clear, it has been proposed that it plays a role similar to that of CD4 and CD8 and that it provides a mechanism for tissue-specific homing (29, 57).
Theileria parva is a tick-borne hemoprotozoan parasite that infects cattle and buffalo in large areas of eastern, central, and southern Africa and in cattle causes East Coast fever (ECF). T. parva sporozoites are deposited during tick feeding and rapidly invade lymphocytes, where their development to the schizont stage is associated with uncontrolled proliferation of the infected cell. Synchronous division of the parasite and host cell ensures that daughter cells retain the infection, resulting in clonal expansion of cells initially infected with the parasite. Subsequent invasion of lymphoid and nonlymphoid tissues by infected cells results in organ dysfunction and severe immunopathological changes (30). Susceptible cattle almost invariably die 2 to 4 weeks after sporozoite inoculation. Cattle that recover from infection or are immunized by infection with sporozoites and simultaneous treatment with long-acting tetracyclines are protected from homologous challenge for up to 3.5 years (8). It has been demonstrated that these animals exhibit strong T. parva-specific MHC-restricted CD4+ and CD8+ T-cell responses (2, 7, 24) and further that protection is mediated by CD8+ cytotoxic T lymphocytes (CTL) (38).
A recombinant subunit vaccine for ECF based on the major surface protein of the sporozoite (43) is currently under field evaluation. The vaccine induces high specific antibody titers and results in complete neutralization of a 70% lethal dose challenge in approximately 30% of immunized cattle. A further 40% develop a mild schizont parasitosis, which they clear in the absence of prior immunological exposure to the schizont stage of the parasite. The remainder succumb to severe disease. We are interested in defining the basis of protection after partial neutralization. In areas where ECF is endemic under conditions of heavy challenge, calves become infected at an early age. In many instances, these animals remain healthy in spite of developing significant parasitosis (39). Given these observations and the predominance of γδ T cells in young cattle, we have investigated the responses to primary infection with T. parva in this population, with a view to understanding the likely performance of subunit ECF vaccines in the field.
MATERIALS AND METHODS
Cattle and immunization with T. parva.
Four male and female Boran (Bos indicus) cattle aged between 3 and 12 months were used for the study. Two of these animals (BJ243 and BJ244) were monozygous twins born of dams implanted with split embryos. All cattle were reared indoors under parasite-free conditions and were clinically normal and negative for T. parva antibody at the outset of the study. Animals BK60 and BJ243 were immunized at the age of 4 to 6 months by subcutaneous inoculation of a sporozoite stabilate of T. parva (Muguga) and simultaneous treatment with long-acting tetracyclines as described previously (48). BL38 was similarly infected at the age of 3 months but was treated with a therapeutic dose of a theilericidal drug, buparvaquone (Butalex) (Pitman-Moore Ltd., Harefield, United Kingdom), 12 and 14 days after sporozoite infection. This was intended to reflect the field situation where cattle develop patent parasitosis prior to recovery. The fourth animal (BJ244) was unimmunized.
Isolation of peripheral blood mononuclear cells (PBMC) and the establishment of T. parva-transformed cell lines.
Cattle were bled before, during, and after immunization as indicated in Results. PBMC were isolated from venous blood collected in Alsever’s solution by flotation on Ficoll-Paque (Pharmacia Fine Chemicals, Uppsala, Sweden) as described previously (23). T. parva-infected lymphoblasts (TpL) were established in vitro by infection of PBMC with sporozoites obtained from triturated salivary glands dissected from infected adult Rhipicephalus appendiculatus ticks as described previously (23). When not stated specifically, TpL were established with T. parva Muguga parasites. To determine the capacity of WC1+ γδ T cells to respond to host cells transformed by different parasite stocks, several cell lines were generated by infecting cloned CD4+ T cells with sporozoites of Muguga, Muguga-Uganda recombinant (42), Muguga-Marikebuni recombinant (42), Mariakani, Marikebuni, Uganda, or Lawrencei parasites. PBMC, short-term cultures, and T-cell lines and clones were maintained in HEPES-free RPMI 1640 medium (Sigma Chemical Co., Poole, Dorset, United Kingdom) supplemented with 10% heat-inactivated fetal calf serum (Life Technologies Ltd., Paisley, Scotland), 5 × 10−5 M 2-mercaptoethanol, 2 mM l-glutamine, and 50 μg of gentamicin per ml (complete medium). TpL were maintained in HEPES-buffered complete RPMI 1640 medium.
Generation of γδ T-cell lines and clones.
A series of WC1+ γδ T-cell lines and clones were generated from PBMC of animal BL38 20 days after infection with T. parva. PBMC were stained with monoclonal antibody (MAb) cc15 and sorted by positive selection with a fluorescence-activated cell sorter (FACS; FACStar; Becton Dickinson, Aalst, Belgium) to obtain WC1+ cells at >95% purity. Aliquots of 200 μl of complete medium with a final concentration of 10% T-cell growth factors (TCGF) containing 104 WC1+ T cells and 104 irradiated autologous TpL were dispensed in wells of 96-well round-bottom plates (Costar) and cultured for 7 days. TCGF were supplied as supernatants from 18-h concanavalin A (ConA)-stimulated PBMC. T-cell cultures were restimulated several times before cloning by limiting dilution (LD). Briefly, T cells were plated at a density of 0.5, 1, and 5 cells/well in 96-well round-bottom plates (10 96-well plates for each dilution) in the presence of irradiated autologous PBMC (2 × 104/well) as filler cells and 104 irradiated autologous TpL, essentially as described previously (23). Growing clones were isolated from plates seeded with cell doses that produced growth in fewer than 15% of the wells, and the probability of clonality of the established T-cell clones was calculated according to the Poisson distribution as 98.7% (35). A panel of eight T-cell clones were established from line 6, and clones F6, F8, and F15 are representative of this panel. The lines and clones were maintained by fortnightly restimulation under similar conditions. For phenotypic analysis by flow cytometry, cells were stained 6 days after restimulation, essentially as described previously (34), with MAbs cc15 (anti-WC1; immunoglobulin G2a [IgG2a]) (11), IL-A43 (anti-CD2; IgG2a) (16), IL-A11 (anti-CD4; IgG2a) (5), IL-A105 (anti-CD8; IgG2a) (37), MM1A (anti-CD3; IgG1) (17), and GB21A (anti-γδ TCR; IgG2b) (18, 36).
Proliferation assays and MAb blocking experiments.
Triplicate cocultures of 104 cloned WC1+ T cells with 104 irradiated TpL, heat-stressed ConA blasts (hsConA blasts), or normal ConA blasts were established in wells of 96-well round-bottom culture plates in complete medium containing a final concentration of 10% TCGF. ConA blasts were heat stressed by incubation for 2 h at 42°C before use in the assay. After 4 to 5 days, cultures were pulsed with 0.25 μCi of [125I]iododeoxyuridine (Amersham, Little Chalfont, United Kingdom) for 8 h and monitored on a gamma counter for incorporation of the radioisotope. The level of proliferation was determined as counts per minute. Cultures of responder cells alone were included to provide control counts per minute values. Where indicated in Results, some data are presented as stimulation index (SI) values, which were calculated as test counts per minute (mean counts per minute of triplicate cultures of γδ T cells plus Ag) divided by control counts per minute (mean counts per minute of triplicate cultures of γδ T cells plus medium). The one-tailed Student t test was used to determine the levels of significance between control and experimental cultures.
Blocking experiments involved coculture of WC1+ T cells with stimulator cells in the presence of MAb GB21A (bovine γδ TCR), IL-A43 (anti-CD2), J11 (IgG1 anti-class II MHC) (4), or IL-A88 (IgG2a anti-class I MHC) (53). MAb J11 has been used previously to block MHC class II-restricted T-cell responses (1). To control for their capacity to block in vitro MHC-restricted T-cell responses, the same batches of IL-A21 and IL-A88 were used in parallel to inhibit MHC class II- and class I-restricted T-cell clones, respectively (15a). Observed blocking ranges normally from 75 to 100%. TpL were preincubated with MAbs for 1 h on ice prior to irradiation and distribution into the wells. In some experiments, responder WC1+ T cells were stained similarly before inclusion in culture. MAbs were also present during the culture period. All MAbs were used at a previously determined saturating dilution of 1:100 ascites fluid. Mouse myeloma-derived IgG1 (MOPC 21 [Sigma]), IgG2a (UPC 10 [Sigma]), and IgG2b (MOPC 141) were included in the experiments at final concentrations of 2 μg/well. The experiments shown were repeated at least three times.
LD assay of WC1+ T cells.
LD microcultures were conducted essentially as described previously (35, 50). Briefly, doubling dilutions of sorted WC1+ T cells from BL38 were distributed in 36 replicate wells of microtiter plates along with 104 irradiated autologous TpL per well in the presence of 10% TCGF. After 8 days of culture, proliferation of individual cultures was determined as described above. The frequencies of proliferating cells were calculated according to the Poisson distribution relationship between responder cells seeded per well and the fraction of nonresponding wells. Individual wells were considered positive only if their counts per minute exceeded the mean of 36 control wells containing WC1+ T cells plus TCGF by at least 3 standard deviations (SDs). Frequencies with P < 0.05 were accepted as accurate.
Cytotoxicity assays.
Bulk cultures of PBMC and TpL were established to determine whether γδ T-cell effectors that can lyse TpL are generated. PBMC obtained from animal BL38 4 to 5 weeks after immunization were stimulated twice with irradiated autologous TpL. Cultured cells were FACS sorted after staining with cc15 to obtain WC1+ γδ T cells of up to 98% purity. The cytolytic activity of these sorted cells was measured by a 4-h 111indium oxine-release assay performed in V-bottom 96-well plates (Greiner). Serial dilutions of effector cells were added to duplicate wells to achieve a range of effector-to-target (E/T) ratios. Target cells were labelled with 111indium oxine (Amersham International, Amersham, United Kingdom; code 1N.15P) as described previously (23) and dispensed in the plates at 5 × 103 cells per well. Percent specific lysis was calculated as (experimental release − spontaneous release/maximum release − spontaneous release) × 100. Maximum release was evaluated by subjecting target cells to two cycles of rapid freezing and slow thawing. Spontaneous release was obtained by incubating target cells in assay medium alone.
The capacity of MAbs specific for monomorphic determinants on bovine class I (IL-A88) and bovine class II (IL-A21; IgG2a) (19) or bovine γδ TCR (GB21A) to inhibit cytotoxic activity of the γδ T-cell effectors was assessed as described previously (23) to determine their MHC restriction and role of the TCR. The MAbs were added to labelled target cells and effector cells and incubated at room temperature for 30 min before distribution into the wells. Isotype-matched mouse myeloma proteins were included in parallel control experiments at concentrations of 2 μg/well.
Treatment of TpL with BW 720C.
T. parva schizonts were eliminated from TpL of BL38 to determine if reactivity of WC1+ T cells to TpL is dependent on the presence of the parasite. TpL were cultured at a density of 2 × 106/well in 24-well culture plates (Costar) in the presence of 10% TCGF, 2.5 μg of ConA per ml, and 50 ng of BW 720C per ml, essentially as described previously (7, 20). Cells were monitored at different time points for the presence of schizont RNA to confirm complete elimination of the parasite. Total RNA from ConA blasts, treated TpL, and untreated TpL was purified according to the protocol of Chomczynski and Sacchi (10). ConA blasts and untreated TpL served as negative and positive controls, respectively. First-strand cDNA was synthesized with the Promega reverse transcription system, PCR related, according to the manufacturer’s instructions. One-tenth of the cDNAs was amplified in 50-μl PCR mixtures containing 5 pM (each) different primer combinations and 2 IU of Taq DNA polymerase (Boehringer Mannheim). Amplification was achieved by 30 cycles of incubation for 2 min at 94°C, 2 min at 55°C, and 2 min at 72°C, and 1/10 of the PCR products was resolved on a 1.5% agarose gel and transferred to Hybond-N membranes (Amersham). PCR primers for mammalian β-actin (21), T. parva β-actin (21), and T. parva-derived hsp 70 protein (15) are described in Table 1. After UV cross-linking, the PCR products were hybridized by standard techniques with 32P-labelled probes described in Table 1. After hybridization at 51°C, the blots were washed for 30 min in 4× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate and then for 15 min in 2× SSC–0.1% sodium dodecyl sulfate at 56°C. Autoradiographs were exposed at −70°C, with Kodak XAR-2 films.
TABLE 1.
Oligonucleotide primer sequences employed in RT-PCR analysis of TpL treated with BW 720C
| Gene | Primer
|
Internal hybridization probe
|
||
|---|---|---|---|---|
| Sequence | Expected size (bp) | Sequence | Size | |
| Mammalian β-actin | 5′ AGC CAT CTC CTG CTC GAA GTC A 3′ | 450 | 5′ CTA CCT GAT GAA AAT CCT CAC TGA GCG TGG 3′ | 30 bases |
| 5′ ACC AAC TGG GAC GAC ATG GAG 3′ | ||||
| T. parva β-actin | 5′ ACT GCT TTA GTT GTG GAC AAT GGT TCC GG 3′ | 1,112 | 5′ GTT CAT GCA GAA GAT TCT GGT TGA ACG TGG 3′ | 30 bases |
| 5′ GCA TTT ACG GTG CAC AAT ATT GGG GCC AC 3′ | ||||
| T. parva hsp 70 | 5′ TGT AAC GAG AAG TTC AGA AGC 3′ | 998 | cDNA of hsp 70 | 1.8 kb |
| 5′ AGG GGC TCC GCC TGG GAA CCC 3′ | ||||
RESULTS
Proliferative responses of naive and immune WC1+ γδ T cells to TpL.
WC1+ cells FACS sorted from peripheral blood obtained from the four cattle were cocultured with autologous TpL, hsConA blasts, or ConA blasts for 5 days, and proliferation was measured by radioisotope incorporation. As shown in Table 2, both naive and immune WC1+ T cells responded to TpL, with SIs ranging from 15 to 40. These cells also proliferated in the presence of hsConA blasts, yielding an SI of 6 to 18. When tested on ConA blasts, only marginal activity (SI, <1) was detected. Thus, both naive and immune WC1+ T cells respond to parasitized cells and hsConA blasts but not ConA blasts. To exclude the possibility that TpL secrete soluble factors that can induce nonspecific proliferative responses, fixed TpL were included as stimulator cells (Table 2). Fixation of TpL resulted in a three- to fourfold reduction in proliferation of WC1+ T cells derived from all the animals.
TABLE 2.
Reactivity of WC1+ T cells freshly isolated from naive and immune cattle
| Animal no.a | SI for stimulator cellsb:
|
|||
|---|---|---|---|---|
| TpL | hsConA blasts | ConA blasts | Fixed TpL | |
| BK60 (i) | 15.4 | 6.7 | 0.6 | 5.5 |
| BJ244 (n) | 22.0 | 7.0 | 0.2 | 7.3 |
| BJ243 (i) | 36.3 | 9.9 | 0.0 | 8.6 |
| BL38 (in) | 40.1 | 18.3 | 0.9 | ND |
Cattle were either immune (i), naive (n), or undergoing infection (in) at the time of sampling. WC1+ T cells (104 cells/well) were FACS sorted from PBMC with MAb cc15 and cocultured with irradiated stimulator cells (104 cells/well) for 5 days in the presence of 10% TCGF.
Results are presented as SIs calculated as described in Materials and Methods. The SD of the mean was always <10% and is not given. Results are representative of three replicate experiments. Control counts per minute of freshly isolated WC1+ T cells cultured with 10% TCGF only were <2,300. Proliferative responses of the WC1+ T cells stimulated with TpL, fixed TpL, and hsConA blasts were shown to be significantly different from the proliferative responses of WC1+ T cells cocultured with ConA blasts (P < 0.05) by the Student one-tailed t test. ND, not determined.
LD analyses were carried out to determine the frequencies of WC1+ cells responding to parasitized lymphoblasts in calf BL38 during the course of a primary T. parva infection. Doubling dilutions of sorted WC1+ T cells were stimulated in vitro with a constant number of autologous ConA blasts, hsConA blasts, or TpL, and the frequencies of responding cells were estimated as described in Materials and Methods. As indicated in Table 3, the frequency of cells responding to TpL prior to infection was 1:13,219, while less than 1:50,000 cells responded to hsConA or ConA blasts. On day 8 after infection, the frequency of TpL-reactive cells had increased considerably to 1:579 while those proliferating to ConA blasts and hsConA blasts had increased to 1:5,328 and 1:1,366, respectively. When measured on day 23, after treatment of infection, TpL-responsive cells were detected at 1:2,296 and those responding to hsConA and ConA blasts were detected at 1:6,438 and 1:4,534, respectively. These findings indicate that WC1+ T cells respond to parasitized lymphoblasts in cattle undergoing a patent infection with T. parva and that a proportion also recognizes uninfected ConA blasts.
TABLE 3.
The frequency and specificity of WC1+ T cells isolated from an animal undergoing T. parva infection
| Infection time pointa | Frequency (1/f)b
|
||
|---|---|---|---|
| TpL | hsConA blasts | ConA blasts | |
| Preinfection | 13,219 | <50,000 | <50,000 |
| 7 days | 636 (517–828) | 3,280 (2,682–4,220) | 13,561 (10,417–19,425) |
| 8 days | 579 (474–742) | 1,366 (1,140–1,704) | 5,328 (4,384–6,791) |
| 10 days | 1,244 (961–1,761) | 5,924 (4,429–8,942) | 13,744 (10,352–20,441) |
| 23 days | 2,296 (1,854–3,015) | 6,438 (5,287–8,231) | 4,534 (3,644–5,996) |
Animal BL38 was drug treated on days 12 and 14 to control infection.
Values of precursor frequencies are presented with their 95% confidence limits shown in parentheses.
Proliferative responses of γδ T-cell clones and lines.
To define more precisely the Ag specificity and MHC restriction of γδ T cells responding to TpL, several WC1+ T-cell lines and clones were established from animal BL38 by restimulation with an autologous parasitized cell line. Clones F6, F8, and F15 were derived from line 6. Analysis of cell surface phenotypes of some of these clones and lines by indirect immunofluorescent staining and flow cytometry with various MAbs demonstrated that these cells express the γδ TCR, CD3, CD2, and WC1 but not the CD4 and CD8 markers (Table 4).
TABLE 4.
Phenotypic analysis of a γδ T-cell line and clones derived from an animal infected with T. parvaa
| Surface marker | Line 6 | F6 | F15 |
|---|---|---|---|
| γδ TCR (GB21A) | 99 | 99 | 99 |
| WC1 (cc15) | 99 | 100 | 99 |
| CD3 (MM1A) | 99 | 100 | 100 |
| CD2 (IL-A43) | 96 | 97 | 99 |
| CD4 (IL-A11) | 0 | 0 | 0 |
| CD8 (IL-A105) | 0 | 0 | 0 |
Cells were stained 6 days after restimulation for phenotypic analysis by flow cytometry essentially as described previously (33). The phenotypes of one uncloned (line 6) and two cloned (F6 and F15) γδ T-cell lines are shown. Results are presented as percentages of positive cells after correcting for control staining with secondary reagent alone. The MAbs used are indicated in parentheses.
Parasite specificity was examined by coculturing these cells with autologous TpL, hsConA blasts, or ConA blasts. As reported in Table 5, one line and three clones reacted to TpL over 10 times more intensely than to hsConA or ConA blasts. Further analysis of Ag specificity was conducted with several γδ T-cell clones and a panel of autologous TpL generated by infecting a cloned CD4+ T-cell line with different T. parva strains. Results of a representative experiment are shown in Table 6. All infected cell lines were capable of stimulating reactivity in the clones, although some were more efficient than others.
TABLE 5.
Parasite specificity of the proliferative response of cloned and uncloned γδ T-cell linesa
| Responder cell population | Result for stimulator cells
|
||
|---|---|---|---|
| TpL | hsConA blasts | ConA blasts | |
| Line 6 | 6.8 | 0.9 | 0.6 |
| clone F6 | 25.3 | 1.6 | 1.7 |
| clone F15 | 35.8 | 2.1 | 1.9 |
| clone A8 | 6.4 | 0.9 | 0.7 |
Results are shown as SIs calculated as described in Materials and Methods. The SD of the mean was always <10% and is not given. The results are representative of three separate experiments. The γδ T-cell line and clones (104 cells/well) were cultured for 5 days with irradiated stimulator cells (104 cells/well) in the presence of 10% TCGF. Control cultures of T cells cultured in the presence of 10% TCGF only showed <2,050 cpm. Proliferative responses of T cells cultured with TpL were shown to be significantly different from proliferative responses of T cells cultured with ConA blasts or hsConA blasts (P < 0.05) by the Student one-tailed t test.
TABLE 6.
Parasite strain specificity of WC1+ T-cell line and clonesa
| Parasite stock | Result for responder cell line
|
||
|---|---|---|---|
| Line 6 | F6 | F15 | |
| Muguga-Uganda recombinant | 11.9 | 59.0 | 36.2 |
| Lawrencei | 14.3 | 94.1 | 29.6 |
| Muguga | 12.1 | 50.4 | 27.9 |
| Muguga-Marikebuni recombinant | 8.5 | 39.0 | 24.7 |
| Mariakani | 8.6 | 29.5 | 20.9 |
| Uganda | 7.7 | 29.4 | 18.9 |
| Marikebuni | 6.8 | 25.2 | 9.7 |
Line 6 and clones F6 and F15 were cocultured with autologous stimulator cells infected with a range of parasite stocks to determine their capacities to respond to multiple stocks. Results of one representative experiment are presented as SIs calculated as described in Materials and Methods. The SD of the mean was always <12% and is not given. In cultures of the γδ T cells stimulated with autologous ConA blasts, SI values ranging between 0.9 and 1.5 could be measured. Control cultures of T cells cultured in the presence of TCGF only showed <1,890 cpm. Proliferative responses of the γδ T cells stimulated by cells infected with a range of parasite stocks were shown to be significantly different from the proliferative responses of T cells cultured with ConA blasts (P < 0.05) by the Student one-tailed t test.
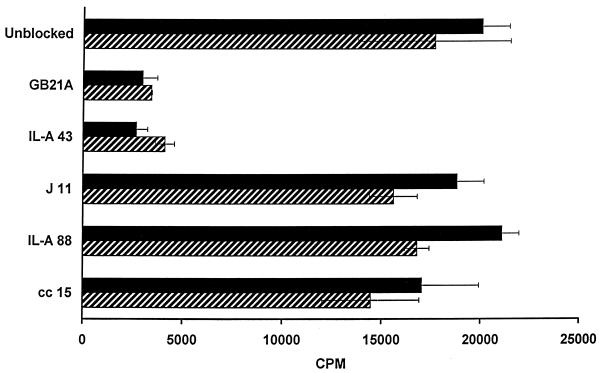
MHC restriction and cognate requirements were evaluated with mismatched TpL and by blocking with MAbs specific for a variety of bovine cell surface markers. A WC1+ T-cell line cocultured with autologous TpL and 11 TpL cell lines derived from randomly selected cattle responded to all of the parasitized cells. In contrast, corresponding cultures incorporating ConA blasts yielded only marginal activity (Table 7). In parallel experiments, two clones were cocultured with autologous TpL in the presence of MAbs specific for the adhesion molecule CD2, WC1, γδ TCR, and class I or class II MHC molecules. Cultures in which no MAb or isotype control MAb was added served as controls. As illustrated in Fig. 1, cultures incorporating MAbs specific for CD2 and γδ TCR failed to proliferate to TpL while the other cultures responded similarly to control cultures. Taken together, these findings indicate that the WC1+ γδ T cells recognize their ligand via the TCR and that CD2 may be involved in contact with the Ag-presenting cells.
TABLE 7.
The reactivity of an uncloned γδ T-cell line to TpL is MHC unrestricteda
| Animal source of stimulator cells | Result for stimulator cells:
|
|
|---|---|---|
| ConA | TpL | |
| BL38 | 0.9 | 9.7 |
| BL384 | 0.8 | 7.5 |
| BL383 | 0.7 | 5.8 |
| BL382 | 0.7 | 3.1 |
| BL380 | 0.7 | 6.4 |
| BL379 | 0.6 | 3.0 |
| BL378 | 1.1 | 7.9 |
| BL377 | 1.0 | 8.5 |
| BL376 | 0.9 | 5.1 |
| G169 | 2.1 | 14 |
| B641 | 2.2 | 6.5 |
| E98 | 1.9 | 12.4 |
Line 6 (104 cells/well) was stimulated with irradiated, autologous, and allogeneic TpL and the corresponding ConA blasts (104 cells/well) derived from randomly chosen animals. Shown are SIs calculated as described in Materials and Methods. The SD of the mean was always <10% and is not given. Control cultures of line 6 with 10% TCGF alone yielded <1,500 cpm. Proliferative responses of line 6 cultured in the presence of autologous or allogeneic TpL were shown to be significantly different from the proliferative responses of line 6 cocultured with ConA blasts (P < 0.05) by the Student one-tailed t test. Clones F6 and F8 responded similarly and are not shown.
FIG. 1.
Inhibition of proliferation of γδ T cells by a cell surface Ag-specific MAb. Two clones, F6 (black bars) and F15 (hatched bars), were cultured with autologous TpL in the absence or presence of MAbs to assess whether these MAbs can block reactivity of γδ T cells to TpL. The MAbs included GB21A (bovine γδ TCR), IL-A43 (CD2), J11 (MHC class II), IL-A88 (MHC class I), and cc15 (WC1). Bars represent mean counts per minute, and error bars show SDs.
Cytolytic responses of γδ T cells.
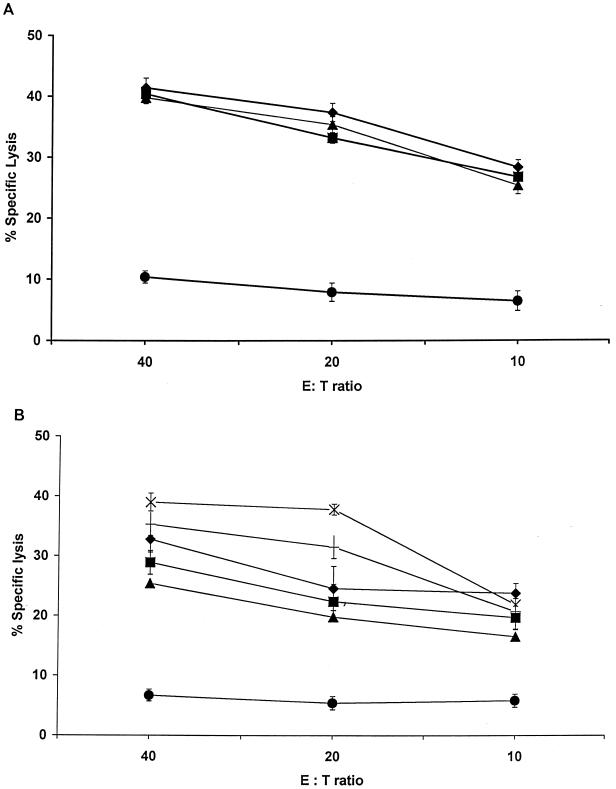
The capacity of responding γδ T cells to lyse parasitized target cells was evaluated. PBMC were stimulated twice with autologous TpL as described in Materials and Methods to generate cytotoxic effector cells. WC1+ γδ T cells were FACS sorted from these cultures and tested directly for their capacity to lyse autologous TpL targets, in the presence or absence of MAbs specific for the γδ TCR or MHC class I or class II molecules. Autologous uninfected ConA blasts were also included as targets. As shown in Fig. 2A, effector γδ T cells were capable of killing TpL in the absence of MAbs (over 40% specific lysis at an E/T ratio of 40:1) but not uninfected targets (less than 5% at an E/T ratio of 40:1). These effectors lysed TpL to the same extent when assayed in the presence of an anti-MHC class I or class II MAb. In contrast, assays performed in the presence of an anti-TCR MAb revealed a fourfold reduction in the level of lysis. Parallel assays were conducted with autologous cells infected with several different T. parva strains as targets. As illustrated in Fig. 2B, effector γδ T cells lysed all of the infected targets to varying degrees. These findings indicate that effector γδ T cells generated in bulk cocultures of immune PBMC with autologous TpL exhibit a non-MHC class I or class II-restricted parasite-specific cytolytic activity extending to targets infected with a variety of parasite strains.
FIG. 2.
(A) The cytolytic activity of γδ T cells is not blocked by MAbs specific for bovine MHC. Effector γδ T cells, generated as described in the text, were assayed for lysis against autologous TpL in the absence (⧫) or presence of MAbs IL-A88 (■), IL-A21 (▴), and GB21A (●) to test whether their cytolytic response to TpL is MHC restricted. The data are presented as the mean (with SD) percent lysis at varying E/T ratios. (B) T. parva-specific γδ T cells lyse targets infected with heterologous parasite populations. Effector γδ T cells were tested for their capacity to kill autologous TpL parasitized with different T. parva stocks including Uganda (×), Muguga (†), Mariakani (⧫), Marikebuni (■), and Lawrencei (▴). The lytic activity was also assayed against autologous uninfected ConA blasts (●). Results are presented as the mean (with SD) percent lysis at varying E/T ratios.
Removal of parasite renders TpL nonstimulatory for γδ T cells.
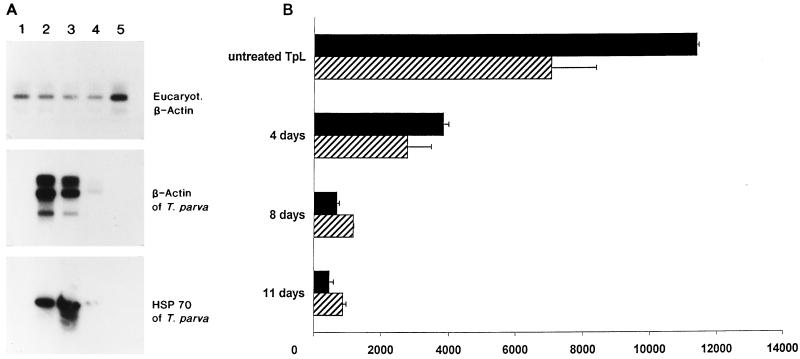
Experiments to determine whether the reactivity of γδ T cells to TpL was dependent on the presence of the parasite utilized stimulator TpL preincubated with a curing agent for varying periods of time. TpL were either untreated or treated for 4, 8, and 11 days and subjected to RT-PCR to verify the disappearance of the parasite. As shown in Fig. 3A, parasite-specific PCR products were only weakly detected with specific probes after 8 days of curing and were completely absent after 11 days of treatment. Coculture of two T-cell clones with the respective TpL revealed that treatment of TpL for 4 days resulted in a threefold reduction in stimulatory capacity and that further treatment for 8 and 11 days completely abrogated stimulation (Fig. 3B). Since loss of the capacity to stimulate coincides with the disappearance of the parasite from TpL, these findings provide strong evidence that γδ T cells recognize a parasite-derived or parasite-induced ligand on TpL.
FIG. 3.
(A) T. parva mRNA is undetectable following treatment of TpL with BW 720C. TpL were cured for 0 (lane 2), 4 (lane 3), 8 (lane 4), and 11 (lane 5) days and subjected to RT-PCR with primers specific for mammalian β-actin, T. parva β-actin, and hsp 70. In lane 1, PCR products derived from ConA blasts were loaded as negative controls. The amplicons were resolved on an agarose gel, transferred to a nylon membrane, and hybridized with 32P-labelled probes as described in the text. (B) Elimination of T. parva by BW 720C abolishes the stimulatory capacity of TpL for γδ T cells. TpL were either untreated or preincubated with the drug for 4, 8, or 11 days before coculture with clone F6 (black bars) and clone F15 (hatched bars) to induce proliferation as described in Materials and Methods. Bars present mean counts per minute corrected for background activity; error bars show SDs.
DISCUSSION
A significant outcome of this study was the observation that bovine γδ T cells recognize T. parva-infected cells and respond to them during the course of primary infection. Although a large body of evidence is consistent with class I and class II MHC-restricted parasite-specific T cells being key mechanisms in immune cattle, it has never been possible to demonstrate these responses during primary infection.
Freshly isolated γδ T cells from both naive and immune animals proliferated in the presence of TpL, and this response was reduced three- to fourfold by fixation of stimulator cells. These cells also reacted to ConA blasts but only after heat shock. This is consistent with at least a portion of the reactivity being due to heat shock proteins; metabolic labelling experiments have confirmed that heat stress induces hsp70 and hsp90 proteins in ConA blasts (data not shown).
The association of γδ T cells with many different infections, particularly those caused by intracellular pathogens, has led to the suggestion that γδ T cells constitute an innate surveillance system that acts as a first line of defense against infectious diseases (31). Therefore, we sought to explore whether bovine γδ T cells respond to cells infected with T. parva during the course of a primary infection. LD analysis revealed a frequency of 1:13,219 γδ T cells reactive to TpL before inoculation of sporozoites. This had increased 20-fold as early as day 7 after infection, at which time class I MHC-restricted CD8+ CTL responses are known to be undetectable (41). Concurrently, frequencies of γδ T cells activated by hsConA blasts and ConA blasts increased by up to 50-fold. The population of autoreactive γδ T cells responding to untreated ConA blasts was not observed in healthy naive or recovered immune animals (Table 2). These cells may represent a component of the γδ T-cell response to T. parva that cross-reacts with normally expressed autoantigen(s). A proportion of the γδ T-cell population responding to autologous ConA blasts appears to recognize stress proteins, as suggested by the slightly higher frequencies of cells stimulated with hsConA blasts. This finding is consistent with previous reports of autoreactive γδ T cells in mice and humans particularly after infections (56). Taken together, these results support the concept of γδ T cells acting as a first line of defense against T. parva infections in young cattle.
The precise role of γδ T cells in infectious and parasitic disease is still controversial. With the exception of mycobacterial heat shock proteins and phosphate bound to alkyl, carbohydrate, or nucleotide residues, the nature of the Ags priming γδ T cells and the restricting elements involved are poorly defined (33). In humans, responses of peripheral T cells from nonexposed donors to malarial Ags were reported to involve γδ T cells (25). Moreover, in humans infected with malaria there is evidence that the number and proportion of γδ T cells are increased in both peripheral blood and spleen (45), although the Ags responsible are unknown. A similar effect was observed in PBMC of healthy tuberculin skin test-negative donors, with 7 days of exposure to mycobacterial lysates resulting in selective expansion of γδ T cells in vitro (46). For cattle, it has been reported that γδ T cells isolated from peripheral blood are stimulated by a cell surface molecule constitutively expressed by mononuclear phagocytes in vivo (44). It has also been observed that γδ T cells expand in cell lines derived from Babesia bovis-immune cattle by stimulation with merozoite fractions (6). The phenotypic characterization of these γδ T-cell lines showed that a subpopulation of cells expressed CD2. However, it is not shown whether these cells coexpress CD2 and WC1. Furthermore, it appeared that the γδ T-cell lines and clones might recognize altered self-proteins, stress proteins, or other autoantigens (6).
In our experiments, bovine γδ T cells did not expand selectively after in vitro stimulation with TpL, although they did respond when isolated from peripheral blood of both immune and naive animals. These observations are consistent with reports that freshly isolated WC1+ γδ T cells derived from Theileria annulata naive cattle proliferated in response to cells transformed by T. annulata. Although these WC1+ cells also responded to fixed T. annulata-infected cells (12), fixation of TpL resulted in a significant reduction in the proliferation of T. parva-reactive γδ T cells (Table 2). While this may reflect, in part, the effect of soluble factors, on the other hand, it is conceivable that a ligand(s) on the cell surface of TpL is modified by fixation. The fact that inclusion of anti-TCR and anti-CD2 MAbs in culture abrogated proliferation is consistent with the latter possibility.
To further characterize the TpL reactivity of bovine WC1+ γδ T cells, we established a panel of γδ T-cell clones and lines from animal BL38. These cells proliferated in the presence of autologous and allogeneic T. parva-transformed cells but not in the presence of the corresponding ConA blasts (Tables 5 and 7). TpL, but not ConA blasts, stimulated proliferation when present in numbers ranging from 105 to 625 cells per well, with highest responses being observed with 1 × 104 to 2 × 104 stimulator cells per well (data not shown). Our data indicate for the first time that CD2 can be expressed on CD4− CD8− WC1+ γδ T cells under stimulation with T. parva. This phenotype was consistently expressed by all γδ T-cell lines and clones which proliferated specifically after stimulation with TpL (Table 4 and data not shown). Whether CD2 is expressed already in vivo by a distinct subset of γδ T cells or whether it is induced in vitro by cocultivation with TpL awaits further investigations. This phenotype is in contrast to data published previously which show that CD4− CD8− WC1+ γδ T cells do not express CD2 (11, 18). However, CD4− CD8− WC1+ γδ T cells have the potential to express CD2 as demonstrated by γδ T-cell lines immortalized with T. parva (3).
The response to TpL was dependent on the parasite, since its removal by culture in the presence of BW 720C abrogated reactivity (Fig. 3). These results suggest that either the ligand(s) responsible is encoded by the parasite or its expression is induced by the infection. Transformation of T and B lymphocytes by T. parva may induce surface expression of stress proteins that can be recognized by γδ T cells, as has been described for other systems (22, 55). Experiments to determine whether MAbs specific for heat shock proteins can inhibit the proliferative response of γδ T cells to TpL may clarify this issue. In any event, the determinant(s) recognized by bovine γδ T cells on TpL is clearly conserved, with reactivity extending to cells infected with a broad range of genetically and immunologically distinct isolates (13, 51) (Table 6 and Fig. 2B). The question of the nature and origin of the ligand(s) stimulating the γδ T-cell clones was addressed in preliminary experiments. Schizonts purified from TpL were included in cultures of γδ T cells with and without autologous monocytes as Ag-presenting cells. Unfortunately, the clones were not stimulated by these conditions (data not shown).
Recognition of the ligand(s) does not appear to be restricted by classical class I and II MHC molecules. This is consistent with observations in other systems where only a few examples of MHC-restricted γδ T-cell responses have emerged (26, 32). It is conceivable that antigenic determinants expressed by T. parva or induced by the transformation process gain access to the surface of the infected cells as processed peptides in association with nonpolymorphic MHC molecules, which have been studied in detail for mouse and human systems, but not for cattle (47, 54). Alternatively, human γδ T cells have been reported to recognize nonpeptide ligands of mycobacteria such as synthetic alkyl phosphates, particularly monoethyl phosphate (40, 49). These Ags are conserved between different mycobacterial isolates although expressed to varying degrees (14).
There is strong evidence that the protective immune response to T. parva is based on elimination of infected cells by parasite-specific class I MHC-restricted CTL (38). In addition, class II MHC-restricted CD4+ T cells with helper and cytolytic activity can be detected after immunization of cattle with T. parva (1, 2, 7, 52). We have now provided evidence that cytotoxic γδ T cells might also directly contribute to protection in young cattle. γδ T cells sorted from cultures of immune PBMC stimulated with TpL specifically lysed parasitized cells but not ConA blasts. The observed killing could not be blocked by MAbs directed against MHC class I or class II molecules and did not distinguish between different parasite stocks (Fig. 2). We were unable to establish cytotoxic γδ T-cell clones, since the lines acquired NK-like nonspecific killing activity after prolonged culture in the presence of TCGF. This may reflect an inability of these cells to adopt a memory phenotype capable of maintaining specificity over multiple restimulations.
In summary, we provide evidence that bovine γδ T cells participate in the early phase of an immune response against T. parva, an intracellular protozoan parasite. These responses are MHC unrestricted and cross-reactive between a broad range of different parasite stocks. This T-cell population therefore shares a number of functional features with human and mouse γδ T cells. These observations may have relevance to the apparent resistance of young calves to ECF under conditions of high endemic challenge. Further, they provide a possible explanation for the ability of a proportion of cattle immunized with prototype subunit-neutralizing vaccine to clear breakthrough infections. We have developed a system based on defined γδ T-cell clones and stimulator cells that will enable an investigation of additional aspects of the biology of bovine γδ T cells. These findings may have implications for a rational design of an effective subunit vaccine against ECF and probably other infectious diseases.
ACKNOWLEDGMENTS
We thank Daniel Ngugi, James Magondu, and Peter Macheru for their valuable technical support. We are also indebted to Inga Melchers for calculation of LD precursor frequencies.
Footnotes
This is ILRI publication no. 98026.
REFERENCES
- 1.Baldwin C L, Goddeeris B M, Morrison W I. Bovine helper T-cell clones specific for lymphocytes infected with Theileria parva (Muguga) Parasite Immunol. 1987;9:499–513. doi: 10.1111/j.1365-3024.1987.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin C L, Iams K P, Brown W C, Grab D J. Theileria parva: CD4+ helper and cytotoxic T-cell clones react with a schizont-derived antigen associated with the surface of Theileria parva-infected lymphocytes. Exp Parasitol. 1992;75:19–30. doi: 10.1016/0014-4894(92)90118-t. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin C L, Black S J, Brown W C, Conrad P A, Goddeeris B M, Kinuthia S W, Lalor P A, MacHugh N D, Morrison W I, Morzaria S P, Naessens J, Newson J. Bovine T cells, B cells, and null cells are transformed by the protozoan parasite Theileria parva. Infect Immun. 1988;56:462–467. doi: 10.1128/iai.56.2.462-467.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldwin C L, Morrison I W, Naessens J. Differentiation antigens and functional characteristics of bovine leukocytes. In: Trnka Z, Miyasaka M, editors. Comparative aspects of differentiation antigens in lympho-haematopoietic tissues. New York, N.Y: Marcel Dekker, Inc.; 1988. pp. 455–465. [Google Scholar]
- 5.Baldwin C L, Teale A J, Naessens J, Goddeeris B M, MacHugh N D, Morrison W I. Characterization of a subset of bovine T lymphocytes that express BoT4 by monoclonal antibodies and function: similarity to lymphocytes defined by human T4 and murine L3T4. J Immunol. 1986;155:4385–4391. [PubMed] [Google Scholar]
- 6.Brown W C, Davis W C, Choi S H, Dobbelaere D A E, Splitter G A. Functional and phenotypic characterization of WC1+ γ/δ T cells isolated from Babesia bovis-stimulated T cell lines. Cell Immunol. 1994;153:9–27. doi: 10.1006/cimm.1994.1002. [DOI] [PubMed] [Google Scholar]
- 7.Brown W C, Sugimoto C, Grab D J. Theileria parva: bovine helper T cell clones specific for both infected lymphocytes and schizont membrane antigens. Exp Parasitol. 1989;69:234–248. doi: 10.1016/0014-4894(89)90070-2. [DOI] [PubMed] [Google Scholar]
- 8.Burridge M J, Morzaria S P, Cunningham M P, Brown C G D. Duration of immunity to East Coast fever (Theileria parva infection of cattle) Parasitology. 1972;64:511–515. doi: 10.1017/s0031182000045571. [DOI] [PubMed] [Google Scholar]
- 9.Chien Y-H, Jores R, Crowley M P. Recognition by γδ T cells. Annu Rev Immunol. 1996;14:511–532. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- 10.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1988;162:156–167. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 11.Clevers H, MacHugh N D, Bensaid A, Dunlap S, Baldwin C L, Kaushal A, Iams K, Howard C J, Morrison W I. Identification of a bovine surface antigen uniquely expressed on CD4−CD8− T cell receptor γ/δ+ T lymphocytes. Eur J Immunol. 1990;20:809–817. doi: 10.1002/eji.1830200415. [DOI] [PubMed] [Google Scholar]
- 12.Collins R A, Sopp P, Gelder K I, Morrison I W, Howard C J. Bovine gamma/delta TcR+ T lymphocytes are stimulated to proliferate by autologous Theileria annulata-infected cells in the presence of interleukin-2. Scand J Immunol. 1996;44:444–452. doi: 10.1046/j.1365-3083.1996.d01-332.x. [DOI] [PubMed] [Google Scholar]
- 13.Conrad P A, Iams K, Brown W C, Sohanpal B, Ole-Moi Yoi O K. DNA probes detect genomic diversity in Theileria parva stocks. Mol Biochem Parasitol. 1987;25:213–226. doi: 10.1016/0166-6851(87)90085-5. [DOI] [PubMed] [Google Scholar]
- 14.Constant P, Poquet Y, Peyrat M A, Davodeau F, Bonneville M, Fournie J J. The antituberculous Mycobacterium bovis BCG vaccine is an attenuated mycobacterial producer of phosphorylated nonpeptidic antigens for human γδ T cells. Infect Immun. 1995;63:4628–4633. doi: 10.1128/iai.63.12.4628-4633.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daubenberger C A, Heussler V T, Gobright E, Wijngaard P L J, Clevers H C, Wells C, Musoke A J, McKeever D J. Molecular characterisation of a cognate 70 kDa heat shock protein of the protozoan Theileria parva. Mol Biochem Parasitol. 1997;85:265–269. doi: 10.1016/s0166-6851(97)02831-4. [DOI] [PubMed] [Google Scholar]
- 15a.Daubenberger, C. A., and E. L. N. Taracha. Unpublished observation.
- 16.Davis W C, Ellis J A, MacHugh N D, Baldwin C L. Bovine pan T-cell monoclonal antibodies reactive with a molecule similar to CD2. Immunology. 1992;63:165–167. [PMC free article] [PubMed] [Google Scholar]
- 17.Davis W C, MacHugh N D, Park Y H, Hamilton M J, Wyatt C R. Identification of a monoclonal antibody reactive with the bovine orthologue of CD3 (BoCD3) Vet Immunol Immunopathol. 1993;39:85–91. doi: 10.1016/0165-2427(93)90167-3. [DOI] [PubMed] [Google Scholar]
- 18.Davis W C, Brown W C, Hamilton M J, Wyatt C R, Orden J A, Khalid A M, Naessens J. Analysis of monoclonal antibodies specific for the gamma delta TcR. Vet Immunol Immunopathol. 1996;52:275–283. doi: 10.1016/0165-2427(96)05578-x. [DOI] [PubMed] [Google Scholar]
- 19.DeMartini J C, MacHugh N D, Naessens J, Teale A J. Differential in vitro and in vivo expression of MHC class II antigens in bovine lymphocytes infected by Theileria parva. Vet Immunol Immunopathol. 1993;35:253–273. doi: 10.1016/0165-2427(93)90038-6. [DOI] [PubMed] [Google Scholar]
- 20.Dobbelaere D A E, Coquerelle T M, Roditi I J, Eichhorn M, Williams R O. Theileria parva infection induces autocrine growth of bovine lymphocytes. Proc Natl Acad Sci USA. 1988;85:4730–4734. doi: 10.1073/pnas.85.13.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehrfeld A Y B. Isolierung und Charakterisierung des Aktin-Gens des intrazellulären Parasiten Theileria parva. Ph.D. thesis. Karlsruhe, Germany: University of Karlsruhe; 1990. [Google Scholar]
- 22.Fisch P, Malkovsky M, Kovats S, Sturm E, Braakman E, Klein B S, Voss S D, Morrissey L W, DeMars R, Welch W J, Bolhuis L H R, Sondel P M. Recognition by human Vγ9/Vδ2 T cells of a GroEL homolog on Daudi Burkitt’s lymphoma cells. Science. 1990;250:1269–1273. doi: 10.1126/science.1978758. [DOI] [PubMed] [Google Scholar]
- 23.Goddeeris B M, Morrison W I. Techniques for the generation, cloning and characterization of bovine cytotoxic T cells specific for the protozoan Theileria parva. J Tissue Cult Methods. 1988;11:101–110. [Google Scholar]
- 24.Goddeeris B M, Morrison W I, Teale A J. Generation of bovine cytotoxic cell lines specific for cells infected with the protozoan parasite Theileria parva and restricted by products of the major histocompatibility complex. Eur J Immunol. 1986;16:1243–1249. doi: 10.1002/eji.1830161010. [DOI] [PubMed] [Google Scholar]
- 25.Goodier M, Fey P, Eichmann K, Langhorne J. Human peripheral blood γδ T cells respond to antigens of Plasmodium falciparum. Int Immunol. 1992;4:33–41. doi: 10.1093/intimm/4.1.33. [DOI] [PubMed] [Google Scholar]
- 26.Guo Y, Ziegler H K, Safley S A, Niesel D W, Vaidya S, Klimpel G R. Human T-cell recognition of Listeria monocytogenes: recognition of listeriolysin O by TcRαβ+ and TcRγδ+ T cells. Infect Immun. 1995;63:2288–2294. doi: 10.1128/iai.63.6.2288-2294.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 28.Hein W R, Dudler L. Divergent evolution of T cell repertoires: extensive diversity and developmentally regulated expression of the sheep γδ T cell receptor. EMBO J. 1993;12:715–724. doi: 10.1002/j.1460-2075.1993.tb05705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hein W R, Mackay C R. Prominence of γδ T cells in the ruminant immune system. Immunol Today. 1991;12:30–34. doi: 10.1016/0167-5699(91)90109-7. [DOI] [PubMed] [Google Scholar]
- 30.Irvin A D, Morrison W I. Immunopathology, immunology and immunoprophylaxis of Theileria infections. In: Soulsby E J L, editor. Immune responses in parasitic infections. Boca Raton, Fla: CRC Press, Inc.; 1987. pp. 223–274. [Google Scholar]
- 31.Kaufmann S H E. Gamma/delta and other unconventional T lymphocytes: what do they see and what do they do? Proc Natl Acad Sci USA. 1996;93:2272–2279. doi: 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozbor D, Trinchieri G, Monos D S, Isobe M, Russo G, Haney J A, Zmijewski C, Croce C E. Human TCR-gamma+/delta+, CD8+ T lymphocytes recognize tetanus toxoid in an MHC-restricted fashion. J Exp Med. 1989;169:1847–1851. doi: 10.1084/jem.169.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kronenberg M. Antigens recognized by γδ T cells. Curr Opin Immunol. 1996;6:64–71. doi: 10.1016/0952-7915(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 34.Lalor P A, Morrison W I, Goddeeris B M, Jack R M, Black S J. Monoclonal antibodies identify phenotypically and functionally distinct cell types in the bovine lymphoid system. Vet Immunol Immunopathol. 1986;13:121–127. doi: 10.1016/0165-2427(86)90054-1. [DOI] [PubMed] [Google Scholar]
- 35.Lefkovitz I, Waldman H. Limiting dilution analysis of cells of the immune system. I. The clonal basis of the immune response. Immunol Today. 1984;5:265–268. doi: 10.1016/0167-5699(84)90137-3. [DOI] [PubMed] [Google Scholar]
- 36.MacHugh N D, Mburu J K, Carol M J, Wyatt C R, Orden J A, Davis W C. Identification of two distinct subsets of bovine γδ T cells with unique cell surface phenotype and tissue distribution. Immunology. 1997;92:340–347. doi: 10.1046/j.1365-2567.1997.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacHugh N D, Taracha E L, Toye P G. Reactivity of workshop antibodies on L cell and COS cell transfectants expressing bovine CD antigens. Vet Immunol Immunopathol. 1993;39:61–67. doi: 10.1016/0165-2427(93)90164-y. [DOI] [PubMed] [Google Scholar]
- 38.McKeever D J, Taracha E L N, Innes E A, MacHugh N D, Awino E, Goddeeris B M, Morrison W I. Adoptive transfer of immunity to Theileria parva in the CD8+ fraction of responding efferent lymph. Proc Natl Acad Sci USA. 1994;91:1959–1963. doi: 10.1073/pnas.91.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moll G, Lohding A, Young A S, Leitch B L. Epidemiology of theileriosis in calves in an endemic area of Kenya. Vet Parasitol. 1986;19:255–273. doi: 10.1016/0304-4017(86)90073-7. [DOI] [PubMed] [Google Scholar]
- 40.Morita C T, Beckman E M, Bukowski J F, Tanaka Y, Band H, Bloom B R, Golan D E, Brenner M B. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 41.Morrison W I, Taracha E L N, McKeever D J. Contribution of T-cell responses to immunity and pathogenesis in infections with Theileria parva. Parasitol Today. 1995;11:14–17. [Google Scholar]
- 42.Morzaria S P, Dolan T T, Norval R A I, Bishop R P, Spooner P R. Generation and characterization of cloned Theileria parva parasites. Parasitology. 1995;111:39–49. doi: 10.1017/s0031182000064581. [DOI] [PubMed] [Google Scholar]
- 43.Musoke A J, Morzaria S P, Nkonge C, Jones E, Nene V. A recombinant sporozoite surface antigen of Theileria parva induces protection in cattle. Proc Natl Acad Sci USA. 1992;89:514–518. doi: 10.1073/pnas.89.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okragly A J, Hanby-Flarida M, Mann D, Baldwin C L. Bovine gamma/delta T-cell proliferation is associated with self-derived molecules constitutively expressed in vivo on mononuclear phagocytes. Immunology. 1996;87:71–79. [PMC free article] [PubMed] [Google Scholar]
- 45.Perera M K, Carter R, Goonewardene R, Mendis K N. Transient increase in circulating γ/δ T cells during Plasmodium vivax malarial paroxysms. J Exp Med. 1994;179:311–315. doi: 10.1084/jem.179.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfeffer K, Schoel B, Gulle H, Kaufmann S H E, Wagner H. Primary responses of human T cells to mycobacteria: a frequent set of γ/δ T cells are stimulated by protease-resistant ligands. Eur J Immunol. 1990;20:1175–1179. doi: 10.1002/eji.1830200534. [DOI] [PubMed] [Google Scholar]
- 47.Porcelli S A, Morita C T, Modlin R L. T-cell recognition of non-peptide antigens. Curr Opin Immunol. 1996;8:510–516. doi: 10.1016/s0952-7915(96)80039-2. [DOI] [PubMed] [Google Scholar]
- 48.Radley D E. Infection and treatment method of immunisation against theileriosis. In: Irvin A D, Cunningham M P, Young A S, editors. Advances in the control of theileriosis. The Hague, The Netherlands: Martinus Nijhoff; 1981. pp. 227–237. [Google Scholar]
- 49.Tanaka Y, Sano S, Nieves E, DeLibero G, Rosa D, Modlin R L, Brenner M B, Bloom B R, Morita C T. Nonpeptide ligands for human γδ T cells. Proc Natl Acad Sci USA. 1994;91:8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taracha E L N, Goddeeris B M, Scott J R, Morrison W I. Standardisation of a technique for analysing the frequency of parasite-specific cytotoxic T lymphocyte precursors in cattle immunised with Theileria parva. Parasite Immunol. 1992;42:143–154. doi: 10.1111/j.1365-3024.1992.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 51.Taracha E L N, Goddeeris B M, Morzaria S P, Morrison W I. Parasite strain specificity of precursor cytotoxic T cells in individual animals correlates with cross-protection in cattle challenged with Theileria parva. Infect Immun. 1995;63:1258–1262. doi: 10.1128/iai.63.4.1258-1262.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taracha E L N, Awino E, McKeever D J. Distinct CD4+ T cell helper requirements in Theileria parva-immune and naive bovine CTL precursors. J Immunol. 1997;159:4539–4545. [PubMed] [Google Scholar]
- 53.Toye P G, MacHugh N D, Bensaid A, Alberti S, Teale A J, Morrison I W. Transfection into mouse L cells of genes encoding two serologically and functionally distinct bovine class I MHC molecules from a MHC-homozygous animal: evidence for a second class I locus in cattle. Immunology. 1990;70:20–26. [PMC free article] [PubMed] [Google Scholar]
- 54.Vidovic D, Roglic M, Mckune K, Guerder S, Mackay C, Dembic Z. Qa-1 restricted recognition of foreign antigen by gamma/delta T-cell hybridoma. Nature. 1989;340:646–650. doi: 10.1038/340646a0. [DOI] [PubMed] [Google Scholar]
- 55.Wei Y, Zhao X, Kariya Y, Fukata H, Teshigawara K, Uchida A. Induction of autologous tumor killing by heat treatment of fresh human tumor cells: involvement of gamma delta T cells and heat shock protein 70. Cancer Res. 1996;56:1104–1110. [PubMed] [Google Scholar]
- 56.Wen L, Hayday A C. Gamma delta T-cell help in responses to pathogens and in the development of systemic autoimmunity. Immunol Res. 1997;16:229–241. doi: 10.1007/BF02786392. [DOI] [PubMed] [Google Scholar]
- 57.Wijngaard P L J, MacHugh N D, Metzelaar M J, Romberg S, Bensaid A, Pepin L, Davis W C, Clevers H. Members of the novel WC1 gene family are differentially expressed on subsets of bovine CD4(−)CD8(−) gamma-delta T lymphocytes. J Immunol. 1994;152:3476–3482. [PubMed] [Google Scholar]