Abstract
Caspase-7 (C7), a cysteine protease involved in apoptosis, is a valuable drug target for its role in human diseases (e.g. Parkinson’s, Alzheimer’s, sepsis). The C7 allosteric site has great potential for small molecule targeting, but numerous drug discovery efforts have identified precious few allosteric inhibitors. Here we present the first selective, drug-like inhibitor of C7 along with several other improved inhibitors based on our previous fragment hit. We also provide a rational basis for the impact of allosteric binding on the C7 catalytic cycle using an integrated approach including X-ray crystallography, stopped-flow kinetics, and molecular dynamics simulations. Our findings suggest allosteric binding disrupts C7 pre-acylation via neutralization of the catalytic dyad, displacement of substrate from the oxyanion hole, and altered dynamics of substrate binding loops. This work advances drug targeting efforts and bolsters our understanding of allosteric structure activity relationships (ASARs).
Keywords: allostery, caspases, drug discovery, enzyme catalysis
Introduction
Caspase-7 (C7), a cysteine protease involved in execution of the apoptotic cascade, is a highly sought-after drug target. C7 activity is endogenously regulated through a number of mechanisms including its translation as a zymogen, procaspase-7 (P7).1–5 During the apoptotic cascade, upstream caspases cleave the L2 loops of P7 to allow for maturation of the dimer to active C7.3–5 Increased C7 activation has been shown to play a role in the etiopathogenesis of several human diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), ischemic reperfusion injuries, and sepsis.6–14 Peptidomimetic inhibitors have shown there is promise in small molecule targeting of C7 for effectively ameliorating these disease states.6,7,11,14 Unfortunately, there are challenges with drug targeting of C7 stemming from the limitations of peptidomimetic inhibitors and poor druggability of the active site.3,15 This is due to i) preferential binding to negatively charged peptides with highest affinity being for DEVD substrates ii) protein-protein interactions in the active site and iii) overlapping substrate specificity with other proteases, in particular caspase-3 (C3).3,15–17
An allosteric site at the C7 dimer interface holds greater potential for the development of small molecule therapeutics compared to the C7 active site.18,19 A few non-drug like allosteric inhibitors of C7 have been discovered, as discussed in Vance et al. (2017).18,20,21 Using a fragment-based drug discovery approach, Vance et al. (2017) found a reversibly-binding, thiophenol-based hit targeting the C7 allosteric site with the greatest tractability of any inhibitor to date.19 The crystal structure of C7 bound to this hit (PDB ID: 5V6U) revealed an expanded allosteric pocket compared to what was previously seen in C7 structures.19 Thus, this fragment hit offered new paths for structure-based drug design, but optimization of the hit was necessary due to its low molecular weight and weak binding.
The structural features observed in C7 allostery include reorientation of the substrate binding loops and rearrangement of the active site, including displacement of the catalytic residues.2,5,18,19 These structural hallmarks of allostery have been observed in crystal structures of C7 bound to our fragment hit, as well as in structures of C7 bound to non-drug-like allosteric small molecule inhibitors.18,19,21 Similar structural features have also been observed in the P7 zymogen, and allosteric modulators are thought to stabilize a “zymogen-like” conformation.2,4,5,22,23 Despite these observations, there remains a significant knowledge gap in establishing a link between binding of allosteric inhibitors and how they affect remote enzymatic catalysis (i.e. the physicochemical basis for how occupancy of small molecules in the allosteric site results in complete inhibition of catalytic turnover is not understood). The studies presented below directly answer this question via a confluence of x-ray crystallography, pre-steady state analysis, inhibitor expansion, and molecular dynamics (MD) simulations.
The chemical mechanism of C7 catalysis can be inferred from previous studies with cysteine proteases including cathepsin C and papain (Fig. S1).24,25 The rate-limiting phase of C7 catalysis has yet to be demonstrated, but is inferred to be deacylation based on experimental findings with other cysteine proteases.24,25 Vance et al. (2017) explored the chemical mechanism of C7 using kinetic solvent isotope effects (KSIEs), which supported a fast acylation phase and a rate-limiting deacylation phase for active C7.19 Three steps important for the initiation of the catalytic cycle have also been identified: i) substrate binding to the L3 loop, ii) activation of the catalytic dyad concurrent with the formation of the oxyanion hole, and iii) nucleophilic attack of substrate by the C186 thiolate.5,26
The chemical understanding of C7 allostery has a limited experimental basis, predominantly informed by experiments with non-drug-like small molecule inhibitors. One such inhibitor is 2-(2,4-Dichlorophenoxy)-N-(2-mercaptoethyl)-acetamide (a.k.a. DICA), which binds covalently to C290 in the C7 allosteric site.18,20 Previous mass spectrometry (MS) data have shown “mutually-exclusive” covalent binding to DICA or an irreversibly binding substrate mimic, carbobenzyloxy-Asp-Glu-Val-Asp-fluoromethyl ketone (Z-DEVD-FMK).18 This study shows DICA binding disrupts C7 catalysis prior to formation of the acylated intermediate.18 Nevertheless, elucidating the link between allosteric binding and catalysis for drug-like C7 inhibition has been difficult due to a lack of potent, drug-like inhibitors targeting the C7 allosteric site.
In this paper, we present several novel, reversibly-binding C7 inhibitors with higher potency, discovered based on the thiophenol fragment hit from our previous publication.19 We also provide detailed insight into allosteric inhibition of C7 using these improved inhibitors. Mechanistic studies show altered active site chemistry, rather than impaired substrate binding (i.e. a physical step), is responsible for small molecule allosteric inhibition of C7. Through integrated crystallographic and computational investigation, we show how structural and dynamic changes induced by inhibitor binding disrupt catalytic dyad activation and nucleophilic attack. Thus, our findings markedly advance inhibitor optimization and provide new insights into C7 catalysis and allosteric inhibition. These structural and mechanistic insights provide allosteric structure-activity relationships (ASARs: this information provides a nexus between inhibitor structure and changes in the receptor structure and reactivity), which may lead to the development of therapeutic drugs for the aforementioned conditions, such as AD and PD.
Results and Discussion
Fragment Expansion and C7 Selectivity
In Vance et al. (2017), structure-activity relationships (SARs) for the published fragment hit (i.e. 2-((2-acetylphenyl)thio)benzoic acid) revealed the thiophenol portion of the compound was critical for inhibition and showed extending the linker from the thiophenol moiety improved the Ki.19 Building upon these findings to expand the thiophenol hit further, four commercially-available expanded fragments with apparent structural similarity to the previously published fragment hit were obtained. These expanded fragments were 33–75 Da larger than the previous fragment hit. Compounds were screened in vitro using fluorometric steady-state kinetics assays to determine the half-maximal inhibitory concentration (IC50) and inhibition constant (Ki) (see Methods). All four of these compounds (1, 3, 4, and 8) had improved IC50 and Ki values compared to the previously published hit (Table S1). Inhibitor 1 was amenable to crystallization with C7 and a structure of 1-bound C7 (PDB ID: 5V6Z) was obtained, as is discussed later in the results section.
A more robust round of fragment expansion was performed by in silico and in vitro screening. Structures of commercially available compounds (from Maybridge Chemicals) retaining the critical thiophenol moiety, were initially screened in silico using the docking utility in Chemical Computing Group’s Molecular Operating Environment (CCG MOE) (see Methods). Fifteen of these compounds were selected for further screening in vitro based on i) docking results (e.g. position in the allosteric pocket and favorable docking score) and ii) increased molecular weight compared to the previous fragment hit (i.e. 33–170 Da larger). Fluorometric steady-state kinetics assays were used to determine the IC50 and Ki (see Methods). Four of these expanded fragments (2, 5, 6, and 7) had improved IC50 and Ki values compared to the previously published hit (Table S1). Inhibitor 2 was amenable to crystallization with C7 and a structure of 2-bound C7 (PDB ID: 8DJ3) was obtained, as discussed later in the results section.
Michaelis-Menten kinetics for C7 with increasing concentrations of inhibitors 1–8 show an apparent decrease in kcat with no change to Km (Fig. S2 and Fig. S4). This suggests a non-competitive kinetic mechanism of inhibition for these compounds. Two of these inhibitors, 2 and 3, were also able to fully inhibit C7 catalysis at high concentrations (Fig. S3 and S5, respectively). Our best inhibitor, 2, was also investigated for inhibition of C3. Inhibitor 2 was shown to have approximately ten-fold better selectivity for C7 compared to C3 (Table 1 and Fig. S6). Michaelis-Menten kinetics for C3 with increasing concentrations of 2 also show an apparent decrease in kcat with no change to Km (Fig. S7).
Table 1.
Inhibitor 2IC50 and Ki values for C7 vs. C3. Ki was determined using GraphPad Prism (version 8.3.2) equation for non-competitive inhibitors.
| Caspase-7 wt | Caspase-3 wt | |
|---|---|---|
|
| ||
| IC50 [a] | 156.8 ± 25.3 μM | 2634 ± 795 μM |
| Ki [a] | 128.8 ± 25.3 μM | 991.7 ± 291.5 μM |
Confidence intervals are reported based on a 95% confidence level
The Impact of Allosteric Inhibition on the Catalytic Cycle by Pre-Steady State Kinetics
To our knowledge, the rate-limiting phase of C7 catalysis (i.e. acylation or deacylation) has heretofore not been established. We developed a fluorometric stopped-flow assay to determine the rate-limiting step of the C7 catalytic cycle, and later to elucidate the mechanism of drug-like allosteric inhibition (see Methods). As expected, the pre-steady state kinetics data are consistent with fast acylation and rate-limiting deacylation for C7 catalysis. This is based on the presence of an exponential pre-steady state burst phase, followed by a linear steady state for uninhibited C7 (Fig. S9).
The covalent allosteric inhibitor DICA was used as a control to develop the stopped-flow assay for drug-like inhibition (see Methods). Based on previous observations of “mutually-exclusive” binding of C7 to DICA or DEVD, we expected a decrease in burst phase amplitude and no change to the burst rate constant (kburst), showing pre-acylation is impaired by DICA binding (i.e. a non-catalytically competent species is formed in lieu of a productive Michaelis complex when DICA is covalently bound to the C7 allosteric site).18 Indeed, we observed a decrease in the burst amplitude and no change to kburst with increasing concentrations of DICA, meaning the concentration of catalytically competent enzyme is diminished (Fig. S10).
The mechanism of allosteric inhibition for 2 was also investigated using the stopped-flow (see Methods). Inhibition of C7 by 2 disrupts catalysis prior to acylation by forming a catalytically incompetent species rather than a productive Michaelis complex (Fig. 1). This is based on the decrease in the burst amplitude and no significant change in the burst phase rate constant with increasing concentrations of inhibitor 2 (Fig. 1C and Fig. 1D). Given these results, drug-like inhibition of C7 by 2 affects either the chemical (i.e. activation of the C186/H144, nucleophilic attack of substrate by C186 thiolate) or physical (i.e. substrate binding in the active site) step of pre-acylation. Taken together with the DICA findings, these results suggest a unified mechanism of small molecule-mediated allostery for C7.
Figure 1. Inhibitor 2 disrupts C7 acylation.
A) Pre-steady state burst phase and steady state rate were measured for C7 wt with increasing concentrations of allosteric inhibitor 2 (6 to 500 uM 2; light pink to dark magenta) and DMSO only (grey). Curves were individually fit to the pre-steady state burst equation at each concentration of 2. B) Residuals for curve fit to pre-steady state burst equation are shown. C) The burst amplitudes and D) burst phase rate constants calculated by the individual fits are shown for 0 to 500 uM inhibitor 2 along with SE bars based on a 95% confidence interval.
Stopped-flow experiments were also attempted with 1 and 3. However, a clear trend was not discernable for the burst rate constant and burst amplitude with 1 because it does not fully inhibit C7 catalysis under these experimental conditions. Despite full inhibition of C7 catalysis by 3 at high concentrations, there remained issues with solubility, potency, and fluorescent interference that limited its use in the stopped-flow assay. Thus, the discovery of 2 was essential for understanding the impact of drug-like allosteric inhibition on the catalytic cycle.
The Impact of Allosteric Inhibition on Substrate Binding
To determine if drug-like C7 inhibition is due to disruption of physical substrate binding, we used differential scanning fluorimetry (DSF, a.k.a. thermal shift) to see if C7 pre-saturated with the irreversible allosteric inhibitor DICA could form a physical enzyme-substrate-inhibitor (ESI) complex with acetyl-Asp-Glu-Val-Asp-chloromethylketone (Ac-DEVD-CMK) (see Methods). Previous experiments by Hardy et al. (2004) show DICA-C7 cannot form a covalently bound ternary complex with Z-DEVD-FMK or turn over the fluorometric substrate, acetyl-Asp-Glu-Val-Asp-7-Amino-4-(trifluoromethyl)coumarin (Ac-DEVD-AFC).18 Prior to DSF, we also confirmed there was no turnover of Ac-DEVD-AFC substrate for DICA-C7 to ensure the enzyme was fully inhibited under our experimental conditions. Surprisingly, we found C7 was able to physically bind substrate and DICA simultaneously, as shown by the additive ESI complex (Fig. S11). Thus, our DSF findings support the idea that changes to structure and reactivity mediate drug-like C7 allostery.
C7 Crystal Structures with Improved Allosteric Inhibitors
We obtained crystal structures of C7 bound to 1 (PDB ID: 5V6Z) and 2 (PDB ID: 8DJ3) to establish a link between structural changes and the mechanism of inhibition for our compounds. The X-ray crystal structures of C7 soaked with inhibitors 1 and 2 were determined at resolutions of 2.6 Å and 3.2 Å, respectively. Data collection and refinement statistics for 5V6Z and 8DJ3 can be found in the Supporting Information (Table S2 and Table S3, respectively). Electron density was fully observed for 1 (Fig. S12), while partial electron density was observed for 2 (Fig. S13). Polder F o−F c omit map for 2 shows the thiophenol end of the compound is well-resolved, while the adamantane moiety is poorly resolved and partially solvent exposed (Fig. S13). As with the previous fragment hit, electron density was observed more clearly in one monomer of C7 for both 1 and 2. 1 is in side I of the allosteric pocket approximately 17 Å from the active site of monomer A, while 2 is in the side II of the allosteric pocket approximately 14 Å from the active site of monomer B (see Fig. S14 for naming and numbering conventions).
Electron density of 1 and 2 was observed in the C7 allosteric site with similar contacts as the previous thiophenol inhibitor fragment. Like the previous fragment, the carboxylate of 1 has an ionic interaction with R167 and a hydrogen bond with T163 (Fig. 2C). The carboxylate of 2 is also near T163 and R167 (Fig. 2D). Additionally, the adamantane and sulfamoyl groups of 2 are interacting with P227 (Fig. 2D). Several residues in the allosteric site of 8DJ3 which may contribute to 2-inhibition have been truncated: E147, N148, and K160 (Fig. 2D). This uncertainty with their position in the crystal may be due to increased dynamics or poor resolution. Compared to apo C7, binding of 1 and 2 in the allosteric site correlated with displacement of the catalytic C186 into the substrate binding pocket, with the displacement being greatest for the strongest inhibitor, 2 (Fig. 2B).
Figure 2. C7 structures bound to inhibitor 1 and 2.
shows several hallmarks of allosteric inhibition A) Chemical structures of 1 and 2 identified from in silico and in vitro screening. B) 1-C7 (PDB ID: 5V6Z, cyan) and 2-C7 (PDB ID: 8DJ3, magenta) show displacement of the catalytic cysteine (C186) compared to apo C7 (PDB ID: 4FDL, white). C) 2-C7 (8DJ3, protein in magenta and 2 in purple) and D) 1-C7 (5V6Z, protein in cyan and 1 in pink) allosteric site residues shown as sticks. Bonds between the inhibitor and residues are shown in yellow. Truncated residues are denoted by asterisks.
Comparison of normalized B-factors from active C7, 1-C7, and 2-C7 underscores the increased mobility of the loop regions in and around the active site when C7 is inhibited (Fig. S15). Notably, we observe increased B-factors in the catalytic H144 when C7 is inhibited by 1 or 2. Presence of 1 or 2 also correlates with the disappearance of electron density near the active site for loops L1, L2/L2’, L3, and L4 (Fig. S15).
Crystal Structure of the C7 Ternary Complex
To further link structural perturbations and the mechanism of drug-like allostery, we obtained a crystal structure of the C7 ternary complex (the C7 dimer plus DEVD-CHO and 1, a.k.a. 1-C7-DEVD, PDB ID: 8DGZ) at a resolution of 2.8 Å. Data collection and refinement statistics for 8DGZ can be found in the Supporting Information (Table S4). Polder F o−F c omit map shows well-defined occupancy of 1 in the C7 ternary complex (Fig. S16A). 2F o−Fc electron density map shows nearly full electron density for DEVD-CHO in the C7 ternary complex (Fig. S16B). DEVD-CHO is observed in both monomers, while 1 is only clearly resolved in side I of the allosteric pocket approximately 17 Å from the active site of monomer A (see Fig. S14 for naming and numbering conventions).
We compared the C7 ternary complex structure to two C7 structures bound to DEVD-CHO (C7-DEVD, PDB IDs: 1F1J and 3H1P). 1F1J is C7 wt bound to DEVD-CHO, while 3H1P is C7 I213A bound to DEVD-CHO. The I213A mutation has been shown to have no effect on the overall fold of the protein.27 Additionally, we observed the same pattern of altered enzyme-substrate interactions and B-factors when comparing 8DGZ to either 1F1J or 3H1P.
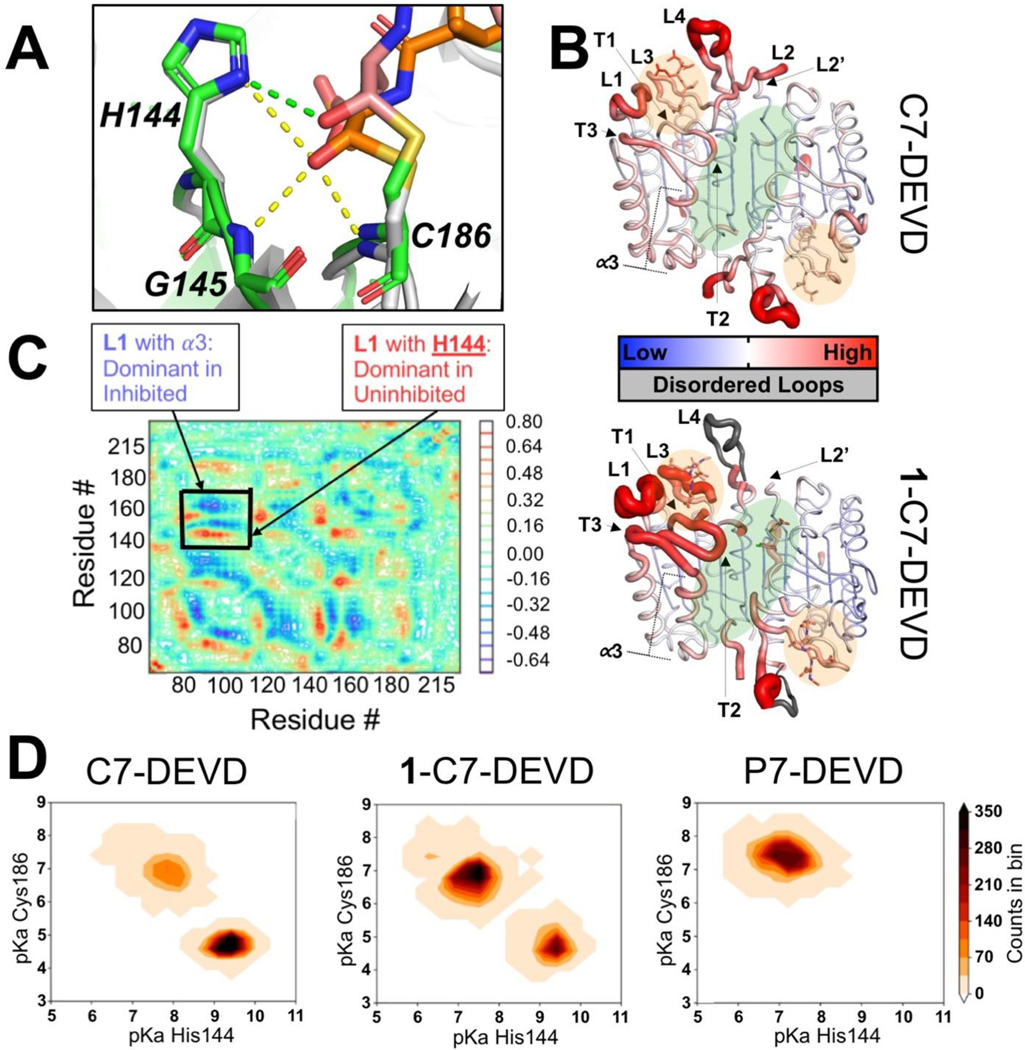
In the C7 ternary complex, many of the important enzyme-substrate interactions in the catalytic dyad activation network identified by Miscione et al. (2010) have been disrupted due to changes in orientation of the substrate and active site residues.26 In the C7 ternary complex, we see the P1 backbone carbonyl oxygen (i.e. residue normally cleaved) of the irreversible substrate has been displaced from the oxyanion hole formed by the NH groups of C186 and G145 (Fig. 3A). Interactions of S231 (carbonyl oxygen) and R87 (NH groups) with the P1 aspartate of the substrate (backbone NH and sidechain carboxylate, respectively) have been altered and there is concurrent reorientation of the substrate in the C7 ternary complex (Fig. S17A and S17C, respectively). The interaction between Q184 (sidechain amide O) and the substrate P1 is absent in the C7 ternary structure and there is concurrent reorientation of the Q184 sidechain (Fig. S17B). Additionally, in the C7 ternary complex there is a new polar interaction formed between Q184 (sidechain amide O) and R87 (sidechain NH) that is not present in C7 bound to DEVD-CHO only (Fig. S17B and Fig. S17C). Compared to C7-DEVD, normalized B-factors are increased in L1, L3, and the β-hairpins with the catalytic H144 for the C7 ternary complex (Fig. 3B and Fig. S18A). Additionally, there is a loss of electron density for the residues of L4 in the C7 ternary complex, which indicates increased dynamics.
Figure 3.
The C7 ternary complex (1-C7-DEVD) provides a rational basis for impaired nucleophilic attack as the mediator of allosteric inhibition. A) Overlay of C7 I213A bound to DEVD-CHO (PDB ID: 3H1P, white/orange elemental) and the C7 ternary complex (PDB ID: 8DGZ, green/pink elemental) show the oxyanion hole is disrupted by 1 binding. For 3H1P; C7 is white, DEVD-CHO is orange, and polar bonds are yellow. For 8DGZ; C7 is green, DEVD-CHO is pink, and polar bonds are green. 3H1P shows the oxyanion hole; formed by the H144 sidechain, G145 backbone, and C186 backbone making polar interactions with the carbonyl oxygen of the substrate. 8DGZ shows the substrate carbonyl oxygen is displaced and no longer making polar interactions with G145 and C186. Polar interactions involving other substrate atoms (i.e. outside the oxyanion hole) have been obscured for clarity. B) The C7 ternary complex (1-C7-DEVD) structure (bottom, 8DGZ) shows increased B-factors in the L1 and L3 loops compared to C7-DEVD (top, 3H1P). Gray loops are used to denote disordered regions not observed/modelled in the crystal structure, which were overlaid from active C7 for reference. The general position of the active sites (orange shadow) and allosteric pocket (green shadow) are indicated on both structures. C) Partial ΔDCCM plot for C7-DEVD vs. the C7 ternary complex (1-C7-DEVD). Numbering begins at residue 58. Residues 197–210, which include the inter subunit linker removed during maturation, are not modeled. The graph shows a limited view of the full ΔDCCM for clarity, capturing the areas with the greatest changes. Residue dynamics that are highly correlated in C7-DEVD, but not 1-C7-DEVD, are shown in red/orange. Residue dynamics that are highly correlated in 1-C7-DEVD, but not C7-DEVD, are shown in blue/purple. D) Histograms showing pKa values of the catalytic dyad residues (C186 and H144) from MD simulations analyzed using PROPKA. These histograms show the pKas for C186 and H144 for C7-DEVD, 1-C7-DEVD, and P7-DEVD. Heat maps were created using all data points from both monomers collected from 100 ns MD simulations performed in triplicate.
The C7 ternary complex structure also shows asymmetry between the two monomers (A and B) of C7 in terms of the protein structure and ligand occupancy. There is more clearly resolved electron density for 1 in the allosteric site side I (see Fig. S14 for naming and numbering conventions). As with C7-DEVD, monomer A of the C7 ternary complex has higher normalized B-factors. Additionally, some of the changes to the catalytic activation network only occur in monomer A (i.e. opposite clearly resolved allosteric inhibitor), such as those involving Q184 and S231. Disruption of substrate orientation in the C7 ternary complex, especially displacement of the P1 backbone carbonyl from the oxyanion hole, and increased normalized B-factors (L1, L3, L4, and turn containing H144) are observed in both monomers.
Computational Investigation of the C7 Ternary Complex
To contextualize these structural data and mechanistic studies, we used several computational approaches to interrogate small molecule-mediated allostery. Molecular dynamics (MD) simulations were performed on energy-minimized structures of C7-DEVD, 1-C7-DEVD (ternary complex), and substrate-bound P7 (P7-DEVD) (see Methods). P7 was included as a native paradigm for allosteric inhibition due to shared structural hallmarks of allostery with small molecule-inhibited C7. For each structure, peptide substrate (DEVD) was modeled in the active site (not covalently bound) to represent the enzyme prior to nucleophilic attack. Data from these simulations were analyzed using dynamic cross-correlation matrices (DCCM) and PROPKA to determine the dynamics and pKa distribution of catalytic residues throughout the simulation, respectively. Normalized B-factors from 3H1P and 8DGZ were compared to normalized root-mean-square fluctuation (RMSF) values from C7-DEVD and 1-C7-DEVD (ternary complex) MDs to ensure these simulations and subsequent analyses accurately represented the crystal data (Fig. S18).
Dynamic cross-correlation matrices (DCCM) for C7-DEVD, 1-C7-DEVD, and P7-DEVD show several changes in correlated motion throughout the protein for 1-C7-DEVD and P7-DEVD compared to C7-DEVD (Fig. S19). For 1-C7-DEVD, we see increased anticorrelation between the nascent L2/L2’ ends and the rest of C7 (Fig. S19). P7-DEVD also shows strong anticorrelation for the L2 loop compared to the rest of the protein. Anticorrelation between L2/L2’ ends and the rest of C7 is not observed in C7-DEVD (Fig. S19). Additionally, strongly correlated regions become more distinct in 1-C7-DEVD and P7-DEVD compared to C7-DEVD (Fig. S19). This suggests allosteric binding adversely influences dynamics by increasing localized regions of anticorrelation and/or by restricting the normal flexibility of the enzyme.
In an effort to clarify important structural changes due to allosteric inhibition, we calculated a delta DCCM (ΔDCCM). ΔDCCM is found by subtracting the inhibited DCCM value from the active DCCM value for each residue: ΔDCCM=DCCMactive-DCCMinhibited. The ΔDCCM plot shows an interaction between L1 and α-helix 3 (α3) is dominant in 1-C7-DEVD compared to C7-DEVD (Fig. 3C, see Fig. S14 for naming and numbering). X-ray crystal structures 5V6Z (1-C7), 8DJ3 (2-C7), and 8DGZ (1-C7-DEVD) show residues of α3 (T163 and R167) have polar interactions with the thiophenol moiety of 1 and 2. Additionally, the resultant ΔDCCM plot shows interactions between the L1 loop (substrate binding) and the catalytic H144 and G145 (oxyanion hole) are dominant in C7-DEVD compared to 1-C7-DEVD (Fig. 3C). H144 and G145 are contained in the first turn (T1) of the β-hairpins from L142-G155. There are also changes in the coupled motion of L1 and β-hairpin turn 2 (T2) containing residue E147, which may interact with the chlorine of 1 (Fig. 3B). Similarly, there are changes in the coupled motion of L1 and β-hairpin turn 3 (T3), which connects directly to α3 (Fig. 3B). These data suggest binding of 1 in the allosteric pocket decouples the motion of the L1 substrate binding loop from the catalytic dyad. Thus, substrate recognition and binding may occur, but proper orientation in the active site may not be achieved.
The pKa of the catalytic residues (H144 and C186) was determined using PROPKA (version 2.0) for each snapshot of the MD simulated structures of C7-DEVD, 1-C7-DEVD, and P7-DEVD (See Methods).28,29 When substrate is bound in the active site prior to acylation, PROPKA data show the catalytic residues of C7-DEVD are mostly ready to initiate nucleophilic attack (Fig. 3D). There are two populations of pKa values. The most abundant population accounts for 75% of the data and has centroid pKa values at 9.2 for H144 and 4.7 for C186, which favors nucleophilic attack. Previous pH rate profiles identified ionization states at 9.0 and 5.6 for active C7, which were attributed to H144 and C186 pKa values, respectively.19 The less abundant population accounts for 25% of the data and has centroid pKa values at 7.8 for H144 and 6.7 for C186, which does not favor nucleophilic attack.
PROPKA data for 1-C7-DEVD show the catalytic dyad is less prepared for nucleophilic attack compared to C7-DEVD (Fig. 3D). As with C7-DEVD, there are two populations of pKa values. The most abundant population accounts for 58% of the data and has centroid pKa values at 7.5 for H144 and 6.8 for C186, which does not favor nucleophilic attack. The less abundant population accounts for 42% of the data and has centroid pKa values at 9.1 for H144 and 4.4 for C186, which favors nucleophilic attack. Disruption of the ionization of the catalytic dyad in 1-C7-DEVD provides support for impairment of the fundamental environment required for acylation as a basis for allosteric inhibition.
For P7-DEVD, PROPKA data show the catalytic residues of the zymogen are not in the correct ionization state to initiate nucleophilic attack (Fig. 3D). There is one population of pKa values for P7-DEVD with centroid pKa values at 7.2 for H144 and 7.3 for C186, which does not favor nucleophilic attack. Previous pH rate profiles identified ionization states at 7.3 and 5.3 for P7, which were attributed to H144 and C186 pKa values, respectively.19
The computationally determined pKa values for the catalytic dyad largely agree with experimentally determined pKa values previously published by Vance et al. (2017). The one exception is the pKa of C186 for the P7 zymogen. This discrepancy may be due to the difficulty in calculating pKa values for cysteine residues. Alternatively, if both the catalytic residues in P7 were mostly neutralized and near the same pKa value (as suggested by PROPKA calculations), then it may be difficult to distinguish their respective ionization states experimentally using pH rate profiles. In any case, disruption of the ionization of the catalytic dyad in P7-DEVD further supports impairment of the fundamental environment required for acylation as a basis for allosteric inhibition.
Conclusion
The improved allosteric inhibitors discovered in this study demonstrate the tractability of the thiophenol moiety for specifically targeting C7. To our knowledge, 2 is the first allosteric inhibitor selective for C7 over C3. These inhibitors could be derivatized to improve inhibition. In particular, increasing contacts along the adamantane moiety of 2 and moving toward the other side of the allosteric pocket would improve inhibition and further resolve specific targeting of C7. Nevertheless, our results support the utility of fragment-based drug design for targeting novel pockets. Our results also underscore the utility of integrating structural, dynamic, and catalytic information to gain insight into the mechanism of inhibition, which is especially important for allosteric leads.
The observed loss of the acylation burst when C7 is inhibited by 2 or DICA must be due to disruption of a chemical or physical step prior to formation of the tetrahedral acylation intermediate. We observe concurrent binding of DEVD to DICA-C7 by DSF, which suggests substrate binding is not the impaired step. Our observations of loss of DEVD-ase activity for DICA-C7, along with previous MS and kinetic experiments, show covalent binding to both DEVD and DICA by C7 does not occur.18 Thus, allosteric binding impacts C7 catalysis either by aberrant substrate formation of the Michaelis complex or, more likely, by chemical means. The atrophied activation of the catalytic dyad is clearly shown by the pKa calculations in Fig. 3D.
Changes in the catalytic dyad further support disruption of the chemical steps prior to acylation as the mechanism of drug-like C7 allostery. X-ray crystal structures of 1-C7 and 2-C7 show displacement of the catalytic C186 upon inhibitor binding, which would disrupt the activation of the catalytic dyad and prevent nucleophilic attack. The X-ray crystal structure of the ternary complex structure also shows changes in key residues which would impair catalytic dyad activation. Displacement of the substrate P1 carbonyl from the oxyanion hole would weaken the electrophilic character of the P1 carbonyl carbon undergoing nucleophilic attack. PROPKA analyses from all-atom MD simulations based on these structures reinforce the dyad neutralization hypothesis, showing the disruption of active site chemistry in the presence of allosteric inhibitor. Notably, these computationally determined pKa values for the catalytic dyad largely agree with experimentally determined pKa values from previously published pH rate profiles by Vance et al. (2018).19 Neutralization of the catalytic dyad in 1-C7-DEVD and P7-DEVD, as compared to C7-DEVD, is unfavorable for initiating nucleophilic attack.
Although the loop regions are known to be associated with substrate binding, changes in the dynamics of the loop regions also supports impairment of pre-acylation as the mechanism of drug-like allostery. This is because proper orientation of substrate in the active site is essential for charging of the catalytic dyad and subsequent nucleophilic attack.5,26 ΔDCCM suggests a decoupling of the motion of the L1 substrate binding loop and T1 containing catalytic H144 and G145 of the oxyanion hole when C7 is allosterically inhibited. The loss of normal coupled motion between L1 and T1 is also accompanied by a new increase in the coupled motion between L1 and α-helix 3 (α3), which contains several residues in the allosteric pocket (e.g. T163 and R167). X-ray crystal structures of 1-C7 and 2-C7 show these allosteric site residues make important polar contacts with the critical thiophenol moiety of these inhibitors. These computational findings are also supported structurally by the ternary complex B-factors, which show increased B-factors in the L1 loop for the ternary complex (1-C7-DEVD) as compared to C7-DEVD. Thus, the presence of a drug-like allosteric inhibitor leads to improper orientation of substrate in the active site via diminished coordination of L1, which in turn disrupts catalysis. Moreover, the pattern of dynamic changes centering around L1 establishes a clear link between the dynamics of the allosteric and active sites.
Crystal structures for our inhibitors (1, 2, and the previous fragment) have consistently shown occupancy in only one of two sides of the allosteric pocket (I and II, see Fig. S14 for naming and numbering). The previous fragment hit and inhibitor 1 favor binding in side I of the allosteric site with more contacts in the more stable monomer B. In the ternary complex, DEVD-CHO is bound to both active sites but is more clearly resolved in monomer B. We observe 2 binding in side II of the allosteric site, but with roughly equivalent contacts to both monomers due to the expanded size of the fragment. This may explain why 2 is now visible in the less stable side of the allosteric site. It remains unclear why we do not observe two bound allosteric ligands in any crystal structure. If the binding of the compounds is 1:1 per monomer, binding in both allosteric sites may not be observed in the crystals for several reasons. Firstly, there may be inherent limitations to ligand occupancy due to poor compound solubility or the use of crystal soaking as an approach. Similarly, there may be occupancy of both sides of the allosteric site but in a small population, which leads to insufficient electron density in the second side. Finally, the asymmetry of apo C7 crystals could favor allosteric binding or improve resolution of allosteric ligands in one pocket over the other. Alternatively, if binding of these compounds is 1:1 per dimer, it indicates there is cooperativity between C7 monomers that has yet to be characterized.
For C7, the integration of experimental structural and computational studies has led to a clear set of ASARs, or patterns that provide a bridge between occupancy at the allosteric pocket and the structure, dynamics, and reactivity at the active site. Recent ASARs for glutamate racemase (MurI)—an enzyme that, like C7, employs a Cys-His catalytic dyad and functions as an obligate dimer—showed a loss of cooperativity and decreased dynamics for allosterically inhibited MurI.30 These findings contrast with our results for C7, which presents distinct patterns of ASARs for C7 and MurI. Although research is still in early stages, ASARs have far-reaching implications for allosteric drug discovery.
Despite the promise of allosteric drugs, less than 1% of FDA approved drugs are known allosteric effectors.31,32 This is likely due to incomplete understanding of the nexus between the remote allosteric site and the active site, which complicates the drug optimization process. ASARs for enzymes (e.g. C7 and MurI) can provide invaluable insights for drug discovery. Indeed, future studies into ASARs may employ a more streamlined all-atom MD to uncover similar patterns linking the allosteric and active site, which may be used for more streamlined drug screening workflows. By building a pattern of ASARs, we may finally realize the full potential of allosteric drugs.
Supplementary Material
Acknowledgements
This work was supported by NIH grants awarded to M. A. Spies (R01-GM09737 and R01-GM138471). Financial support for student fellowships to K. F. Hobbs and N. R. Vance are acknowledged from the Center for Biocatalysis and Bioprocessing at the University of Iowa and the NIH Predoctoral Training Program in Biotechnology (grant number T32-GM008365). We thank Nicholas Schnicker, Jay Nix, and Grant T. Cooling for assistance with x-ray crystallography efforts. This research used the resources of i) Carver College of Medicine’s Protein and Crystallography Facility at the University of Iowa, ii) the Advanced Light Source at Lawrence Berkeley National Laboratory, and iii) the Advanced Photon Source at Argonne National Laboratory. Beamline 4.2.2 of the Advanced Light Source, a U.S. DOE Office of Science User Facility under Contract No. DE-AC02-05CH11231, is supported in part by the ALS-ENABLE program funded by the National Institutes of Health (NIH), National Institute of General Medical Sciences, grant P30 GM124169-01. The Advanced Photon Source is a U.S. Department of Energy (DOE) Office of Science user facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supporting Information
Additional references cited within the Supporting Information.[33–49]
References
- [1].Riedl SJ, Fuentes-Prior P, Renatus M, Kairies N, Krapp S, Huber R, Salvesen GS, Bode W, Proc. Natl. Acad. Sci. U. S. A 2001, 98, 26, 14790–14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Thomsen ND, Koerber JT, Wells JA, Proc. Natl. Acad. Sci. U. S. A 2013, 110, 21, 8477–8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pop C, Salvesen GS, J. Biol. Chem 2009, 284, 33, 21777–21781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Donepudi M, Grütter MG, Biophys. Chem 2002, 101–102, 145–153. [DOI] [PubMed] [Google Scholar]
- [5].Fuentes-Prior P, Salvesen GS, Biochem. J 2004, 384, 201–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, Cano J, Brundin P, Englund E, Venero JL, Joseph B, Nature 2011, 472, 7343, 319–324. [DOI] [PubMed] [Google Scholar]
- [7].Chaudhary S, Madhukrishna B, Adhya AK, Keshari S, Mishra SK, Oncogenesis 2016, 5, 4, e219–e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lamkanfi M, Kanneganti T-D, Int. J. Biochem. Cell Biol 2010, 42, 1, 21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McIlwain DR, Berger T, Mak TW, Cold Spring Harb. Perspect. Biol 2013, 5, a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gervais F, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeg LHT, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni R, Roy S, Nicholson DW, Cell 1999, 97, 395–406. [DOI] [PubMed] [Google Scholar]
- [11].Wellington CL, Singaraja R, Ellerby L, Savill J, Roy S, Leavitt B, Cattaneo E, Hackam A, Sharp A, Thornberry N, Nicholson DW, Bredesen DE, Hayden MR, J. Biol. Chem 2000, 275, 26, 19831–19838. [DOI] [PubMed] [Google Scholar]
- [12].Wellington CL, Ellerby LM, Gutekunst C-A, Rogers D, Warby S, Graham RK, Loubser O, van Raamsdonk J, Singaraja R, Yang Y-Z, Gafni J, Bredesen D, Hersch SM, Leavitt BR, Roy S, Nicholson DW, Hayden MR, J. Neurosci 2002, 22, 18, 7862–7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wellington CL, Ellerby LM, Hackam AS, Margolis RL, Trifiro MA, Singaraja R, McCutcheon K, Salvesen GS, Propp SS, Bromm M, Rowland KJ, Zhang T, Rasper D, Roy S, Thornberry N, Pinsky L, Kakizuka A, Ross CA, Nicholson DW, Bredesen DE, Hayden MR J. Biol. Chem 1998, 273, 15, 9158–9167. [DOI] [PubMed] [Google Scholar]
- [14].Aziz M, Jacob A, Wang P, Cell Death Dis. 2014, 5, 11, e1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Poreba M, Szalek A, Kasperkiewicz P, Rut W, Salvesen GS, Drag M, Chem. Rev 2015, 115, 22, 229–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, Chapman KT, Nicholson DW, J. Biol. Chem 1997, 272, 29, 17907–17911. [DOI] [PubMed] [Google Scholar]
- [17].Dhani S, Zhao Y, Zhivotovsky B, Cell Death Dis. 2021, 12, 10, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hardy JA, Lam J, Nguyen JT, O’Brien T, Wells JA, Proc. Natl. Acad. Sci. U. S. A 2004, 101, 34, 12461–12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vance NR, Gakhar L, Spies MA, Angew. Chem. Int. Ed Engl 2017, 56, 46, 14443–14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hardy JA, Wells JA, J. Biol. Chem 2009, 284, 38, 26063–26069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Feldman T, Kabaleeswaran V, Jang SB, Antczak C, Djaballah H, Wu H, Jiang X, Mol. Cell 2012, 47, 4, 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Scheer JM, Romanowski MJ, Wells JA, Proc. Natl. Acad. Sci. U. S. A 2006, 103, 20, 7595–7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Häcker H-G, Sisay MT, Gütschow M, Pharmacol. Ther 2011, 132, 2, 180–195. [DOI] [PubMed] [Google Scholar]
- [24].Schneck JL, Villa JP, McDevitt P, McQueney MS, Thrall SH, Meek TD, Biochemistry 2008, 47, 33, 8697–8710. [DOI] [PubMed] [Google Scholar]
- [25].Hussain S, Pinitglang S, Bailey TSF, Reid JD, Noble MA, Resmini M, Thomas EW, Greaves RB, Verma CS, Brocklehurst K, Biochem. J 2003, 372, 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Miscione GP, Calvaresi M, Bottoni A, J. Phys. Chem. B 2010, 114, 4637–4645. [DOI] [PubMed] [Google Scholar]
- [27].Witkowski WA, Hardy JA, Protein Sci. 2009, 18, 7, 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Søndergaard CR, Olsson MHM, Rostkowski M, Jensen JH, Chem J. Theory Comput. 2011, 7, 7, 2284–2295. [DOI] [PubMed] [Google Scholar]
- [29].Olsson MHM, Søndergaard CR, Rostkowski M, Jensen JH, Chem J. Theory Comput. 2011, 7, 525–537. [DOI] [PubMed] [Google Scholar]
- [30].Chheda PR, Cooling GT, Dean SF, Propp J, Hobbs KF, Spies MA Commun. Chem 2021, 4, 1, 1–13. [DOI] [PubMed] [Google Scholar]
- [31].Amamuddy OS, Veldman W, Manyumwa C, Khairallah A, Agajanian S, Oluyemi O, Verkhivker GM, Bishop ÖT, Int. J. Mol. Sci 2020, 21, 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wishart D, Knox C, Guo A, Shrivastava S, Hassanali M, Stothard P, Chang Z, Woolsey J, Nucleic Acids Res. 2006, 34, D668–D672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhou Q, Snipas S, Orth K, Muzio M, Dixit VM, Salvesen GS, J. Biol. Chem 1997, 272, 12, 7797–7800. [DOI] [PubMed] [Google Scholar]
- [34].Pop C, Chen YR, Smith B, Bose K, Bobay B, Tripathy A, Franzen S, Clark AC, Biochemistry 2001, 40, 47, 14224–14235. [DOI] [PubMed] [Google Scholar]
- [35].Kabsch W, Acta Cryst. 2010, 66, 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ, J. Appl. Cryst 2007, 40, 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liebschner D, et al. Acta Cryst. 2019, 75, 861–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Emsley P, Lohkamp B, Scott WG, Cowtan K, Acta Cryst. 2010, 66, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wei Y, Fox T, Chambers SP, Sintchak J, Coll JT, Golec JMC, Swenson L, Wilson KP, Charifson PS, Chem. Biol 2000, 7, 6, 423–432. [DOI] [PubMed] [Google Scholar]
- [40].Dean SF, Whalen KL, Spies MA, ACS Cent. Sci 2015, 1, 7, 364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hengel SR, Malacaria E, da S Constantino LF, Bain FE, Diaz A, Koch BG, Yu L, Wu M, Pichierri P, Spies MA, Spies M, eLife 2016, 5, e14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Maier JA, Martinez C, Kasavajhala K, Wickstrom L, Hauser KE, Simmerling C, Chem J. Theory Comput. 2015, 11, 8, 3696–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG, J. Chem. Phys 1998, 103, 19, 8577–8593. [Google Scholar]
- [44].Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA, J. Comput. Chem 2004, 25, 1157–1174. [DOI] [PubMed] [Google Scholar]
- [45].Jakalian A, Jack DB, Bayly CI, J. Comput. Chem 2002, 23, 1623–1641. [DOI] [PubMed] [Google Scholar]
- [46].Canutescu AA, Dunbrack RL, Protein Sci. 2003, 12, 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Krieger E, Joo K, Lee J, Raman S, Thompson J, Tyka M, Baker D, Karplus K, Proteins 2009, 77, 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hooft RW, Vriend G, Sander C, Abola EE, Nature 1996, 381, 6580, 272. [DOI] [PubMed] [Google Scholar]
- [49].Chai J, Shiozaki E, Srinivasula SM, Wu Q, Dataa P, Alnemri ES, Shi Y, Cell 2001, 104, 769–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.