Abstract
Background:
Bone transport in distraction osteogenesis is an effective, well-known procedure. However, bone compression is an aspect of this technique for which there is no objective information. The lack of direct bone compression measurements may result in a lack of uniformity in the bone transport process, which can result in its ineffective application and may be contributing to its underutilization. This study describes the results of applying objectively measured compressions to achieve a distraction regeneration zone and docking site consolidation during bone transport in distraction osteogenesis.
Methods:
This prospective study describes the results of a single cohort of 32 patients who underwent distraction osteogenesis with bone transport utilizing a combination of a minimally invasive rail plate and monolateral external fixation. The patients were categorized into 2 groups: (1) those with hypertrophic, atrophic, or infectious pseudarthrosis-nonunion (the pseudarthrosis-nonunion group), and (2) those with bone loss due to trauma or osteomyelitis (the bone loss group). The initial bone compression was measured during the latency phase, and the final compression was measured during the distraction phase. The healing index, external fixation index, healing time, consolidation time, and docking time were calculated for each patient. The Mann-Whitney U and Kruskal-Wallis tests were used for comparisons between and within groups.
Results:
In this study, 28 (88%) of the patients were male. The mean patient age was 44.93 ± 16.21 years. The median values were 3.2 Nm for the initial compression and 3.4 Nm for the final compression, with no significant difference between or within groups of patients. The osseous results were excellent in 29 patients (91%), and the functional results were good or excellent in 31 patients (97%).
Conclusions:
This study is the first to objectively measure compression in the bone transport process. Our findings showed that all patients who had an initial compression of ≥3.2 Nm achieved 100% consolidation of the distraction regeneration zone, and those who had a final compression of ≥2.9 Nm achieved complete docking site consolidation without complications. These 2 values thus represent effective compression and highlight the role of bone compression in bone transport.
Level of Evidence:
Therapeutic Level II. See Instructions for Authors for a complete description of levels of evidence.
Bone transport in distraction osteogenesis is an effective, well-known procedure that has been widely used since it was first described1-17. The application of bone transport in distraction osteogenesis should consider the treatment time (bone healing and distraction degeneration zone) and the time to final bone healing1,6,10,18-24 as well as the development of possible complications, including those due to prolonged use of the external fixator (pin-track infection, joint instability, and stiffness), refracture after removal of the fixator1,17,20,22-36, failure in the distraction regeneration zone (DRZ), and nonunion or the need for new surgical procedures to achieve docking site consolidation (DSC)9,17,22,24,37-40.
The utility of bone compression in external fixation for the management of pseudarthrosis-nonunion (PN), infection, or bone loss or for the reestablishment of bone continuity has been discussed since 194841. Bone compression has been described as stimulating the bone-healing process in infectious and non-infectious PN15,17,21,25-32,38,42-53. However, until now, compression in bone transport has been applied using mostly pragmatic criteria, rather than based on objective information. Previous studies have not directly measured the effect of the magnitude of the force used for compression on the success of bone transport or correlated the variations in the magnitude of bone compression with the clinical result of bone transport for distraction osteogenesis. This lack of scientific, evidence-based surgical practice can contribute to the underutilization of this technique for a variety of conditions2,5,7,10-12,14-16,31,42.
A minimally invasive rail plate (MIRP) consists of a special low-contact plate with a rail, holes for locking screws, and holes for conventional screws. It is used with monolateral external fixation (MEF) (similar to the Orthofix Monolateral External Fixator to Limb Reconstruction and Bone Elongation) and a locking Schanz pin system (Fig. 1) and is placed with a minimally invasive technique. At each end of the rail plate is a locking hole for the locking Schanz pin. This allows the rail to be used to guide the initial placement of the MEF and to maintain the same orientation until the end of the docking process. C-arm fluoroscopy is used during placement (Figs. 2-A, 2-B, and 2-C). MIRP and MEF for bone transport can provide measurements of the compression that are specified in the 2 specific points of transport, the initial and final compression values, in Nm, during the treatment of PN (infected and non-infected) and of bone loss (due to trauma or osteomyelitis).
Fig. 1.
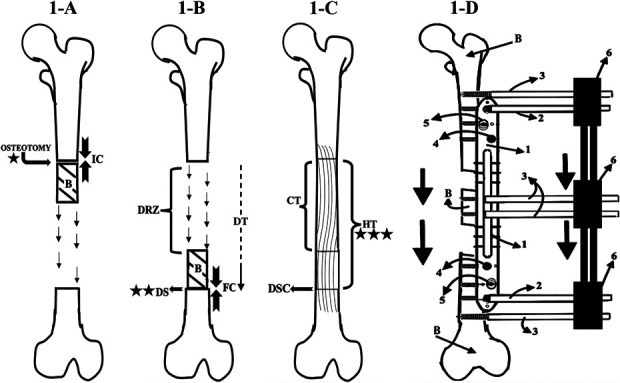
Figs. 1-A through 1-D Bone transport with an MIRP. Radiographic follow-up was first performed at the beginning of the latency phase (time 0; star), continued at the docking site during the distraction phase (2 stars) and the consolidation phase (allowing determination of the HT), and for an additional 6 months (3 stars). B = bone. Fig. 1-A Latency phase: This phase begins with osteotomy and measurement of the initial compression (IC), in Nm (defined as the torque resulting from a force of 1 Newton applied perpendicular to the end of a moment arm that is 1 meter long5,5). Its duration is the number of days for which the initial compression was applied. Fig. 1-B Distraction phase: At the end of this phase, the bone has been transported to the docking site (DS), and the measurement of the final compression (FC) in Nm is performed. The duration of this phase is the docking time (DT). DRZ = distraction regeneration zone. Fig. 1-C Consolidation phase. The duration of this phrase is the regenerate consolidation time (CT). The healing time (HT), from osteotomy to when consolidation of all bone has been completed, is the sum of the latency, distraction, and consolidation phases. Docking site consolidation = DSC. Fig. 1-D Diagram illustrating the bone transport process. 1 = rail plate, 2 = locking Schanz pin, 3 = simple Schanz pins, 4 = locking screw, 5 = conventional screw, and 6 = MEF (similar to the Orthofix product Monolateral External Fixator to Limb Reconstruction and Bone Elongation).
Fig. 2.
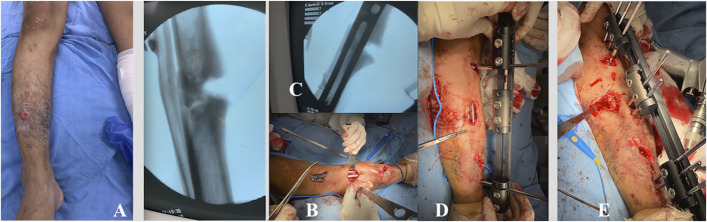
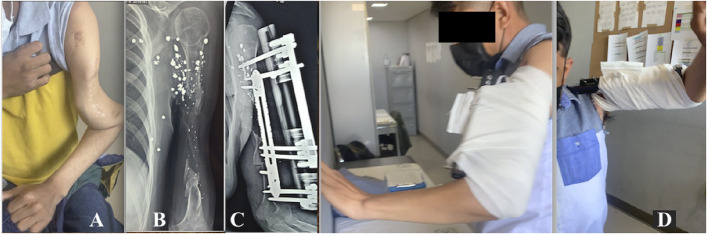
Figs. 2-A through 2-E Patient 1. Fig. 2-A An infected PN in the right tibia. Figs. 2-B and 2-C The minimally invasive technique for percutaneous plate insertion. Fig. 2-D Placement of the locking Schanz pin to serve as a guide for external fixator placement, and Gigli saw osteotomy. Fig. 2-E Completion of the MIRP and MEF application.
To our knowledge, this study is the first to describe the results of applying objectively measured compression to achieve a DRZ and DSC in patients undergoing bone transport. We hypothesized that it is possible to obtain a DRZ and DSC with fewer complications using objectively measured compression applied under appropriate conditions during the phases of bone transport.
Materials and Methods
Study Population and Data Sources
This was a single-center, prospective study of 32 patients undergoing distraction osteogenesis with bone transport in the lower extremity (femur or tibia) or upper extremity (humerus). The study was conducted between March 2019 and November 2021. All patients were adequately informed about the study’s objectives, procedures, and risks. Informed consent was obtained from all patients, and the Helsinki statement guidelines were followed54.
The participating patients included 2 groups: (1) patients with PN, which includes hypertrophic, atrophic, and infected or septic forms; and (2) patients with bone loss, due to osteomyelitis (requiring resection of the involved bone) and traumatic loss (involving open fractures). The inclusion criteria were PN or bone loss involving a long bone (tibia, humerus, or femur). For the PN group, ≥1 surgical procedures must have failed previously (Table I).
TABLE I.
Patient Sociodemographic and Clinical Characteristics*
| Patient No. | Age (yr) | Sex | Bone | Side | Group | Location | Osteotomy Method | No. of Previous Surgeries | Type of BT | DRL (cm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 26 | Male | Tibia | Right | SPS | Medial | Gigli saw | 1 | Proximal-distal | 2.4 |
| 2 | 56 | Male | Tibia | Left | OBL | Distal | Reciprocating saw | 2 | Proximal-distal | 7.4 |
| 3 | 46 | Female | Humerus | Right | APS | Medial | Gigli saw | 1 | Distal-proximal | 2.5 |
| 4 | 43 | Male | Tibia | Right | TBL | Medial | Reciprocating saw | 2 | Proximal-distal | 6.5 |
| 5 | 23 | Male | Tibia | Left | HPS | Medial | Gigli saw | 1 | Proximal-distal | 2 |
| 6 | 20 | Male | Tibia | Right | HPS | Medial | Gigli saw | 1 | Distal-proximal | 1.8 |
| 7 | 51 | Male | Tibia | Right | SPS | Medial | Gigli saw | 1 | Distal-proximal | 1.5 |
| 8 | 45 | Male | Tibia | Left | HPS | Medial | Gigli saw | 1 | Distal-proximal | 1 |
| 9 | 76 | Male | Femur | Right | TBL | Proximal | Reciprocating saw | 2 | Distal-proximal | 5 |
| 10 | 24 | Male | Tibia | Right | APS | Distal | Gigli saw | 1 | Proximal-distal | 3 |
| 11 | 23 | Male | Tibia | Right | HPS | Medial | Gigli saw | 1 | Proximal-distal | 1.2 |
| 12 | 43 | Male | Tibia | Right | APS | Distal | Reciprocating saw | 2 | Distal-proximal | 1.8 |
| 13 | 20 | Male | Tibia | Right | HPS | Medial | Gigli saw | 2 | Distal-proximal | 1.5 |
| 14 | 54 | Male | Tibia | Left | SPS | Medial | Reciprocating saw | 1 | Distal-proximal | 1.1 |
| 15 | 57 | Male | Tibia | Left | SPS | Distal | Reciprocating saw | 1 | Proximal-distal | 1.5 |
| 16 | 32 | Male | Femur | Right | TBL | Distal | Reciprocating saw | 0 | Proximal-distal | 8.5 |
| 17 | 42 | Male | Humerus | Left | HPS | Medial | Gigli saw | 2 | Distal-proximal | 0.8 |
| 18 | 61 | Male | Tibia | Right | HPS | Medial | Reciprocating saw | 2 | Proximal-distal | 1 |
| 19 | 43 | Female | Humerus | Right | HPS | Medial | Gigli saw | 3 | Distal-proximal | 1.4 |
| 20 | 61 | Male | Tibia | Right | HPS | Medial | Reciprocating saw | 3 | Distal-proximal | 1 |
| 21 | 51 | Male | Tibia | Right | HPS | Distal | Osteotome | 4 | Proximal-distal | 1.1 |
| 22 | 50 | Male | Tibia | Right | OBL | Proximal | Gigli saw | 2 | Distal-proximal | 6 |
| 23 | 52 | Female | Humerus | Left | APS | Proximal | Gigli saw | 2 | Distal-proximal | 1.2 |
| 24 | 22 | Male | Humerus | Left | TBL | Medial | Gigli saw | 2 | Distal-proximal | 10.5 |
| 25 | 56 | Male | Tibia | Left | TBL | Distal | Reciprocating saw | 2 | Proximal-distal | 15.2 |
| 26 | 45 | Male | Tibia | Left | HPS | Medial | Reciprocating saw | 1 | Distal-proximal | 1.3 |
| 27 | 23 | Male | Tibia | Right | HPS | Medial | Reciprocating saw | 2 | Distal-proximal | 2.4 |
| 28 | 41 | Male | Tibia | Left | OBL | Distal | Reciprocating saw | 1 | Proximal-distal | 1.2 |
| 29 | 64 | Male | Femur | Left | TBL | Medial | Reciprocating saw | 2 | Distal-proximal | 6.4 |
| 30 | 60 | Male | Femur | Left | HPS | Medial | Reciprocating saw | 1 | Proximal-distal | 8 |
| 31 | 48 | Male | Tibia | Left | TBL | Distal | Reciprocating saw | 2 | Proximal-distal | 1 |
| 32 | 80 | Female | Tibia | Right | HPS | Proximal | Reciprocating saw | 1 | Proximal-distal | 3 |
BT = bone transport, DRL = distraction regenerate length, SPS = septic pseudarthrosis, OBL = bone loss due to osteomyelitis, APS = atrophic pseudarthrosis, TBL = traumatic bone loss, and HPS = hypertrophic pseudarthrosis.
Bone compression was measured in Nm (defined as the torque resulting from a force of 1 Newton applied perpendicular to the end of a moment arm that is 1 meter long55), using a digital dynamometer at 2 points on the bone transport (by having sufficient resistance when the bones come into contact): initial compression immediately after the osteotomy (Fig. 1-A), compression during the latency phase, and final compression when bone-to-bone contact (docking) has been achieved at the end of the distraction phase (Fig. 1-B).
Surgical Procedure
All patients underwent bone transport using a combination of an MIRP and MEF (Fig. 1). Osteotomies were performed with a reciprocating saw, a Gigli saw, or an osteotome (Table I). All patients in the PN group had undergone at least 1 previous treatment, which had failed.
For the PN group (Figs. 2 through 5), the MIRP placement was preceded by simple lavage and the preservation of existing tissue and cells (previously present and newly formed: fibrocartilaginous tissue, inflammatory cells, and undifferentiated cells15,29,30,43,44,46,52) and prophylactic antibiotic treatment. The MIRP system was applied and controlled compression over the docking site was used until consolidation was obtained17,21,24,25,32,41-48,56. In cases in which a previous osteosynthesis attempt had been performed, only the material that hindered MIRP placement, bone shaft alignment, and osteotomy was removed (Figs. 4 and 5).
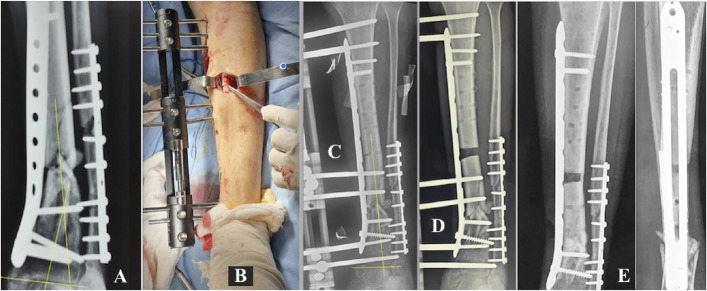
Fig. 5.
Figs. 5-A through 5-E Patient 10. Fig. 5-A Atrophic PN of the right tibia, secondary to failure of fixation with an intramedullary nail. Fig. 5-B Initiation of bone transport with the MIRP. Fig. 5-C Docking at the end of the bone transport process is controlled by means of the MIRP rail, and final compression of 4 Nm and dynamization of the external fixator have been performed. Fig. 5-D Healing of the PN has been achieved by effective compression. Fig. 5-E Completely healed bone after removal of the MIRP.
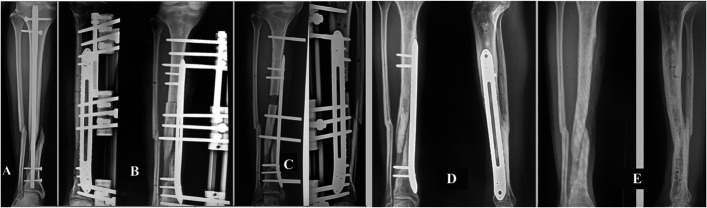
Fig. 4.
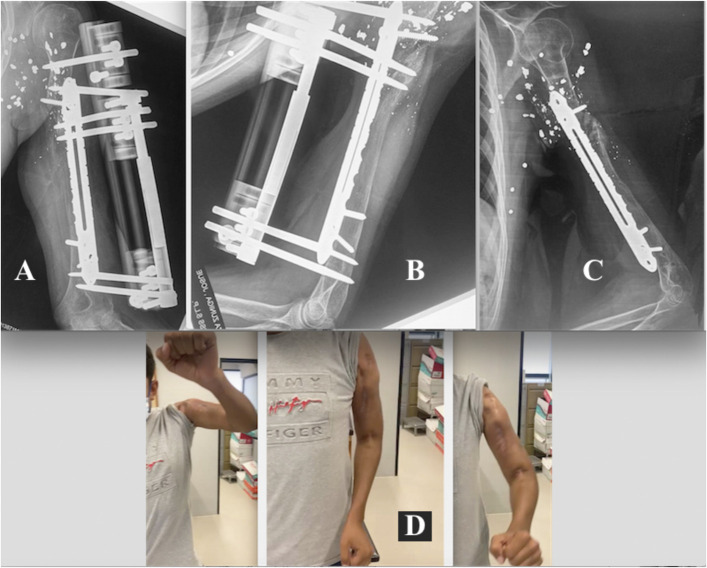
Figs. 4-A through 4-E Patient 15. Fig. 4-A Infected nonunion of the distal third of the left tibia. A previous osteosynthesis attempt failed, with 24° varus deviation of the distal fragment and joint. Fig. 4-B Removal of osteosynthesis material and placement of the MIRP (minimally invasive technique) and the initiation of osteotomy. Fig. 4-C Immediate correction of the tibial and joint axes. Fig. 4-D Control of docking by means of the MIRP rail, with final compression on the infected PN. Fig. 4-E Removal of the device and docking site consolidation healing of the infected PN (4.4-Nm final compression), and formation of osseous trabeculation in the distraction regeneration zone.
For patients with bone loss due to trauma, surgical management did not involve the manipulation of the edges of the affected bone and the tissue interposed between the osseous fragments (Figs. 6, 7, 8, and 9); however, it is an advantage of the technique if the angulation of the fracture is corrected so that the axis of the bone is normalized. For patients with osteomyelitis (Fig. 10), resection of the diseased bone was performed.
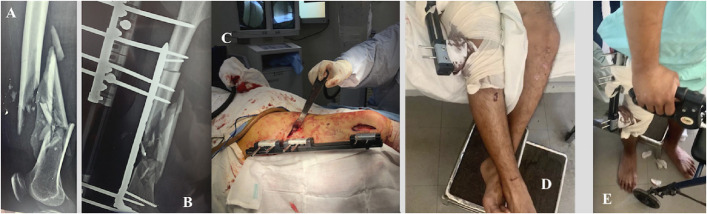
Fig. 6.
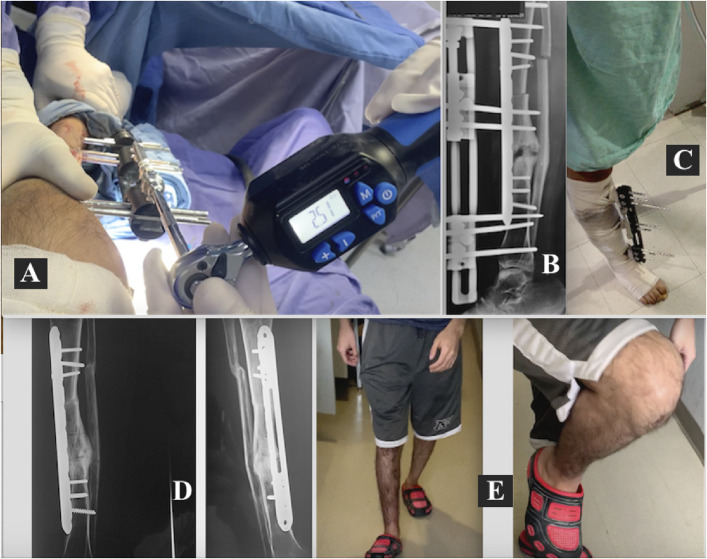
Figs. 6-A through 6-E Patient 16. Fig. 6-A Bone loss (8.5 cm) caused by a firearm projectile in the distal third of the right femur. Fig. 6-B MIRP and MEF application, resulting in immediate correction of femoral length and orientation. Fig. 6-C The minimally invasive technique. Fig. 6-D Passive knee motion is begun 24 hours postoperatively. Fig. 6-E Partial weight-bearing with a walker.
Fig. 7.
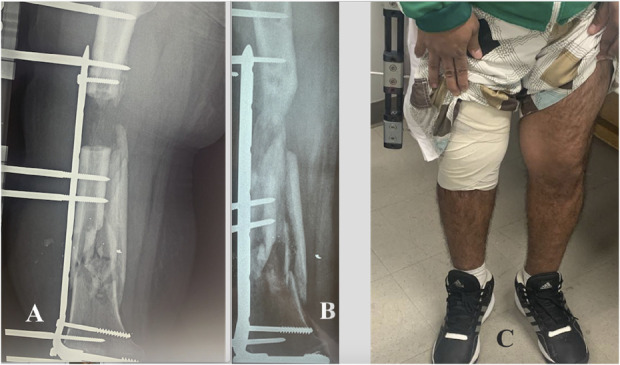
Figs. 7-A, 7-B, and 7-C Patient 16. Fig. 7-A Bone arrival at the docking site 135 days after the surgical procedure. The final compression is 3.4 Nm. Fig. 7-B Bone trabeculation has formed in the DRZ when dynamization begins, separating the external fixator from the bone and then continuing to progressively remove the Schanz pins. Fig. 7-C Recovery of normal leg length, axis, and function, including gait that allowed the patient to carry out activities of daily living with partial support.
Fig. 8.
Figs. 8-A through 8-D Patient 24. Figs. 8-A and 8-B Bone loss (10.5 cm) in the left humerus due to a firearm projectile. Fig. 8-C Initial compression is applied in the latency phase after placement of the MIRP, which has resulted in immediate recovery of bone length, orientation, and stability. The mechanobiological principle refers to the fact that the mechanical stability offered by MIRP does not interrupt the biological process of distraction osteogenesis. Fig. 8-D Immediate rehabilitation with the external fixator.
Fig. 9.
Figs. 9-A through 9-D Patient 24. Fig. 9-A Bone transport has ended with docking at the destination. Final compression is 3.2 Nm. Fig. 9-B The distraction regeneration zone and docking site have consolidated. Fig. 9-C Consolidation has resulted in completely healed bone with normal length and alignment. Fig. 9-D The patient begins to recover the function of the arm progressively. Previously, the arm did not have any function.
Fig. 10.
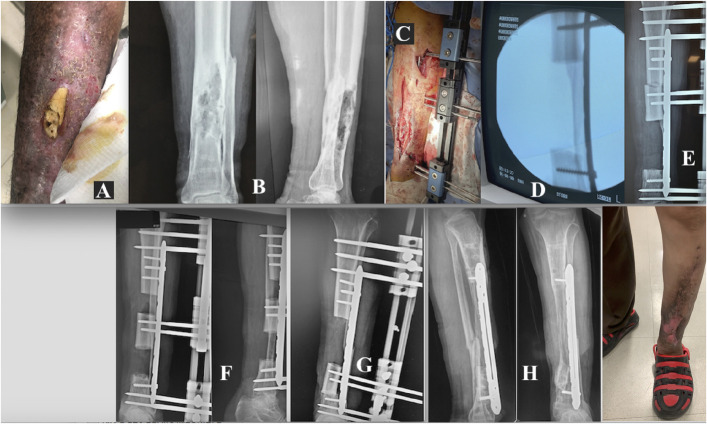
Figs. 10-A through 10-H Patient 2. Fig. 10-A Osteomyelitis in the distal third of the left tibia. Fig. 10-B The involved bone segment. Fig. 10-C Placement of the MIRP. Fig. 10-D Resection of infected and necrotic bone tissue. Fig. 10-E Initial compression of 3.5 Nm. Fig. 10-F The MIRP rail is used for transport of the bone to its final destination and control of its docking. Fig. 10-G Completion of bone transport, with a final compression of 3.1 Nm. Fig. 10-H Complete consolidation has resulted in functional, uninfected bone.
All surgical procedures for both groups of patients were performed by 2 surgeons on the same surgical team of 5 surgeons. All patients underwent early rehabilitation beginning at 24 hours postoperatively (Figs. 3-C, 6-D, 6-E, and 8-D).
Fig. 3.
Figs. 3-A through 3-E Patient 1. Fig. 3-A Compression measurement (in Nm) with a digital dynamometer. Fig. 3-B Initial compression in the process of bone transport has resulted in immediate recovery of the alignment and length of the bone. Fig. 3-C Early rehabilitation, with partial weight-bearing support with a crutch 24 hours after the surgical procedure. Fig. 3-D Eradication of infection and completely healed bone with trabeculae in the DRZ (achieved with 3.2-Nm initial compression) and the DSC (achieved with 3.5-Nm final compression). Fig. 3-E Complete recovery of normal gait and leg shape and length.
Bone transport began at the end of the latency period, at a rate of 1 mm/day (0.25 mm every 6 hours). Once the bone docked at its destination, compression force was increased until resistance to it increased and was measured with an objective measurement to achieve DSC (Fig. 1). The separation of the external fixator from the bone is part of the dynamization57 that began after the arrival of the bone at the docking site. The removal of the external fixator was performed once the bone transport was concluded and bone mineralization began in the DRZ, indicating the beginning of the DSC (Figs. 3, 4-D, 4-E, 5-C, 5-D, 7, 9-A, 9-B, and 9-C, 10-G, and 10-H). External fixator dynamization and removal occurred in the surgeon’s office.
Primary Outcome
The clinical team in charge of the surgical procedures performed data collection and evaluation of the intervention outcomes. In all cases, rehabilitation and follow-up were possible until complete resolution of the condition that had prompted the surgical procedure. The postoperative data collected included whether the involved location was in the proximal, medial, or distal portion of the bone (Table I); the direction of the bone transport (proximal to distal or vice versa); initial compression (Fig. 1-A); and final compression (Fig. 1-B).
Anteroposterior and lateral radiographs were made every 3 to 4 weeks during the distraction period (Fig. 1-B) and every month during the consolidation period (Fig. 1-C) until bone continuity was reestablished. Bone continuity was defined as sufficient consolidation in the DRZ and solid docking site union with signs of osseous consolidation in at least 3 cortices (Figs. 1-C, 3-D, 5-D, 5-E, 9-C, and 10-H).
Functional, clinical, and radiographic outcomes were evaluated as excellent, good, fair, or poor based on the Association for the Study and Application of the Method of Ilizarov (ASAMI) criteria established by Paley et al.22,37-40,42,49,58-61 (Table II). Complications were categorized as minor or major22,23. Each patient underwent an additional 6-month follow-up after the consolidation time (CT) of the regenerate was reached (Fig. 1-C, Table III).
TABLE II.
ASAMI Score for Functional-Clinical and Osseous Results in the Limbs Evaluated in the Study (Tibia, Femur, and Humerus)*,†
| Score | Osseous Result | Functional Result |
|---|---|---|
| Excellent | Osseous union, no infection, deformity <7°, limb-length discrepancy <2.5 cm | Ability to perform previous activities of daily living, no pain or mild pain |
| Good | Osseous union, failure to meet 1 of the other criteria | No limp, no soft-tissue sympathetic dystrophy, knee or ankle or shoulder or elbow joint contracture <5°, loss of joint motion <15° |
| Fair | Osseous union, failure to meet 2 of the other criteria | Ability to perform most activities of daily living with minimal difficulty, no pain or mild pain, failure to meet 2 of the other criteria |
| Poor | Nonunion or refracture, failure to meet 3 of the other criteria | Significant pain requiring narcotics, failure to meet 3 of the other criteria |
Data in this table were obtained from: Paley D, Catagni MA, Argnani F, Villa A, Benedetti GB, Cattaneo R. Ilizarov treatment of tibial nonunions with bone loss. Clin Orthop Relat Res. 1989 Apr;(241):146-6590.
The upper limb included the humerus.
TABLE III.
Outcomes of the Patients
| Patient No. | Total Follow-up (days) | Functional Result | Osseous Result | Complications* |
|---|---|---|---|---|
| 1 | 363 | Excellent | Excellent | None |
| 2 | 421 | Excellent | Excellent | Minor |
| 3 | 362 | Excellent | Excellent | None |
| 4 | 401 | Excellent | Excellent | None |
| 5 | 297 | Excellent | Excellent | None |
| 6 | 266 | Excellent | Excellent | Minor |
| 7 | 347 | Excellent | Excellent | None |
| 8 | 345 | Excellent | Excellent | Minor |
| 9 | 354 | Good | Excellent | None |
| 10 | 402 | Excellent | Excellent | Minor |
| 11 | 272 | Good | Excellent | Minor |
| 12 | 357 | Excellent | Excellent | None |
| 13 | 314 | Excellent | Excellent | None |
| 14 | 371 | Excellent | Excellent | Minor |
| 15 | 327 | Excellent | Excellent | None |
| 16 | 606 | Excellent | Good | None |
| 17 | 285 | Excellent | Excellent | None |
| 18 | 315 | Excellent | Excellent | Minor |
| 19 | 374 | Excellent | Excellent | None |
| 20 | 294 | Excellent | Excellent | None |
| 21 | 283 | Poor | Poor | Major |
| 22 | 385 | Good | Excellent | None |
| 23 | 299 | Good | Good | None |
| 24 | 478 | Excellent | Excellent | None |
| 25 | 541 | Excellent | Excellent | None |
| 26 | 327 | Excellent | Excellent | None |
| 27 | 323 | Excellent | Excellent | None |
| 28 | 371 | Excellent | Excellent | None |
| 29 | 428 | Excellent | Excellent | None |
| 30 | 281 | Excellent | Excellent | None |
| 31 | 328 | Excellent | Excellent | None |
| 32 | 290 | Excellent | Excellent | None |
Classified according to the ASAMI criteria.
Secondary Outcomes
In addition to the functional and radiographic results, we recorded the docking time, defined as when the transported bone reaches its final destination (the culmination of the distraction phase, and the initiation of the final compression). The CT is the time that it takes to consolidate the DRZ, and starts at the end of the distraction phase. The external fixation time (EFT) is the time spent in the fixator before its removal—in our study, until DSC occurs. The DSC time was recorded as the time (in days) that it takes to consolidate the docking site, measured from when the final compression is begun at the docking site until DSC is obtained. The healing time (HT) was recorded as the time elapsed between the placement of the external fixator and the end of the consolidation phase (marked by callus in at least 3 cortices at the docking site and distraction zone callus). The HT is the sum of the latency phase (days of initial compression), the distraction phase, and the consolidation phase1,22,37-39,42,49,58-61 (Fig. 1).
Finally, we calculated (1) the external fixation index (EFI) as the duration of external fixation in months divided by the lengthening in centimeters (EFT/distraction regenerate length [DRL]), and (2) the healing index (HI) as the time for complete bone healing in months divided by the lengthening in centimeters (HT/DRL)1,22,37-39,42,49,58-61 (Table IV).
TABLE IV.
Compression, Docking, and Healing Indices of the Patients*
| Patient No. | IC (Nm) | FC (Nm) | DT (days) | DSCT (days) | EFT (days) | HT (days) | CT (days) | EFI (mo/cm) | HI (mo/cm) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.2 | 3.5 | 65 | 58 | 133 | 183 | 108 | 1.84 | 2.5 |
| 2 | 3.5 | 3.1 | 126 | 39 | 175 | 241 | 105 | 0.79 | 1.08 |
| 3 | 3.2 | 3.5 | 55 | 34 | 122 | 182 | 117 | 1.62 | 2.42 |
| 4 | 3.0 | 3.2 | 110 | 40 | 160 | 221 | 101 | 0.82 | 1.13 |
| 5 | 3.1 | 3 | 30 | 29 | 69 | 117 | 84 | 1.15 | 1.95 |
| 6 | 2.2 | 3.8 | 16 | 20 | 56 | 86 | 129 | 1.03 | 1.59 |
| 7 | 3.2 | 4.0 | 42 | 55 | 107 | 167 | 115 | 2.3 | 3.7 |
| 8 | 2.3 | 3.2 | 15 | 33 | 58 | 165 | 140 | 1.93 | 5.5 |
| 9 | 3.5 | 3.8 | 74 | 30 | 114 | 174 | 90 | 0.76 | 1.1 |
| 10 | 3.3 | 4.0 | 66 | 66 | 142 | 222 | 146 | 1.57 | 2.4 |
| 11 | 3.1 | 3.0 | 15 | 30 | 56 | 92 | 69 | 1.5 | 2.5 |
| 12 | 2.2 | 3.8 | 42 | 56 | 108 | 177 | 125 | 2.0 | 3.2 |
| 13 | 4.1 | 4.2 | 25 | 34 | 74 | 134 | 99 | 1.64 | 2.9 |
| 14 | 2.4 | 2.9 | 29 | 70 | 116 | 191 | 150 | 3.5 | 5.7 |
| 15 | 3.7 | 4.4 | 25 | 50 | 90 | 147 | 110 | 2.0 | 3.2 |
| 16 | 5.0 | 3.4 | 135 | 62 | 220 | 426 | 273 | 0.86 | 1.6 |
| 17 | 3.3 | 3.0 | 10 | 30 | 50 | 105 | 85 | 2.0 | 4.3 |
| 18 | 2.4 | 3.0 | 15 | 30 | 55 | 135 | 132 | 1.83 | 4.5 |
| 19 | 4.7 | 3.4 | 37 | 30 | 84 | 194 | 140 | 2.0 | 4.6 |
| 20 | 3.6 | 4.5 | 15 | 28 | 53 | 114 | 89 | 1.7 | 3.8 |
| 21 | 3.0 | 2.3 | 17 | NA | 65 | 103 | 76 | 1.9 | 3.0 |
| 22 | 3.4 | 3.2 | 99 | 40 | 149 | 205 | 96 | 0.82 | 1.1 |
| 23 | 3.2 | 2.9 | 27 | 22 | 60 | 119 | 90 | 1.6 | 3.3 |
| 24 | 3.3 | 3.2 | 179 | 55 | 248 | 298 | 105 | 0.78 | 0.94 |
| 25 | 3.5 | 3.1 | 165 | 66 | 256 | 361 | 180 | 0.56 | 0.79 |
| 26 | 4.0 | 3.2 | 18 | 32 | 70 | 147 | 109 | 1.79 | 3.7 |
| 27 | 3.5 | 3.4 | 14 | 30 | 59 | 143 | 102 | 1.96 | 4.7 |
| 28 | 3.2 | 3.1 | 96 | 40 | 147 | 191 | 85 | 0.76 | 0.9 |
| 29 | 3.1 | 3.2 | 128 | 50 | 192 | 248 | 110 | 0.8 | 1.0 |
| 30 | 2.8 | 3.2 | 16 | 30 | 56 | 101 | 75 | 1.86 | 3.3 |
| 31 | 2.9 | 3.2 | 48 | 30 | 88 | 148 | 90 | 0.97 | 1.6 |
| 32 | 3.0 | 3.5 | 8 | 35 | 53 | 110 | 92 | 2.5 | 5.2 |
IC = initial compression, FC = final compression, DT = docking time, DSCT = docking site consolidation time, EFT = external fixation time, HT = healing time, CT = consolidation time of the regenerate, EFI = external fixation index, HI = healing index, and NA = not applicable.
Statistical Analysis
We used the Mann-Whitney U and Kruskal-Wallis tests to compare continuous variables between and within groups. The Pearson r value was used to evaluate correlations. In all analyses, the significance level used was p < 0.05. The analysis was performed using RStudio (version 2023.06.1+524; The R Foundation).
Results
The study included 32 patients; 28 (88%) were male, and 4 (13%) were female. The patient ages ranged from 20 to 80 years, with a mean (and standard deviation) of 44.93 ± 16.21 years. The group with bone loss included 10 patients (31%), all male. Seven patients in this group had traumatic bone loss, and the remaining 3 had osteomyelitis. The PN group included 22 patients, of whom 14 had the hypertrophic form, 4 had the atrophic form, and 4 had the infectious form. The involved bone was the tibia in 23 patients (72%), the humerus in 5 (16%), and the femur in 4 (13%) (Table I).
Primary Outcomes: Compression Values and Functional and Radiographic Outcomes
The median initial compression was 3.2 Nm and the median final compression was 3.4 Nm, with no significant difference between the 2 patient groups or among their subgroups (Table V). Radiographic results were good or excellent in 31 patients (97%) and poor in 1 patient (Patient 21; 3%). Functional results were excellent in 27 patients (84%), good in 4 patients (13%), and poor in 1 patient (3%). Seven minor complications were observed: delayed consolidation in the DRZ in 4 patients (13%) and lack of skin closure of <2 cm in the wound when inserting the plate resulting in MIRP removal after complete healing of the bone in 3 patients (9%) (Table III).
TABLE V.
Comparison of Parameters Between and Among Groups of Patients*
| Bone Loss Group | PN Group | Between-Group P Value‡ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OBL | TBL | P Value† | Group Median | APS | HPS | SPS | P Value† | Group Median | ||
| ICBT (Nm) | 3.4 | 3.3 | 0.8175 | 3.35 | 3.2 | 3.1 | 3.2 | 0.9338 | 3.2 | 0.2885 |
| FCBT (Nm) | 3.1 | 3.2 | 0.1390 | 3.2 | 3.65 | 3.2 | 3.75 | 0.5504 | 3.4 | 0.4351 |
| DT (days) | 99 | 128 | 0.5167 | 118 | 48.5 | 15.5 | 35.5 | 0.0029 § | 21.5 | <0.001 § |
| DSCT (days) | 40 | 50 | 0.4875 | 40 | 45 | 30 | 56.5 | 0.0099 § | 32 | 0.0962 |
| EFT (days) | 149 | 192 | 0.5167 | 167.5 | 115 | 57 | 111.5 | 0.0022 § | 67 | <0.001 § |
| HT (days) | 205 | 248 | 0.5167 | 231 | 179.5 | 115.5 | 175 | 0.0147 § | 139 | 0.0002 § |
| CT (days) | 96 | 105 | 0.3021 | 103 | 121 | 95.5 | 112.5 | 0.1826 | 108.5 | 0.9838 |
| EFI (mg/cm) | 0.79 | 0.8 | 0.8186 | 0.795 | 1.61 | 1.845 | 2.15 | 0.0875 | 1.85 | <0.001 § |
| HI (mg/cm) | 1.08 | 1.1 | 0.5664 | 1.09 | 2.81 | 3.75 | 3.45 | 0.3080 | 3.3 | <0.001 § |
PN = pseudarthrosis-nonunion, OBL = bone loss due to osteomyelitis, TBL = traumatic bone loss, APS = atrophic pseudarthrosis, HPS = hypertrophic pseudarthrosis, SPS = septic pseudarthrosis, ICBT = initial compression during bone transport, FCBT = final compression during bone transport, DT = docking time, DSCT = docking site consolidation time, EFT = external fixation time, HT = healing time, CT = consolidation time, EFI = external fixation index, and HI = healing index.
Mann-Whitney U test.
Kruskal-Wallis test.
Significant.
It is important to note that 6 (19%) of our 32 patients were ≥60 years of age. All of them underwent treatment for conditions in the lower limbs and had excellent osseous results and good or excellent functional results. Only 1 of these patients had a minor complication that did not require a new surgical procedure.
Only 1 patient had a major complication (Patient 21, Tables I and IV). This patient did not achieve DSC because he did not have alignment in the bone axis, had final compression of the bone that was <2.9 Nm, and had segments with <50% of the contact surface in the docking site. As a result, it was necessary to repeat the surgical procedure to correct the alignment of the bone. The final compression during the repat procedure was 3.9 Nm, and DSC was achieved, with consolidation involving >50% of the contact surface at the docking site.
Secondary Outcomes
The docking time (p < 0.001), healing time (p < 0.001), and EFT (p = 0.0002) were significantly shorter for the patients in the PN group compared with those in the bone loss group. The HI and EFI were significantly higher in the patients in the PN group (p < 0.001 for both) (Table V).
Within the bone loss group, no significant differences were observed between patients with trauma and those with osteomyelitis. However, within the PN group, the docking time, DSC time, EFT, and healing time were significantly lower in patients with hypertrophic PN (Tables IV and V).
Discussion
Although bone transport has been effectively applied for quite some time, direct measurement of compression applied at the beginning and end of bone transport can be challenging. Currently, the compressive force applied during the bone transport process is based mainly on subjective criteria, which may lead to a higher risk of treatment failure due to a defective DRZ and a failure to achieve DSC1,9,17,20-24,34-37,40. To our knowledge, this is the first study to report direct compression measurements during the bone transport process.
This study reports on the use of bone transport for the surgical management of 2 distinct pathologies: PN and bone loss. In both, the bone transport goes through the same phases of latency, distraction, and consolidation, and it is therefore possible to optimize their management by taking advantage of the stability provided by an MIRP. Likewise, these common characteristics make it possible to study the effect of bone compression on achieving the DRZ and DSC. The objective of bone transport is to achieve the DSC; however, in the treatment of PN, maintaining compression in the final phase of distraction, until achieving the DSC, produces healing and consolidation. However, this compression must have the characteristics of what we call effective compression.
In our study, we identified effective initial and final bone compression levels objectively as those that always produced 2 results during bone transport: (1) consolidation of the DRZ without complications (which was found to occur consistently at an initial compression of ≥3.2 Nm), and (2) achieving the DSC when there was adequate alignment of the bone axis, stability during distraction, and contact at >50% of the docking site (which occurred consistently at a final compression of ≥2.9 Nm). All patients achieved the DSC without subsequent procedures when the identified effective compression level of ≥2.9 Nm was applied during the final stage of distraction. Effective bone compression also led to few complications and subsequent surgical procedures compared with previous studies17,22-24,40,62-65.
The EFT was shorter among patients with bone loss than that reported for the Ilizarov technique. Moreover, the mean EFI of patients with bone loss was 0.79 months/cm, <50% of the 1.7 months/cm reported for the use of the Ilizarov circular ring45,58 and comparable with that reported for bone transport using an intramedullary nail61,66 or minimally invasive plate osteosynthesis37,46,59.
Notably, we observed that the placement of this device is well tolerated even among elderly patients. The 6 patients between 60 and 80 years of age had no loosening of the pins, which can be attributed to the adequate distribution of loads (by the MIRP and MEF) and the improvements in angular stability offered by the locking screw and the locking Schanz pin67-69.
Aside from allowing the direct measurement of compression applied during bone transport, the MIRP-MEF combination facilitates control and stability at the proximal and distal sites in the bone. The combination provides direct fixation, neutralizing the loads during the distraction phase and the negative effect of cantilever bending that would deliver asymmetric compression to the fracture site52,57,70-76. Furthermore, these characteristics spare the patient from pain and promote fibrous union during consolidation, resulting in the prevention of sagittal deformity by adequately protecting the distraction callus during early mobilization37,58-63,77-82.
An additional benefit of MIRP is the transport of the bone segment from its initial site to its final site on a rail, which avoids deviation from the bone axis and thus displacement at the docking site, and enables an uninterrupted distraction osteogenesis cascade37,70,71,83-89. That additional advantage, added to its compact design (which reduces the patient’s loss of mobility), makes this combination a better alternative than the Ilizarov device for patients needing bone transport in an upper limb20,22-24,34,54,63,66,75,80.
A potential source of bias in this study is the evaluation of patients by the surgeons who performed the procedures. However, radiographic evidence confirmed the results of the evaluation. Despite limitations such as a small sample size and the lack of comparison with traditional treatment, the results suggest that combining MIRP and MEF in distraction osteogenesis, along with the use of compression forces within specific ranges, offers several advantages over other procedures, including structural stability, ease of use, lower failure rates, more reliable achievement of the DSC, and shorter treatment times. However, these conclusions should be corroborated in further studies comparing the MIRP-MEF combination with the conventional technique (the Ilizarov circular ring).
In conclusion, all patients who received initial compression of ≥3.2 Nm had consolidation of the DRZ without complications such as delayed consolidation, a distraction zone defect, axial deviation, retraction of the transported segment, or refracture. Additionally, our data suggest that the application of this device with a minimum final compression of ≥2.9 Nm achieves the DSC in patients who have adequate alignment of the bone axis, stability during distraction, and contact at >50% of the docking site, and avoids the development of complications such as delayed consolidation, nonunion, and retraction of the transported segment6-9,20,22,24,35,37,40,58,60,73,89. The initial and final compression values of ≥3.2 and ≥2.9 Nm can therefore be termed effective compression levels.
We hope to contribute to optimizing a surgical technique described >40 years ago, making it more attractive to a new generation of orthopaedists to resolve complications related to fracture (PN, osteomyelitis, and bone loss).
Footnotes
Investigation performed at the Orthopedic Surgery Department, Hospital Cl 50, Mexican Social Security Institute (IMSS) and TUORTOPEDISTA, Medical Orthopaedic Specialty Group, San Luis Potosí, México
Disclosure: No external funding was received for this work. The Article Processing Charge for open access publication was funded by a donation from S.R. Jorge Trujillo. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSOA/A716).
Contributor Information
E.P. López Avendaño, Email: edgardopaul@gmail.com.
I. Arámbula Rodríguez, Email: montsesamurai@yahoo.com.
C. García López, Email: drcgarciatyo@gmail.com.
L. Flores Huerta, Email: chivas.lf71@gmail.com.
References
- 1.Noonan KJ, Leyes M, Forriol F, Cañadell J. Distraction osteogenesis of the lower extremity with use of monolateral external fixation. A study of two hundred and sixty-one femora and tibiae. J Bone Joint Surg Am. 1998 Jun;80(6):793-806. [DOI] [PubMed] [Google Scholar]
- 2.Ilizarov GA. The principles of the Ilizarov method. Bull Hosp Joint Dis Orthop Inst. 1988. Spring;48(1):1-11. [PubMed] [Google Scholar]
- 3.Cierny G, Zorn KE. Segmental tibial defects. Comparing conventional and Ilizarov methodologies. Clin Orthop Relat Res. 1994 Apr;(301):118-23. [PubMed] [Google Scholar]
- 4.Green SA. Skeletal defects. A comparison of bone grafting and bone transport for segmental skeletal defects. Clin Orthop Relat Res. 1994 Apr;(301):111-17. [PubMed] [Google Scholar]
- 5.Aronson J. Limb-lengthening, skeletal reconstruction, and bone transport with the Ilizarov method. J Bone Joint Surg Am. 1997 Aug;79(8):1243-58. [DOI] [PubMed] [Google Scholar]
- 6.de Pablos J, Barrios C, Alfaro C, Cañadell J. Large experimental segmental bone defects treated by bone transportation with monolateral external distractors. Clin Orthop Relat Res. 1994 Jan;(298):259-65. [PubMed] [Google Scholar]
- 7.Baumgart R, Schuster B, Baumgart T. [Callus distraction and bone transport in the treatment of bone defects]. Orthopade. 2017 Aug;46(8):673-80. German. [DOI] [PubMed] [Google Scholar]
- 8.Veselý R, Procházka V. [Callus distraction in the treatment of post-traumatic defects of the femur and tibia]. Acta Chir Orthop Traumatol Cech. 2016;83(6):388-92. Czech. [PubMed] [Google Scholar]
- 9.Sigmund IK, Ferguson J, Govaert GAM, Stubbs D, McNally MA. Comparison of Ilizarov bifocal, acute shortening and relengthening with bone transport in the treatment of infected, segmental defects of the tibia. J Clin Med. 2020 Jan 28;9(2):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aronson J, Johnson E, Harp JH. Local bone transportation for treatment of intercalary defects by the Ilizarov technique. Biomechanical and clinical considerations. Clin Orthop Relat Res. 1989 Jun;(243):71-9. [PubMed] [Google Scholar]
- 11.Murray JH, Fitch RD. Distraction histiogenesis: principles and indications. J Am Acad Orthop Surg. 1996 Nov;4(6):317-27. [DOI] [PubMed] [Google Scholar]
- 12.Einhorn TA, Lee CA. Bone regeneration: new findings and potential clinical applications. J Am Acad Orthop Surg. 2001 May-Jun;9(3):157-65. [DOI] [PubMed] [Google Scholar]
- 13.Watson JT. Distraction osteogenesis. J Am Acad Orthop Surg. 2006;14(10):S168-74. [DOI] [PubMed] [Google Scholar]
- 14.Funk JF, Krummrey G, Perka C, Raschke MJ, Bail HJ. Distraction osteogenesis enhances remodeling of remote bones of the skeleton: a pilot study. Clin Orthop Relat Res. 2009 Dec;467(12):3199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marongiu G, Dolci A, Verona M, Capone A. The biology and treatment of acute long-bones diaphyseal fractures: overview of the current options for bone healing enhancement. Bone Rep. 2020 Jan 28;12:100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gubin AV, Borzunov DY, Marchenkova LO, Malkova TA, Smirnova IL. Contribution of G.A. Ilizarov to bone reconstruction: historical achievements and state of the art. Strategies Trauma Limb Reconstr. 2016 Nov;11(3):145-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmal H, Brix M, Bue M, Ekman A, Ferreira N, Gottlieb H, Kold S, Taylor A, Toft Tengberg P, Ban I; Danish Orthopaedic Trauma Society. Nonunion - consensus from the 4th Annual Meeting of the Danish Orthopaedic Trauma Society. EFORT Open Rev. 2020 Jan 29;5(1):46-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lasanianos NG, Kanakaris NK, Giannoudis PV. Current management of long bone large segmental defects. Orthop Trauma. 2010;24(2):149-63. [Google Scholar]
- 19.Reichert JC, Saifzadeh S, Wullschleger ME, Epari DR, Schütz MA, Duda GN, Schell H, van Griensven M, Redl H, Hutmacher DW. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials. 2009 Apr;30(12):2149-63. [DOI] [PubMed] [Google Scholar]
- 20.Spiegl U, Pätzold R, Friederichs J, Hungerer S, Militz M, Bühren V. Clinical course, complication rate and outcome of segmental resection and distraction osteogenesis after chronic tibial osteitis. Injury. 2013 Aug;44(8):1049-56. [DOI] [PubMed] [Google Scholar]
- 21.Tetsworth K, Paley D, Sen C, Jaffe M, Maar DC, Glatt V, Hohmann E, Herzenberg JE. Bone transport versus acute shortening for the management of infected tibial non-unions with bone defects. Injury. 2017 Oct;48(10):2276-84. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Yushan M, Liu Z, Liu J, Ma C, Yusufu A. Complications of bone transport technique using the Ilizarov method in the lower extremity: a retrospective analysis of 282 consecutive cases over 10 years. BMC Musculoskelet Disord. 2020 Jun 6;21(1):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paley D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop Relat Res. 1990 Jan;(250):81-104. [PubMed] [Google Scholar]
- 24.Iacobellis C, Berizzi A, Aldegheri R. Bone transport using the Ilizarov method: a review of complications in 100 consecutive cases. Strategies Trauma Limb Reconstr. 2010 Apr;5(1):17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilizarov GA. [Basic principles of transosseous compression and distraction osteosynthesis]. Ortop Travmatol Protez. 1971 Nov;32(11):7-15. Russian. [PubMed] [Google Scholar]
- 26.Ilizarov GA, Kaplunov AG, Degtiarev VE, Lediaev VI. Lechenie lozhnykh sustavov i nesrosshikhsia perelomov, oslozhnennykh gnoĭnoĭ infektsieĭ, metodom kompressionno-distraktsionnogo osteosinteza [Treatment of pseudarthroses and ununited fractures, complicated by purulent infection, by the method of compression-distraction osteosynthesis]. Ortop Travmatol Protez. 1972 Nov;33(11):10-14. Russian. [PubMed] [Google Scholar]
- 27.Lavini F, Dall’Oca C, Bartolozzi P. Bone transport and compression-distraction in the treatment of bone loss of the lower limbs. Injury. 2010 Nov;41(11):1191-5. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan A, Pamecha C, Patwa JJ. Modified Ilizarov technique for infected nonunion of the femur: the principle of distraction-compression osteogenesis. J Orthop Surg (Hong Kong). 2006 Dec;14(3):265-72. [DOI] [PubMed] [Google Scholar]
- 29.Rajiv K, Neha A, Girish M, Manish P. Management of infected nonunions of the femur and tibia with compression-distraction osteogenesis coupled with modern methods at a tertiary military establishment: a prospective case series. Curr Orthop Pract. 2021 Jan/Feb;32(1):23-31. [Google Scholar]
- 30.Subramanyam KN, Mundargi AV, Reddy PS, Bhoskar RN. Compression osteosynthesis - an effective solution for hypertrophic nonunion of tibia in children. J Orthop Case Rep. 2018 Nov-Dec;8(6):61-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magadum MP, Basavaraj Yadav CM, Phaneesha MS, Ramesh LJ. Acute compression and lengthening by the Ilizarov technique for infected nonunion of the tibia with large bone defects. J Orthop Surg (Hong Kong). 2006 Dec;14(3):273-79. [DOI] [PubMed] [Google Scholar]
- 32.Meselhy MA, Kandeel M, Halawa AS, Siger MS. Infected tibial nonunion: assessment of compression distraction Ilizarov technique without debridement. Orthop Traumatol Surg Res. 2021 Dec;107(8):102881. [DOI] [PubMed] [Google Scholar]
- 33.Ma H, Zhao L, Long X, Liu X, Liu L. Ilizarov technique with compression and distraction osteogenesis for treatment of traumatic femoral shaft defects. Chinese Journal of Trauma. 2021;(12):708-14. [Google Scholar]
- 34.Wu Y, Yin Q, Rui Y, Sun Z, Gu S. Ilizarov technique: bone transport versus bone shortening-lengthening for tibial bone and soft-tissue defects. J Orthop Sci. 2018 Mar;23(2):341-45. [DOI] [PubMed] [Google Scholar]
- 35.Simpson AH, Kenwright J. Fracture after distraction osteogenesis. J Bone Joint Surg Br. 2000 Jul;82(5):659-65. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Liu T, Li Z, Peng W. Reconstruction with callus distraction for nonunion with bone loss and leg shortening caused by suppurative osteomyelitis of the femur. J Bone Joint Surg Br. 2007 Nov;89(11):1509-14. [DOI] [PubMed] [Google Scholar]
- 37.Oh CW, Apivatthakakul T, Oh JK, Kim JW, Lee HJ, Kyung HS, Baek SG, Jung GH. Bone transport with an external fixator and a locking plate for segmental tibial defects. Bone Joint J. 2013 Dec;95-B(12):1667-72. [DOI] [PubMed] [Google Scholar]
- 38.Yin P, Zhang L, Li T, Zhang L, Wang G, Li J, Liu J, Zhou J, Zhang Q, Tang P. Infected nonunion of tibia and femur treated by bone transport. J Orthop Surg Res. 2015 Apr 10;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sigmund IK, Ferguson J, Govaert GAM, Stubbs D, McNally MA. Comparison of Ilizarov bifocal, acute shortening and relengthening with bone transport in the treatment of infected, segmental defects of the tibia. J Clin Med. 2020 Jan 28;9(2):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu JZ, Shi ZY, Yang CZ, Wang RC, Wu H, Zhu CM, Xie Y, Mao CH. Clinical study of bone transport combined with bone graft and internal fixation at the docking site in the treatment of large segmental bone defect in lower limb. Chinese Journal of Orthopaedics. 2018;12:280-7. [Google Scholar]
- 41.Greifenteiner H, Klarmann O, Wustmann O. Osteosynthesis by double-wire clamp for the treatment of pseudarthroses. Zenttralbl Chir. 1948;73:959-70. [Google Scholar]
- 42.Yin P, Ji Q, Li T, Li J, Li Z, Liu J, Wang G, Wang S, Zhang L, Mao Z, Tang P. A systematic review and meta-analysis of Ilizarov methods in the treatment of infected nonunion of tibia and femur. PLoS One. 2015 Nov 3;10(11):e0141973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989 Jan;(238):249-81. [PubMed] [Google Scholar]
- 44.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989 Feb;(239):263-85. [PubMed] [Google Scholar]
- 45.Zhang Q, Zhang W, Zhang Z, Zhang L, Chen H, Hao M, Deng J, Tang P. Femoral nonunion with segmental bone defect treated by distraction osteogenesis with monolateral external fixation. J Orthop Surg Res. 2017 Nov 25;12(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber BG, Cech O. Pseudarthrosis: Pathophysiology Biomechanics Therapy Results. Bern: Hans Huber; 1976. [Google Scholar]
- 47.Paley D, Catagni MA, Argnani F, Villa A, Benedetti GB, Cattaneo R. Ilizarov treatment of tibial nonunions with bone loss. Clin Orthop Relat Res. 1989 Apr;(241):146-65. [PubMed] [Google Scholar]
- 48.Cattaneo R, Catagni M, Johnson EE. The treatment of infected nonunions and segmental defects of the tibia by the methods of Ilizarov. Clin Orthop Relat Res. 1992 Jul;(280):143-52. [PubMed] [Google Scholar]
- 49.Dendrinos GK, Kontos S, Lyritsis E. Use of the Ilizarov technique for treatment of non-union of the tibia associated with infection. J Bone Joint Surg Am. 1995 Jun;77(6):835-46. [DOI] [PubMed] [Google Scholar]
- 50.Xie J, Zhao G, Yasheng T, Chen H, Amuti N, Maimaitirexiati M, Yibulayinmu A, Cao M, Yusufu A. Ilizarov bone transport to treat infected nonunion of long bones: a multicenter retrospective cohort study. J Int Med Res. 2021 Mar;49(3):3000605211002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Catagni MA, Guerreschi F, Holman JA, Cattaneo R. Distraction osteogenesis in the treatment of stiff hypertrophic nonunions using the Ilizarov apparatus. Clin Orthop Relat Res. 1994 Apr;(301):159-63. [PubMed] [Google Scholar]
- 52.García-Cimbrelo E, Martí-González JC. Circular external fixation in tibial nonunions. Clin Orthop Relat Res. 2004 Feb;(419):65-70. [PubMed] [Google Scholar]
- 53.Schuelke J, Meyers N, Reitmaier S, Klose S, Ignatius A, Claes L. Intramembranous bone formation after callus distraction is augmented by increasing axial compressive strain. PLoS One. 2018 Apr 6;13(4):e0195466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013 Nov 27;310(20):2191-4. [DOI] [PubMed] [Google Scholar]
- 55.Knight RD. Work. In: Physics for Scientists and Engineers: A Strategic Approach. 2nd ed. San Francisco: Pearson Addison-Wesley; 2008. [Google Scholar]
- 56.Ilizarov GA. Transosseous Osteosynthesis: Theoretical and Clinical Aspects of the Regeneration and Growth of Tissue. New York: Springer; 1992. [Google Scholar]
- 57.Fragomen AT, Rozbruch SR. The mechanics of external fixation. HSS J. 2007 Feb;3(1):13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Apivatthakakul T, Arpornchayanon O. Minimally invasive plate osteosynthesis (MIPO) combined with distraction osteogenesis in the treatment of bone defects. A new technique of bone transport: a report of two cases. Injury. 2002 Jun;33(5):460-5. [DOI] [PubMed] [Google Scholar]
- 59.Oh CW, Song HR, Kim JW, Choi JW, Min WK, Park BC. Limb lengthening with a submuscular locking plate. J Bone Joint Surg Br. 2009 Oct;91(10):1394-9. [DOI] [PubMed] [Google Scholar]
- 60.Lu Y, Ma T, Ren C, Li Z, Sun L, Xue H, Li M, Zhang K, Zhang C, Wang Q. Treatment of segmental tibial defects by bone transport with circular external fixation and a locking plate. J Int Med Res. 2020 Apr;48(4):300060520920407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paley D, Herzenberg JE, Paremain G, Bhave A. Femoral lengthening over an intramedullary nail. A matched-case comparison with Ilizarov femoral lengthening. J Bone Joint Surg Am. 1997 Oct;79(10):1464-80. [DOI] [PubMed] [Google Scholar]
- 62.Aronson J, Harp JH. Mechanical forces as predictors of healing during tibial lengthening by distraction osteogenesis. Clin Orthop Relat Res. 1994 Apr;(301):73-9. [PubMed] [Google Scholar]
- 63.Fischgrund J, Paley D, Suter C. Variables affecting time to bone healing during limb lengthening. Clin Orthop Relat Res. 1994 Apr;(301):31-7. [PubMed] [Google Scholar]
- 64.Hantes ME, Malizos KN, Xenakis TA, Beris AE, Mavrodontidis AN, Soucacos PN. Complications in limb-lengthening procedures: a review of 49 cases. Am J Orthop (Belle Mead NJ). 2001 Jun;30(6):479-83. [PubMed] [Google Scholar]
- 65.Ma Y, Yin Q, Wu Y, Wang Z, Sun Z, Gu S, Rui Y, Han X. Retraction of transporting bone segment during Ilizarov bone transport. BMC Musculoskelet Disord. 2020 Oct 26;21(1):704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oh CW, Song HR, Roh JY, Oh JK, Min WK, Kyung HS, Kim JW, Kim PT, Ihn JC. Bone transport over an intramedullary nail for reconstruction of long bone defects in tibia. Arch Orthop Trauma Surg. 2008 Aug;128(8):801-8. [DOI] [PubMed] [Google Scholar]
- 67.Haidukewych GJ. Innovations in locking plate technology. J Am Acad Orthop Surg. 2004 Jul-Aug;12(4):205-12. [DOI] [PubMed] [Google Scholar]
- 68.Ring D, Kloen P, Kadzielski J, Helfet D, Jupiter JB. Locking compression plates for osteoporotic nonunions of the diaphyseal humerus. Clin Orthop Relat Res. 2004 Aug;(425):50-4. [DOI] [PubMed] [Google Scholar]
- 69.Gardner MJ, Griffith MH, Demetrakopoulos D, Brophy RH, Grose A, Helfet DL, Lorich DG. Hybrid locked plating of osteoporotic fractures of the humerus. J Bone Joint Surg Am. 2006 Sep;88(9):1962-7. [DOI] [PubMed] [Google Scholar]
- 70.Cross AR, Lewis DD, Murphy ST, Rigaud S, Madison JB, Kehoe MM, Rapoff AJ. Effects of ring diameter and wire tension on the axial biomechanics of four-ring circular external skeletal fixator constructs. Am J Vet Res. 2001 Jul;62(7):1025-30. [DOI] [PubMed] [Google Scholar]
- 71.Podolsky A, Chao EY. Mechanical performance of Ilizarov circular external fixators in comparison with other external fixators. Clin Orthop Relat Res. 1993 Aug;(293):61-70. [PubMed] [Google Scholar]
- 72.Paley D, Fleming B, Catagni M, Kristiansen T, Pope M. Mechanical evaluation of external fixators used in limb lengthening. Clin Orthop Relat Res. 1990 Jan;(250):50-7. [PubMed] [Google Scholar]
- 73.Wolfson N, Hearn TC, Thomason JJ, Armstrong PF. Force and stiffness changes during Ilizarov leg lengthening. Clin Orthop Relat Res. 1990 Jan;(250):58-60. [PubMed] [Google Scholar]
- 74.Goodship AE, Watkins PE, Rigby HS, Kenwright J. The role of fixator frame stiffness in the control of fracture healing. An experimental study. J Biomech. 1993 Sep;26(9):1027-35. [DOI] [PubMed] [Google Scholar]
- 75.Fleming B, Paley D, Kristiansen T, Pope M. A biomechanical analysis of the Ilizarov external fixator. Clin Orthop Relat Res. 1989 Apr;(241):95-105. [PubMed] [Google Scholar]
- 76.Ilizarov GA. The apparatus: components and biomechanical principles of application. In: Transosseous Osteosynthesis. Theoretical and Clinical Aspects of the Regeneration and Growth of Tissue. New York: Springer; 1992. [Google Scholar]
- 77.Iobst CA, Dahl MT. Limb lengthening with submuscular plate stabilization: a case series and description of the technique. J Pediatr Orthop. 2007 Jul-Aug;27(5):504-9. [DOI] [PubMed] [Google Scholar]
- 78.Farouk O, Krettek C, Miclau T, Schandelmaier P, Guy P, Tscherne H. Minimally invasive plate osteosynthesis: does percutaneous plating disrupt femoral blood supply less than the traditional technique? J Orthop Trauma. 1999 Aug;13(6):401-6. [DOI] [PubMed] [Google Scholar]
- 79.Baumgaertel F, Buhl M, Rahn BA. Fracture healing in biological plate osteosynthesis. Injury. 1998;29(Suppl 3):C3-6. [DOI] [PubMed] [Google Scholar]
- 80.Saini P, Kumar R, Shekhawat V, Joshi N, Bansal M, Kumar S. Biological fixation of comminuted subtrochanteric fractures with proximal femur locking compression plate. Injury. 2013 Feb;44(2):226-31. [DOI] [PubMed] [Google Scholar]
- 81.Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br. 2002 Nov;84(8):1093-110. [DOI] [PubMed] [Google Scholar]
- 82.Zhang SB, Zhang YB, Wang SH, Zhang H, Liu P, Zhang W, Ma JL, Wang J. Clinical efficacy and safety of limited internal fixation combined with external fixation for pilon fracture: a systematic review and meta-analysis. Chin J Traumatol. 2017 Apr;20(2):94-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Emara KM, Ghafar KA, Al Kersh MA. Methods to shorten the duration of an external fixator in the management of tibial infections. World J Orthop. 2011 Sep 18;2(9):85-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kadhim M, Holmes L, Jr, Gesheff MG, Conway JD. Treatment options for nonunion with segmental bone defects: systematic review and quantitative evidence synthesis. J Orthop Trauma. 2017 Feb;31(2):111-9. [DOI] [PubMed] [Google Scholar]
- 85.Glatt V, Evans CH, Tetsworth K. A concert between biology and biomechanics: the influence of the mechanical environment on bone healing. Front Physiol. 2017 Jan 24;7:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wolff J. The Law of Bone Remodelling. New York: Springer; 1986. [Google Scholar]
- 87.Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987 Sep;219(1):1-9. [DOI] [PubMed] [Google Scholar]
- 88.Perren SM. The biomechanics and biology of internal fixation using plates and nails. Orthopedics. 1989 Jan;12(1):21-34. [DOI] [PubMed] [Google Scholar]
- 89.Meyers N, Schülke J, Ignatius A, Claes L. Evolution of callus tissue behavior during stable distraction osteogenesis. J Mech Behav Biomed Mater. 2018 Sep;85:12-19. [DOI] [PubMed] [Google Scholar]
- 90.Paley D, Catagni MA, Argnani F, Villa A, Benedetti GB, Cattaneo R. Ilizarov treatment of tibial nonunions with bone loss. Clin Orthop Relat Res. 1989 Apr;(241):146-65. [PubMed] [Google Scholar]