Abstract
Background and Objectives: Due to its link with the SARS-CoV-2, Multisystem Inflammatory Syndrome in Children (MIS-C) gained global attention as a serious condition that requires hospital care. Our study aimed to present the clinical and laboratory characteristics of MIS-C patients by age group and intensive care unit (ICU) admission status and assess early echocardiographic changes. Materials and Methods: A single-center partly retrospective, partly prospective observational cohort study was performed from December 2020 to June 2024. The study included 42 patients aged between 1 month and 18 years who were diagnosed with MIS-C and gave informed consent. Results: The median age was 6.5 years (IQR 2.0–9.3). The predominant symptoms were cardiovascular (88.1%), mucocutaneous (85.7%) and gastrointestinal (76.2%). Five children (11.9%) developed shock. About two-thirds of patients (66.7%) were admitted to the ICU. Adolescents (≥12 years) were less likely to exhibit mucocutaneous or cardiovascular symptoms and thus less frequently having Kawasaki—like disease symptoms compared with other age groups (<5 years or 5–11 years). Lymphopenia was more common among patients aged 5 years and older. Adolescents had higher procalcitonin (PCT) and a lower estimated glomerular filtration rate. Troponin I and B-type natriuretic peptide (BNP) levels were higher in children aged 5–11 years, while ferritin levels were lower among the youngest (<5 years). Patients treated at the ICU were more likely to have cardiovascular and respiratory symptoms, as well as a history of symptomatic COVID-19, higher C-reactive protein (CRP), PCT, BNP and lower albumin levels. Echocardiographic abnormalities were found in 71.4% of cases. During hospitalization, left ventricular ejection fraction values increased significantly (p < 0.001) over 12 (IQR 9.0–14.0) days. Conclusions: Symptoms and laboratory markers of MIS-C vary according to age. Higher CRP, PCT, BNP and hypoalbuminemia are predictors of MIS-C severity. Cardiovascular involvement is common and might be severe, but rapid resolution is encouraging.
Keywords: MIS-C, severity, intensive care unit (ICU), cardiac involvement, echocardiography
1. Introduction
First described in the United Kingdom in April 2020 [1], Multisystem Inflammatory Syndrome in Children (MIS-C) was soon identified in many countries, gaining global attention as a serious condition that requires hospital care [2,3]. Lithuania also encountered this problem, but the first case was diagnosed relatively late, at the end of 2020, therefore officially registered only in 2021 [4]. Later, the number of cases increased, and MIS-C became part of routine clinical practice.
MIS-C is characterized by fever, elevated inflammatory markers, evidence of SARS-CoV-2 infection, and multisystem organ involvement, with no other alternative diagnosis [5,6]. The syndrome can present with a wide range of clinical features, affecting the gastrointestinal, mucocutaneous, cardiovascular, respiratory, hematological, neurological and renal systems [7,8,9,10]. Diagnosing MIS-C requires thorough investigation, including a series of laboratory and instrumental tests. One of the most challenging aspects of managing MIS-C is its impact on the cardiovascular system, which necessitates extensive testing and ongoing monitoring [11,12,13].
The syndrome primarily affects children and young adults up to 21 years, with the highest risk of MIS-C between the ages of 5 and 11 years [9,14]. Limited data are reported regarding age differences in the presentation. The severity varies from milder cases to severe complications requiring intensive care [9,10]. Around half or more of MIS-C patients typically require admission to an intensive care unit (ICU), and the mortality rate can reach up to 5% [8,9,10,15,16,17]. There is variability in the data regarding factors associated with severe outcomes [8,11,16,17,18]. Our study aims to present the clinical and laboratory characteristics of MIS-C patients by age group and ICU admission status and assess early echocardiographic changes in a single large tertiary care center.
2. Materials and Methods
A single-center, partly retrospective, partly prospective observational cohort study was performed from December 2020 to June 2024. Patients were recruited at the Vilnius University Hospital Santaros Klinikos, designated as a major hospital for admissions of patients with MIS-C in the northeast region of Lithuania. This study was approved by the Vilnius Regional Biomedical Research Ethics Committee (No. 2022/3-1419-888) and conducted in accordance with the principles of the World Medical Association Helsinki Declaration as well as local law.
The study included patients from 1 month to 18 years who met the national diagnostic criteria of MIS-C [19], adapted from the WHO and CDC [5,6]: fever ≥ 38.0 °C for ≥24 h; new onset manifestations in at least two different organ systems (gastrointestinal, mucocutaneous, cardiovascular, respiratory, renal, neurological and hematological); evidence of COVID-19 (RT-PCR or serology positive); laboratory evidence of inflammation; exclusion of an alternative diagnosis.
Patients were excluded if there was no written informed consent form.
Detailed data of each patient were obtained from patient electronic medical records:
-
-
basic characteristics (age, gender, race, height, weight, date of hospitalization);
-
-
epidemiological data (known previous COVID-19 infection, vaccination status against SARS-CoV-2);
-
-
clinical data (gastrointestinal, mucocutaneous, cardiovascular, respiratory, renal, neurological and other symptoms);
-
-
comorbidities;
-
-
laboratory data (SARS-CoV-2 RT-PCR or serology, full blood count (FBC), C-reactive protein (CRP), procalcitonin (PCT), interleukin 6 (IL-6), ferritin, troponin I, B-type natriuretic peptide (BNP), D-Dimer, albumin and estimated glomerular filtration rate (eGFR, which was based on the revised Schwartz equation [20]);
-
-
echocardiographic initial findings and early outcome on discharge (left ventricular ejection fraction (LVEF), coronary artery involvement, valvular regurgitation, pericardial effusion);
-
-
electrocardiographic (ECG) findings (conduction abnormalities such as atrioventricular (AV) block, bundle branch blocks; tachyarrhythmias, including premature beats; significant repolarization abnormalities, including ST deviation > 1 mm, negative T waves at I, II, aVF, V5-6; QTc prolongation);
-
-
treatment (oxygen therapy, vasoactive drugs, intravenous immunoglobulin (IVIG), glucocorticoids, other immunomodulators);
-
-
outcome data (duration of hospitalization, admissions to ICU, recovered/deceased).
Clinical data were defined by selecting the most prominent signs and symptoms: gastrointestinal (abdominal pain, diarrhea, vomiting, abnormal liver function tests, colitis, ileitis and ascites), mucocutaneous (conjunctivitis, periorbital swelling/redness, mucus membrane changes, strawberry tongue, rash, lymphadenopathy, swollen hands and feet), cardiovascular (tachycardia, high blood pressure, arterial hypotension, shock), respiratory (cough, sore throat, oxygen requirement, patchy infiltrates, pleural effusion), neurological (headache, confusion, irritability, reduced level of consciousness/lethargy, syncope), renal (renal function impairment, decreased diuresis, urine sedimentation abnormalities), other (arthralgia, myalgia).
Based on the clinical presentation, MIS-C patients were divided into three subgroups [7] as follows: MIS-C with shock; Kawasaki—like disease (KD; patients with fever, lymphadenopathy, mucocutaneous and cardiovascular involvement); undifferentiated MIS-C (patients with fever and inflammation who did not meet either KD criteria or symptoms of shock).
Nasopharyngeal swabs were tested using real-time reverse-transcriptase polymerase chain reaction (RT-PCR) tests for SARS-CoV-2, using the Xpert Xpress plus (Cepheid, Sunnyvale, CA, USA) with 100% specificity and 100% sensitivity. COVID-19 serology was tested by quantification of SARS-CoV-2 anti-RBD IgG antibodies against the RBD domain of the spike protein.
Cardiac echocardiography was performed by two specialists using an ultrasound system Samsung HS7A74L/WR (Suwon-si, Republic of Korea). A normal LVEF was defined as an LVEF of 55% or higher. Coronary artery involvement was classified according to the American Heart Association (AHA) guidelines, using the z-score system: z-score < 2—no involvement; z-score ≥ 2 to <2.5—dilation; z-score ≥ 2.5—aneurysm [21]. AV or other valve regurgitation or pericardial effusion were deemed abnormal or not on the basis of the echocardiography report.
Categorical data were presented as frequencies and percentages and analyzed using Fisher’s exact test. For continuous data, medians/interquartile range (IQR) were calculated. A one-way analysis of variance (ANOVA) was used to compare the three age groups (<5 years, 5–11 years and >12 years) with possible covariates. Initial and follow-up echocardiographic findings were compared using McNemar’s test for categorical variables and paired-t-tests for continuous variables. Student’s t-test and Kruskal–Wallis H test were used for categorical–continuous variable pairs. Statistical analyses were performed with IBM SPSS Statistics 22.0 (Chicago, IL, USA). A p value < 0.05 was considered significant.
3. Results
3.1. Study Sample
A total of 51 patients have been diagnosed with MIS-C over 3.5 years. Forty-two children were included in the final analysis. Nine patients were excluded as their parents did not provide written informed consent. Of these, three patients with comorbidities died.
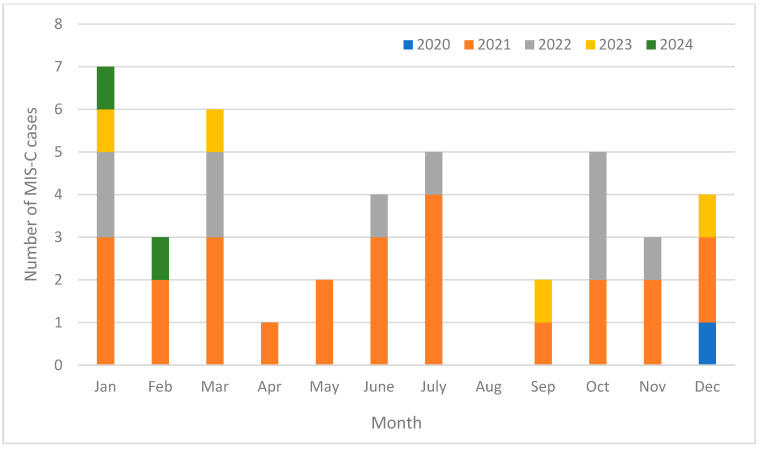
The first case of MIS-C was diagnosed in December 2020. The majority of cases were in 2021 (n = 25) with a declining number of cases in subsequent years (ten cases in 2022, four cases in 2023 and two cases during the first half of 2024). The monthly distribution of cases showed slight increases in January and March, while August had no cases at all (Figure 1).
Figure 1.
Monthly distribution of MIS-C, December 2020–June 2024.
All enrolled patients were Caucasian, with more boys (73.8%, n = 31) than girls. The youngest patient was 9 months old, and the oldest was 16 years. The median age was 6.5 years (IQR 2.0–9.3). All patients had fever. The predominant symptoms were cardiovascular (88.1%, n = 37), mucocutaneous (85.7%, n = 36) and gastrointestinal (76.2%, n = 32). Eight patients (19.1%) had two organ systems involved. About two thirds of patients (69.0%, n = 29) had symptoms involving three or four organ systems, while the remaining five patients (11.9%) had more extensive involvement.
The most common MIS-C phenotype was KD, seen in 73.8% of cases (n = 31). Five children had shock symptoms (11.9%), and six children presented with undifferentiated MIS-C (14.3%). Adolescents (≥12 years) were less likely to exhibit mucocutaneous or cardiovascular symptoms and thus less frequently having KD symptoms compared with other age groups (<5 years or 5–11 years; Table 1).
Table 1.
Demographic and clinical characteristics at admission of MIS-C patients by age group.
| All Cases | <5 Years | 5–11 Years | ≥12 Years | p-Value | |
|---|---|---|---|---|---|
| Counts (%) | 42 (100) | 18 (42.9) | 18 (42.9) | 6 (14.3) | |
| Male sex | 31 (73.8) | 13 (72.2) | 13 (72.2) | 5 (83.3) | 0.858 |
| Comorbidities | 9 (21.4) | 3 (16.7) | 3 (16.7) | 3 (50.0) | 0.193 |
| A known history of symptomatic COVID-19 |
18 (42.9) | 5 (27.8) | 8 (44.4) | 5 (83.3) | 0.058 |
| Signs and symptoms | |||||
| Gastrointestinal symptoms | 32 (76.2) | 14 (77.8) | 12 (66.7) | 6 (100) | 0.260 |
| Mucocutaneous symptoms | 36 (85.7) | 16 (88.9) | 18 (100) | 2 (33.3) | 0.000 |
| Cardiovascular symptoms | 37 (88.1) | 17 (94.4) | 17 (94.4) | 3 (50.0) | 0.006 |
| Respiratory symptoms | 18 (42.9) | 7 (38.9) | 9 (50.0) | 2 (33.3) | 0.716 |
| Neurological symptoms | 13 (31.0) | 7 (38.9) | 4 (22.2) | 2 (33.3) | 0.571 |
| Renal symptoms | 8 (19.0) | 4 (22.2) | 3 (16.7) | 1 (16.7) | 0.909 |
| Other symptoms * | 3 (7.1) | 2 (11.1) | 1 (5.6) | 0 (0) | 0.638 |
| MIS-C phenotype | |||||
| Kawasaki-like disease | 31 (73.8) | 15 (83.3) | 14 (77.8) | 2 (33.3) | 0.047 |
| MIS-C with shock; | 5 (11.9) | 0 (0) | 4 (22.2) | 1 (16.7) | 0.116 |
| Undifferentiated MIS-C | 6 (14.3) | 3 (16.7) | 0 (0) | 3 (50.0) | 0.007 |
| Echocardiography and electrocardiography at admission | |||||
| Abnormal echocardiogram | 30 (71.4) | 13 (72.2) | 15 (83.3) a | 2 (33.3) a | 0.064 |
| Abnormal ECG | 22 (52.4) | 9 (50.0) | 10 (55.6) | 3 (50.0) | 0.943 |
| Laboratory test results at admission (normal range), peak value: median (IQR) | |||||
| White blood cell count (4.5–12.0 × 109/L; n = 42) |
15.4 (10.9–20.1) |
16.8 (11.7–25.1) |
14.5 (10.9–17.7) |
15.1 (8.2–16.8) |
0.801 |
|
Absolute lymphocyte count
(1.5–6.8 × 109/L; n = 42) |
2.2
(1.2–4.7) |
3.9
(2.0–9.2) |
1.3
(0.9–3.1) |
1.3
(0.8–4.6) |
0.008 |
| Absolute neutrophil count (1.5–7.5 × 109/L; n = 42) |
10.9 (7.7–14.1) |
11.1 (8.1–14.1) |
11.0 (7.7–15.5) |
9.3 (7.0–14.8) |
0.641 |
| Platelets (140–450 × 109/L; n = 42) |
352.5 (137.3–612.8) |
442.5 (138.0–657.0) |
371.0 (137.3–570.0) |
162.5 (100.3–367.0) |
0.169 |
| C-reactive protein (<5 mg/L; n = 42) |
158.6 (100.7–228.5) |
148.7 (115.9–230.9) |
170.9 (61.6–218.5) |
165.5 (87.5–256.4) |
0.801 |
| Procalcitonin (<0.05 ng/mL; n = 41) |
3.4 (1.6–7.4) |
3.5 (1.4–7.1) |
3.4 b (1.4–5.1) |
12.0 b (1.6–31.0) |
0.983 |
| Interleukin–6 (<5.9 ng/L; n = 23) |
103.0 (28.6–238.0) |
142.0 (34.8–395.8) |
46.0 (20.5–190.8) |
49.3 (49.3–49.3) |
0.142 |
|
Troponin I
(<16 ng/L; n = 40) |
12.5
(2.0–70.3) |
2.5
(1.0–13.0) |
50.0
(6.0–225.5) |
14.0
(4.0–439.0) |
0.036 |
| B-type natriuretic peptide (<100 ng/L; n = 35) |
207.3 (71.1–960.2) |
113.4 (17.5–794.9) |
611.4 c (88.5–965.5) |
141.9 c (47.1–434.8) |
0.276 |
|
Ferritin
(7–140 μg/L; n = 41) |
375.5
(252.7–501.7) |
289.0
(212.6–386.9) |
384.6
(252.4–846.6) |
481.6
(361.5–1247.8) |
0.038 |
| D-Dimer (45–280 μg/L; n = 42) |
2102.5 (1283.8–3170.0) |
2007.5 (1502.5–2721.3) |
2142.5 (907.5–4906.3) |
1990.0 (1113.8–7519.5) |
0.801 |
| Albumin (38–54 g/L, n = 34) |
29.64 (25.6–34.3) |
28.4 (26.5–35.5) |
29.0 (24.4–34.2) |
32.7 (29.5–38.4) |
0.567 |
| Estimated glomerular filtration rate (n = 41) | 108.4 (83.5–123.8) |
114.4 (95.5–129.6) |
110.3 (88.1–121.7) |
56.2 (42.9–94.0) |
0.012 |
| Management | |||||
| Intravenous immunoglobulin | 39 (92.9) | 17 (94.4) | 17 (94.4) | 5 (83.3) | 0.638 |
| Glucocorticoids | 34 (81.0) | 13 (72.2) | 16 (88.9) | 5 (83.3) | 0.458 |
| Oxygen therapy | 11 (26.2) | 3 (16.7) | 7 (38.9) | 1 (16.7) | 0.284 |
| Vasoactive drugs | 5 (11.9) | 0 (0) | 4 (22.2) | 1 (16.7) | 0.116 |
| ICU treatment | 28 (66.7) | 11 (61.1) | 13 (72.2) | 4 (66.7) | 0.792 |
* arthralgia, myalgia. a p = 0.038; b p = 0.018; c p = 0.038. Significant differences (p < 0.05) among age groups (<5 Years; 5–11 Years; ≥12 Years) are bolded.
The majority of patients presented with leucocytosis (>12 × 109/L; 69.0%), neutrophilia (>7.5 × 109/L; 78.6%) and lymphocytopenia (<1.5 × 109/L; 44.1%). Thrombocytosis (>450 × 109/L) was more common than thrombocytopenia (<140 × 109/L; 40.0% and 27.5%, respectively). All inflammatory markers increased, most notably CRP and IL-6: 76.2% (32/42) patients had CRP >100 mg/L and 52.2% (12/23) had IL-6 >100 ng/L. An increase in BNP was higher and more common compared to troponin I: 62.9% (22/35) had BNP > 100 ng/L and 41.0% (16/39) had troponin I > 16 ng/L. Most patients had significantly elevated ferritin and D-dimer levels: ferritin > 300 μg/L in 63.4% (26/41) and D-dimer > 1000 μg/L in 80.5% (33/41) of cases. Hypoalbuminemia (<38 g/L) was seen in 84.8% (28/33) of patients and reduced eGFR (<90 mL/min/1.73m2)—in 29.3% (12/41) of cases.
Laboratory tests revealed age-related differences in several parameters (Table 1). Lymphopenia was more common among patients aged 5 years and older. Adolescents (≥12 years) had higher PCT and lower eGFR. Troponin I and BNP levels were higher in children aged 5–11 years, while ferritin levels were lower among the youngest (<5 years).
None of the patients were vaccinated against COVID-19. Almost two-thirds (62.8%, n = 27) of the patients were not vaccinated, as there was no age-appropriate SARS-CoV-2 vaccine at the time. Data about eight patients, who could have been vaccinate were missing.
All tested children (n = 41) had a positive SARS-CoV-2 serology. One patient was not tested but had a recent history of COVID-19. Additionally, thirty-eight children were tested for SARS-CoV-2 PCR, and the test was positive in six cases (14.3%).
3.2. Echocardiography Findings
All children included in the analysis underwent cardiac ultrasound at least twice. We compared initial echocardiographic findings with discharge data. A follow-up echocardiogram was performed with a median of 12.0 (IQR 9.0–14.0) days after the initial echocardiogram.
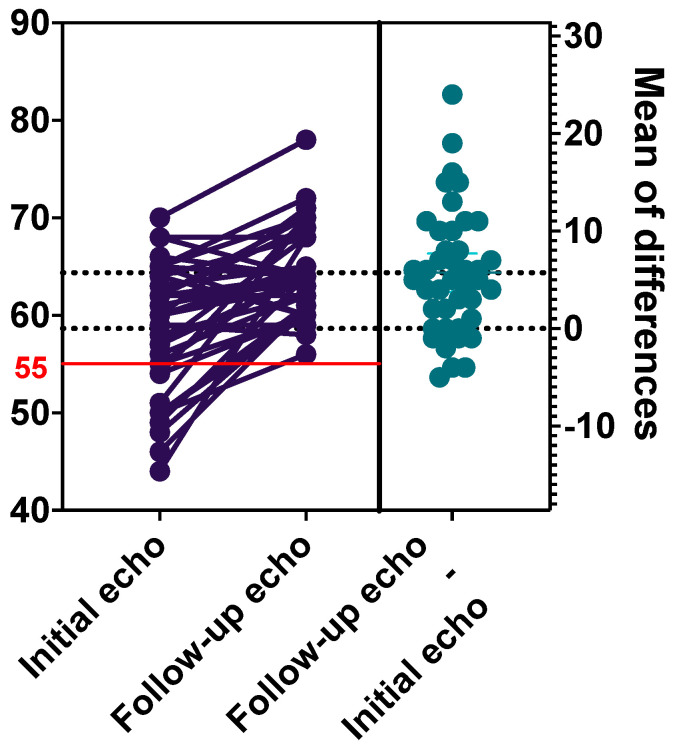
Initially, 30 patients (71.4%) had echocardiographic abnormalities. The most common findings were pericardial effusion (64.3%, n = 27) and valvular regurgitation (54.8%, n = 23). Atrioventricular valve regurgitation was the most common (47.6%, n = 20). Mitral valve and tricuspid valve regurgitation was described in 31.0% of cases (n = 13). Pulmonary valve regurgitation was detected in 14.3% (n = 6) and aortic valve regurgitation—in 4.8% (n = 2). A decreased LVEF <55% was found in 26.2% patients (n = 11). There were no coronary artery aneurysms, only one patient had coronary artery dilation. Echocardiographic parameters improved during early follow-up (Table 2), including LVEF values, which significantly increased during the treatment (p < 0.001; Figure 2).
Table 2.
Initial and early follow-up echocardiographic findings of MIS-C patients.
| Echocardiographic Findings | Initial Findings, n (%) | Early Outcome on Discharge, n (%) | p-Value |
|---|---|---|---|
| Left ventricular ejection fraction < 55% | 11 (26.2) | 0 (0) | N/A |
| Coronary artery dilation | 1 (2.4) | 0 (0) | N/A |
| Mitral valve regurgitation | 13 (31.0) | 4 (9.5) | 0.012 |
| Tricuspid valve regurgitation | 13 (31.0) | 3 (7.1) | 0.013 |
| Pericardial effusion | 27 (64.3) | 6 (14.3) | 0.000 |
p values less than 0.05 are bolded; N/A—not applicable.
Figure 2.
Left ventricular systolic dysfunction (LVEF): initial data and early follow-up changes at hospital discharge. A normal LVEF was defined as an LVEF of 55% or higher. The left side shows LVEF initial findings and follow-up changes at hospital discharge. The right side shows the mean of LVEF differences.
3.3. Treatment and Outcome Data
Children were hospitalized from 5 to 43 days. The average duration of hospitalization was 13 days (IQR 9.75–15.25). About two-thirds of patients (66.7%, n = 28) were admitted to ICU and treated there for 2 to 8 days. The average duration in ICU was 4 days (IQR 3.0–5.0). Patients treated at the ICU were more likely to have cardiovascular and respiratory symptoms, as well as a history of symptomatic COVID-19, higher CRP, PCT, BNP and lower albumin levels (Table 3). The percentage of comorbidities was the same in both ICU and non-ICU patients.
Table 3.
Comparison between MIS-C patients admitted or not admitted to the intensive care unit (ICU).
| Non-ICU Patients | ICU Patients | p-Value | |
|---|---|---|---|
| Counts (%) | 14 (33.3) | 28 (66.7) | |
| <5 years | 7 (50.0) | 11 (39.3) | 0.369 |
| 5–11 years | 5 (35.7) | 13 (46.4) | 0.373 |
| ≥12 years | 2 (14.3) | 4 (14.3) | 0.666 |
| Male sex | 10 (71.4) | 21 (75.0) | 0.541 |
| Comorbidities | 3 (21.4) | 6 (21.4) | 1.000 |
| A known history of symptomatic COVID-19 | 3 (21.4) | 15 (53.6) | 0.047 |
| Signs and symptoms | |||
| Gastrointestinal symptoms | 12 (85.7) | 20 (71.4) | 0.267 |
| Mucocutaneous symptoms | 12 (85.7) | 24 (85.7) | 1.000 |
| Cardiovascular symptoms | 10 (71.4) | 27 (96.4) | 0.035 |
| Respiratory symptoms | 3 (21.4) | 15 (53.6) | 0.047 |
| Neurological symptoms | 4 (28.6) | 9 (32.1) | 0.553 |
| Renal symptoms | 2 (14.3) | 6 (21.4) | 0.457 |
| Other symptoms * | 2 (14.3) | 1 (3.6) | 0.254 |
| MIS-C phenotype | |||
| Kawasaki-like disease | 11 (78.6) | 20 (71.4) | 0.459 |
| MIS-C with shock | 0 (0) | 5 (17.9) | 0.116 |
| Undifferentiated MIS-C | 3 (21.4) | 3 (10.7) | 0.311 |
| Echocardiography and electrocardiography at admission | |||
| Abnormal echocardiogram | 9 (64.3) | 21 (75.0) | 0.353 |
| Abnormal ECG | 7 (50.0) | 15 (53.6) | 0.543 |
| Laboratory test results, peak value: median (IQR) | |||
| White blood cell count (n = 42) | 15.0 (10.2–18.0) | 15.4 (11.4–21.7) | 0.545 |
| Absolute lymphocyte count (n = 42) | 1.9 (1.3–3.6) | 2.2 (1.2–5.5) | 0.901 |
| Absolute neutrophil count (n = 42) | 10.2 (7.6–13.1) | 11.0 (8.8–15.2) | 0.289 |
| Platelets (n = 42) | 457 (236.8–587.3) | 252 (127.5–614.3) | 0.712 |
| C-reactive protein (n = 42) | 97.0 (28.6–145.1) | 196.8 (138.2–249.8) | 0.000 |
| Procalcitonin (n = 41) | 1.5 (0.7–3.1) | 4.0 (2.6–15.4) | 0.003 |
| Interleukin-6 (n = 23) | 49.2 (24.6–298.6) | 117.5 (28.8–245.8) | 0.656 |
| Troponin I (n = 40) | 3.0 (1.3–27.0) | 13.5 (2.3–79.3) | 0.466 |
| B-type natriuretic peptide (n = 35) | 82.4 (10.7–418.8) | 351.9 (95.0–965.4) | 0.041 |
| Ferritin (n = 41) | 313.5 (202.6–382.3) | 390.6 (266.6–635.9) | 0.125 |
| D-Dimer (n = 42) | 1900 (600.0–2302.5) | 2190 (1367.5–5058.8) | 0.316 |
| Albumin (n = 34) | 38.3 (34.0–40.0) | 27.6 (24.4–32.0) | 0.000 |
| Estimated glomerular filtration rate (n = 41) | 110.7 (92.1–126.1) | 103.8 (103.8–123.0) | 0.118 |
| Management | |||
| Intravenous immunoglobulin | 11 (78.6) | 28 (100) | 0.032 |
| Glucocorticoids | 11 (78.6) | 23 (82.1) | 0.543 |
| Oxygen therapy | 0 (0) | 11 (39.3) | 0.005 |
| Vasoactive drugs | 0 (0) | 5 (17.9) | 0.116 |
* arthralgia, myalgia; Significant differences (p < 0.05) between Non-ICU and ICU patients are bolded.
Most patients were treated with IVIG (92.9%, n = 39) and GCC (81.0%, n = 34). A combination of IVIG and GCC was given to 32 patients (76.2%), 7 patients received IVIG alone, 2 patients received GCC alone and 1 patient received only symptomatic treatment. Eleven patients (26.2%) required oxygen therapy, which lasted on average from 1 to 5 days. Vasoactive drugs were administered also from 1 to 5 days in five children (11.9%). Treatment differences between age groups and ICU/non-ICU patients are shown in Table 1 and Table 3. All study patients were discharged.
4. Discussion
This study covers the entire period of MIS-C history in our pediatric center, as it analyses data from the first case of MIS-C in 2020 up to summer 2024. A notable peak in MIS-C cases occurred in 2021, coinciding with the prevalence of the SARS-CoV-2 Alpha and Delta variants in Europe [22]. Subsequent years showed a gradual decline in cases, mirroring trends observed internationally [23,24], possibly due to increased community exposure to other COVID-19 variants like Omicron and rising vaccination rates [23,25,26,27].
While some regions reported a lag between COVID-19 peaks and subsequent rises in MIS-C cases [28,29,30], our data did not show any clear seasonal trends or direct correlations with spikes in COVID-19 infections. For instance, despite a surge in SARS-CoV-2 infections among children in our hospital from January to March 2022 [31], MIS-C cases remained scattered throughout the year.
Our cohort was exclusively Caucasian, preventing any assessment of racial influences on MIS-C, although it is known that Black and Hispanic children appear to be disproportionally affected, while Asian children account for only a small number of cases [32]. Males predominated, consistent with global observations [9,25,33]. Sex differences are possibly due to heightened immune responses in males leading to more severe inflammatory reactions [33]. The median age of the MIS-C patients was 6.5 years, aligning with global data [7,15,17,25], and we observed notable clinical and laboratory differences across age groups. For example, lymphopenia was less common in the youngest cohort (under 5 years), who also exhibited lower ferritin levels. In contrast, school-aged children (5–11 years) showed elevated levels of Troponin I and BNP, indicative of more significant cardiovascular involvement—a potential factor in the higher ICU admission rates observed in this age group. Other studies have also pointed out that children of this age are at higher risk of being admitted to the ICU [16,17].
Adolescents (12 years and older) were less prone to mucocutaneous or cardiovascular symptoms and abnormal echocardiographic findings but exhibited higher PCT rates and reduced eGFR. Most of the cases with reduced eGFR were of prerenal cause and lasted just a few days till fluid balance was restored [34]. Adolescents showed fewer Kawasaki-like symptoms, in accordance with other reports [10]. This may be related to the fact that classic KD typically affects infants and young children [35].
Severity predictors for MIS-C are diverse. Data suggest that symptoms such as shortness of breath, abdominal pain, seizures and renal failure are more likely to precipitate the ICU admissions [8,16,17]. In our study, patients requiring treatment at the ICU more frequently presented with cardiovascular and respiratory symptoms and demonstrated higher CRP, PCT, BNP, and lower albumin levels—trends consistent with global observations [11,16,17,18]. Furthermore, a constellation of abnormal laboratory markers, including reduced platelet or lymphocyte counts, increased troponin, ferritin, D-dimer, fibrinogen and IL-6 were identified in other studies as correlates of ICU necessity, emphasizing that both inflammatory and cardiac markers serve as crucial severity predictors for MIS-C [8,11,16,17,18]. In our study group, comorbidities did not have a clear impact on the course of the disease, except that we cannot speculate about excluded patients who had comorbidities and died.
Cardiovascular involvement, a critical concern in MIS-C, was prominent among our patients, with a majority exhibiting echocardiographic abnormalities. ICU patients commonly showed heightened cardiovascular symptoms and higher BNP levels. Fortunately, cardiac abnormalities in MIS-C cases tend to resolve swiftly, as indicated by the significant improvement in our echocardiographic findings at discharge, although some patients showed lingering issues such as valve regurgitation and pericardial effusion. It is hypothesized that myocardial injury in MIS-C results from transient myocardial edema caused by intense inflammation rather than direct viral damage, with most cases of LVEF normalizing within 1 to 2 weeks [12,13,15,36]. However, MRIs performed several months post-diagnosis revealed persistent cardiac abnormalities in some cases [37], highlighting the need for extended follow-up.
Our study has few limitations, as it is a single-center study with a relatively small number of patients, but the results are presented as real data and reflect the situation in Eastern Europe. Another limitation is that patients who died were not included in the study because the parents did not give permission.
5. Conclusions
This study illustrates variability in MIS-C symptoms and laboratory markers across different age groups and identifies severity predictors—higher CRP, PCT, BNP and hypoalbuminemia. Our data confirm that focus on the cardiovascular disorders among MIS-C cases is essential as they are quite common and might be severe. While the rapid resolution of cardiac abnormalities post-treatment is encouraging, long-term follow-up and continuous research at both national and international levels remain crucial.
Acknowledgments
Special thanks to the Vilnius University Hospital Santaros Klinikos multidisciplinary medical staff for their contribution to the diagnostics and therapeutic management of the patients presented in this study.
Author Contributions
Conceptualization, A.J. and I.I.; methodology, I.S., A.J. and I.I.; software, I.S.; formal analysis, I.S.; investigation, O.K. and L.K.; writing—original draft preparation, I.S.; writing—review and editing, A.J., I.I. and I.S.; visualization, I.S.; supervision, A.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Vilnius Regional Biomedical Research Ethics Committee (No. 2022/3-1419-888, 15 March 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data set could be available with reasonable request presenting data analysis plan to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) COVID Data Tracker. [(accessed on 22 August 2024)]; Available online: https://covid.cdc.gov/covid-data-tracker.
- 3.Molloy M.J., Auger K.A., Hall M., Shah S.S., Schondelmeyer A.C., Parikh K., Kazmier K.M., Katragadda H., Jacob S.A., Jerardi K.E., et al. Epidemiology and Severity of Illness of MIS-C and Kawasaki Disease During the COVID-19 Pandemic. Pediatrics. 2023;152:e2023062101. doi: 10.1542/peds.2023-062101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higienos Instituto Duomenys, Paskaičiuoti iš Privalomojo Sveikatos Draudimo Informacinės Sistemos. [(accessed on 16 August 2024)]. Available online: https://stat.hi.lt/
- 5.World Health Organization (WHO) Multisystem Inflammatory Syndrome in Children and Adolescents Temporally Related to COVID-19. [(accessed on 26 August 2024)]. Available online: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19.
- 6.Centers for Disease Control and Prevention (CDC) Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with SARS-CoV-2 Infection 2023 Case Definition. [(accessed on 26 August 2024)]; Available online: https://ndc.services.cdc.gov/case-definitions/multisystem-inflammatory-syndrome-in-children-mis-c-2023/
- 7.Alvarado-Gamarra G., del Aguila O., Dominguez-Rojas J., Chonlon-Murillo K., Atamari-Anahui N., Borcic A., Sánchez S., Huamani-Echaccaya P., Garcés-Ghilardi R., Estupiftan-Vigil M., et al. Clinical phenotypes of multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. Andes Pediatr. 2022;93:841–850. doi: 10.32641/andespediatr.v93i6.4084. [DOI] [PubMed] [Google Scholar]
- 8.Varga P., Balajthy A., Biró E., Bíró B., Reiger Z., Szikszay E., Mogyorósy G., Káposzta R., Szabó T. Multicolored MIS-C, a single-centre cohort study. BMC Pediatr. 2023;23:190. doi: 10.1186/s12887-023-03997-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbas Q., Ali H., Amjad F., Hussain M.Z.H., Rahman A.R., Khan M.H., Padhani Z.A., Abbas F., Imam D., Alikhan Z., et al. Clinical presentation, diagnosis and management of multisystem inflammatory syndrome in children (MIS-C): A systematic review. BMJ Paediatr. Open. 2024;8:e002344. doi: 10.1136/bmjpo-2023-002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J., Barranco M.A., Maxted A.M., Rosenberg E.S., Easton D., et al. Multisystem Inflammatory Syndrome in Children in New York State. N. Engl. J. Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uka A., Bressieux-Degueldre S., Buettcher M., Kottanattu L., Plebani M., Niederer-Loher A., Schöbi N., Hofer M., Tomasini J., Trück J., et al. Cardiac involvement in children with paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 (PIMS-TS): Data from a prospective nationwide surveillance study. Swiss Med. Wkly. 2023;153:40092. doi: 10.57187/smw.2023.40092. [DOI] [PubMed] [Google Scholar]
- 12.Ludwikowska K.M., Moksud N., Tracewski P., Sokolski M., Szenborn L. Cardiac Involvement in Patients with Multisystem Inflammatory Syndrome in Children (MIS-C) in Poland. Biomedicines. 2023;11:1251. doi: 10.3390/biomedicines11051251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman K.G., Harrild D.M., Newburger J.W. Cardiac Dysfunction in Multisystem Inflammatory Syndrome in Children. J. Am. Coll. Cardiol. 2020;76:1962–1964. doi: 10.1016/j.jacc.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhedin S., Lundholm C., Horne A., Smew A.I., Osvald E.C., Haddadi A., Alfvén T., Kahn R., Król P., Brew B.H., et al. Risk factors for multisystem inflammatory syndrome in children—A population-based cohort study of over 2 million children. Lancet Reg. Health Eur. 2022;19:100443. doi: 10.1016/j.lanepe.2022.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapoor R., Chandra T., Singh C.P., Singh R., Pandey I. Multisystem Inflammatory Syndrome in Children (MIS-C) Related to SARS-CoV-2 and 1-Year Follow-up. Indian. J. Pediatr. 2023;90:1008–1012. doi: 10.1007/s12098-022-04385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abrams J.Y., Oster M.E., Godfred-Cato S.E., Bryant B., Datta S.D., Campbell A.P., Leung J.W., Tsang C.A., Pierce T.J., Kennedy J.L., et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: A retrospective surveillance study. Lancet Child. Adolesc. Health. 2021;5:323–331. doi: 10.1016/S2352-4642(21)00050-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernández-Sarmiento J., Acevedo L., Niño-Serna L.F., Boza R., García-Silva J., Yock-Corrales A., Yamazaki-Nakashimada M.A., Faugier-Fuentes E., Del Águila O., Camacho-Moreno G., et al. Risk Factors Associated with Intensive Care Admission in Children with Severe Acute Respiratory Syndrome Coronavirus 2-Related Multisystem Inflammatory Syndrome (MIS-C) in Latin America: A Multicenter Observational Study of the REKAMLATINA Network. J. Intensive Care Med. 2024;39:785–793. doi: 10.1177/08850666241233189. [DOI] [PubMed] [Google Scholar]
- 18.Tran D.M., Pham D.V., Cao T.V., Hoang C.N., Nguyen H.T.T., Nguyen G.D., Le C.N., Thieu Q.Q., Ta T.A., Dau H.V., et al. Severity predictors for multisystemic inflammatory syndrome in children after SARS-CoV-2 infection in Vietnam. Sci. Rep. 2024;14:15810. doi: 10.1038/s41598-024-66891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lietuvos sveikatos mokslų universiteto Medicinos akademijos Vaikų ligų klinika, Vilniaus universiteto Medicinos fakulteto Klinikinės medicinos instituto Vaikų ligų klinika Vaikų COVID-19 infekcija: Diagnostikos ir gydymo metodinės rekomendacijos. Kaunas. 2022. [(accessed on 26 August 2024)]. pp. 1–38. Available online: https://sam.lrv.lt/uploads/sam/documents/files/2022%20Vaiku%20COVID-19%20infekcija%20-%20diag-ir-gyd-metod-rekom_Uzs79%20--%20eISBN%20(lock).pdf.
- 20.Schwartz G.J., Muñoz A., Schneider M.F., Mak R.H., Kaskel F., Warady B.A., Furth S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCrindle B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M., Baker A.L., Jackson M.A., Takahashi M., Shah P.B., et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 22.Ptak K., Szymońska I., Olchawa-Czech A., Kukla K., Cisowska M., Kwinta P. Comparison of the course of multisystem inflammatory syndrome in children during different pandemic waves. Eur. J. Pediatr. 2023;182:1647–1656. doi: 10.1007/s00431-022-04790-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yousaf A.R., Lindsey K.N., Wu M.J., Shah A.B., Free R.J., Simeone R.M., Zambrano L.D., Campbell A.P. Notes from the Field: Surveillance for Multisystem Inflammatory Syndrome in Children—United States, 2023. MMWR Morb Mortal Wkly Rep. 2024;73:225–228. doi: 10.15585/mmwr.mm7310a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy N., Koppel J.H., Kaplan O., Yechiam H., Shahar-Nissan K., Cohen N.K., Shavit I. Severity and Incidence of Multisystem Inflammatory Syndrome in Children During 3 SARS-CoV-2 Pandemic Waves in Israel. JAMA. 2022;327:2452–2454. doi: 10.1001/jama.2022.8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ooms C., Mossong J., Vergison A., Biver A., Wagner K., Niel O., Parrish A., Abdelrahman T.T., de la Fuente Garcia I. Multisystem inflammatory syndrome in children during the first two years of the COVID-19 pandemic in Luxembourg. Front. Pediatr. 2023;11:1141074. doi: 10.3389/fped.2023.1141074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pino R., Antoñanzas J.M., Paredes-Carmona F., Perramon A., Rivière J.G., Coma M., Martínez-Mejías A., Ripoll F., López N., Conti R., et al. Multisystem inflammatory syndrome in children and SARS-CoV-2 variants: A two-year ambispective multicentric cohort study in Catalonia, Spain. Eur. J. Pediatr. 2023;182:1897–1909. doi: 10.1007/s00431-023-04862-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamad Saied M., van der Griend L., van Straalen J.W., Wulffraat N.M., Vastert S., Jansen M.H.A. The protective effect of COVID-19 vaccines on developing multisystem inflammatory syndrome in children (MIS-C): A systematic literature review and meta-analysis. Pediatr. Rheumatol. Online J. 2023;21:80. doi: 10.1186/s12969-023-00848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.La Torre F., Elicio M.P., Monno V.A., Chironna M., Moramarco F., Campanozzi A., Civino A., Cecinati V., Vairo U., Giordano M., et al. Incidence and Prevalence of Multisystem Inflammatory Syndrome in Children (MIS-C) in Southern Italy. Children. 2023;10:766. doi: 10.3390/children10050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fliesler N. MIS-C: New Findings Bring COVID-Related Syndrome into Focus. [(accessed on 20 July 2024)]. Available online: https://answers.childrenshospital.org/mis-c-case-series/
- 30.Nygaard U., Holm M., Hartling U.B., Glenthøj J., Schmidt L.S., Nordly S.B., Matthesen A.T., Linstow M.L., von Espenhain L. Incidence and clinical phenotype of multisystem inflammatory syndrome in children after infection with the SARS-CoV-2 delta variant by vaccination status: A Danish nationwide prospective cohort study. Lancet Child. Adolesc. Health. 2022;6:459–465. doi: 10.1016/S2352-4642(22)00100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stacevičienė I., Ivaškevičienė I., Burokienė S., Steponavičienė A., Vaičiūnienė D., Tarutytė G., Jankauskienė A. Epidemiological changes of acute respiratory infections in children: A single-center experience after COVID-19 lockdown. PLoS ONE. 2024;19:e0300877. doi: 10.1371/journal.pone.0300877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stierman B., Abrams J.Y., Godfred-Cato S.E., Oster M.E., Meng L., Yip L., Patel P., Balachandran N., Prezzato E., Pierce T., et al. Racial and Ethnic Disparities in Multisystem Inflammatory Syndrome in Children in the United States, March 2020 to February 2021. Pediatr. Infect. Dis. J. 2021;40:e400–e406. doi: 10.1097/INF.0000000000003294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajamanickam A., Kumar N.P., Venkataraman A., Varadarjan P., Selladurai E., Sankaralingam T., Thiruvengadam K., Selvam R., Thimmaiah A., Natarajan S., et al. Sex-specific differences in systemic immune responses in MIS-C children. Sci. Rep. 2024;14:1720. doi: 10.1038/s41598-024-52116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart D.J., Mudalige N.L., Johnson M., Shroff R., du Pré P., Stojanovic J. Acute kidney injury in paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) is not associated with progression to chronic kidney disease. Arch. Dis. Child. 2022;107:e21. doi: 10.1136/archdischild-2021-322866. [DOI] [PubMed] [Google Scholar]
- 35.Yeom J.S., Woo H.O., Park J.S., Park E.S., Seo J.H., Youn H.S. Kawasaki disease in infants. Korean J. Pediatr. 2013;56:377–382. doi: 10.3345/kjp.2013.56.9.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das N., Hill R., Trivedi M., Kenkre T.S., Alsaied T., Feingold B., Harris T.H., Christopher A.B. Longitudinal Assessment of Cardiac Function Following Multisystem Inflammatory Syndrome in Children Associated with COVID-19. Pediatr. Cardiol. 2023;44:607–617. doi: 10.1007/s00246-022-02972-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arslan S.Y., Bal Z.S., Bayraktaroglu S., Ozenen G.G., Bilen N.M., Levent E., Ay O., Ozkaya P.Y., Ozkinay F., Cicek C., et al. Cardiac Assessment in Children with MIS-C: Late Magnetic Resonance Imaging Features. Pediatr. Cardiol. 2023;44:44–53. doi: 10.1007/s00246-022-02977-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set could be available with reasonable request presenting data analysis plan to the corresponding author.