Abstract
Ehrlichia chaffeensis and E. sennetsu are genetically divergent obligatory intracellular bacteria of human monocytes and macrophages, and the human granulocytic ehrlichiosis (HGE) agent is an obligatory intracellular bacterium of granulocytes. Infection with both E. chaffeensis and E. sennetsu, but not HGE agent, in the acute monocytic leukemia cell line THP-1 almost completely inhibited by treatment with deferoxamine, a cell-permeable iron chelator. Transferrin receptors (TfRs) accumulated on both E. chaffeensis and E. sennetsu, but not HGE agent, inclusions in THP-1 cells or the cells of the promyelocytic leukemia cell line HL-60. Reverse transcription-PCR showed an increase in the level of TfR mRNA 6 h postinfection which peaked at 24 h postinfection with both E. chaffeensis and E. sennetsu infection in THP-1 or HL-60 cells. In contrast, HGE agent in THP-1 or HL-60 cells induced no increase in TfR mRNA levels. Heat treatment of E. chaffeensis or the addition of monodansylcadaverine, a transglutaminase inhibitor, 3 h prior to infection inhibited the up-regulation of TfR mRNA. The addition of oxytetracycline 6 h after E. chaffeensis infection caused a decrease in TfR mRNA which returned to the basal level by 24 h postinfection. These results indicate that both internalization and continuous proliferation of ehrlichial organisms or the production of ehrlichial proteins are required for the up-regulation of TfR mRNA. Results of electrophoretic mobility shift assays showed that both E. chaffeensis and E. sennetsu infection increased the binding activity of iron-responsive protein 1 (IRP-1) to the iron-responsive element at 6 h postinfection and remained elevated at 24 h postinfection. However, HGE agent infection had no effect on IRP-1 binding activity. This result suggests that activation of IRP-1 and subsequent stabilization of TfR mRNA comprise the mechanism of TfR mRNA up-regulation by E. chaffeensis and E. sennetsu infection.
Ehrlichia chaffeensis, E. sennetsu, and human granulocytic ehrlichiosis (HGE) agent are three major human ehrlichiosis agents which belong to the family Rickettsiaceae (29–31). They are obligatory intracellular, gram-negative, pleomorphic cocci which infect leukocytes. E. chaffeensis and E. sennetsu infect monocytes-macrophages and are genetically divergent (14.4% 16S rRNA gene sequence difference [1]), while HGE agent infects granulocytes (9). The extents of 16S rRNA gene sequence divergence of HGE agent from E. chaffeensis and E. sennetsu are 7.5 and 14.7%, respectively (9). E. chaffeensis was first isolated in 1990 at Fort Chaffee, Arkansas, from a patient with human monocytic ehrlichiosis (HME) (11). Since the first report of this disease in the United States in 1987 (21), more than 460 cases of HME have been confirmed in 30 states (26). Serologic evidence suggests the presence of HME in Europe (Spain [17], Portugal [24], and Belgium [28]) and Africa (38). Clinical signs include fever, headache, myalgia, arthralgia, nausea, vomiting, anorexia, chills, and sometimes rash (13, 26, 39). Elevations in serum hepatic aminotransferases, leukopenia, thrombocytopenia, and rebound leukocytosis are noted. The severity of the disease can range from asymptomatic infection to severe morbidity and death (13, 26, 39).
HGE agent is the etiologic agent of HGE, an emerging tick-borne disease (2, 4, 9). HGE was first found in 12 patients in Minnesota and Wisconsin in 1994 (2, 9). Since then more than 400 cases of HGE have been reported in the northeast and upper midwestern United States (2, 42), and recently HGE has been reported in Europe (7) (Sweden [14], United Kingdom [35], and Norway [4]). Clinical signs and laboratory findings for HGE patients are similar to those for HME patients (3, 13, 42). HGE agent is almost identical to E. equi and E. phagocytophila in 16S rRNA gene sequence and antigenic composition.
E. sennetsu, the etiologic agent of Sennetsu ehrlichiosis in Japan and Malaysia, was first isolated in 1953 (15, 29, 31). Sennetsu ehrlichiosis is characterized by acute fever, lethargy, generalized lymphadenopathy, and increases in serum transaminases, leukopenia, and lymphocytosis.
E. chaffeensis and HGE agent reside in membrane-bound inclusions which contain multiple ehrlichiae per inclusion (or morula), whereas E. sennetsu inclusions generally contain only a single or a few ehrlichiae per inclusion (29). We have shown previously that E. chaffeensis in the acute monocytic leukemia cell line THP-1 occupy a membrane-bound compartment which is positive for transferrin receptor (TfR) and do not fuse with lysosomes (6). We have also shown that E. risticii, the causative agent of Potomac horse fever and closely related to E. sennetsu, selectively prevents lysosomal fusion with ehrlichia-containing inclusions in P388D1 cells (41). The extreme sensitivity of two genetically divergent monocytic ehrlichiae (E. chaffeensis and E. risticii) to iron depletion caused by the cell-permeable iron chelator deferoxamine (5, 27) suggests that cytoplasmic iron is essential for ehrlichiae and that these Ehrlichia spp. do not possess iron-uptake mechanisms with an affinity for iron greater than that of deferoxamine. We have previously shown that E. chaffeensis up-regulates the expression of host TfR mRNA in THP-1 cells (6). How E. chaffeensis up-regulates TfR mRNA is unknown. A cytoplasmic protein, iron-responsive protein (IRP), is known to regulate TfR mRNA levels by preventing TfR mRNA degradation (16, 19). In this study, we compared the above three Ehrlichia spp. for dependency on cytoplasmic labile iron pool and for effects on the localization of TfR, TfR mRNA levels, and IRP activity.
MATERIALS AND METHODS
Ehrlichia culture.
E. chaffeensis Arkansas (11), E. sennetsu (Miyayama) (43), and HGE agent HZ (32) were propagated in THP-1 cells (37), kindly provided by M. D. Wewers (The Ohio State University, Columbus, Ohio), in RPMI 1640 medium (GIBCO-BRL, Grand Island, N.Y.) supplemented with 10% fetal bovine serum (Atlanta Biological, Norcross, Ga.) and 4 mM l-glutamine (GIBCO-BRL) or in the human promyelocytic leukemia cell line HL-60 (10) in RPMI 1640 medium supplemented with 5% fetal bovine serum 1% l-glutamine, 1% sodium pyruvate (GIBCO-BRL), and 1% nonessential amino acids (GIBCO-BRL) (32) at 37°C in 5% CO2–95% air without antibiotics.
Preparation of host cell-free ehrlichiae.
When more than 90% of THP-1 cells were highly infected with E. chaffeensis, E. sennetsu, or HGE agent, as determined by examination of cytocentrifuged (Cytospin 2; Shandon, Inc., Pittsburgh, Pa.) cells stained with Diff-Quik (Baxter Scientific Products, Obetz, Ohio), the infected cells were suspended in 5 ml of culture medium at 106 cells per ml, sonicated at a setting of 2 at 20 kHz for 7 to 8 s in an model W-380 ultrasonic processor (Heat Systems, Farmingdale, N.Y.), and then centrifuged at 500 × g for 5 min. The supernatant containing the host cell-free ehrlichiae was centrifuged at 10,000 × g for 10, min and the pellet was used to infect THP-1 or HL-60 cells. For most experiments, a ratio of 1 uninfected cell to 2 infected cells was used.
Effect of deferoxamine on ehrlichial infection.
THP-1 cells were plated at 104 cells/well in a 96-well flat-bottom plate, pretreated with or without deferoxamine mesylate (15 μM; Sigma, St. Louis, Mo.) 24 h before infection, and then infected with host cell-free E. chaffeensis, E. sennetsu, or HGE agent derived from 105 heavily infected cells per well. After 4 days of incubation at 37°C, 100 μl of medium from each well containing infected cells was cytocentrifuged onto a glass slide. The cells were stained with Diff-Quik and observed at a magnification of ×1,000 to quantitate infectivity.
Quantitation of infectivity.
The percentage of infected cells and the number of ehrlichial organisms were scored in 100 cells per well from three wells as previously described (5, 27). Briefly, since E. chaffeensis and HGE agent are minute cocci which grow in a membrane-bound colony called a morula, it is impossible to accurately count individual organisms, especially when the cells are heavily infected. Therefore, morulae of infected cells were assigned to one of three size categories (1 to 10, 11 to 50, or 51 to 100 organisms per morula), and the number of morulae of each size per cell was determined. Total numbers of ehrlichial organisms per 100 monocytes were calculated by multiplying the mean number of organisms per morula in each category (5, 30, or 75 organisms per morula) by the total number of morulae in that category and adding all of them together. E. sennetsu organisms generally grow as individual cocci in THP-1 cells and were counted individually. All points were done in triplicate. The data were compared with the control by Student’s t test.
Double immunofluorescence labeling.
THP-1 or HL-60 cells at 3 to 4 days postinfection were cytocentrifuged at 250 × g and fixed for 10 s in Diff-Quik stain fixative containing methanol. The cells were then incubated with an appropriate primary antibody (dog anti-E. chaffeensis [6], rabbit anti-E. sennetsu [43], or horse anti-HGE agent [kindly provided by J. E. Madigan, University of California, Davis]) at 1:100 dilution in phosphate-buffered saline (PBS; 0.17 M NaCl, 0.003 M KCl, 0.01 M Na2HPO4, 0.002 M KH2PO4 [pH 7.4]) for 1 h at 37°C and then washed three times with PBS. All primary ehrlichial immune sera were preabsorbed with uninfected THP-1 or HL-60 cells at 106 cells/ml of serum at 37°C for 1 h. Fluorescein isothiocyanate (FITC)-conjugated anti-dog immunoglobulin G (IgG), anti-rabbit IgG, or anti-horse IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) at 15 μg/ml was used to label the dog, rabbit, or horse IgG, respectively at 37°C for 1 h. The cells were then incubated with primary mouse monoclonal antibody against human TfR (Immunotech, Westbrook, Maine) at 1:5 dilution of original solution in PBS for 1 h at 37°C and washed three times with PBS. Lissamine rhodamine-conjugated anti-mouse IgG at 15 μg/ml (Jackson) was used to label the mouse antibody. Negative controls consisted of uninfected THP-1 cells incubated with dog anti-E. chaffeensis, rabbit anti-E. sennetsu, or horse anti-HGE agent serum and FITC-conjugated anti-dog IgG, FITC-conjugated anti-rabbit IgG, or FITC-conjugated anti-horse IgG, respectively. Also, infected THP-1 cells were incubated with secondary conjugated antibodies alone or with preimmune dog, rabbit, or horse serum and FITC-conjugated anti-dog IgG, anti-rabbit IgG, or anti-horse IgG, respectively. The labeled cells were then covered with a semipermanent mounting medium containing Mowiol 4-88 (Calbiochem, La Jolla, Calif.) (6) and viewed by epifluorescence microscopy.
TfR mRNA levels in cells infected with E. chaffeensis, E. sennetsu, and HGE agent.
Briefly, infected THP-1 or HL-60 cells (5 × 106 in 25-cm2 flasks, seven flasks for each group) were cultured as described previously and harvested at 0, 6, 12, 18, 24, 48, and 72 h postinfection. The cells were pelleted by centrifugation for 5 min at 500 × g and total RNA was isolated; cDNA was synthesized by reverse transcription (RT), and relative amounts of TfR mRNA were determined by RT-PCR as described previously (6). Approximate amounts of TfR mRNA in the cDNA specimens were estimated by using a TfR cDNA positive control (ClonTech, Palo Alto, Calif.) as a standard. The Pearson’s correlation coefficient values between intensities of bands of PCR products versus amounts of cDNA were determined by a statistical program, StatView 4.11 (Abacus Concepts, Inc., Berkeley, Calif.).
Determination of whether ehrlichial viability, internalization, or protein synthesis is required for TfR mRNA upregulation.
The host cell-free E. chaffeensis derived from 107 infected cells in 1 ml of RPMI medium without serum was boiled in a water bath for 10 min to kill the ehrlichiae and denature proteins. This preparation (1 ml) was incubated with 5 × 106 THP-1 cells in a 25-cm2 flask for 3 h at 37°C, and then 4 ml of medium was added. This was done for each time point (seven flasks in total). THP-1 cells were pretreated with the transglutaminase inhibitor monodansylcadaverine (MDC; Sigma) at a concentration of 250 μM 3 h prior to addition of host cell-free ehrlichiae or were treated at 6 h postinfection with oxytetracycline (10 μg/ml; Sigma). THP-1 cells at 5 × 106 per flask were then harvested at 0, 6, 12, 18, 24, 48, and 72 h after addition of the E. chaffeensis derived from 107 infected cells per flask. At each time point, total RNA was isolated for RT-PCR. Sonicated uninfected THP-1 cell lysate was used as a negative control to evaluate the influence of host cell materials in the ehrlichial preparation.
Cytoplasmic extract preparation and RNA electrophoretic mobility shift assay (EMSA).
To prepare cytoplasmic extracts which contain IRP, 5 × 106 infected THP-1 cells were harvested at 0, 6, 12, and 24 h postinfection, lysed for 15 min at 4°C with modified lysis buffer containing 40 mM KCl, 25 mM Tris-HCl, 1% Triton X-100, 10 μg of leupeptin per ml, 25 μM p-nitrophenyl-p′-guanidinobenzoate, and 0.1 mM phenylmethylsulfonyl fluoride as described by Hentze et al. (19), and then centrifuged at 10,000 × g for 4 min. The protein concentration of the supernatants was determined by the Bradford method (Bio-Rad [Hercules, Calif.] protein assay), and the extracts were stored at −80°C. Radiolabeled RNA probe was generated from plasmid I-12.CAT (kindly provided by M. W. Hentze, European Molecular Biology Laboratory, Heidelberg, Germany), which encodes the human ferritin iron-responsive element (IRE) (16). The plasmid was linearized with restriction endonuclease XbaI and labeled with [32P]CTP with a Riboprobe in vitro transcription kit (Promega, Madison, Wis.) with T7 RNA polymerase. The transcript (58 bp) has the sequence 5′-GGGCGAAUUCGAGCU CGGUACCCGGGGAUCCUGCUUCAACAGUGCUUGGACGGAUCCU-3′, where the unpaired C residue and the loop of the IRE are underlined. The radiolabeled probe was purified as previously described (16) by excising the RNA band on the gel, mincing the gel, and extracting the RNA in 200 μl of elution buffer (0.5 M ammonium acetate, 1 mM EDTA, 0.1% sodium dodecyl sulfate) overnight at 37°C after separating the labeled probe on a 5% denaturing polyacrylamide gel at 200 V for 3 h. The cytoplasmic extract at 30 μg was incubated with the purified radiolabeled RNA probe (40,000 cpm), 1 U of RNase inhibitor (GIBCO-BRL), and 4 μl of binding buffer (125 mM HEPES, 750 mM potassium acetate, 7.5 mM MgCl2, 25% glycerol) for 30 min at 25°C; then 4 μl of heparin (final concentration, 5 mg/ml) was added, and the reaction mixture (20 μl, final volume) was incubated for additional 10 min. To determine total IRP activity, the cytoplasmic extracts were preincubated with 2% (final concentration) 2-mercaptoethanol (2-ME) 10 min prior to addition of the RNA probe. Purified ehrlichiae (E. sennetsu, E. chaffeensis, or HGE agent), 30 μg of protein per lane, were run to examine the presence of IRP activity, and purified recombinant IRP-1 (rIRP-1) at 0.5 μg/lane (Maria Polycarpou-Schwarz and MBI Fermentas, Vilnius, Lithuania) was used as a positive control. RNA-protein complexes were resolved by 5% nondenaturing polyacrylamide gel electrophoresis at 8 V/cm (120 V in total) for 3 h, dried, and exposed to X-ray film (X-Omat AR; Kodak, Rochester, N.Y.) at 27°C for 3 to 4 h. The band intensities in the original gel were registered with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) and analyzed with image analysis software (ImageQuant; Molecular Dynamics). All data points were done in triplicate.
RESULTS
Localization of TfR in ehrlichial inclusions.
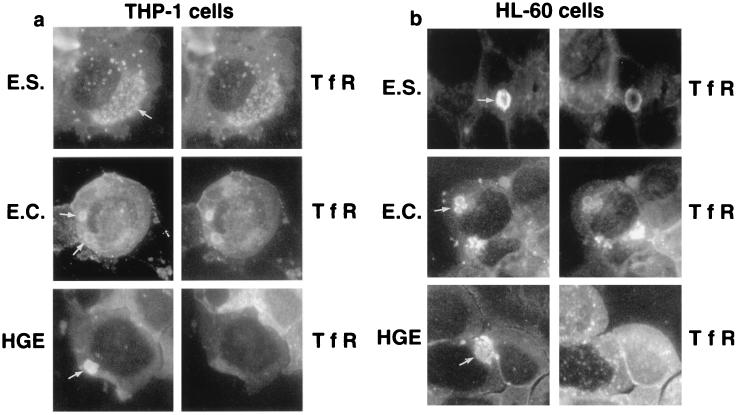
Ehrlichia spp. are generally host cell specific (29). Monocytic (monocyte-tropic) ehrlichiae such as E. chaffeensis and E. sennetsu are seen primarily in cells of monocyte and macrophage lineage, and granulocytic (granulocyte-tropic) ehrlichiae such as HGE agent are seen in granulocytes in the peripheral blood of severely ill patients. Double immunofluorescence labeling for E. chaffeensis, E. sennetsu, or HGE agent and TfR was performed to see whether TfR colocalizes with ehrlichial inclusions in THP-1 or HL-60 cells. THP-1 is a human acute monocytic leukemia cell line (37) previously used to cultivate E. chaffeensis (5). HL-60 is a human promyelocytic leukemia cell line (10) used to isolate and cultivate HGE agent (32). Previously we found that E. chaffeensis proliferates in HL-60 cells as well as HGE agent (25). In the present study, we found that HGE agent and E. sennetsu proliferate in THP-1 cells as well as E. chaffeensis. Previously we found that E. chaffeensis colocalizes with TfR in THP-1 (6) and HL-60 (25) cells and that E. sennetsu and TfR colocalize in the murine macrophage cell line P388D1 (44). Inclusions of E. sennetsu strongly colocalized with TfR in THP-1 cells (Fig. 1a) and in HL-60 cells (Fig. 1b). However, HGE agent inclusions did not colocalize with TfR in THP-1 cells (Fig. 1a) or in HL-60 cells (25) (Fig. 1b). In addition, TfR labeling intensity was much stronger in E. chaffeensis- and E. sennetsu-infected THP-1 cells than in uninfected THP-1 cells, suggesting up-regulation of TfR. These results indicate that the colocalization of TfR with monocytic ehrlichial inclusion is likely ehrlichia specific and not influenced by types of the host cells they infect, since the labeling of TfRs in the ehrlichial inclusions did not change in different cell lines. E. sennetsu, however, grew substantially more slowly in HL-60 cells than in THP-1 cells and grew in morulae (Fig. 1b) instead of remaining as single organisms as in THP-1 or P388D1 cells; as a result, E. sennetsu-infected HL-60 cells were not used in the time course experiments. Negative controls as described in Materials and Methods did not label either ehrlichiae or any cellular structures.
FIG. 1.
Double immunofluorescence labeling of E. sennetsu, E. chaffeensis, HGE agent, and human TfR in THP-1 and HL-60 cells. Cells were infected with host cell-free ehrlichiae and harvested 3 (6 for E. sennetsu in HL-60 cells) days postinfection. Paired photomicrographs show FITC-labeled E. chaffeensis (E.C.), E. sennetsu (E.S.), or HGE agent (HGE) (white arrows) on the left and lissamine rhodamine-labeled human TfR on the right. Results are representative of three independent labeling experiments. Magnification, ×812.
Effect of deferoxamine on ehrlichial infection.
Deferoxamine, a siderophore from Actinomyces sp., is a cell-permeable iron chelator of the labile iron pool (22). To compare the sensitivities of E. sennetsu, E. chaffeensis, and HGE agent to intracellular iron depletion, THP-1 cells were pretreated for 24 h with 15 μM deferoxamine. Infection by E. chaffeensis and E. sennetsu in THP-1 cells was almost completely inhibited by treatment with deferoxamine. However, the HGE agent in THP-1 cells was only partially inhibited by deferoxamine treatment (Table 1). This finding suggests that HGE agent can gain access to iron from other sources in addition to iron of the labile iron pool which is susceptible to deferoxamine, or HGE agent might produce a siderophore with greater iron affinity than deferoxamine. Treatment with deferoxamine at 15 μM for more than 3 days was toxic to the HL-60 cells; therefore, HL-60 cells could not be used for the deferoxamine study. THP-1 cells, however, showed no morphologic evidence of toxicity after the same treatment.
TABLE 1.
Inhibitory effect of deferoxamine on ehrlichial proliferation in THP-1 cells
| Infection | Effecta
|
|||
|---|---|---|---|---|
| With 15 μM deferoxamine
|
Without deferoxamine (control)
|
|||
| % Infected | No. of ehrlichiae/100 cells | % infected | No. of ehrlichiae/100 cells | |
| E. chaffeensis | 3 ± 2 | 18 ± 14 | 91 ± 4 | 6,102 ± 525 |
| E. sennetsu | 3 ± 1 | 97 ± 23 | 91 ± 3 | 7,289 ± 923 |
| HGE agent | 30 ± 4 | 1,602 ± 480 | 97 ± 2 | 7,869 ± 480 |
Mean ± standard deviation of three independent experiments (in triplicate) determined 4 days postinfection.
Time course of TfR mRNA levels.
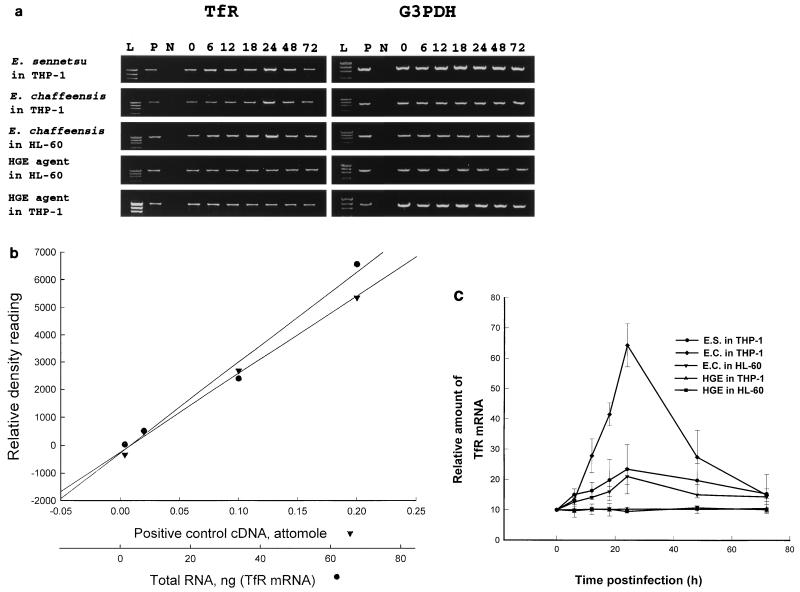
Since the immunofluorescence labeling of TfR was much stronger in E. chaffeensis- and E. sennetsu-infected cells than in uninfected cells, we examined by RT-PCR whether the increase in intensity of TfR labeling after E. chaffeensis and E. sennetsu infection is correlated with an increase in the steady-state level of TfR mRNA. Figure 2a shows the time course of TfR mRNA expression in THP-1 or HL-60 cells infected with three Ehrlichia spp. Low levels of TfR mRNA were constitutively expressed in uninfected THP-1 and HL-60 cells. The expression of glyceraldehyde-3-phosphate dehydrogenase (G3PDH) mRNA served as a control for the amount of input RNA across the samples. All samples showed comparable levels of G3PDH expression in THP-1 and HL-60 cells. With increase in infection time, up to 24 h postinfection with E. chaffeensis, there is a gradual 7-fold increase in the TfR mRNA levels compared to that at 0-h infection in E. chaffeensis-infected THP-1 cells and a 2.5-fold increase in E. sennetsu-infected THP-1 cells, which after 24 h steadily declined to baseline levels (Fig. 2c). Additionally, E. chaffeensis-infected HL-60 cells show a gradual 2.5-fold increase in TfR mRNA compared to the 0-h time point. In contrast, HGE agent in THP-1 or HL-60 cells did not show any increase in the TfR mRNA levels throughout infection. These results suggests that only E. chaffeensis and E. sennetsu, not HGE agent, up-regulate TfR mRNA expression. The percentages of infected cells and (in parentheses) numbers of ehrlichiae per 100 cells at 3 days after infection with E. chaffeensis in THP-1 and HL-60 cells, HGE agent in THP-1 and HL-60 cells, and E. sennetsu in THP-1 cells were 64% ± 3.05% (4,588 ± 533), 67% ± 4.58% (4,665 ± 922), 62% ± 3.51% (4,613 ± 101), 64% ± 4.35% (4,743 ± 375), and 69% ± 2.64% (4,914 ± 191), respectively (n = 3 for each infection). These results indicate that up-regulation of TfR mRNA is not simply the result of ehrlichial growth or iron uptake but is specific to these two species of monocytic ehrlichiae, E. chaffeensis and E. sennetsu. Additionally, there was no change in the TfR mRNA levels with uninfected host cell lysate alone controls, indicating that the up-regulation of the TfR mRNA is not due to a component of the host cell lysate (data not shown).
FIG. 2.
TfR mRNA expression in THP-1 cells at various times after infection with different Ehrlichia spp. (a) THP-1 cells (5 × 106) were infected with host cell-free E. sennetsu, E. chaffeensis, or HGE agent (derived from 107 infected cells), and HL-60 cells (5 × 106) were infected with host cell-free E. chaffeensis or HGE agent (derived from 107 infected cells). After 0, 6, 12, 18, 24, 48, or 72 h of infection, total RNA was extracted and cDNA was synthesized. An aliquot of the PCR product (9 μl) was visualized on a 1.5% ethidium bromide-agarose gel. Lanes: L, DNA ladder (φX174 replicative-form DNA digested with HaeIII); P, TfR (1,347 bp) or G3PDH (983-bp) positive control; N, negative control; 4 to 10, 0, 6, 12, 18, 24, 48, and 72 h. (b) Dose-response standard curve for TfR mRNA, determined as described below. The Pearson’s correlation coefficient values between relative density versus TfR cDNA (Clontech) and versus cDNA from our study at 24 h were 0.996 (P < 0.01) and 0.979 (P < 0.05), respectively. cDNA (1 μl) derived from 34 ng of total RNA at 24 h postinfection corresponds approximately to 0.1 amol of TfR cDNA (Clontech). (c) Relative TfR mRNA concentrations at 0, 3, 6, 12, 18, 24, 48, and 72 h based on a standard curve. The positive control for TfR mRNA was obtained from a human TfR control amplimer set (Clontech). Values are the means and standard deviations of three independent experiments for each Ehrlichia sp. (abbreviated as for Fig. 1).
Effect of heat treatment of ehrlichiae, MDC, and oxytetracycline treatment on TfR mRNA up-regulation by E. chaffeensis.
Throughout the experiment, heat-killed E. chaffeensis did not up-regulate TfR mRNA in THP-1 cells (Fig. 3), indicating that viable organisms or intact proteins of E. chaffeensis are required for the up-regulation. MDC does not inhibit binding of E. risticii to P388D1 cells but blocks internalization of E. risticii into P388D1 cells (23). At 3 days postinfection, the control culture was 64% ± 3% infected with E. chaffeensis and there were 4,648 ± 157 ehrlichiae per 100 cells (n = 3). However, in the presence of 250 μM MDC added at 3 h prior to infection, no THP-1 cells (n = 3) were infected. MDC inhibited up-regulation of TfR mRNA levels throughout the incubation period (Fig. 3), indicating that ehrlichial internalization is required to up-regulate TfR mRNA. With oxytetracycline (10 μg/ml added at 6 h postinfection), there was an increase in TfR mRNA in THP-1 cells up to 6 h, which sharply returned to the baseline (0-h) mRNA level by 24 h postinfection (Fig. 3). Taken together, these results suggest that the E. chaffeensis-induced up-regulation of TfR mRNA is dependent on viable ehrlichiae which must enter into the host cell and that ehrlichial protein synthesis is required to maintain elevated TfR mRNA levels up to 24 h.
FIG. 3.
Effects of heat treatment, MDC, and oxytetracycline on TfR mRNA expression. (a) THP-1 cells (5 × 106) were treated with MDC (250 μM, 3 h preinfection; bottom row) or oxytetracycline (10 μg/ml, 6 h postinfection; middle row) and incubated with live host-cell free E. chaffeensis (derived from 107 infected cells) or were incubated with heat-killed E. chaffeensis (top row). After incubation, total RNA was extracted and cDNA was synthesized as described in Materials and Methods. An aliquot of the PCR product (9 μl) was visualized on a 1.5% ethidium bromide-agarose gel. Lanes are as in Fig. 2a. (b) Approximate TfR mRNA concentrations at 0, 3, 6, 12, 18, 24, 48, and 72 h based on a standard curve. Values are the means and standard deviations of three independent experiments for each Ehrlichia species.
IRP in ehrlichia-infected THP-1 cells.
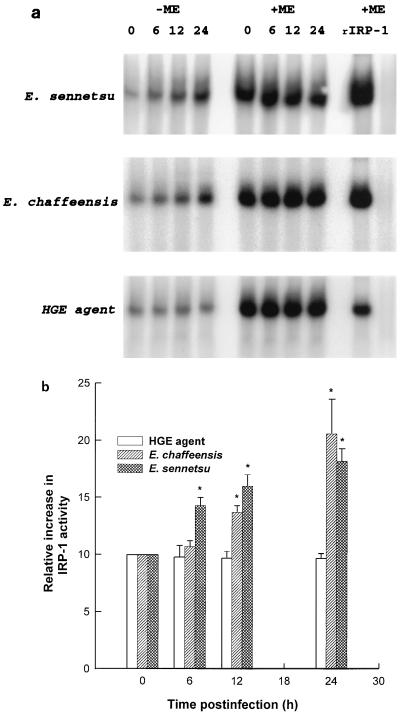
TfR mRNA stability is known to be regulated by reversible binding of iron-regulatory factors or IRPs present in the cytoplasm to evolutionarily conserved cis-acting specific mRNA sequences called IREs (14a, 16, 19). IREs are present in the five stem-loop structures within 3′ untranslated region of TfR mRNA. When the iron level is low, IRPs bind to IREs; when levels of cellular iron rise, IRP converts into the enzyme aconitase (iron-sulfur cluster protein) and loses its ability to bind to IREs (16, 19). Thus, assembly-disassembly of the cluster is the sensing signal that is transduced into regulated binding of IRP. IRP requires free sulfhydryl groups for its specific binding to IREs. Reducing conditions increase binding activity, and oxidizing conditions inhibit binding (19). Iron status operates as a sulfhydryl switch by reversible oxidation or reduction of critical sulfhydryl group or groups in the IRP. The binding of IRPs to an IRE prevents degradation of TfR mRNA. As a result, it causes accumulation of TfR mRNA. Presently, at least two IRPs are known: IRP-1 and IRP-2 (molecular masses 97 and 105 kDa, respectively) (18, 34). Whether E. chaffeensis, E. sennetsu, or HGE agent infection modulates IRP binding to the IRE was examined in uninfected THP-1 cells by EMSA. The EMSA, by using a radioactive RNA probe which contains a characteristic six-membered IRE loop (CAGUGN), can determine by autoradiography levels of reduced IRP (active IRP) present in the cytosol due to the slower migration (migration shift) of the IRP-bound IRE compared to free IRE probe in the gel electrophoresis. Purified rIRP-1 was used as a positive control to identify the position of IRP-1 in the EMSA. Both E. chaffeensis and E. sennetsu infection increased the binding of the IRP-1 to the IRE, as seen by an up to twofold increase in the intensity of the band at the same position as rIRP-1, whereas HGE agent infection did not change the IRP-1 binding activity throughout the 24-h infection period (Fig. 4). IRP-2 activity, which is expected to run slower than IRP-1 due to its larger mass, was not detected throughout any of the time course experiments. The addition of 2-ME fully activated the IRP-1 in E. chaffeensis-, E. sennetsu-, and HGE agent-infected THP-1 cells up to three times the basal (0-h) level without 2-ME (Fig. 4) and showed that the total amount of IRP-1 in the cytosol did not change throughout the 24-h infection period with any of the Ehrlichia spp. This approximately threefold increase is similar to what was found in HL-60 cells after treatment with 2-ME (14a). No IRP activity was detected in purified E. sennetsu, E. chaffeensis, or HGE agent.
FIG. 4.
EMSA of IRP in the cytoplasm of THP-1 cells infected with three Ehrlichia spp. (a) Cytoplasmic extracts from infected THP-1 cells harvested at 0, 6, 12, and 24 h postinfection were incubated with 32P-labeled IRE probe in binding buffer for 30 min at 25°C; then heparin was added at 5 mg/ml, and the reaction mixture was incubated for additional 10 min. To determine total IRP activity, cell lysates were preincubated with 2% 2-ME 10 min prior to addition of the IRE probe. Purified E. sennetsu, E. chaffeensis, or HGE agent (30 μg/lane) was run to examine the presence of IRP activity, and purified rIRP-1 (0.5 μg/lane) was the positive control. RNA-protein complexes were resolved by 5% nondenaturing polyacrylamide gel electrophoresis and exposed to X-ray film. (b) Analysis of the gel with a PhosphorImager and of band intensities with image analysis software. An asterisk indicates that the IRP-1 activity is significantly (P < 0.05) different from 0-h control level by Student’s t test. Data are expressed as means and standard deviations of three independent experiments. Based on the density analysis data obtained with the rIRP-1 as a standard, the basal IRP-1 activity without 2-ME in uninfected THP-1 cells was approximately 110 ± 55 ng/30 μg of cytosolic protein (n = 9) and the total IRP-1 activity in the presence of 2-ME was approximately 315 ± 70 ng/30 μg of cytosolic protein (n = 9).
DISCUSSION
E. chaffeensis, E. sennetsu, and HGE agent are obligatory intracellular pathogens that are divergent in their 16S rRNA gene sequences, protein compositions, and antigenic compositions (30, 31), yet all of them occupy and replicate in membrane-lined inclusions in the cytoplasm of leukocytes, which are primary effector cells of antimicrobial defense. Therefore, all ehrlichiae must create a compartment conducive not only for survival but also for replication in a usually inhospitable environment. For obligatory intracellular bacteria such as ehrlichiae, the maintenance of the inclusion environment is expected to be more stringent than with facultative intracellular bacteria. We have shown that replicative inclusions of E. chaffeensis in THP-1 cells are early endosomes positive for TfR, Rab5, and early endosomal antigen 1 but negative for lysosomal glycoproteins (6, 25). When E. chaffeensis initially bound to THP-1 cells at 4°C, TfR colocalization was not seen (6). During a subsequent 12-h infection period at 37°C, increasing amounts of numerous small TfR-positive granules were seen in the peripheral cytoplasm. Following 1 to 3 days of infection, these small TfR-positive granules disappeared and virtually all cytoplasmic TfR molecules were localized on ehrlichial inclusions. At the same time, labeling intensity for TfR protein was increased in E. chaffeensis inclusions, and TfR mRNA levels also increased (6). The inclusion accumulated exogenous FITC-labeled transferrin (FITC-Tf) (6). Although TfR localization in or around inclusions of several other intracellular organisms has been reported, we are not aware of any other organism which up-regulates TfR mRNA and accumulates Tf in its inclusion. The fact FITC-Tf accumulates in E. chaffeensis inclusions indicate the compartment is connected with the endocytic pathway of TfR. How much of the Tf in E. chaffeensis inclusions remains loaded with iron is unknown. Since (i) iron molecules are dissociated at the acidic pH of the endosome, at pH 6.5 (one Fe) and pH 6.0 (2 Fe) and apo-Tf (Tf free of iron) and TfR cycle back to the cell surface for more iron uptake (25a) and (ii) the E. chaffeensis inclusion is slightly acidic (6), we expect that at least a part of Tf in the inclusion is iron free. Whether the recycling of TfR is inhibited or delayed by E. chaffeensis infection has not been determined.
We have found that the HGE agent inclusion in THP-1 cells does not accumulate TfR. Parallel studies in our laboratory revealed that replicative inclusions of HGE agent are distinct from those of E. chaffeensis in HL-60 cells, not only with respect to TfR but also in the early endosomal markers Rab5 and early endosomal antigen 1, suggesting that replicative inclusions of HGE agent are separated from the endosomal network (25). The result is in agreement with recent study of Webster et al. (39a) with respect to the absence of colocalization of TfR and the lysosomal membrane glycoprotein LAMP 1 or lack of accumulation of acidic markers in HGE agent inclusions. But in several aspects, such as our finding of the absence of colocalization of cation-dependent mannose 6-phosphate receptor and our conclusion that the replicative inclusion of HGE agent is disconnected from the endosome-lysosome pathway (25), our study is in contradiction to theirs, which implicated the HGE agent inclusion as an endosomal compartment (39a). TfR-Tf cycles between plasma membrane and early endosomes (33); i.e., the cytoplasmic compartment lined by the membrane, which contains TfR regardless of whether the receptor is bound to iron-Tf or iron-free Tf, is excluded from lysosomes. Thus, we speculate that monocytic ehrlichiae may avoid lysosomal fusion by being able to fuse with TfR endosomes and retain TfR. HGE agent inclusions also do not fuse with lysosomes (25) but must utilize a different mechanism to avoid lysosomal fusion, since these inclusions lack TfR. The results of our study suggest that monocytic and granulocytic Ehrlichia species use different receptors for internalization or intracellular membrane traffic.
As for E. risticii in murine peritoneal macrophages (27) or E. chaffeensis in phorbol myristate acetate-treated THP-1 cells (5), the addition of 15 μM deferoxamine completely inhibited the survival of intracellular E. sennetsu in THP-1 cells, while HGE agent was partially resistant to the effect of deferoxamine in THP-1 cells. The partial resistance of HGE agent could be due to the use by the HGE agent of a mechanism of iron acquisition independent of the labile iron pool. Iron might be essential for ehrlichial growth, since evidence suggests that E. risticii and E. sennetsu lack a conventional glycolytic pathway, and thus the electron transport chain consisting of cytochrome enzymes may be their sole mechanism of ATP generation (40). Whether E. chaffeensis and HGE agent lack a conventional glycolytic pathway has not been determined. The iron dependency of both ehrlichiae, however, suggests that this may be the case.
Although E. chaffeensis and E. sennetsu accumulate TfR on their inclusions, how the ehrlichiae acquire iron within the host cell is unknown. The present results suggest that that intracellular, labile iron dependency and accumulation of TfRs in ehrlichial inclusions might be a universal phenomenon among the monocytic Ehrlichia spp. Deferoxamine chelates iron in the labile iron pool, and this labile iron pool consists of iron that is immediately available to the cell for metabolic processes (20). Such iron is in a readily transportable form rather than in storage compounds such as ferritin or hemosiderin. Iron released from endocytosed Tf immediately enters this pool before it is used for metabolic processes or bound to ferritin. The fact that deferoxamine inhibits E. chaffeensis, E. risticii, and E. sennetsu indicates that these ehrlichiae, like Legionella pneumophila (8), derive iron from the labile iron pool. Monocytic ehrlichiae might acquire iron released from Tf in the slightly acidic pH within the inclusion (6) before the iron is transported to the host cytosol across the inclusion membrane.
The up-regulation of TfR mRNA by E. chaffeensis and E. sennetsu may simply be the passive result of the mode of competitive iron acquisition and uptake by the monocytic ehrlichiae which limits the available iron to the host cell, or it may be part of an active novel mechanism for the acquisition of iron needed for monocytic ehrlichial survival. This also implies that iron uptake by HGE agent does not compete with host cell iron acquisition. TfR expression in the host cell is regulated primarily by the stability of levels of TfR mRNA, which is dependent on the iron levels of the cell (16, 19). We found that both E. chaffeensis and E. sennetsu infections increased the binding of the IRP-1 to the IRE, whereas HGE infection did not change the IRP-1 binding activity. E. chaffeensis and E. sennetsu, which are monocytic ehrlichiae, may sequester iron from the labile iron pool, which would reduce the cytoplasmic iron level and as a result augment IRP binding to the IRE. Alternatively, these monocytic ehrlichiae may modulate the redox potential within the cell and increase the affinity of the IRP for the IRE. The production of nitric oxide (NO) or the addition of chemicals which are able to release NO have been shown to transform IRP from an aconitase to a high-affinity IRE-binding form (12). However, the production of NO is likely not the cause for increased IRP activity in E. chaffeensis- and E. sennetsu-infected THP-1 cells since infected THP-1 cells were shown not to produce NO (5). The fact that HGE agent did not alter IRP-1 binding activity suggests that the HGE agent might acquire iron from sources other than the labile iron pool. Whether ehrlichiae or any other bacteria have an IRP has not been reported. The lack of IRP activity in any of host cell-free Ehrlichia spp. indicates that the IRP-1 activity observed in this study is the host origin but not derived from intracellular ehrlichiae.
Recently IRP-2, an approximately 105-kDa protein which has 57% amino acid identity with IRP-1 (97 kDa) and affinity similar to that of as IRP-1, was discovered. The levels of IRP-2 are inversely regulated by iron levels due to its own degradation via the proteasome pathway. In addition to changes in total amounts of IRP-2, the IRE binding activity of IRP-2 can also vary up to fourfold in the absence of any changes in IRP-2 protein levels (34). IRP-2 activity is high in the brain and intestine but low in lymph nodes and spleen and is not detectable in log-phase-growing or deferoxamine-treated HL-60 cells (18). Also, IRP-2 binding activity was shown not to change after the addition of a reducing agent such as 2-ME (18, 34). The findings that in ehrlichia-infected samples IRP binding to the IRE probe migrated the same distance as the purified IRP-1 and that 2-ME treatment increased the binding of IRP to the IRE indicate that IRP-1 is the major IRP expressed in THP-1 cells.
TfR accumulation and TfR mRNA up-regulation were different in a granulocytic ehrlichia (HGE agent) and monocytic ehrlichiae (E. sennetsu and E. chaffeensis). Since these monocytic ehrlichiae and HGE agent were found in different types of host cells in patients and were cultivated in different cell lines, it is unclear whether this difference is due the host cell type or to the species of Ehrlichia involved. Our results for TfR mRNA and immunofluorescent antibody labeling were in agreement for THP-1 cells and HL-60 cells, indicating that these changes are not related to the type of host cell. These results suggest that the HGE agent utilizes a mechanism of iron acquisition which is disconnected from the IRP-mediated TfR mRNA stabilization mechanism. This is the first demonstration of a difference between monocytic and granulocytic ehrlichia modulation of host cell IRP-1 activity and expression of TfR mRNA, a critical protein required by the host cell. Further study of the mechanism of up-regulation of TfR mRNA and iron acquisition by ehrlichiae is under way.
ACKNOWLEDGMENTS
We thank Yilan Zhang for preliminary work with double immunofluorescence labeling of E. sennetsu inclusions for TfR in P388D1 cells, Ning Zhi for assistance in 32P-labeling of the IRE probe, and Jason Mott for the HGE agent culture. We also thank Maria Polycarpou-Schwarz for guidance on IRE probe purification and for generously supplying purified rIRP-1 to use as a positive control.
This research was supported by grants ROI AI30010 and F32AI09177 from the National Institutes of Health.
REFERENCES
- 1.Anderson B E, Dawson J E, Jones D C, Wilson K E. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakken J S, Dumler J S, Chen S M, Eckman M R, van Etta L L, Walker D H. Human granulocytic ehrlichiosis in the upper Midwest United States. a new species emerging? JAMA. 1994;272:212–218. [PubMed] [Google Scholar]
- 3.Bakken J S, Krueth J, Wilson-Nordskogg C, Tilden R L, Asanovich K, Dumler J S. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. [PubMed] [Google Scholar]
- 4.Bakken J S, Krueth J, Tilden R L, Dumler J S, Kristansen R E. Serological evidence of human granulocytic ehrlichiosis in Norway. Eur J Clin Microbiol Infect Dis. 1997;15:829–832. doi: 10.1007/BF01701530. [DOI] [PubMed] [Google Scholar]
- 5.Barnewall R E, Rikihisa Y. Abrogation of gamma interferon-induced inhibition of Ehrlichia chaffeensis infection in human monocytes with iron transferrin. Infect Immun. 1994;62:4804–4810. doi: 10.1128/iai.62.11.4804-4810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnewall R E, Rikihisa Y, Lee E H. Ehrlichia chaffeensis inclusions are early endosomes which selectively accumulate transferrin receptor. Infect Immun. 1997;65:1455–1461. doi: 10.1128/iai.65.4.1455-1461.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brouqui P, Dumler J S, Lienhard R, Brossard M, Raoult D. Human granulocytic ehrlichiosis in Europe. Lancet. 1995;346:782–783. doi: 10.1016/s0140-6736(95)91544-3. [DOI] [PubMed] [Google Scholar]
- 8.Byrd T F, Horowitz M A. Interferon gamma-activated human monocytes down regulate transferrin receptors and inhibit the intracellular multiplication of Legionela pneumophila by limiting the availability of iron. J Clin Investig. 1989;83:1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S M, Dumler J S, Bakken J S, Walker D H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins S J, Gallo R C, Gallagher R E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977;270:347–350. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- 11.Dawson J E, Anderson B E, Fishbein D, Sanchez J, Goldsmith C, Wilson K, Duntly C. Isolation and characterization of an Ehrlichia sp. from a patient with human ehrlichiosis. J Clin Microbiol. 1991;29:2741–2745. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drapier J C, Hirling H, Wietzerbin J, Aldy P, Kuhn L. Biosynthesis of nitric oxide activates iron regulatory factor in macrophages. EMBO J. 1993;12:3643–3649. doi: 10.1002/j.1460-2075.1993.tb06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumler J S. Human ehrlichioses: clinical, laboratory, epidemiologic, and pathologic considerations. In: Kazar J, Toman R, editors. Proceedings of the Vth International Symposium on Rickettsiae and Rickettsial Diseases. Bratislava, Slovak Republic: Slovak Academy of Sciences; 1996. pp. 287–302. [Google Scholar]
- 14.Dumler J S, Dotevall L, Gustafson R, Granstrom M. A population-based seroepidemiologic study of human granulocytic ehrlichiosis and Lyme borreliosis on the west coast of Sweden. J Infect Dis. 1997;175:720–722. doi: 10.1093/infdis/175.3.720. [DOI] [PubMed] [Google Scholar]
- 14a.Eisenstein R S, Tuazon P T, Schalinske K L, Anderson S A, Traugh J A. Iron-responsive element-binding protein: phosphorylation by protein kinase C. J Biol Chem. 1993;268:27363–27370. [PubMed] [Google Scholar]
- 15.Fukuda T, Kitao T, Keida Y. Studies on the causative agent of “Hyuganestsu” disease. I. Isolation of the agent and its inoculation trial in human beings. Med Biol. 1954;23:200–209. [Google Scholar]
- 16.Gray N K, Quick S, Goossen B, Constable A, Hirling H, Kuhn L C, Hentze M W. Recombinant iron-regulatory factor functions as an iron-responsive-element binding protein, a translational repressor and an aconitase: a functional assay for translational repression and direct demonstration of the iron switch. Eur J Biochem. 1993;218:657–667. doi: 10.1111/j.1432-1033.1993.tb18420.x. [DOI] [PubMed] [Google Scholar]
- 17.Guerrero A, Fishbein D B, Mesa E, Escudero R. Human infection by Ehrlichia canis in Spain? Med Clin (Barcelona) 1991;96:236–237. [PubMed] [Google Scholar]
- 18.Henderson B R, Seiser C, Kuhn L C. Characterization of a second RNA-binding protein in rodents with specificity for iron-responsive elements. J Biol Chem. 1993;268:27327–27334. [PubMed] [Google Scholar]
- 19.Hentze W M, Rouault T A, Harford J B, Klausner R D. Oxidation-reduction and the molecular mechanism of a regulatory RNA-protein interaction. Science. 1989;244:357–359. doi: 10.1126/science.2711187. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs A. Low molecular weight intracellular iron transport compounds. Blood. 1977;50:433–439. [PubMed] [Google Scholar]
- 21.Maeda K, Markowitz N, Hawley R C, Ristic M, Cox D, McDate J E. Human infection with Ehrlichia canis, a leukocytic rickettsia. N Engl J Med. 1987;316:853–856. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- 22.Martinez J L, Delgado Iribarren A, Baquero F. Mechanisms of iron acquisition and bacterial virulence. FEMS Microbiol Rev. 1990;6:45–56. doi: 10.1111/j.1574-6968.1990.tb04085.x. [DOI] [PubMed] [Google Scholar]
- 23.Messick J B, Rikihisa Y. Characterization of Ehrlichia risticii binding, internalization, and proliferation in host cells by flow cytometry. Infect Immun. 1993;61:3803–3810. doi: 10.1128/iai.61.9.3803-3810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morais J D, Dawson J E, Greene C, Filipe A R, Galhardas L C, Bacellar F. First European cases of ehrlichiosis. Lancet. 1991;338:633–634. doi: 10.1016/0140-6736(91)90644-5. [DOI] [PubMed] [Google Scholar]
- 25.Mott J, Barnewall R E, Rikihisa Y. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect Immun. 1999;67:1368–1378. doi: 10.1128/iai.67.3.1368-1378.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Nunez M-T, Gaete V, Watkins J A, Glass J. Mobilization of iron from endocytic vesicles. J Biol Chem. 1990;265:6688–6692. [PubMed] [Google Scholar]
- 26.Paddock C D, Sumner J W, Merrill Shore G M, Bartley D C, Elie R C, McQuade J G, Martin C R, Goldsmith C S, Childs J E. Isolation and characterization of Ehrlichia chaffeensis strains from patients with fatal ehrlichiosis. J Clin Microbiol. 1997;35:2496–2502. doi: 10.1128/jcm.35.10.2496-2502.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park J, Rikihisa Y. l-Arginine-dependent killing of intracellular Ehrlichia risticii by macrophages treated with gamma interferon. Infect Immun. 1992;60:3504–3508. doi: 10.1128/iai.60.9.3504-3508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierard D, Levtchenko E, Dawson J E, Lauwers S. Ehrlichiosis in Belgium. Lancet. 1995;346:1233–1234. doi: 10.1016/s0140-6736(95)92943-6. [DOI] [PubMed] [Google Scholar]
- 29.Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 1991;4:286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rikihisa Y. Ehrlichiae. In: Kazar J, Toman R, editors. Proceedings of the Vth International Symposium on Rickettsiae and Rickettsial Diseases. Blatislava, Slovak Republic: Slovak Academy of Sciences; 1996. pp. 272–286. [Google Scholar]
- 31.Rikihisa Y. Ehrlichiae, emerging human pathogens. In: Yanagihara Y, Masuzawa T, editors. Proceedings of the 2nd International Symposium on Lyme Disease in Shizuoka. Emerging and Re-Emerging Diseases Transmitted by Arthropod Vectors and Rodents. Shizuoka, Japan: University of Shizuoka; 1997. pp. 332–345. [Google Scholar]
- 32.Rikihisa Y, Zhi N, Wormser G P, Wen B, Horowitz H M, Hechemy K E. Ultrastructural and antigenic characterization of granulocytic ehrlichia directly isolated and cultivated from a patient in New York State. J Infect Dis. 1996;175:210–213. doi: 10.1093/infdis/175.1.210. [DOI] [PubMed] [Google Scholar]
- 33.Rothenberger S, Lacopetta B J, Kuhn L C. Endocytosis of the transferrin receptor requires the cytoplasmic domain but not its phosphorylation site. Cell. 1987;49:423–431. doi: 10.1016/0092-8674(87)90295-9. [DOI] [PubMed] [Google Scholar]
- 34.Samaniego F, Chin J, Iwai K, Rouault T A, Klausner R D. Molecular characterization of a second iron-responsive element binding protein, iron regulatory protein 2. J Biol Chem. 1994;269:30904–30910. [PubMed] [Google Scholar]
- 35.Sumpton K J, Wright J M, Cutler S J, Dale B S S. Human ehrlichiosis in the UK. Lancet. 1995;346:1487–1488. doi: 10.1016/s0140-6736(95)92502-3. [DOI] [PubMed] [Google Scholar]
- 36.Telford S R, III, Dawson J E, Warner C K, Kolbert C P, Persing D H. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuchiya S, Yamake M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of human monocytic leukemia cell line (THP-1) Int J Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 38.Uhaa I J, MacLean J D, Green C R, Fishbein D B. A case of human ehrlichiosis acquired in Mali: clinical and laboratory findings. Am J Trop Med Hyg. 1992;46:161–146. doi: 10.4269/ajtmh.1992.46.161. [DOI] [PubMed] [Google Scholar]
- 39.Walker D H, Dumler J S. Emergence of the ehrlichioses as human health problems. Emerg Infect Dis. 1996;2:18–29. doi: 10.3201/eid0201.960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39a.Webster P J, Ijdo W, Chicoine L M, Fikrig E. The agent of human granulocytic ehrlichiosis resides in an endosomal compartment. J Clin Investig. 1998;101:1932–1941. doi: 10.1172/JCI1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss E, Dasch G A, Kang Y-H, Westfall H N. Substrate utilization by Ehrlichia sennetsu and Ehrlichia risticii separated from host constituents by Renografin gradient centrifugation. J Bacteriol. 1988;170:5012–5017. doi: 10.1128/jb.170.11.5012-5017.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wells M Y, Rikihisa Y. Lack of lysosomal fusion with phagosome containing Ehrlichia risticii in P388D1 cells: abrogation of inhibition with oxytetracycline. Infect Immun. 1988;56:3209–3215. doi: 10.1128/iai.56.12.3209-3215.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wormser G, Mckenna D, Aguero-Rosenfeld M, et al. Human granulocytic ehrlichiosis-New York. Morbid Mortal Weekly Rep. 1995;44:593–595. [PubMed] [Google Scholar]
- 43.Zhang Y, Ohashi N, Lee E H, Tamura A, Rikihisa Y. Ehrlichia sennetsu groE operon and antigenic properties of the GroEL homolog. FEMS Immunol Med Microbiol. 1997;18:39–46. doi: 10.1111/j.1574-695X.1997.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhang, Y., and Y. Rikihisa. Unpublished data.