Table 1.
Overview of the synthetic access and chemical uses for 1,1′-bispnictogen-substituted dppf analogs.
| Group 15 Elements Substituting 1,1′-Ferrocenes | Precursors for Syntheses | Chemical Applications and Complexes |
|---|---|---|
| 1,1′-Symmetrically substituted systems: 1,1′-diaminoferrocenes | ||
|
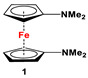
C5H4LiNMe2 and FeCl2 (Scheme 2A) [49] | Isolated complexes of 1 are reported with TiCl2 [50]. Compound 1 has frequently been used for the purpose of electrochemical measurements [51,52,53]. |
|
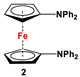
Fc’Br2, NaNPh2, and CuI [54] | Compound 2 has been used for electrochemical measurements [51,53,54]. |
|
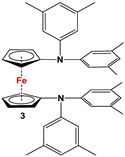
Fc’(NH2)2, 3,5-Me2-C6H3-Br, and PdBINAP [55] | No application reported [55]. |
|
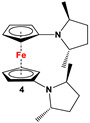
Fc’(NH2)2 and (2R,5R)-2,5-hexanediol cyclic sulfate [56] | Used for comprehending N-CpFc electron donation [56]. |
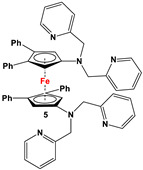
|
C5H4LiN(CH2Py)2 and FeCl2 (Scheme 2B) [57] | Isolated complex of 5 is reported with ZnBr2, Zn(CF3SO3)2, and Co(CF3SO3)2 [58,59,60]. Compound 5 has further been used for synthesizing redox-switchable complexes. |
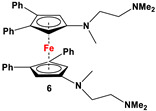
|
C5H4LiNMe(CH2CH2NMe2) and FeCl2 (Scheme 2B) [57] | Compound 6 has been used for electrochemical measurements [58]. |
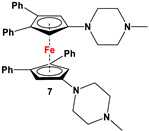
|
C5H4LiN(CH2)4NMe and FeCl2 (Scheme 2B) [57,58] | Compound 7 was used for electrochemical measurements [58]. |
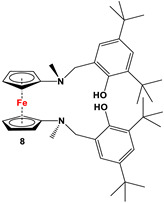
|
Fc’(NH2)2 and 3,5-di-tert-butyl-2-hydroxybenzaldehyde [61]. | Isolated complexes of 8 are reported with Zr(OtBu)2, which was further used in ROP for L-lactide and ε-caprolactone [61]. |
|
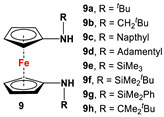
Fc’(NH2)2 and aldehydes or silylchlorides or respective ketones (with p-toluene sulfonic acid monohydrate) [62,63,64,65,66]. | Different variations of 9 were used for electrochemical measurements and computational purposes [64], and to act as substituents for carbenes, stannylenes, germylenes [62,63,67], Zr(Bn)2, Mg(THF)2, TiCl2, and TiMe2 [68]. Germylenes with deprotonated 9a, 9d, 9e, and 9h were further explored for oxidation reactions with S, Se, and (PhSe)2 [69]. Isolated complexes of [M(CH2Ar)(THF)] (M = Sc, Y, La, Lu) with 9d and 9f were used for dearomatization and ring-opening reactions [70,71,72,73,74]. |
|
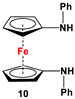
Fc’(NH2)2, PhBr, and Pd2(dba)3 (similar to Scheme 2D) [75]. | Compound 10 was used to synthesize zirconium chelates [76]. |
|
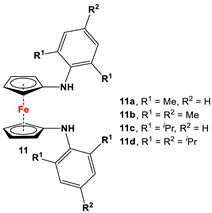
Fc’(NH2)2, respective arylbromides, and Pd2(dba)3 (similar to Scheme 2D) [65,75,77]. | 11b and 11c were used to synthesize N-heterocyclic silylenes [78], germylenes, and stannylenes [79]. Germylenes of 11b and 11c were further explored for oxidation reactions with S, Se, and (PhSe)2 [69]. Isolated complexes of 11c were reported with Al(III) [77]. Isolated complexes of 11d were reported with Zr(NMe2)2 and Zr(Bz)2 [76]. |
|
Fc’(NH2)2, 3,5-Me2-C6H3-Br, and PdBINAP [55]. | No application reported [55]. |
| 1,1′-Symmetrically substituted systems: 1,1′-diimidazoliumferrocenes | ||
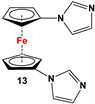
|
Fc’I2, imidazole and CuI [80]. | Compound 13 was used to synthesize ferrocene-based redox-responsive receptors [80]. |
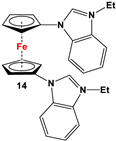
|
Fc’(NH2)2 and 2-fluoronitrobenzene [81]. | Isolated complexes of compound 14 were reported with Ir(cod), where “cod” stands for 1,5-cyclooctadiene [81]. |
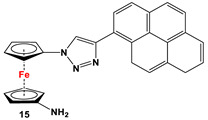
|
Fc’(N3)2, ethynylpyrene and CuSO4 [82]. | Compound 15 was used to synthesize ion-pair recognition receptors [82]. |
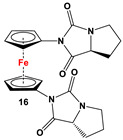
|
M and L-proline (Scheme 2E) [31]. | No application reported [31]. |
| 1,1′-Symmetrically substituted systems: 1,1′-diiminoferrocenes | ||
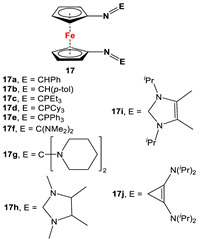
|
Fc’(NH2)2 and respective arylaldehydes [83,84,85,86,87]. | Isolated complex of 17a was reported with PdCl2 and PdMeCl, which have further been used for catalytic purposes [88,89]. Reduced versions of 17 were used for complexation with Zr(Bz)4 [90]. Cationic Ni(II) and Pd(II) complexes are reported with 17c–17i [84,85,86,87]. |
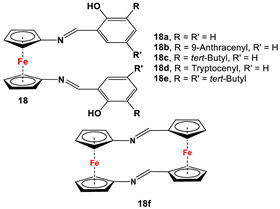
|
Fc’(NH2)2 and respective arylaldehydes (for 18a–18e) [89,91]; Fc’(N3)2, PPh3, and Fc’(CHO)2 (for 18f) [92]. | Isolated complexes for different variations of 18 were reported with Zr(Bz)2 [91], Mg(THF)2 [91], TiCl2 [89], Ti(OiPr)2 [89,93,94], Ce(OtBu)2 [95], In(OtBu) [94,96], Zn [97], Co [97], Zr(OiPr)2 [98], Zr(OtBu)2 [94,98], and Al(OiPr) [94]. Some of these complexes were further used for ethylene, lactone, and lactide polymerizations [89,93,94,95,96,98]. |
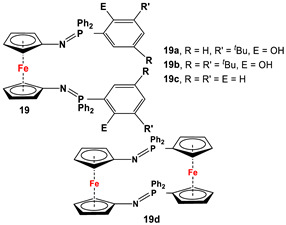
|
Fc’(N3)2 and respective arylphosphine (Scheme 2F) [92,99,100]. | Isolated complexes with 19 are reported with Ce(OtBu)2 [99], Ce(OtBu)THF [99], CeCl(THF) [99], CeI(THF) [99], YCl [99], Y(OtBu) [99], YCl [101], Y(CH2Ph) [101], and Y(CH2SiMe3) [101]. |
| 1,1′-Symmetrically substituted systems: 1,1’-diarsanylferrocenes | ||
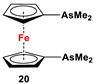
|
Fc’Li2·2/3tmeda and Me2AsCl (Scheme 3A) [2]. | Isolated complexes with compounds 20 are reported with Cr(CO)4 [102], Mo(CO)4 [102], W(CO)4 [102], PdCl2 [103], PdBr2 [103], PtCl2 [103], PtBr2 [103], PtI2 [103], Ni(CO)2 [103], Ni(CO)I2 [103], NiBr2 [104], and [Cu(MeCN)2]BF4 [105]. |
|
1,1′-bis(benzodithiaarsole) ferrocene and excess CyMgCl (Scheme 3E) [106]. | 21 was reported for in situ complexation with Pd2(dba)3, which was further used as an arsa-Buchwald ligand for catalytic purposes [106]. |
|
Fc’Li2·2/3tmeda and Ph2AsCl (Scheme 3A) [2]. | Isolated complexes with compounds 20 are reported with Cr(CO)4 [102], Mo(CO)4 [102], W(CO)4 [102], Ni(CO)2 [103], Ni(CO)I2 [103], (η2-C60)Pt [107], and PdCl2 [108]. |
|
(α-CH2NMe2, β-SiMe3-C5H3)(C5H5)Fe, nBuLi, and Ph2AsCl (Scheme 3C) [109]. | Fungicidal activity of compound 23 for crop plants was examined against fusarium head blight of wheat, early blight of tomato, wilt disease of cotton, ring-rot disease of apple, and brown blotch disease of peanut [109]. |
| 1,1′-Symmetrically substituted systems: 1,1′-distibanylferrocene | ||
|
Fc’Br2, nBuLi, and Ph2SbCl (Scheme 3B) [110]. | Isolated complexes with compounds 24 are reported with AgClO4 [110]. Compound 24 was further oxidized to stiboranes, which was eventually converted to a rare SbOSb [3]FCP [110]. |
| 1,1′-Symmetrically substituted systems: 1,1′-dibismuthanylferrocenes | ||
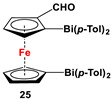
|
(α-CHO-C5H4)(C5H5)Fe, Me2N(CH2)2NMeLi, and nBuLi, and (p-Tol)2BiCl in the next step [111]. | No application reported for 25 [111]. |
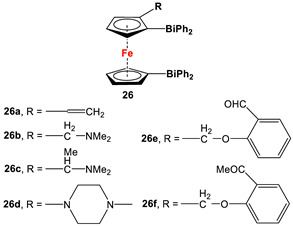
|
(α-CH2NMe2-C5H3)(C5H5)Fe, nBuLi, and Ph2BiCl (Scheme 3D) for 26b, [112] which acted as a precursor for other species. | No application reported for 26 [112]. |
| 1,1′-Unsymmetrically substituted systems: 1,1′-aminophosphanylferrocenes | ||
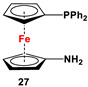
|
Fc’(PPh2·BH3)N3 and DABCO (Scheme 4A) [113]. | No application reported for 27 [113]. |
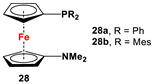
|
Fc’(NMe2)Br, nBuLi, and R2PCl (R = Ph and Mes) [114]. | Isolated complexes with compound 28a were reported with PdCl2, PdCl(SbF6), PPh3(BF4)2, PdPPh2Cp(SbF6)2, [28a·Pd(PPh2)Fc’(NMe2)], and P(p-OMe-C6H4)3(BF4)2 [114]. |
|
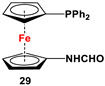
Fc’(PPh2·S)N3, Raney Ni, and HCOOAc (Scheme 4B) [113]. | Compound 29 was used to synthesize 32a [113]. |
|
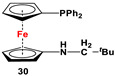
30·S and Raney Ni [115]. | No application reported for 30 [115]. |
|
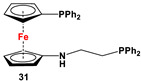
Fc’(PPh2·BH3)NH2 and Ph2PCH2CO2H·BH3, followed by DABCO [116]. | Isolated complexes with compounds 31 are reported with PdCl2, PdCl(SbF6), and PdCl(SbF6)2 [116]. |
|
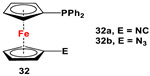
29 and BOP/DBU for 32a [113], Fc’(Ph2P·BH3)Br or Fc’(Ph2P·S)Br, and nBuLi and TsN3 for 32b, followed by removal of BH3 or S [113]. | Isolated complexes with compound 32a are reported with AgCl [113], Ag(SbF6) [113], Ag(Me2CO)(SbF6) [113], AuCl [113], (AuCl)2 [113], (AuCN)2(SbF6)2 [113], and (AuCN)2(NTf)2 [113]. Au-complexes of 32a were used for cyclodimerization of enynol [113]. Fischer-type and Mesoionic carbenes were also synthesized from 32a and 32b, respectively [117,118]. |
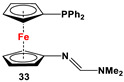
|
Fc’(PPh2)NH2 and Me2NCH(OMe)2 [119]. | Isolated complexes with compounds 33 are reported with PdCl2, PdCl(BARF) (BARF = B(3,5-(CF3)2C6H3)4), (η6–1-Me, 3-iPr-C6H4)Ru, and (η6-C5Me5)Rh [119]. |
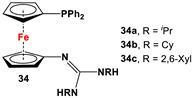
|
Fc’(PPh2)NH2, nBuLi, and respective RNCNR [120,121]. | Isolated complexes with compounds 34 are reported with PdCl2 [120], PdCl(SbF6) [120], PdCl(BF4) [120], PtCl2 [121], PdCl(BARF) [121], PdBr(4-CN-C6H4) [121], and Pd(4-CN-C6H4)SbF6 [121]. Isolated complexes with [34·H]SbF6 were reported with PdCl2 and μ-Pd2Cl4 [120]. |
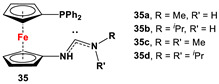
|
35·PdCl2: Fc’(PPh2)NC, PdCl2(cod), and respective RR’NH [118]. | Isolated complexes with compound 35 are reported with PdCl2, which was further reported with Miyaura borylation [118]. |
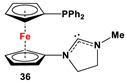
|
36·PdCl2: Fc’(PPh2)NC, PdCl2(cod), and respective [Cl(CH2)2NH2Me]Cl [118]. | Isolated complexes with compound 36 are reported with PdCl2, which was further reported with Miyaura borylation [118]. |
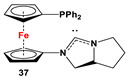
|
37·PdCl2: Fc’(PPh2)NC, PdCl2(cod), and (S)-2-(chloromethyl)pyrrolidine [118]. | No application reported for 37 [118]. |
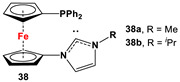
|
38·PdCl2: Fc’(PPh2)NC, PdCl2(cod), and respective (MeO)2CHCH2NHR (R = Me, iPr) [118]. | Isolated complexes with compound 38 are reported with PdCl2, which was further reported with Miyaura borylation [118]. |
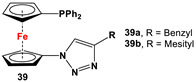
|
Fc’(PPh2·BH3)N3, RC≡CH (R = Bz, Mes), and CuSO4·5H2O [122]. | Isolated complexes with compound 39 are reported with MeBF4, PdCl2(MeBF4), AuiPr(MeBF4), and [PdCl2(AuiPr)2(MeBF4)2]1/2 [122]. |
| 1,1’-Unsymmetrically substituted systems: 1,1’-arsanylphosphanylferrocenes | ||
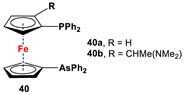
|
[1]PhosphaFCP and nBuLi, followed by Ph2AsCl [123,124]. | No application reported for 37 [123,124,125]. |
| 1,1′-Unsymmetrically substituted systems: 1,1′-phosphanylstibanylferrocene | ||
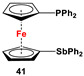
|
Fc’(PPh2)Br and nBuLi, followed by Ph2SbCl [126]. | Isolated complexes with compound 41 are reported with AuCl, which was further treated with 3,5-di-tert-butyl-o-benzoquinone for further complexation with Sb. Both complexes were eventually used for gold catalysis [127]. |
