Abstract
The connection between Primary sclerosing cholangitis (PSC) and lymphoma remains uncertain. To address this, Mendelian randomization (MR) was utilized to investigate the potential causal links between PSC and lymphoma. A 2-sample MR analysis was conducted utilizing summary-level data obtained from genome-wide association study datasets. Four complementary MR methods were performed, including inverse-variance weighting (IVW), MR-Egger, weighted median, and MR-robust adjusted profile score (MR-RAPS). Several sensitivity analyses were also employed to further validate the results. The results of IVW estimates showed that there was a potential causal association between PSC and diffuse large B-cell lymphoma (DLBCL) risk (OR = 1.138, 95% CI = 1.052–1.230, P = .001). No causal association was observed between PSC and other lymphoma subtypes. No horizontal and directional pleiotropy was observed in the MR studies. This study represents the inaugural utilization of MR analysis to investigate the causal associations between PSC and DLBCL. Further investigation is warranted to elucidate the underlying mechanism of this causal relationship.
Keywords: diffuse large B-cell lymphoma, genome-wide association studies, Mendelian randomization, primary sclerosing cholangitis, single-nucleotide polymorphisms
1. Introduction
Malignant lymphomas are classified based on their pathological characteristics into Hodgkin lymphoma(HL) and non-HL (NHL), with the latter encompassing B-cell and NK/T-cell lymphomas. In Western populations, non-HLs represent approximately 90% of all lymphomas, with the most prevalent subtype being diffuse large B-cell lymphoma (DLBCL) accounting for 30% to 40% of cases. The development of lymphoma is influenced by various factors, such as genetics, immune dysregulation, and chronic inflammation. The pathogenesis of primary sclerosing cholangitis (PSC), an autoimmune liver disorder characterized by persistent biliary inflammation and fibrosis, may be linked to immune dysregulation-induced breakdown of self-tolerance to autoantigens, along with genetic predisposition.[1]
Lymphoma is diagnosed in <1% of patients with autoimmune diseases, yet it is linked to an increased susceptibility to NHL including DLBCL.[2] This correlation may be attributed to factors such as inflammation, immune system dysregulation, and the administration of certain medications.[3,4] However, there is currently a lack of empirical evidence establishing a direct causal connection between PSC and lymphoma.
Randomized controlled trials (RCTs) are widely regarded as the highest quality form of clinical randomized evidence. However, they are not without limitations, including challenges related to implementation and ethical constraints. Observational studies, on the other hand, are often subject to bias and are considered less reliable. Mendelian randomization (MR) studies offer a solution to these limitations by utilizing single-nucleotide polymorphisms (SNPs) as a proxy for exposure, enabling the assessment of causality between exposure and the outcome of interest while minimizing the influence of confounding variables. MR studies also help in avoiding reverse causality and allow for the examination of long-term effects of exposure on outcomes without being hindered by ethical concerns or practical obstacles, thus facilitating direct implementation.
The objective of this research was to examine the unintended impact of PSC on the likelihood of malignant lymphoma development using MR analysis of aggregated data from genome-wide association study (GWAS) datasets. This was done to pinpoint particular risk populations for targeted early intervention and preventive measures.
2. Materials and methods
2.1. Data sources for PSC and lymphoma GWAS
The PSC data were obtained from the IPSCSG, comprising a cohort of 2871 patients and 12,019 control participants.[5] The GWAS datasets are publicly accessible and can be acquired from the OPEN GWAS website at https://gwas.mrcieu.ac.uk/. The GWAS repositories for lymphoma were accessible through the FinnGen website at https://www.finngen.fi/en. The GWAS dataset linked to HL included 846 cases and 324,650 controls. The GWAS data associated with NHL encompassed various subtypes such as diffuse large B-cell lymphoma (DLBCL; 1050 cases), follicular lymphoma (1181 cases), mantle cell lymphoma (210 cases), marginal zone lymphoma (202 cases), and mature T/NK-cell lymphomas (363 cases).We did not need to perform any additional ethical reviews because informed permission and ethical approval had already been obtained for each of the initial studies.
2.2. Genetic instrumental variant selection for PSC
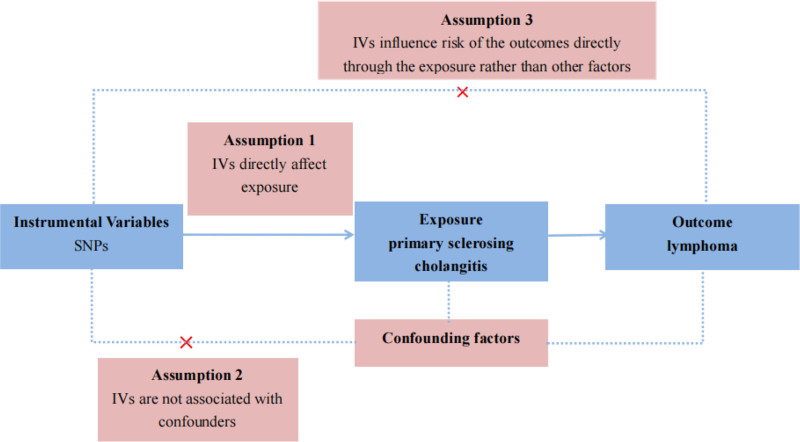
The design of MR studies must adhere to 3 key assumptions: a strong association exists between instrumental variants (IVs) and exposure factors; the IVs are independent of confounding factors that may influence the relationship between exposure and outcome; and genetic variants affect the outcome solely through the exposure and not through other pathways. The 3 aforementioned assumptions were depicted in Figure 1. To fulfill the mentioned core assumptions, first, the IVs with genome-wide significance (P < 5 × 10−8) were extracted from the PBC and PSC GWAS. The linkage disequilibrium of r2 = 0.01 and clumping distance = 10,000. Then, palindromic SNPs were excluded when harmonizing the PSC and malignant lymphoma GWAS. Next, MR Pleiotropy RESidual Sum and Outlier (MR-PRESSO) test was employed to discern potential outlier SNPs for correcting potential horizontal pleiotropy.[6,7] Finally, the remaining SNPs were utilized for MR analysis. And the F-statistic was calculated for each leftover SNP to measure the strength of genetic IVs. IVs with F-statistic > 10 indicate not a weak genetic instrument.
Figure 1.
Three core assumptions of Mendelian randomization (MR) analysis. Three fundamental principles of MR analysis are as follows: (A) The correlation assumption posits a robust linkage between instrumental variants (IVs) and exposure factors; (B) The independence assumption asserts that IVs are unrelated to confounding variables influencing the exposure-outcome association; and (C) The exclusivity assumption stipulates that genetic variants solely impact the outcome via the exposure and not through alternative pathways.
2.3. MR analysis
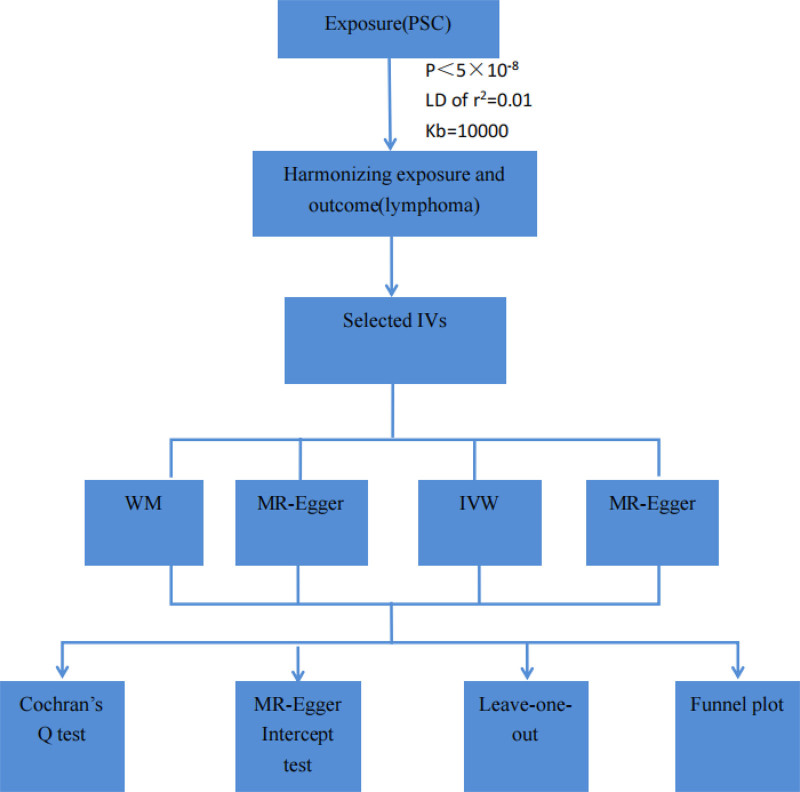
Four MR methods including MR-Egger, inverse-variance-weighting (IVW), weighted median (WM), and MR-robust adjusted profile score (MR-RAPS) were applied for the estimation of the causality of PSC and lymphoma. The IVW model assumes that all enrolled SNPs are valid genetic IVs and no pleiotropic effects exist.[8] If the estimates of causal effects from the 3 models are inconsistent, the IVW methods are considered the primary outcome. The odds ratio (OR) was utilized as the effect size to determine causality direction, where an OR > 1 indicated the exposure as a risk factor for the outcome. Statistical significance of causality was determined by P-values < .05. Heterogeneity among genetic IVs was assessed using Cochran Q test. Sensitivity analysis primarily employed the leave-one-out method. The MR-Egger intercept test was utilized to identify directional pleiotropy, with a P-value < .05 indicating its presence. Funnel plots were generated to evaluate directional pleiotropy, akin to its role in meta-analysis for assessing publication bias. The MR analysis was conducted using R software (version 4.3.3) with the R packages “TwoSampleMR” and “MRPRESSO.” The study’s flow schematic was depicted in Figure 2.
Figure 2.
The flow diagram of the MR study. PSC = primary sclerosing cholangitis, LD = linkage disequilibrium, IVs = instrumental variants, WM = weighted median, IVW = inverse-variance-weighting, MR-RAPS = MR-robust adjusted profile score.
3. Results
3.1. Genetic IVs for PSC
Initially, we identified 26 SNPs that were significantly (5 × 10−8) and independently (LDr2 < 0.01) associated with PSC. Subsequently, we calculated F-statistics, which ranged from 27 to 835, indicating the lack of weak instruments. This was followed by the integration of PSC data with lymphoma data, and the combined datasets for PSC and lymphoma are detailed in Table S1, Supplemental Digital Content, http://links.lww.com/MD/N913.
3.2. MR estimates and sensitivity analyses
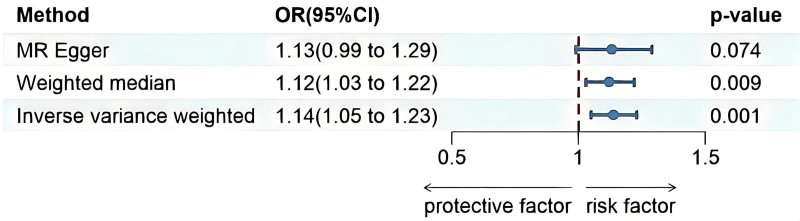
The MR results are shown in Figure 3. The results of IVW estimates showed that there was a potential causal association between PSC and DLBCL risk (OR = 1.138, 95% CI = 1.052–1.230, P = .001). The WM method also revealed a significant genetic correlation between PSC and DLBCL risk (OR = 1.121, 95% CI = 1.029–1.222, P = .009). And the MR-RAPS method also revealed a significant genetic correlation between PSC and DLBCL risk (OR = 1.140, 95% CI = 1.080–1.204, P < .001). However, no such association was found using the MR-Egger method (OR = 1.132, 95% CI = 0.994–1.291, P = .074). The Cochran Q test results suggested that heterogeneity was observed in the MR study (P = .001). The MR-Egger intercept test indicating that there was no horizontal pleiotropy(P = .932).
Figure 3.
The forest plots revealed the causal association of primary sclerosing cholangitis with diffuse large B-cell lymphoma (DLBCL). 95% CI = 95% confidence interval of OR, OR = odds ratio, PSC = primary sclerosing cholangitis.
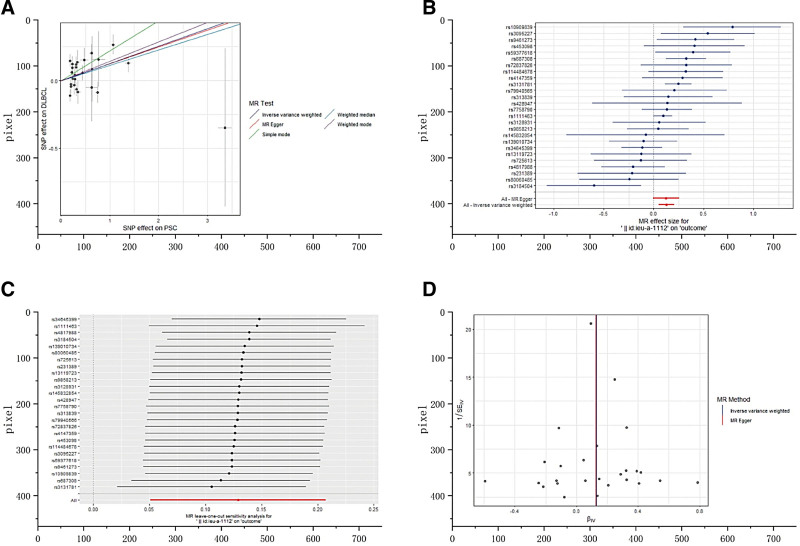
The scatterplot (Fig. 4A) also showed an elevated risk of DLBCL in patients with PSC. The forest plot showed the effect sizes and their 95% CIs for each of the independent SNPs in the PSC GWAS, as well as the overall causal estimates from the MR-Egger and IVW models (Fig. 4B). As shown in Figure 4C, the sensitivity analyses were performed using a “leave-one-out” approach, and the results showed that the causal estimation conclusions of PSC for DLBCL remained stable and reliable when any of the selected SNPs were removed. The funnel plot (Fig. 4D) was approximately symmetric, indicating the absence of directional pleiotropy. PSC has no significant causal relationship with other malignant lymphomas. The results of IVW estimates showed that there was no potential causal association between them (Table S2, Supplemental Digital Content, http://links.lww.com/MD/N914).
Figure 4.
(A) The scatter plot from genetically predicted primary sclerosing cholangitis (PSC) on diffuse large B-cell lymphoma (DLBCL). (B) The forest plot of causality effect sizes of both single and merged single-nucleotide polymorphisms for PSC on DLBCL. (C) The results of leave-one-out methods for sensitivity analysis. (D) The funnel plot from genetically predicted PSC on DLBCL.
4. Discussion
PSC can be asymptomatic in its early stages. The presence of antineutrophil cytoplasmic antibodies (ANCA) can be identified in 88% of individuals with PSC.[9,10] Patients diagnosed with PSC commonly display abnormal liver biochemical markers. Imaging studies typically reveal multifocal bile duct narrowing in PSC cases. The genetic association of PSC is predominantly linked to the human leukocyte antigen complex located on chromosome 6.[11] Research has indicated a stronger genetic correlation between PSC and ulcerative colitis compared to Crohn disease,[5] which may explain the high prevalence of inflammatory bowel disease (IBD), particularly ulcerative colitis, in PSC patients. Additionally, around 25% of individuals with PSC also suffer from other autoimmune conditions.[12] PSC patients face an elevated risk of developing cholangiocarcinoma and colorectal cancer. Presently, there are no pharmaceutical interventions capable of completely halting the progression of PSC. Liver transplantation is often necessary for the majority of PSC patients, although some individuals experience relapses posttransplantation.[13] The exact pathogenesis of PSC remains unclear. However, chronic inflammatory responses and immune dysregulation are believed to be pivotal in the initiation and advancement of the disease. Two proposed hypotheses suggest that pro-inflammatory agents like lipopolysaccharides activate innate immunity through TOLL-like receptor signaling pathways in the portal circulation, and that microbial-induced antigenic stimulation prompts T-cell proliferation and differentiation in the intestines. These T-cells migrate to the liver, stimulating B-cell proliferation and differentiation into plasma cells, which release inflammatory mediators leading to bile duct damage.[14] Studies have linked the development of colorectal cancer in PSC patients to adaptive immunity, involving clonally expanded IL-17A + FOXP3 + T-cells and IgG-secreting plasma cell immune responses. The intestinal inflammation observed in PSC patients is driven by antigen-specific immune reactions.[15] Notably, a study involving 13 PSC patients revealed a higher total B-cell count in PSC-derived peripheral blood mononuclear cells compared to healthy individuals.[16]
DLBCL is the most prevalent form of malignant lymphoma, characterized by significant diversity in both phenotypic and genetic characteristics. It can be classified into 2 main subtypes, namely activated B-cell-like (ABC-DLBCL) and germinal center B-cell-like (GCB-DLBCL), based on their cellular origins. GCB-DLBCL is closely linked to the follicular T-cells and epigenetic regulation of immune recognition molecules, while ABC-DLBCL is associated with frequent genetic aberrations affecting the class I major histocompatibility complex.[17] Patients with DLBCL often exhibit a high incidence of autoimmune diseases,[18] particularly B-cell-mediated conditions such as rheumatoid arthritis, systemic lupus erythematosus, and Sjögren’s syndrome. Most patients present with lymphoma after the diagnosis of autoimmune disease.[19] These patients are predominantly older women and usually have a poor prognosis, with high serum lactate dehydrogenase levels, and a high risk of developing febrile neutropenia.[20–23] The Genomic and transcriptomic analyses of these patients revealed dysregulated cell cycle and immune responses, activation of oxidative phosphorylation pathways, and elevated Th1/Th2 and Th17/Treg ratios.[24]
A range of B-cell signaling pathway alterations are observed in both autoimmune diseases and DLBCL, including B-cell receptor activation, activation of the NF-κB pathway, elevation of interleukin (IL)-10, dysregulation of the BAFF and APRIL pathways, and dysregulation of IRF8 activity.[25] R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) chemical Immunotherapeutic regimens are fundamental in the first-line treatment of DLBCL.[26] Patients with both autoimmune diseases and DLBCL constitute a subgroup with a distinct disease pathogenesis. In such cases, the use of B-cell specific drugs, such as immunosuppressive drugs, has therapeutic benefits for both conditions. Evidence suggests that the R-CHOP regimen provides palliative effects for autoimmune diseases and lymphomas. Current DLBCL treatment strategies emphasize subtype-specific treatments.[27,28] Patients with both conditions may tailor their treatment based on the specific disease pathogenesis. Limited observational studies exist on the correlation between PSC and malignant lymphoma, with only a few case reports of PSC combined with lymphomas like HL, DLBCL, Burkitt lymphoma, and enteropathy-associated T-cell lymphoma.[29–32] Lymphoma complicating cholestasis necessitates investigation into common causes such as extrahepatic obstruction due to enlarged lymph nodes or direct lymphoma invasion of the liver.[33] In cases where no cause is identified, factors related to immune dysregulation, release of toxic cytokines from lymphoma cells causing paraneoplastic syndromes, and pharmacological treatment should be considered. A recent cohort study involving 13,492 patients with IBD assessed the risk of lymphoma in patients with IBD. The study revealed that the risk of lymphoma was more than tripled in patients with comorbid PSC, as well as in those treated with anti-TNF-α therapy and immunosuppressive therapy with thiopurines.[34]
Observational studies and RCTs have inherent limitations, necessitating the accumulation of a sufficient number of cases and prolonged follow-up periods. MR studies offer a means to establish causality by leveraging genetic variant susceptibility loci associated with a particular disease. This study represents the first 2-sample MR investigation into the causal impact of PSC on malignant lymphoma. Through MR analysis, the study assessed the evidence supporting a causal link between PSC and malignant lymphoma, identifying 26 distinct and highly correlated SNPs in PSC. The MR findings revealed a 13.8% increased risk of DLBCL among PSC patients, suggesting PSC as an independent risk factor for DLBCL development (ORIVW = 1.138, 95% CI = 1.052–1.230, P = .001). However, no causal association was observed between PSC and other lymphoma subtypes. Notably, limitations of the study include the predominantly European ancestry of the study population, cautioning against generalizing the findings to other ethnic groups. Second, the sample size is relatively small and the rigor of the conclusions is lacking. Third, NHL is a heterogeneous group of hematologic disorders with many subtypes. We have used the most reliable dataset of NHL subtypes (i.e., FinnGen). It is still possible that the estimates are biased. Fourth, while the selected genetic instruments exhibited strong predictive capabilities for exposure, uncertainties remain regarding the validation of correlation hypotheses and exclusion restrictions. Additionally, long-term use of immunosuppressive and biological agents has been linked to an increased risk of malignant lymphoma, potentially influenced by chronic inflammatory processes or treatment regimens in PSC patients. The absence of GWAS pertaining to specific treatments precludes the validation of causal relationships through MR studies, suggesting the need for future investigations in this area.
5. Conclusions
In conclusion, our MR study offers empirical backing for a potential causal association between PSC and the onset of DLBCL from a genetic standpoint. The underlying mechanism of this causal link warrants additional exploration. Conducting further investigations among affected individuals would be advantageous in reinforcing the evidential basis. These findings underscore the importance for healthcare practitioners to prioritize monitoring patients with autoimmune conditions for early detection of lymphoma.
Acknowledgments
The referenced studies or consortiums are gratefully acknowledged by the authors for contributing open-access datasets for the analysis.
Author contributions
Conceptualization: Zhengyang Miao.
Formal analysis: Zhengyang Miao.
Funding acquisition: Hailin Chen.
Investigation: Hailin Chen.
Methodology: Zhengyang Miao, Hailin Chen.
Project administration: Zhengyang Miao.
Resources: Zhengyang Miao.
Software: Hailin Chen.
Supervision: Hailin Chen.
Validation: Zhengyang Miao, Hailin Chen.
Visualization: Zhengyang Miao.
Writing – original draft: Zhengyang Miao, Yong-Ming Zhou.
Writing – review & editing: Yong-Ming Zhou.
Supplementary Material
Abbreviations:
- DLBCL
- diffuse large B-cell lymphoma
- GWAS
- genome-wide association study
- IVs
- instrumental variables
- IVW
- inverse-variance-weighted
- MR
- Mendelian randomization
- MR-Egger
- The Mendelian randomization-Egger
- MR-RAPS
- MR-robust adjusted profile score
- OR
- odds ratio
- PSC
- primary sclerosing cholangitis
- SNP
- single-nucleotide polymorphism
- WM
- weighted median
This study was funded by National Administration of Traditional Chinese Medicine National Studio for Heritage of Famous Traditional Chinese Medicine Experts under grant number [2022]75, Shanghai Clinical Key Specialty Construction Project under Grant number SHSLCZDZK 05201, State Administration of Traditional Chinese Medicine High-level Chinese Medicine Key Discipline Construction Project (zyyzdxk-202365), Shanghai University of Traditional Chinese Medicine Summit Plateau Team Project (30304114341), Shanghai Famous Elderly Chinese Medicine Doctor Academic Experience Research Studio Construction Project (SHGZS-2017019).
Because all of the data used in our MR analysis were drawn from previously published summary data, participant consent, and ethical approval were not needed for this investigation.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
How to cite this article: Miao Z, Chen H, Zhou Y. Causal effect of primary sclerosing cholangitis on diffuse large B-cell lymphoma: A two-sample Mendelian randomized study. Medicine 2024;103:47(e40542).
Contributor Information
Zhengyang Miao, Email: 1023743504@qq.com.
Hailin Chen, Email: chenhailinyyyy@163.com.
References
- [1].Dyson JK, Beuers U, Jones DEJ, Lohse AW, Hudson M. Primary sclerosing cholangitis. Lancet. 2018;391:2547–59. [DOI] [PubMed] [Google Scholar]
- [2].Koshiol J, Lam TK, Gridley G, Check D, Brown LM, Landgren O. Racial differences in chronic immune stimulatory conditions and risk of non-Hodgkin’s lymphoma in veterans from the United States. J Clin Oncol. 2011;29:378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Klein A, Polliack A, Gafter-Gvili A. Rheumatoid arthritis and lymphoma: Incidence, pathogenesis, biology, and outcome. Hematol Oncol. 2018;36:733–9. [DOI] [PubMed] [Google Scholar]
- [4].Baecklund E, Smedby KE, Sutton LA, Askling J, Rosenquist R. Lymphoma development in patients with autoimmune and inflammatory disorders – what are the driving forces? Semin Cancer Biol. 2014;24:61–70. [DOI] [PubMed] [Google Scholar]
- [5].Ji SG, Juran BD, Mucha S, et al.; UK-PSC Consortium. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat Genet. 2017;49:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rosoff DB, Clarke TK, Adams MJ, et al. Educational attainment impacts drinking behaviors and risk for alcohol dependence: results from a two-sample Mendelian randomization study with ~780,000 participants. Mol Psychiatry. 2021;26:1119–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pagoni P, Dimou NL, Murphy N, Stergiakouli E. Using Mendelian randomisation to assess causality in observational studies. Evid Based Ment Health. 2019;22:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Trivedi PJ, Adams DH. Mucosal immunity in liver autoimmunity: a comprehensive review. J Autoimmun. 2013;46:97–111. [DOI] [PubMed] [Google Scholar]
- [10].Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3781–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14:279–95. [DOI] [PubMed] [Google Scholar]
- [12].Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis - a comprehensive review. J Hepatol. 2017;67:1298–323. [DOI] [PubMed] [Google Scholar]
- [13].Tan N, Lubel J, Kemp W, Roberts S, Majeed A. Current therapeutics in primary sclerosing cholangitis. J Clin Transl Hepatol. 2023;11:1267–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kim YS, Hurley EH, Park Y, Ko S. Primary sclerosing cholangitis (PSC) and inflammatory bowel disease (IBD): a condition exemplifying the crosstalk of the gut-liver axis. Exp Mol Med. 2023;55:1380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shaw DG, Aguirre-Gamboa R, Vieira MC, et al. Antigen-driven colonic inflammation is associated with development of dysplasia in primary sclerosing cholangitis. Nat Med. 2023;29:1520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang J, Zhang W, Leung PS, et al. Ongoing activation of autoantigen-specific B cells in primary biliary cirrhosis. Hepatology. 2014;60:1708–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Takahara T, Nakamura S, Tsuzuki T, Satou A. The immunology of DLBCL. Cancers (Basel). 2023;15:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li H, Yu L, Zhang X, Shang J, Duan X. Exploring the molecular mechanisms and shared gene signatures between rheumatoid arthritis and diffuse large B cell lymphoma. Front Immunol. 2022;13:1036239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim JS, Kim IH, Byun JM, Chang JH. Population-based study on the association between autoimmune disease and lymphoma: National Health Insurance Service-National Sample Cohort 2002-2015 in Korea. J Autoimmun. 2021;121:102647. [DOI] [PubMed] [Google Scholar]
- [20].Koff JL, Rai A, Flowers CR. Characterizing autoimmune disease-associated diffuse large B-cell lymphoma in a SEER-medicare cohort. Clin Lymphoma Myeloma Leuk. 2018;18:e115–21. [DOI] [PubMed] [Google Scholar]
- [21].Kleinstern G, Averbuch M, Abu Seir R, Perlman R, Ben Yehuda D, Paltiel O. Presence of autoimmune disease affects not only risk but also survival in patients with B-cell non-Hodgkin lymphoma. Hematol Oncol. 2018;36:457–62. [DOI] [PubMed] [Google Scholar]
- [22].Kleinstern G, Maurer MJ, Liebow M, et al. History of autoimmune conditions and lymphoma prognosis. Blood Cancer J. 2018;8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mörth C, Valachis A, Abu Sabaa A, et al. Autoimmune disease in patients with diffuse large B-cell lymphoma: occurrence and impact on outcome. Acta Oncol. 2019;58:1170–7. [DOI] [PubMed] [Google Scholar]
- [24].Sheng L, Fu D, Cao Y, et al. Integrated genomic and transcriptomic analyses of diffuse large B-cell lymphoma with multiple abnormal immunologic markers. Front Oncol. 2022;12:790720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Koff JL, Flowers CR. B cells gone rogue: the intersection of diffuse large B cell lymphoma and autoimmune disease. Expert Rev Hematol. 2016;9:553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med. 2021;384:842–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Voulgarelis M, Giannouli S, Anagnostou D, Tzioufas AG. Combined therapy with rituximab plus cyclophosphamide/doxorubicin/vincristine/prednisone (CHOP) for Sjögren’s syndrome-associated B-cell aggressive non-Hodgkin’s lymphomas. Rheumatology (Oxford). 2004;43:1050–3. [DOI] [PubMed] [Google Scholar]
- [28].Voulgarelis M, Giannouli S, Tzioufas AG, Moutsopoulos HM. Long term remission of Sjögren’s syndrome associated aggressive B cell non-Hodgkin’s lymphomas following combined B cell depletion therapy and CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone). Ann Rheum Dis. 2006;65:1033–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Majid N, Bernoussi Z, Mrabti H, Errihani H. Celiac disease, enteropathy-associated T-cell lymphoma, and primary sclerosing cholangitis in one patient: a very rare association and review of the literature. Case Rep Oncol Med. 2013;2013:838941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Abedi SH, Ghassami M, Molaei M, Mohsenifar Z, Mohammad Alizadeh AH. Secondary Sclerosing Cholangitis and Hodgkin’s lymphoma. Clin Med Insights Case Rep. 2015;8:83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Van Hauwaert V, Meers S, Verhoef G, et al. Rectal non-Hodgkin’s lymphoma in an infliximab treated patient with ulcerative colitis and primary sclerosing cholangitis. J Crohns Colitis. 2010;4:683–6. [DOI] [PubMed] [Google Scholar]
- [32].Lu G, Qiao L, Li D, Liu Z, Zhao F, Yu D. Concurrent lymphoma and hemophilia B in a pediatric patient: a case report. Medicine (Baltimore). 2019;98:e15474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shaikh H, Umar S, Sial M, Christou A, Kulkarni A. A case of secondary sclerosing cholangitis in the setting of non-Hodgkin’s lymphoma. Cureus. 2019;11:e4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yu J, Refsum E, Wieszczy P, et al. Risk of malignant lymphomas in patients with inflammatory bowel disease: a population-based cohort study. BMJ Open Gastroenterol. 2023;10:e001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.