Abstract
Two novel bacterial species were isolated from the rhizosphere of Solanum lycopersicum (tomato plant), both exhibiting plant growth-promoting properties. Two isolated strains, Rhodanobacter lycopersici sp. nov. Si-cT and Rhodanobacter geophilus sp. nov. S2-gT, were classified through a polyphasic approach, confirming their novel status within the Rhodanobacter genus. The strains demonstrated a remarkable tolerance to extreme pH conditions, with R. lycopersici Si-cT surviving in pH 3.0–13.0 and R. geophilus S2-gT tolerating pH 2.0–13.0. Additionally, both strains exhibited multiple plant growth-promoting traits, including indole-3-acetic acid and ammonia production, phosphate solubilization, and siderophore formation. These characteristics suggest that the two strains may play an important role in promoting plant growth, especially in soils with variable pH levels. However, since the direct impact on plant growth was not experimentally tested, the potential of these bacteria for agricultural applications remains to be confirmed through further research. This study expands our understanding of the diversity within the Rhodanobacter genus and provides insights into the potential use of these novel species in sustainable agriculture.
Keywords: Rhodanobacter, plant growth-promoting bacteria, pH tolerance, genomic analysis, rhizosphere, novel species, polyphasic taxonomy
1. Introduction
In response to the increasing global population and rising demand for crops, there is growing attention on sustainable farming practices [1]. The overuse of chemical fertilizers has caused significant soil degradation and environmental pollution, raising concerns about ecosystem health and food security [2]. As a result, plant growth-promoting bacteria (PGPB) have emerged as a promising solution, offering eco-friendly alternatives to enhance plant growth without the harmful effects of chemical inputs [3,4]. Several genera, including Acinetobacter, Agrobacterium, Arthrobacter, Bacillus, Burkholderia, Enterobacter, Flavobacterium, Lysinibacillus, Paenibacillus, and Pseudomonas, have been reported to contain PGPB that positively influence plant health and growth [5]. Certain PGPB are known to thrive under harsh conditions, such as salt, temperature, and drought stress [6,7,8]. PGPB act directly as biofertilizers by stimulating root growth, restoring root nodules, controlling plant stress, and breaking down heavy metals in the soil while also contributing indirectly through antimicrobial activity, induction of systemic resistance, and nutrient competition [9,10].
Plant growth-promoting rhizobacteria (PGPR), a subset of PGPB, are soil bacteria that specifically colonize the plant root surface and rhizosphere, contributing to plant growth [3,11]. These bacteria actively interact with the plants in the root environment, promoting growth through mechanisms such as enhancing nutrient uptake, suppressing pathogens, and producing hormones [12]. Rhizobacteria in the rhizosphere can colonize both the root surface and surrounding areas, influencing plant growth under suitable soil and environmental conditions [5,13,14,15].
In this context, this study focused on isolating and identifying efficient strains of rhizobacteria from the tomato rhizosphere, aiming to secure natural microbial resources. Two novel Rhodanobacter species were isolated, and their phylogenetic, physiological, and chemotaxonomic attributes were assessed. These strains were evaluated for key plant growth-promoting traits, such as indole-3-acetic acid (IAA) production, ammonia production, siderophore production, nitrogen fixation, and phosphate solubilization. Although these properties were evaluated in vitro, further research is required to assess their effectiveness in vivo. Despite the need for additional studies, this research marks an important step toward uncovering novel microbial bioresources and expanding our understanding of Rhodanobacter species. It lays the groundwork for developing strains that could contribute to sustainable and eco-friendly agricultural practices.
2. Materials and Methods
2.1. Isolation and Culture Conditions
Strains Si-cT and S2-gT were isolated from the rhizosphere of potted tomato plants at Dongguk University, Ilsan, Republic of Korea (37°40′42.8″ N, 126°48′24.9″ E). Before planting in a pot, tomato seeds were surface sterilized following the previously described method [16]. Five seeds were planted in a round pot (diameter, 18.5 cm; height, 5 cm; base, 13 cm) with 400 g of Plant World-Mix soil substrate (Nongwoo Bio Co., Ltd., Suwon, Republic of Korea). The soil contained 49.876% coco peat, 25% peat moss, 12% perlite, 7% vermiculite, 6% zeolite, and 0.11% NPK fertilizer containing 0.187 g/kg of nitrogen, phosphorus, and potassium. The soil pH is 5.5–6.0. One week after planting, all seedlings were removed, leaving the healthiest seedling. The remaining seedling was grown with regular irrigation using tap water for six months.
For screening bacterial colonies, the soil samples were prepared using previously described methods [17,18]. The tomato plant was uprooted from the pot, and the soil on the roots was vigorously shaken off and washed with running tap water to remove any non-adhering soil. A total of 2 g of soil surrounding the roots was transferred into a 50 mL conical tube along with 5 mL of sterilized 0.85% (w/v) NaCl. Subsequently, the rotated mixture was diluted to 10−1–10−4 using the same reagent, and 100 μL of each dilution was spread on Reasoner’s 2A (R2A; MB Cell, Seoul, Republic of Korea) agar that was incubated at 30 °C for 3 days. Strains Si-cT and S2-gT were repeatedly re-streaked on the same medium to ensure purity. Pure colonies of each strain were stored at −80 °C in a 25% (v/v) glycerol mixture, prepared by combining R2A broth and 50% glycerol in a 1:1 ratio for long-term preservation. Strains Si-cT and S2-gT have been deposited in culture collections under the accession numbers KACC 23732T and TBRC 19125T for Si-cT and KACC 23733T and TBRC 19012T for S2-gT.
2.2. Genomic Analysis and Genome Annotation
The genomic DNA of strains Si-cT and S2-gT was extracted using the Maxwell® RSC Tissue DNA Kit (Promega, Madison, WI, USA). The samples were then prepared for sequencing with the TruSeq Nano DNA Library Prep Kit (Illumina, Inc., San Diego, CA, USA) and sequenced on the Illumina NovaSeq 6000 platform. The sequencing data were assembled using the SPAdes ver. 3.15.0. de novo assembler [19] at Macrogen (Seoul, Republic of Korea). The completeness and contamination rates of the genomes were identified using the CheckM bioinformatics tool (https://ecogenomics.github.io/CheckM, accessed on 10 September 2024) [20]. The phylogenomic trees were reconstructed using the 92 up-to-date bacterial core gene sets (UBCG) and EzAAI [21,22]. The draft genomes were annotated using both the NCBI Prokaryotic Genome Automatic Annotation Pipeline (PGAP) and the Rapid Annotation using Subsystem Technology (RAST) online server [23,24]. Genes related to motility, pH tolerance, and plant growth-promoting traits were identified through the PGAP platform. Prokka [25] was used to annotate the genome and identify coding sequences (CDSs) and features, such as mobile genetic elements, comprehensive antibiotic resistance genes, CRISPRs, and phages [26,27,28]; these were visualized using a circular genomic map created with the Proksee online tool (https://proksee.ca/, accessed on 31 July 2024) [29]. Orthologous clusters (OCs) were compared between the isolated strains and closely related species using the OrthoVenn 3.0 online platform [30], with protein sequences from the annotated genomes processed using PGAP. The antibiotics and Secondary Metabolite Analysis Shell (antiSMASH) ver. 7.1.0. was employed to identify biosynthetic gene clusters (BGCs) associated with secondary metabolite compounds in the novel strains using the relaxed setting [31]. The abbreviations used for interpreting the antiSMASH results are defined in the antiSMASH glossary (https://docs.antismash.secondarymetabolites.org/glossary/, accessed on 11 September 2024). The overall genome relatedness index (OGRI) between the isolated strains and closely related Rhodanobacter species was calculated by using EzAAI for average amino acid identity (AAI), OrthoANI (www.ezbiocloud.net/tools/orthoani, accessed on 24 June 2024) for average nucleotide identity (ANI), and the Genome-to-Genome Distance Calculator ver. 3.0 (https://ddgc.dsmz.de/, accessed on 24 June 2024) for digital DNA–DNA hybridization (dDDH) [22,32,33]. The Whole Genome Shotgun projects for strains Si-cT and S2-gT have been deposited at DDBJ/ENA/GenBank under the accession numbers JBFOHK000000000 and JBFOHL000000000, respectively.
2.3. 16S rRNA Gene Sequencing and Phylogenetic Analysis
For phylogenetic analysis, this study sequenced the 16S rRNA genes of Si-cT and S2-gT using the Sanger method and retrieved the full-length 16S rRNA sequences from the genomes of these strains. The genome-derived 16S rRNA sequences were used for all phylogenetic analyses. The 16S rRNA genes of strains Si-cT and S2-gT were amplified using universal primer set 27F (5′-AGA GTT TGA TCC TGG CTC AG-3′)/1492R (5′-GGT TAC CTT GTT ACG ACT T-3′) [34] and 518F (5′-CCA GCA GCC GCG GTA ATA C-3′)/805R (5′-GAC TAC CAG GGT ATC TAA TC-78 3′) [35] and sequenced by Sanger method at Solgent Co., Ltd. (Daejeon, Republic of Korea). PCR was performed on an ABI PRISM 3730XL DNA Analyzer (Applied Biosystems, Waltham, MA, USA) with 30 cycles of denaturation at 96 °C for 10 s, annealing at 50 °C for 5 s, and extension at 60 °C for 4 min. The reaction mixture contained 4 µL of Terminator Ready Reaction Mix, 1 µL of cleaned PCR product (20–40 ng), 1 µL each of forward and reverse primers (5 pmol), and sterile water to a final volume of 10 µL. The amplicons were compiled using SeqMan software version 5.0 (DNASTAR, Madison, WI, USA). Nearly full-length 16S rRNA gene sequences of the two strains were obtained using the Sanger method, and full-length 16S rRNA gene sequences were retrieved from the draft genomes using ContEst16S [36]. The sequences from both methods were compared using the NCBI nucleotide BLAST server [37]. The 16S rRNA gene sequences obtained from the genome were uploaded to the EzBioCloud server (www.ezbiocloud.net/, accessed on 10 September 2024) [38] to identify phylogenetic neighbors based on 16S rRNA gene sequences of type species with valid names. Phylogenetic analysis was performed using phylogenetic trees constructed with MEGA version X software [39] by employing the maximum likelihood (ML), neighbor-joining (NJ), and maximum parsimony (MP) algorithms [40,41,42]. The confidence level of tree topologies was determined through bootstrap analysis with 1000 replicates [43]. Xanthomonas campestris ATCC 33913T was used as an outgroup. The GenBank accession numbers for the 16S rRNA gene sequences of strain Si-cT are PP647361 (Sanger method) and PP946760 (whole genome). For strain S2-gT, the accession numbers are PP647365 (Sanger method) and PP946762 (whole genome).
2.4. Physiological and Morphological Analysis
Physiological and chemotaxonomic analyses were performed using fully grown bacterial cells cultured on R2A agar for 5 days at 30 °C. Except for temperature tests, all other experiments were performed under identical conditions. To determine the optimal growth medium, strains Si-cT and S2-gT were cultured on R2A agar, marine agar (MB Cell), nutrient agar (Difco, Becton, Dickinson and Company, Franklin Lakes, NJ, USA), tryptic soy agar (MB Cell), and Luria–Bertani agar (MB Cell) containing 1.5% agar powder (Duksan, Ansan-si, Republic of Korea). The strains were also grown on R2A agar at different temperatures (4, 10, 15, 20, 25, 28, 30, 35, 37, 40, and 42 °C) and pH levels (pH 1.0–14.0, at pH 1.0 intervals). Salinity tolerance was determined in R2A agar plates supplemented with different concentrations of NaCl (0–5%, at 1.0% intervals). The pH was adjusted using the following buffers before sterilization: hydrochloric acid buffer (pH 1.0–2.0), citrate/NaH2PO4 buffer (pH 3.0–5.0), phosphate buffer (pH 6.0–8.0), Tris buffer (pH 9.0–10.0), and Na2HPO4–NaOH buffer (pH 11.0–14.0). Transmission electron microscopy at 80 kV (TEM; JEM1010, JEOL, Akishima, Japan) was performed to assess the cell morphology, as described previously [44]. In brief, the cells were suspended in autoclaved distilled water, placed on a grid, and negatively stained with uranyl acetate for 5–10 s. Motility was assessed by stabbing a needle contaminated with bacterial cells into R2A medium containing 0.4% agar. Anaerobic growth was assessed by culturing the strains on R2A agar at 30 °C for 14 days in an AnaeroPack rectangular system with oxygen absorber strips (Mitsubishi Gas Chemical Company, Tokyo, Japan) to eliminate oxygen. Hydrolytic activities were assessed using R2A agar containing casein (1%, w/v, skim milk; Biopure, GenomicBase, Namyangju-si, Republic of Korea), carboxymethyl cellulose (CM cellulose, 1%, w/v; Duksan), chitin (2%, w/v; Tokyo Chemical Industry Co., Ltd., Tokyo, Japan), and Tween 20 and 80 (1.5%; Samchun, Seoul, Republic of Korea) [45]. DNase activity was confirmed on DNase agar (MB Cell). Gram reactions were assessed using the standard Gram staining method and the non-staining KOH lysis method (3% KOH) [46]. Catalase activity was assessed using a hydrogen peroxide solution (3%, v/v); the production of bubbles indicated a positive result. Furthermore, oxidase activity was assessed using a tetramethyl-p-phenylenediamine solution (1%, w/v; bioMérieux, Marcy-l’Étoile, France); a color change to purple indicated a positive result. Enzymatic and metabolic activities were assessed using API 20NE strips (bioMérieux).
2.5. Chemotaxonomic Analysis
Fatty acids were extracted through saponification, methylation, and extraction, as described previously [47]. The extracts were identified using the Sherlock Microbial Identification System version 6.3 and RTSBA6 libraries. Polar lipids were extracted and developed using two-dimensional thin-layer chromatography [48]. A mixture of chloroform/methanol/water in a ratio of 65:25:4 (v/v/v) was used in the first dimension, and a mixture of chloroform/methanol/acetic acid/water in a ratio of 80:15:12:4 (v/v/v/v) was used in the second dimension. Spots were visualized using the following reagents: 5% molybdophosphoric acid (Sigma-Aldrich, Burlington, MA, USA) for total lipids, 0.2% ninhydrin reagent (Sigma-Aldrich) for amino lipids, α-naphthol reagent for glycolipids, and Zinzadze’s reagent (molybdenum blue spray reagent, 1.3%; Sigma-Aldrich) for phospholipids. Isoprenoid quinones were extracted using a mixture of chloroform and methanol in a ratio of 2:1 (v/v), as reported previously [49].
2.6. Plant Growth-Promoting Traits
2.6.1. Quantification of Indole Acetic Acid (IAA)
IAA was detected and quantified using a previously described method [5]. Strains Si-cT and S2-gT were grown in R2A broth for 3 days at 30 °C with shaking at 180 rpm. The absorbance of the bacterial cells was measured at 600 nm, and the OD at 600 nm was adjusted to 1. Subsequently, 100 μL of the bacterial suspension was inoculated into 10 mL of R2A broth supplemented with 0.1% tryptophan. The medium was prepared by autoclaving the R2A broth, adding tryptophan, and sterilizing the filter.
The inoculated medium was incubated at 30 °C with shaking at 180 rpm for 5 days. The bacterial cells were centrifuged at 10,000 rpm for 5 min, and 1 mL of the bacterial supernatant was then mixed with 2 mL of Salkowski’s reagent (98 mL of 35% perchloric acid and 2 mL of 0.5 M FeCl3). The mixture was incubated at 30 °C with shaking at 180 rpm for 30 min, and the absorbance was spectrophotometrically measured at 530 nm. The experiment was performed in triplicate for each strain. IAA production was confirmed by a change in color from yellow to pink. The IAA concentration was determined using an IAA standard curve.
2.6.2. Nitrogen Fixation
The nitrogen-fixing ability of the isolated bacterial strains was confirmed using Jensen’s medium (sucrose 20 g, K2HPO4 1 g, MgSO4 0.5 g, NaCl 0.5 g, FeSO4 0.1 g, Na2MoO4 0.005 g, CaCO3 2 g, agar 15 g, and distilled water 1 L) [50]. The bacterial strains were inoculated on Jensen’s medium and incubated at 30 °C for one week. Abundant growth on the medium indicated that the strains were capable of fixing atmospheric nitrogen.
2.6.3. Siderophore Production
Isolated strains were grown on chrome azurol sulfonate (CAS) agar plates to assess siderophore production as described previously [50]. Cultures were incubated at 30 °C for one week. Siderophore production was indicated by a color change in the medium around the bacterial colonies, demonstrating the presence of iron-chelating activity.
2.6.4. Phosphate Solubilization
The phosphate solubilization ability of bacterial strains was evaluated using Pikovskaya’s agar [51]. Bacterial strains were inoculated onto the agar plates (yeast extract 0.5 g, glucose 10 g, Ca3(PO4)2 5 g, (NH4)2SO4 0.5 g, KCl 0.2 g, MgSO4·7H2O 0.1 g, MnSO4·H2O 0.002 g, NaCl 0.2 g, FeSO4 0.002 g, agar 15 g, and distilled water 1 L, pH 7.0) with 0.025 g bromophenol blue. The plates were incubated at 30 °C for one week. Phosphate solubilization was assessed by observing the formation of clear halos around inoculated spots.
2.6.5. Quantification of Ammonia
To quantify ammonia production, 100 uL of bacterial cultures adjusted to OD600 = 1.0 was inoculated into 10 mL of peptone water (NaCl 10 g, peptone 5 g, distilled water 1 L) [52]. The inoculum was incubated at 30 °C for 2 days in a shaking incubator set to 180 rpm. After incubation, 0.5 mL of Nessler’s reagent was added to the supernatant of each sample to detect ammonia production. The experiment was conducted in triplicate for each strain. The amount of ammonia was quantified using a standard curve created with ammonium sulfate. Positive results were indicated by a color change to brown or orange, reflecting the presence of ammonia.
3. Results and Discussion
3.1. Phylogenetic Analysis Based on 16S rRNA Gene Sequences
A comparison of the 16S rRNA gene sequences obtained using the Sanger method and the ContEst16S tool revealed 100% identity for both strains (Si-cT and S2-gT). The sequence lengths based on the Sanger method and ContEst16S tool were 1465 and 1541 bp for strain Si-cT and 1468 and 1541 bp for strain S2-gT, respectively. The similarity between strains Si-cT and S2-gT was 99.0%. Comparative analysis of 16S rRNA gene sequences using the EzBioCloud server revealed that strain Si-cT exhibited the highest similarity to R. humi RS22T (99.1%), R. denitrificans 2APBS1T (98.4%), and R. thiooxydans LCS2T (98.2%), while strain S2-gT exhibited the highest similarity to R. humi RS22T (98.6%), R. aciditrophus sjH1T (98.0%), and R. denitrificans 2APBS1T (97.7%). The phylogenetic trees based on the 16S rRNA gene sequences revealed that strains Si-cT and S2-gT clustered well with species belonging to the genus Rhodanobacter (Figure 1 and Figure S1).
Figure 1.
Phylogenetic tree constructed based on 16S rRNA gene sequences using the maximum likelihood algorithm. The tree was rooted using Xanthomonas campestris ATCC 33913T (X95917) as an outgroup. The taxonomic relationships between strains Si-cT and S2-gT and other closely related species are depicted. Bootstrap values (>50%) based on 1000 replications are shown at the branch points. Bar, 0.020 substitutions per nucleotide position.
Notably, in all trees, the two novel bacterial strains exhibited a close relationship with R. humi RS22T and R. aciditrophus sjH1T. Strains Si-cT and S2-gT clustered effectively with members of the genus Rhodanobacter and formed a distinct lineage with their closest relatives. Based on consistent genealogical evidence, these strains should be classified as novel species within the genus Rhodanobacter.
3.2. Genomic Analysis and Genome Annotation
The genome of strain Si-cT had a total size of 4320329 bp with a G + C content of 67.0 mol%. It consisted of 15 contigs, 3821 genes, 3734 protein-coding genes, 47 tRNAs, three rRNAs, and 33 total pseudogenes. The genome coverage was 146.3×. The 16S rRNA gene sequences obtained from the genome were 1541 bp in length and matched 100% with those obtained using the Sanger sequencing method.
The genome of strain S2-gT had a total size of 3752455 bp with a G + C content of 68.5 mol%. It consisted of 31 contigs, 3376 genes, 3281 protein-coding genes, 47 tRNAs, three rRNAs, and 41 total pseudogenes. The genome coverage was 145.8×. The 16S rRNA gene sequences obtained from the genome were 1541 bp in length and matched 100% with those obtained using the Sanger sequencing method.
The completeness and contamination values were 99.9% and 1.7% for strain Si-cT and 99.9% and 0.1% for strain S2-gT, respectively, as affirmed by CheckM results. Phylogenomic trees constructed based on the UBCG and AAI revealed that the two isolated strains were positioned within the genus Rhodanobacter (Figure 2 and Figure S2); this finding is consistent with the results obtained from the 16S rRNA-based trees.
Figure 2.
Phylogenetic tree using a set of 92 bacterial core genes. Xanthomonas campestris ATCC 33913T was selected as an outgroup. The numbers at the nodes represent the gene support index, with the maximum value being 92. Bar, 0.10 substitutions per nucleotide position.
Through the NCBI annotation, several genes were identified that contribute to motility, pH tolerance, and plant growth-promoting traits. Motility-related genes such as flg, flh, fli, and motD were detected, which are known to contribute to flagellar assembly. Notably, the fliL gene, found in both isolates but absent in the reference strains, was also identified. Flagella-related genes for isolated strains and the reference strains are presented in Table S1.
Additionally, several genes related to pH tolerance were identified, including atp, pho, cyo, cco, clp, and pst. These genes play critical roles in energy metabolism, phosphate transport, and stress responses, helping the bacteria adapt to varying pH conditions (Table S2). In terms of plant growth-promoting traits, genes associated with IAA production, such as trpABCDE, were found. Siderophore production was supported by the detection of two genes, a siderophore-interacting protein, and the catecholate siderophore receptor Fiu, both involved in siderophore synthesis and transport [53]. Phosphate solubilization-related genes, including ppk, pho, and pst, and PQQ-dependent enzymes were detected. Specifically, ppk contributes to polyphosphate synthesis, enabling phosphate storage and utilization, while pho and pst are involved in phosphate transport and regulation, allowing phosphate acquisition under limiting conditions [54]. Additionally, PQQ-dependent enzymes enhance phosphate solubilization by producing organic acids, which lower the pH and convert insoluble phosphates into soluble forms usable by plants [55]. These genes indicate a strong potential for phosphate solubilization, improving phosphorus availability in soil environments. Ammonia production genes such as glnA, glnE, hutH, and ilvA were also identified. glnA and glnE are involved in nitrogen assimilation and ammonia synthesis, while hutH and ilvA contribute to ammonia production through histidine and threonine degradation, respectively. These genes underscore the strains’ strong ammonia production capabilities, enhancing nitrogen availability for plant growth [56]. Although no nitrogen fixation genes were identified in either strain, several nitrogen metabolism-related genes were detected. Both strains contained glnK, ntrC, and gltB, which are involved in nitrogen regulation and assimilation. In strain Si-cT, additional genes such as narHIJ and nirK were found, facilitating nitrate reduction and denitrification processes. Other relevant genes, including nitric-oxide reductase, nitrilase-related carbon–nitrogen hydrolase, and nitrate/nitrite transporter, highlighted the ability to metabolize nitrogen efficiently. These pathways enabled the strains to convert nitrate into ammonia and assimilate it, allowing for limited growth even in the absence of fixed nitrogen sources by facilitating ammonia assimilation and recycling through nitrate reduction and nitrogen assimilation mechanisms [57]. The genes related to plant growth-promoting traits are listed in Table 1.
Table 1.
Plant growth-promoting traits-related genes identified in strains (A) Si-cT and (B) S2-gT. The table lists genes involved in IAA production, nitrogen metabolism, siderophore production, phosphate solubilization, and ammonia production. These genes were annotated using NCBI annotation platform. +, the forward transcription strand; −, the reverse transcription strand.
| (A) Si-cT | |||||||
| Traits | Gene | Protein Name | Accession | Start | Stop | Strand | Length (aa) |
| IAA Production |
trpA | Tryptophan synthase subunit alpha | JBFOHK010000001 | 582,844 | 583,644 | − | 266 |
| trpB | Tryptophan synthase subunit beta | JBFOHK010000001 | 583,641 | 584,852 | − | 403 | |
| trpE | Anthranilate synthase component I | JBFOHK010000002 | 726,143 | 727,618 | + | 491 | |
| trpD | Anthranilate phosphoribosyltransferase | JBFOHK010000002 | 748,695 | 749,750 | + | 351 | |
| trpC | Indole-3-glycerol phosphate synthase TrpC | JBFOHK010000002 | 749,819 | 750,613 | + | 264 | |
| Tryptophan--tRNA ligase | JBFOHK010000001 | 1,478,563 | 1,479,909 | + | 448 | ||
| Tryptophan 2,3-dioxygenase | JBFOHK010000001 | 1,487,847 | 1,488,695 | − | 282 | ||
| Phosphoribosylanthranilate isomerase | JBFOHK010000001 | 586,128 | 586,745 | − | 205 | ||
| Nitrogen Metabolism |
narH | Nitrate reductase subunit beta | JBFOHK010000004 | 23,785 | 25,326 | − | 513 |
| narI | Respiratory nitrate reductase subunit gamma | JBFOHK010000004 | 22,432 | 23,139 | − | 235 | |
| narJ | Nitrate reductase molybdenum cofactor assembly chaperone | JBFOHK010000004 | 23,168 | 23,785 | − | 205 | |
| nirK | Copper-containing nitrite reductase | JBFOHK010000001 | 244,694 | 246,220 | + | 508 | |
| Nitric-oxide reductase large subunit | JBFOHK010000001 | 239,631 | 241,928 | − | 765 | ||
| Nitrilase-related carbon–nitrogen hydrolase | JBFOHK010000003 | 308,876 | 310,303 | + | 475 | ||
| Nitrate/nitrite transporter | JBFOHK010000004 | 29,107 | 30,357 | − | 416 | ||
| glnK | P-II family nitrogen regulator | JBFOHK010000002 | 417,051 | 417,389 | − | 112 | |
| P-II family nitrogen regulator | JBFOHK010000004 | 437,822 | 438,160 | + | 112 | ||
| Carbon–nitrogen hydrolase | JBFOHK010000001 | 189,566 | 190,456 | − | 296 | ||
| ntrC | Nitrogen regulation protein NR(I) | JBFOHK010000002 | 88,709 | 90,127 | − | 472 | |
| Nitrogen regulation protein NR(II) | JBFOHK010000002 | 90,124 | 91,131 | − | 335 | ||
| gltB | Glutamate synthase large subunit | JBFOHK010000005 | 185,142 | 189,584 | + | 1480 | |
| FMN-binding glutamate synthase family protein | JBFOHK010000001 | 1,075,646 | 1,077,154 | − | 502 | ||
| Siderophore Production | Siderophore-interacting protein | JBFOHK010000001 | 740,009 | 740,797 | + | 262 | |
| Catecholate siderophore receptor Fiu | JBFOHK010000004 | 298,978 | 301,326 | + | 782 | ||
| Phosphate Solubilization | PQQ-dependent sugar dehydrogenase | JBFOHK010000001 | 290,539 | 291,759 | − | 406 | |
| PQQ-binding-like beta-propeller repeat protein | JBFOHK010000001 | 448,189 | 449,700 | − | 503 | ||
| ppk1 | Polyphosphate kinase 1 | JBFOHK010000004 | 105,024 | 107,168 | + | 714 | |
| ppk1 | Polyphosphate kinase 1 | JBFOHK010000004 | 423,147 | 425,255 | + | 702 | |
| ppk2 | Polyphosphate kinase 2 | JBFOHK010000001 | 1,336,814 | 1,337,578 | + | 254 | |
| phoB | Phosphate regulon transcriptional regulator PhoB | JBFOHK010000004 | 421,015 | 421,704 | + | 229 | |
| phoR | Phosphate regulon sensor histidine kinase PhoR | JBFOHK010000004 | 422,042 | 423,091 | + | 349 | |
| phoU | Phosphate signaling complex protein PhoU | JBFOHK010000001 | 638,194 | 638,925 | + | 243 | |
| pstA | Phosphate ABC transporter permease PstA | JBFOHK010000001 | 636,381 | 637,250 | + | 289 | |
| pstB | Phosphate ABC transporter ATP-binding protein PstB | JBFOHK010000001 | 637,243 | 638,058 | + | 271 | |
| pstC | Phosphate ABC transporter permease subunit PstC | JBFOHK010000001 | 635,410 | 636,381 | + | 323 | |
| pstS | Phosphate ABC transporter substrate-binding protein PstS | JBFOHK010000001 | 634,202 | 635,218 | + | 338 | |
| Ammonia Production | glnA | Type I glutamate--ammonia ligase | JBFOHK010000002 | 96,336 | 97,745 | − | 469 |
| glnE | Bifunctional [glutamate--ammonia ligase]-adenylyl-L-tyrosine phosphorylase/[glutamate--ammonia-ligase] adenylyltransferase | JBFOHK010000005 | 351,827 | 354,676 | + | 949 | |
| Ammonium transporter | JBFOHK010000004 | 438,147 | 439,490 | + | 447 | ||
| L-serine ammonia-lyase | JBFOHK010000002 | 861,277 | 862,659 | − | 460 | ||
| hutH | Histidine ammonia-lyase | JBFOHK010000003 | 215,896 | 217,797 | − | 633 | |
| ilvA | Threonine ammonia-lyase, biosynthetic | JBFOHK010000005 | 59,564 | 61,132 | − | 522 | |
| (B) S2-gT | |||||||
| Traits | Gene | Protein Name | Accession | Start | Stop | Strand | Length (aa) |
| IAA Production |
trpB | Tryptophan synthase subunit beta | JBFOHL010000002 | 114,807 | 116,018 | + | 403 |
| trpA | Tryptophan synthase subunit alpha | JBFOHL010000002 | 116,015 | 116,815 | + | 266 | |
| trpC | Indole-3-glycerol phosphate synthase TrpC | JBFOHL010000018 | 31,276 | 32,070 | − | 264 | |
| trpD | Anthranilate phosphoribosyltransferase | JBFOHL010000018 | 32,135 | 33,184 | − | 349 | |
| trpE | Anthranilate synthase component I | JBFOHL010000018 | 49,841 | 51,316 | − | 491 | |
| Tryptophan--tRNA ligase | JBFOHL010000019 | 4284 | 5630 | + | 448 | ||
| Tryptophan 2,3-dioxygenase | JBFOHL010000019 | 12,868 | 13,716 | − | 282 | ||
| Phosphoribosylanthranilate isomerase | JBFOHL010000002 | 112,135 | 112,770 | + | 211 | ||
| Nitrogen Metabolism |
glnK | P-II family nitrogen regulator | JBFOHL010000001 | 297,354 | 297,692 | + | 112 |
| P-II family nitrogen regulator | JBFOHL010000012 | 49,216 | 49,554 | − | 112 | ||
| Carbon–nitrogen hydrolase | JBFOHL010000017 | 32,018 | 32,908 | − | 296 | ||
| ntrC | Nitrogen regulation protein NR(I) | JBFOHL010000011 | 96,362 | 97,741 | + | 459 | |
| Nitrogen regulation protein NR(II) | JBFOHL010000011 | 95,248 | 96,255 | + | 335 | ||
| gltB | Glutamate synthase large subunit | JBFOHL010000008 | 141,185 | 145,627 | − | 1480 | |
| FMN-binding glutamate synthase family protein | JBFOHL010000006 | 52,390 | 53,901 | − | 503 | ||
| Siderophore Production |
Catecholate siderophore receptor Fiu | JBFOHL010000003 | 276,284 | 278,641 | + | 785 | |
| Siderophore-interacting protein | JBFOHL010000004 | 8785 | 9567 | + | 260 | ||
| Phosphate Solubilization |
PQQ-binding-like beta-propeller repeat protein | JBFOHL010000002 | 269,737 | 270,987 | + | 416 | |
| PQQ-binding-like beta-propeller repeat protein | JBFOHL010000004 | 101,633 | 102,091 | − | 152 | ||
| PQQ-dependent sugar dehydrogenase | JBFOHL010000002 | 386,884 | 388,104 | − | 406 | ||
| ppk1 | Polyphosphate kinase 1 | JBFOHL010000003 | 117,749 | 119,893 | + | 714 | |
| ppk1 | Polyphosphate kinase 1 | JBFOHL010000012 | 60,438 | 62,531 | − | 697 | |
| phoB | Phosphate regulon transcriptional regulator PhoB | JBFOHL010000012 | 65,413 | 66,102 | − | 229 | |
| phoR | Phosphate regulon sensor histidine kinase PhoR | JBFOHL010000012 | 64,073 | 65,371 | − | 432 | |
| phoU | Phosphate signaling complex protein PhoU | JBFOHL010000012 | 71,639 | 72,370 | − | 243 | |
| pstA | Phosphate ABC transporter permease PstA | JBFOHL010000002 | 73,333 | 74,202 | − | 289 | |
| pstB | Phosphate ABC transporter ATP-binding protein PstB | JBFOHL010000002 | 72,513 | 73,340 | − | 275 | |
| pstC | Phosphate ABC transporter permease subunit PstC | JBFOHL010000002 | 74,202 | 75,173 | − | 323 | |
| pstS | Phosphate ABC transporter substrate-binding protein PstS | JBFOHL010000002 | 75,395 | 76,429 | − | 344 | |
| Ammonia Production |
glnA | Type I glutamate--ammonia ligase | JBFOHL010000011 | 88,689 | 90,098 | + | 469 |
| glnE | Bifunctional [glutamate--ammonia ligase]-adenylyl-L-tyrosine phosphorylase/[glutamate--ammonia-ligase] adenylyltransferase | JBFOHL010000015 | 31,987 | 34,836 | + | 949 | |
| Ammonium transporter | JBFOHL010000012 | 47,901 | 49,226 | − | 441 | ||
| L-serine ammonia-lyase | JBFOHL010000016 | 57,527 | 58,909 | − | 460 | ||
| hutH | Histidine ammonia-lyase | JBFOHL010000009 | 170,273 | 172,174 | − | 633 | |
| ilvA | Threonine ammonia-lyase, biosynthetic | JBFOHL010000013 | 65,891 | 67,462 | − | 523 | |
RAST analysis revealed that strains Si-cT and S2-gT had the highest number of genes in the following categories in the given order: amino acids and derivatives, protein metabolism and cofactors, vitamins, prosthetic groups, and pigments. This order aligns with that of other closely related species. Specifically, strain Si-cT had 230, 182, and 158 genes associated with these categories, while strain S2-gT had 234, 178, and 144 genes in these categories, respectively. Species with a high number of genes in these categories likely exhibit efficient metabolic pathways. Details of the RAST analysis are provided in Table S3. A set of tools integrated into the Proksee online server was used for comprehensive genome annotation and visualization of the circular genomic map (Figure S3). Prokka pipeline analysis revealed that strain Si-cT contained a total of 3771 features, including 3716 CDSs, 51 tRNAs, three rRNAs, and one tmRNA. Moreover, it contained 11 CRISPRs, two CARDs, and 79 genes predicted using Alien Hunter. MobileOG-db revealed 91 genomic features related to the replication/recombination/repair, integration/excision, phase, transfer, and stability/transfer/defense of mobile genetic elements. For strain S2-gT, Prokka pipeline analysis revealed a total of 3344 features, including 3289 CDSs, 55 tRNAs, three rRNAs, and one tmRNA. This strain also contained seven CRISPRs, two CARDs, and 99 genes predicted using Alien Hunter. MobileOG-db revealed a total of 73 genomic features associated with the replication/recombination/repair, integration/excision, phase, transfer, and stability/transfer/defense of mobile genetic elements. Moreover, analysis using OrthoVenn 3.0 software revealed a total of 4012 OCs, including 2103 core OCs shared by all strains, 1926 accessory OCs shared by more than two but not all strains, and 73 unique OCs. A total of 24 OCs were unique to strain Si-cT, while 8 OCs were unique to strain S2-gT. These findings highlight the common genetic features between these strains and the distinct genetic traits that differentiate strains Si-cT and S2-gT (Figure S4). Using antiSMASH 7.1.0, several BGCs were predicted in the genomes of strains Si-cT and S2-gT. Six BGCs were identified in strain Si-cT, and seven were identified in strain S2-gT. The genome of strain Si-cT contained the following BGCs: aryl polyene (BGCs 1 and 3), terpene (BGC 2), RiPP-like (BGC 4), NRPS, T1PKS (BGC 5), and lasso peptide (BGC 6). On the other hand, the genome of strain S2-gT contained the following BGCs: aryl polyene (BGCs 1 and 2), terpene (BGC 3), T1PKS, NRPS-like, NRPS (BGC 4), NRPS, NRPS-like (BGC 5), lasso peptide (BGC 6), and NRPS (BGC 7). The BGC types identified in the genomes of these two strains exhibited notable similarities, including the presence of aryl polyene, terpene, T1PKS, NRPS, and lasso peptide BGCs. Both strains shared these BGC types, suggesting similar biosynthetic capabilities. However, strain Si-cT contained an additional RiPP-like BGC that was not found in strain S2-gT, while strain S2-gT contained NRPS-like BGCs that were absent in strain Si-cT. Thus, while the two strains have a similar core set of BGCs, there are distinct differences in their biosynthetic potential. Among all BGCs in the novel strains, only one BGC exhibited more than 50% similarity to a known BGC. It was 100% identical to a rhizomide A BGC from Paraburkholderia rhizoxinica HKI 454T (NC_014718.1). The other BGCs showed only minimal or no similarity to previously identified BGCs, suggesting that the novel strains Si-cT and S2-gT have significant potential for producing new natural products. Details regarding the BGCs present in the genomes of the two novel strains and their functions are presented in Table S4. The calculated OGRI for strain Si-cT ranged from 75.4% to 89.5% for AAI, 79.2% to 89.4% for ANI, and 22.4% to 37.3% for dDDH. For strain S2-gT, the calculated OGRI ranged from 75.5% to 89.5% for AAI, 79.5% to 89.4% for ANI, and 22.5% to 37.3% for dDDH. All these values met the recognized cutoff thresholds for distinguishing bacterial species (95–96% for ANI, 95%–96% for AAI, and 70% for dDDH) [58,59,60]. The OGRI values between the two novel bacterial strains and recognized Rhodanobacter species are presented in Table 2.
Table 2.
Overall genome relatedness index (OGRI) between strains Si-cT and S2-gT and other Rhodanobacter species (AAI, ANI, and dDDH values).
| Si-cT | S2-gT | |||||
|---|---|---|---|---|---|---|
| AAI (%) | ANI (%) | dDDH (%) | AAI (%) | ANI (%) | dDDH (%) | |
| R. lycopersici Si-cT | 100 | 100 | 100 | 89.5 | 89.4 | 37.3 |
| R. geophilus S2-gT | 89.5 | 89.4 | 37.3 | 100 | 100 | 100 |
| R. caeni MJ01T | 75.6 | 80.0 | 23.1 | 75.8 | 80.2 | 23.1 |
| R. denitrificans 2APBS1T | 77.0 | 81.4 | 24.1 | 77.4 | 81.8 | 24.6 |
| R. fulvus Jip2T | 75.5 | 79.2 | 22.5 | 75.6 | 79.6 | 22.7 |
| R. ginsenosidimutans Root627 | 75.4 | 79.2 | 22.4 | 75.5 | 79.5 | 22.5 |
| R. glycinis MO64T | 77.9 | 80.7 | 23.7 | 77.7 | 80.9 | 23.7 |
| R. humi C06 | 87.9 | 88.0 | 33.9 | 88.6 | 88.8 | 35.6 |
| R. lindaniclasticus DSM 17932 | 75.8 | 80.2 | 23.4 | 76.1 | 80.6 | 23.5 |
| R. panaciterrae KCTC 22232T | 76.8 | 79.5 | 22.5 | 76.8 | 79.7 | 22.6 |
| R. soli JCM 16126T | 77.3 | 80.9 | 23.5 | 77.4 | 81.2 | 23.8 |
| R. spathiphylli B39T | 76.2 | 80.3 | 23.2 | 76.3 | 80.7 | 23.4 |
| R. thiooxydans LCS2T | 77.2 | 81.2 | 24.3 | 77.5 | 81.8 | 24.7 |
3.3. Physiological and Chemotaxonomic Analysis
Cells of strains Si-cT and S2-gT were Gram-negative, oxidase-positive, aerobic, and motile by means of flagella. They had a rod-shaped morphology (Figure S5) and formed visible yellow colonies on R2A agar at 30 °C within 3 days. Strain Si-cT was catalase-positive, whereas strain S2-gT was catalase-negative. Flagella were detected by TEM. The differential physiological and biochemical features of the two novel strains compared with other closely related species within the genus Rhodanobacter are presented in Table 3.
Table 3.
Phenotypic features of strains Si-cT, S2-gT, and type strains of closely related species belonging to the genus Rhodanobacter. Strains: 1, Si-cT (data from this study); 2, S2-gT (data from this study); 3, R. humi RS22T [61]; 4, R. denitrificans 2APBS1T [62]; and 5, R. thiooxydans LCS2T [63]. All strains exhibited positive results for oxidase activity; esculin hydrolysis; β-galactosidase activity; and D-glucose, N-acetylglucosamine, and D-maltose assimilation but negative results for indole production; arginine hydrolyze and urease activities; and L-arabinose, D-mannitol, potassium gluconate, capric acid, adipic acid, malate, trisodium citrate, and phenylacetic acid assimilation. +, positive; −, negative; w, weakly positive; ND, no data.
| Characteristic | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Growth temperature (°C) | 10–37 | 10–42 | 15–35 | 10–35 | 10–40 |
| pH range | 3.0–13.0 | 2.0–13.0 | 4.5–11.0 | 4.0–8.0 | 5.0–8.0 |
| Salt tolerance (%, w/v) | 0–4 | 0–4 | 0–4 | 0–2 | 0–2 |
| Catalase | + | − | + | + | + |
| Motility | + | + | − | + | − |
| Length | 1–1.1 | 1.2–1.6 | 1.8–2.9 | 3.0–5.0 | 1.5–3.0 |
| Width | 0.5–0.6 | 0.4–0.5 | 0.8–1.2 | 0.3–0.5 | 0.6–0.8 |
| Casein | + | − | − | + | − |
| Chitin | − | − | − | ND | ND |
| CM cellulose | + | − | − | ND | ND |
| DNA | − | − | + | − | w |
| Tween 20 | − | − | ND | ND | ND |
| Tween 80 | − | − | − | ND | ND |
| Nitrate reduction | + | − | − | + | + |
| Glucose fermentation | − | − | + | − | − |
| Gelatin hydrolysis | − | − | − | + | + |
| D-Mannose | + | w | − | − | − |
Both strains exhibited vigorous growth on R2A agar, nutrient agar, and tryptic soy agar; slight growth on marine agar; and no growth on Luria–Bertani agar. On R2A media, strain Si-cT grew at temperatures of 10–37 °C (optimum, 28–30 °C), at pH levels of 3.0–13.0 (optimum, pH 6.0–7.0), and in the presence of up to 4% NaCl (optimum, 0%), while strain S2-gT grew at temperatures of 10–42 °C (optimum, 28–30 °C), at pH levels of 2.0–13.0 (optimum, pH 7.0–8.0), and in the presence of up to 4% NaCl (optimum, 0%). Strain S2-gT hydrolyzed casein and CM cellulose; however, strain Si-cT did not hydrolyze any of the tested substrates. In the API 20NE tests, both strains exhibited positive results for esculin hydrolysis; β-galactosidase activity; and D-glucose, D-mannose, N-acetylglucosamine, and D-maltose assimilation but negative results for indole production; glucose fermentation; L-arginine hydrolysis; urease activity; gelatin hydrolysis; and L-arabinose, D-mannitol, potassium gluconate, capric acid, adipic acid, malate, trisodium citrate, and phenylacetic acid assimilation.
The major fatty acids in strains Si-cT and S2-gT were iso-C15:0 (17.3% and 18.6%, respectively), iso-C16:0 (13.6% and 9.6%, respectively), iso-C17:0 (15.8% and 10.6%, respectively), and summed feature 9 (iso-C17:1 ω9c and/or C16:0 ω6c 10-methyl; 19.4% and 21.3%, respectively). The fatty acid profiles of strains Si-cT and S2-gT were generally consistent with those of closely related Rhodanobacter species, although slight deviations were noted in detailed proportions. Both strains exhibited lower proportions of iso-C15:0 and summed feature 9 than the reference strains. They also exhibited lower proportions of summed feature 3 (C16:1 ω7c and/or C16:1 ω6c; 2% in strain Si-cT and 3.2% in strain S2-gT). The detailed fatty acid profiles are presented in Table S5.
The polar lipid profiles of both strains consisted of phosphatidylethanolamine, diphosphatidylglycerol, phosphatidyl-N-methylethanolamine, and phosphatidylglycerol, which are commonly reported as polar lipids in the genus Rhodanobacter. However, strain Si-cT was distinguished from recognized Rhodanobacter species by the presence of five unknown aminophosphoglycolipids, two unknown aminophospholipids, two unknown phosphoglycolipids, two unknown glycolipids, and three unknown phospholipids, in addition to their respective positions on the chromatogram. Compared with strain Si-cT, strain S2-gT also contained an additional unknown phosphoglycolipid and two unknown glycolipids, with differences being observed in the precise positions of each spot on the chromatogram (Figure S6).
3.4. Plant Growth-Promoting Traits
According to Salkowski’s test, strain Si-cT produced 19.3 µg/mL of IAA, which was greater than the 13.1 µg/mL produced by strain S2-gT (Figure 3a). These levels were comparable with the IAA production of the PGPB strain Pseudomonas azotoformans NBRC 12693T [64]. In the ammonia production assay, Si-cT produced 0.39 µmol/mL, while S2-gT produced 0.31 µmol/mL (Figure 3b), values comparable to those of the PGPB strain Enterobacter hormaechei J146 [65].
Figure 3.
Quantification of IAA (a) and ammonia (b) of strains Si-cT and S2-gT. The values represent the mean and standard deviation from three replicates.
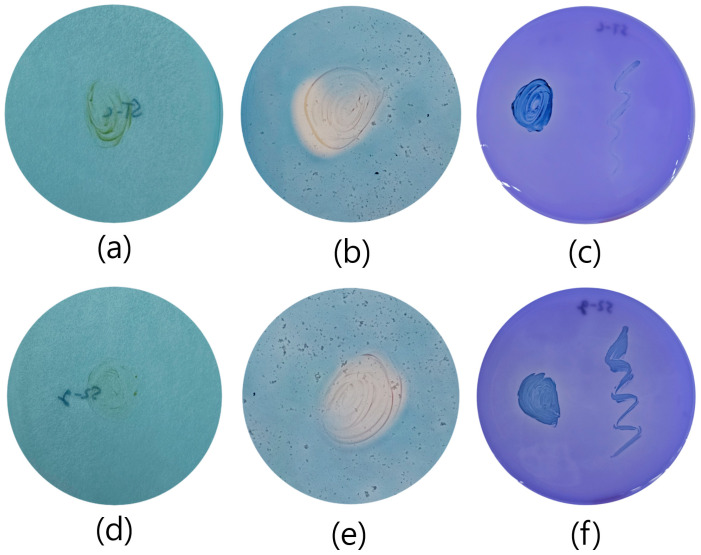
Both strains exhibited weak growth on the Jensen medium; however, since nitrogen fixation genes were not detected, this growth cannot be attributed to nitrogen fixation. For siderophore production and phosphate solubilization, clear zones were observed around both strains, confirming their capabilities (Figure 4).
Figure 4.
Results of the plant growth-promoting traits, including nitrogen fixation, siderophore production, and phosphate solubilization. Strains Si-cT and S2-gT are shown in (a–f), respectively. (a,d) represent nitrogen fixation; (b,e) represent siderophore production; (c,f) represent phosphate solubilization.
The two isolated strains possess various plant growth-promoting traits and related genes, and their ability to survive in extreme pH environments indicates their potential as valuable contributions to plant growth. However, since only in vitro experiments have been conducted, further validation in practical applications is needed.
4. Conclusions
This study identified two novel bacterial species, Rhodanobacter lycopersici sp. nov. and Rhodanobacter geophilus sp. nov., isolated from the rhizosphere of Solanum lycopersicum. Both strains exhibited broad pH tolerance and key plant growth-promoting traits, including IAA production, siderophore production, ammonia production, and phosphate solubilization.
The discovery of these novel species not only broadens our understanding of the genus Rhodanobacter but also offers promising potential for their application in sustainable agriculture, particularly in soils with fluctuating pH levels. While this study was limited to in vitro experiments, further pot experiments will provide valuable insights into the practical agricultural applications of these strains. The isolation and characterization of these two novel strains provide valuable insights into their functional traits and provide a solid starting point for future studies focused on promoting plant growth and soil health under diverse environmental conditions.
4.1. Description of Rhodanobacter lycopersici sp. nov.
R. lycopersici (ly.co.per’si.ci. N.L. gen. n. lycopersici, of the plant genus Lycopersicon).
Cells are Gram-negative, aerobic, motile, oxidase-positive, and catalase-positive. On TEM analysis, the cells appear rod-shaped and flagellated, measuring 1.0–1.1 µm in length and 0.5–0.6 µm in width. Colonies on R2A agar are pale yellow, raised, opaque with a translucent edge, entire, smooth, and circular. Growth is abundant on R2A agar, nutrient agar, and tryptic soy agar; weak on marine agar; and absent on Luria–Bertani agar. The growth conditions are as follows: temperature, 15–37 °C (optimum, 28–30 °C); pH, 3.0–13.0 (optimum, pH 6.0–7.0); and NaCl concentration, 0–4.0% (w/v; optimum, 0%). The results of hydrolysis tests are negative. In the API 20NE tests, the cells are positive for the reduction of nitrate; hydrolysis of esculin and β-galactosidase; and assimilation of D-glucose, D-mannose, N-acetylglucosamine, and D-maltose; all other results are negative. The predominant cellular fatty acid profiles are iso-C15:0, iso-C16:0, iso-C17:0, and summed feature 9 (comprising iso-C17:1 ω9c and/or C16:0 ω6c 10-methyl). Q-8 is the predominant respiratory quinone. The polar lipid profiles consist of phosphatidylethanolamine, diphosphatidylglycerol, phosphatidyl-N-methylethanolamine, phosphatidylglycerol, five unknown aminophosphoglycolipids, two unknown aminophospholipids, two unknown phosphoglycolipids, two unknown glycolipids, and three unknown phospholipids.
The type strain Si-cT (=KACC 23732T = TBRC 19125T) was isolated from the rhizosphere of a tomato plant in Ilsan, Republic of Korea.
4.2. Description of Rhodanobacter geophilus sp. nov.
R. geophilus (ge.o’phi.lus. Gr. n. ge, earth; Gr. suff. -philos, loving; N.L. masc. adj. geophilus, earth loving).
Cells are Gram-negative, aerobic, motile, oxidase-positive, and catalase-negative. On TEM analysis, the cells appear rod-shaped and flagellated, measuring 1.2–1.6 µm in length and 0.4–0.5 µm in width. Colonies on R2A agar are initially yellow and gradually turn pale brown. The colonies appear raised, opaque with a translucent edge, entire, smooth, and circular. Growth is abundant on R2A agar, nutrient agar, and tryptic soy agar; weak on marine agar; and absent on Luria–Bertani agar. The growth conditions are as follows: temperature, 15–42 °C (optimum, 28–30 °C); pH, 2.0–13.0 (optimum, pH 7.0–8.0); and NaCl concentration, 0–4% (w/v; optimum, 0%). The results of hydrolysis tests are all negative. In the API 20NE tests, the cells are positive for the hydrolysis of esculin and β-galactosidase and assimilation of D-glucose, D-mannose, N-acetylglucosamine, and D-maltose; all other results are negative. The predominant cellular fatty acid profiles are iso-C15:0, iso-C17:0, and summed feature 9 (comprising iso-C17:1 ω9c and/or C16:1 ω6c 10-methyl). Q-8 is the predominant respiratory quinone. The polar lipid profiles consist of phosphatidylethanolamine, diphosphatidylglycerol, phosphatidyl-N-methylethanolamine, phosphatidylglycerol, five unknown aminophosphoglycolipids, two unknown aminophospholipids, three unknown phosphoglycolipids, four unknown glycolipids, and three unknown phospholipids.
The type strain S2-gT (=KACC 23733T = TBRC 19012T) was isolated from the rhizosphere of a tomato plant in Ilsan, Republic of Korea.
Acknowledgments
We thank Bernhard Schink (University of Konstanz, Konstanz, Germany) for suggesting the species names.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms12112227/s1, Figure S1: Phylogenetic trees constructed using 16S rRNA gene sequences with (a) NJ and (b) MP algorithms; Figure S2: AAI tree generated using EzAAI illustrating the phylogenetic positions of the strains Si-cT, S2-gT, and their closely related species; Figure S3: Graphical circular genomic map generated using the Proksee online server, displaying the genome views for strains (a) Si-cT and (b) S2-gT; Figure S4: Venn diagrams built using the OrthoVenn3 online server, exhibiting the number of shared and unique orthologous gene clusters of strains Si-cT, S2-gT, and other closely related species; Figure S5: Cell morphology under the transmission electron microscope for (a) Si-cT and (b) S2-gT; Figure S6: Polar lipid profiles of strains (a) Si-cT and (b) S2-gT; Table S1: Overview of flagella-related genes in the genomes of strains Si-cT, S2-gT, and closely related species within the genus Rhodanobacter; Table S2: Genes related to pH tolerance of strains (a) Si-cT and (b) S2-gT identified with NCBI annotation systems; Table S3: Comparison of subsystem category distribution and feature counts for strains Si-cT, S2-gT, and other closely related taxa using RAST analysis; Table S4: Overview of identified BGCs in the (a) Si-cT and (b) S2-gT genome predicted by antiSMASH; Table S5: Fatty acid compositions (% of the total) of strains Si-cT, S2-gT, and closely related taxa.
Author Contributions
Conceptualization, H.W. and T.S.; Methodology, H.W., I.K., G.C. and S.P.; Formal analysis, H.W., H.L. and S.Y.; Writing—original draft preparation, H.W.; Writing—review and editing, H.W., I.K., S.P. and T.S.; Supervision, T.S. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The DDBJ/ENA/GenBank accession numbers for the Whole Genome Shotgun project, 16S rRNA gene sequence obtained using the Sanger method, and 16S rRNA gene sequence obtained from the whole genome are as follows: Si-cT: JBFOHK000000000 (Whole Genome Shotgun), PP647361 (Sanger method 16S rRNA), and PP946760 (whole genome 16S rRNA). S2-gT: JBFOHL000000000 (Whole Genome Shotgun), PP647365 (Sanger method 16S rRNA), and PP946762 (whole genome 16S rRNA).
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Funding Statement
This work was supported by a grant from the National Institute of Biological Resources (NIBR) funded by the Ministry of Environment (MOE) of the Republic of Korea (NIBR202402203) and by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT; 2022R1F1A1070108).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.van Dijk M., Morley T., Rau M.L., Saghai Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food. 2021;2:494–501. doi: 10.1038/s43016-021-00322-9. [DOI] [PubMed] [Google Scholar]
- 2.Gerhardson B. Biological substitutes for pesticides. Trends Biotechnol. 2002;20:338–343. doi: 10.1016/S0167-7799(02)02021-8. [DOI] [PubMed] [Google Scholar]
- 3.Compant S., Duffy B., Nowak J., Clément C., Barka E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005;71:4951–4959. doi: 10.1128/AEM.71.9.4951-4959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kisvarga S., Hamar-Farkas D., Ördögh M., Horotán K., Neményi A., Kovács D., Orlóci L. The Role of the Plant–Soil Relationship in Agricultural Production—With Particular Regard to PGPB Application and Phytoremediation. Microorganisms. 2023;11:1616. doi: 10.3390/microorganisms11061616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chhetri G., Kim I., Kang M., So Y., Kim J., Seo T. An Isolated Arthrobacter sp. Enhances Rice (Oryza sativa L.) Plant Growth. Microorganisms. 2022;10:1187. doi: 10.3390/microorganisms10061187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sánchez P., Castro-Cegrí A., Sierra S., Garrido D., Llamas I., Sampedro I., Palma F. The Synergy of galotolerant PGPB and mauran mitigates salt stress in tomato (Solanum lycopersicum) via osmoprotectants accumulation. Physiol. Plant. 2023;175:e14111. doi: 10.1111/ppl.14111. [DOI] [PubMed] [Google Scholar]
- 7.Mahreen N., Yasmin S., Asif M., Yahya M., Ejaz K., Mehboob-ur-Rahman, Yousaf S., Amin I., Zulfiqar S., Imran A., et al. Mitigation of water scarcity with sustained growth of Rice by plant growth promoting bacteria. Front. Plant Sci. 2023;14:1081537. doi: 10.3389/fpls.2023.1081537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitra D., Díaz Rodríguez A.M., Parra Cota F.I., Khoshru B., Panneerselvam P., Moradi S., Sagarika M.S., Anđelković S., de los Santos-Villalobos S., Das Mohapatra P.K. Amelioration of thermal stress in crops by plant growth-promoting rhizobacteria. Physiol. Mol. Plant Pathol. 2021;115:101679. doi: 10.1016/j.pmpp.2021.101679. [DOI] [Google Scholar]
- 9.Rahman N.S.N.A., Hamid N.W.A., Nadarajah K. Effects of Abiotic Stress on Soil Microbiome. Int. J. Mol. Sci. 2021;22:9036. doi: 10.3390/ijms22169036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caracciolo A.B., Terenzi V., Saccà L., Manici L.M. Rhizosphere Microbial Communities and Heavy Metals. Microorganisms. 2021;9:1462. doi: 10.3390/microorganisms9071462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glick B.R. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 2011;41:109–117. doi: 10.1139/m95-015. [DOI] [Google Scholar]
- 12.Ahemad M., Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014;26:1–20. doi: 10.1016/j.jksus.2013.05.001. [DOI] [Google Scholar]
- 13.Li H., Qiu Y., Yao T., Ma Y., Zhang H., Yang X. Effects of PGPR microbial inoculants on the growth and soil properties of Avena Sativa, Medicago Sativa, and Cucumis Sativus seedlings. Soil Tillage Res. 2020;199:104577. doi: 10.1016/j.still.2020.104577. [DOI] [Google Scholar]
- 14.Pathania P., Gulati D., Setia H., Bhatia R. Characterization and performance evaluation of plant growth promoting bacteria in tomato rhizosphere. S. Afr. J. Bot. 2023;161:388–394. doi: 10.1016/j.sajb.2023.08.037. [DOI] [Google Scholar]
- 15.Sandhya V., SK. Z. A., Grover M., Reddy G., Venkateswarlu B. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol. Fertil. Soils. 2009;46:17–26. doi: 10.1007/s00374-009-0401-z. [DOI] [Google Scholar]
- 16.Subramanian P., Mageswari A., Kim K., Lee Y., Sa T. Psychrotolerant Endophytic Pseudomonas sp. Strains OB155 and OS261 Induced Chilling Resistance in Tomato Plants (Solanum lycopersicum Mill.) by Activation of Their Antioxidant Capacity. Mol. Plant-Microbe Interact. 2015;28:1073–1081. doi: 10.1094/MPMI-01-15-0021-R. [DOI] [PubMed] [Google Scholar]
- 17.Chhetri G., Kim I., Kim J., So Y., Park S., Jung Y., Seo T. Paraburkholderia tagetis sp. nov., a novel species isolated from roots of Tagetes Patula enhances the growth and yield of Solanum lycopersicum L. (Tomato) Front. Microbiol. 2023;14:1140484. doi: 10.3389/fmicb.2023.1140484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woo H., Chhetri G., Kim I., So Y., Park S., Jung Y., Seo T. Pedobacter rhodius sp. nov. and Pedobacter punctiformis sp. nov., isolated from soil. Antonie van Leeuwenhoek. 2024;117:72. doi: 10.1007/s10482-024-01963-z. [DOI] [PubMed] [Google Scholar]
- 19.Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., Lesin V.M., Nikolenko S.I., Pham S., Prjibelski A.D., et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parks D.H., Imelfort M., Skennerton C.T., Hugenholtz P., Tyson G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Na S.I., Kim Y.O., Yoon S.H., Ha S.m., Baek I., Chun J. UBCG: Up-to-date bacterial core gene set and pipeline for phylogenomic tree reconstruction. J. Microbiol. 2018;56:281–285. doi: 10.1007/s12275-018-8014-6. [DOI] [PubMed] [Google Scholar]
- 22.Kim D., Park S., Chun J. Introducing EzAAI: A pipeline for high throughput calculations of prokaryotic average amino acid identity. J. Microbiol. 2021;59:476–480. doi: 10.1007/s12275-021-1154-0. [DOI] [PubMed] [Google Scholar]
- 23.Aziz R.K., Bartels D., Best A., DeJongh M., Disz T., Edwards R.A., Formsma K., Gerdes S., Glass E.M., Kubal M., et al. The RAST Server: Rapid Annotations Using Subsystems Technology. BMC Genom. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatusova T., Dicuccio M., Badretdin A., Chetvernin V., Nawrocki E.P., Zaslavsky L., Lomsadze A., Pruitt K.D., Borodovsky M., Ostell J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44:6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seemann T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 26.Couvin D., Bernheim A., Toffano-Nioche C., Touchon M., Michalik J., Eron B.N., Rocha E.P.C., Vergnaud G., Gautheret D., Pourcel C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018;46:W246–W251. doi: 10.1093/nar/gky425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vernikos G.S., Parkhill J. Interpolated variable order motifs for identification of horizontally acquired DNA: Revisiting the Salmonella pathogenicity islands. Bioinformatics. 2006;22:2196–2203. doi: 10.1093/bioinformatics/btl369. [DOI] [PubMed] [Google Scholar]
- 28.Brown C.L., Mullet J., Hindi F., Stoll J.E., Gupta S., Choi M., Keenum I., Vikesland P., Pruden A., Zhang L. MobileOG-Db: A Manually Curated Database of Protein Families Mediating the Life Cycle of Bacterial Mobile Genetic Elements. Appl. Environ. Microbiol. 2022;88:e00991-22. doi: 10.1128/aem.00991-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grant J.R., Enns E., Marinier E., Mandal A., Herman E.K., Chen C.Y., Graham M., Van Domselaar G., Stothard P. Proksee: In-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. 2023;51:W484–W492. doi: 10.1093/nar/gkad326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun J., Lu F., Luo Y., Bie L., Xu L., Wang Y. OrthoVenn3: An integrated platform for exploring and visualizing orthologous data across genomes. Nucleic Acids Res. 2023;51:W397–W403. doi: 10.1093/nar/gkad313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blin K., Shaw S., Augustijn H.E., Reitz Z.L., Biermann F., Alanjary M., Fetter A., Terlouw B.R., Metcalf W.W., Helfrich E.J.N., et al. antiSMASH 7.0: New and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 2023;51:W46–W50. doi: 10.1093/nar/gkad344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee I., Kim Y.O., Park S.C., Chun J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2016;66:1100–1103. doi: 10.1099/ijsem.0.000760. [DOI] [PubMed] [Google Scholar]
- 33.Meier-Kolthoff J.P., Carbasse J.S., Peinado-Olarte R.L., Göker M. TYGS and LPSN: A database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acids Res. 2022;50:D801–D807. doi: 10.1093/nar/gkab902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson K.H., Blitchington R.B., Greene R.C. Amplification of bacterial 16S ribosomal DNA with polymerase chain reaction. J. Clin. Microbiol. 1990;28:1942–1946. doi: 10.1128/jcm.28.9.1942-1946.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X., Wang Y., Hou Q., Liao X., Zheng X., Dong W., Wang J., Zhang X. Significant correlations between heavy metals and prokaryotes in the Okinawa Trough hydrothermal sediments. J. Hazard. Mater. 2024;479:135657. doi: 10.1016/j.jhazmat.2024.135657. [DOI] [PubMed] [Google Scholar]
- 36.Lee I., Chalita M., Ha S.M., Na S.I., Yoon S.H., Chun J. ContEst16S: An algorithm that identifies contaminated prokaryotic genomes using 16S RNA gene sequences. Int. J. Syst. Evol. Microbiol. 2017;67:2053–2057. doi: 10.1099/ijsem.0.001872. [DOI] [PubMed] [Google Scholar]
- 37.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 38.Kim O.S., Cho Y.J., Lee K., Yoon S.H., Kim M., Na H., Park S.C., Jeon Y.S., Lee J.H., Yi H., et al. Introducing EzTaxon-e: A prokaryotic 16s rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol. 2012;62:716–721. doi: 10.1099/ijs.0.038075-0. [DOI] [PubMed] [Google Scholar]
- 39.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Felsenstein J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981;17:368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- 41.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.A040454. [DOI] [PubMed] [Google Scholar]
- 42.Fitch W.M. Toward Defining the Course of Evolution: Minimum Change for a Specific Tree Topology. Syst. Biol. 1971;20:406–416. doi: 10.1093/sysbio/20.4.406. [DOI] [Google Scholar]
- 43.Felsenstein J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution. 1985;39:783. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- 44.Woo H., Chhetri G., Kim I., So Y., Park S., Jung Y., Seo T. Roseateles subflavus sp. nov. and Roseateles aquae sp. nov., isolated from artificial pond water and Roseateles violae sp. nov., isolated from a Viola mandshurica root. Int. J. Syst. Evol. Microbiol. 2024;74:006426. doi: 10.1099/ijsem.0.006426. [DOI] [PubMed] [Google Scholar]
- 45.Smibert R.M. Methods for General and Molecular Bacteriology. American Society of Microbiology; Washington, DC, USA: 1994. Phenotypic Characterization; pp. 607–654. [Google Scholar]
- 46.Buck J.D. Nonstaining (KOH) method for determination of gram reactions of marine bacteria. Appl. Environ. Microbiol. 1982;44:992–993. doi: 10.1128/aem.44.4.992-993.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuykendall L.D., Roy M.A., O’Neill J.J., Devine T.E. Fatty Acids, Antibiotic Resistance, and Deoxyribonucleic Acid Homology Groups of Bradyrhizobium japonicum. Int. J. Syst. Bacteriol. 1988;38:358–361. doi: 10.1099/00207713-38-4-358. [DOI] [Google Scholar]
- 48.Komagata K., Suzuki K.I. 4 Lipid and Cell-Wall Analysis in Bacterial Systematics. Methods Microbiol. 1988;19:161–207. doi: 10.1016/S0580-9517(08)70410-0. [DOI] [Google Scholar]
- 49.Collins M.D., Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol. Rev. 1981;45:316–354. doi: 10.1128/mr.45.2.316-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jimtha J.C., Smitha P.V., Anisha C., Deepthi T., Meekha G., Radhakrishnan E.K., Gayatri G.P., Remakanthan A. Isolation of endophytic bacteria from embryogenic suspension culture of banana and assessment of their plant growth promoting properties. Plant Cell Tissue Organ Cult. 2014;118:57–66. doi: 10.1007/s11240-014-0461-0. [DOI] [Google Scholar]
- 51.Mehta S., Nautiyal C.S. An Efficient Method for Qualitative Screening of Phosphate-Solubilizing Bacteria. Curr. Microbiol. 2001;43:51–56. doi: 10.1007/s002840010259. [DOI] [PubMed] [Google Scholar]
- 52.Fahsi N., Mahdi I., Mesfioui A., Biskri L., Allaoui A. Phosphate solubilizing rhizobacteria isolated from jujube ziziphus lotus plant stimulate wheat germination rate and seedlings growth. PeerJ. 2021;9:e11583. doi: 10.7717/peerj.11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Page M.G.P. The Role of Iron and Siderophores in Infection, and the Development of Siderophore Antibiotics. Clin. Infect. Dis. 2019;69:S529–S537. doi: 10.1093/cid/ciz825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wan W., Qin Y., Wu H., Zuo W., He H., Tan J., Wang Y., He D. Isolation and Characterization of Phosphorus Solubilizing Bacteria With Multiple Phosphorus Sources Utilizing Capability and Their Potential for Lead Immobilization in Soil. Front. Microbiol. 2020;11:510229. doi: 10.3389/fmicb.2020.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagh J., Shah S., Bhandari P., Archana G., Kumar G.N. Heterologous expression of pyrroloquinoline quinone (pqq) gene cluster confers mineral phosphate solubilization ability to Herbaspirillum seropedicae Z67. Appl. Microbiol. Biotechnol. 2014;98:5117–5129. doi: 10.1007/s00253-014-5610-1. [DOI] [PubMed] [Google Scholar]
- 56.Harper C.J., Hayward D., Kidd M., Wiid I., van Helden P. Glutamate dehydrogenase and glutamine synthetase are regulated in response to nitrogen availability in Myocbacterium smegmatis. BMC Microbiol. 2010;10:138. doi: 10.1186/1471-2180-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Durand S., Guillier M. Transcriptional and Post-transcriptional Control of the Nitrate Respiration in Bacteria. Front. Mol. Biosci. 2021;8:667758. doi: 10.3389/fmolb.2021.667758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Auch A.F., von Jan M., Klenk H.P., Göker M. Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand Genomic Sci. 2010;2:117–134. doi: 10.4056/sigs.531120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richter M., Rosselló-Móra R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA. 2009;106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Konstantinidis K.T., Tiedje J.M. Towards a genome-based taxonomy for prokaryotes. J. Bacteriol. 2005;187:6258–6264. doi: 10.1128/JB.187.18.6258-6264.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dahal R.H., Kim J. Rhodanobacter humi sp. nov., an acid-tolerant and alkalitolerant gammaproteobacterium isolated from forest soil. Int. J. Syst. Evol. Microbiol. 2017;67:1185–1190. doi: 10.1099/ijsem.0.001786. [DOI] [PubMed] [Google Scholar]
- 62.Prakash O., Green S.J., Jasrotia P., Overholt W.A., Canion A., Watson D.B., Brooks S.C., Kostka J.E. Rhodanobacter denitrificans sp. nov., isolated from nitrate-rich zones of a contaminated aquifer. Int. J. Syst. Evol. Microbiol. 2012;62:2457–2462. doi: 10.1099/ijs.0.035840-0. [DOI] [PubMed] [Google Scholar]
- 63.Lee C.S., Kim K.K., Aslam Z., Lee S.T. Rhodanobacter thiooxydans sp. nov., isolated from a biofilm on sulfur particles used in an autotrophic denitrification process. Int. J. Syst. Evol. Microbiol. 2007;57:1775–1779. doi: 10.1099/ijs.0.65086-0. [DOI] [PubMed] [Google Scholar]
- 64.Vaitiekūnaitė D., Kuusienė S., Beniušytė E. Oak (Quercus robur) Associated Endophytic Paenibacillus sp. Promotes Poplar (Populus spp.) Root Growth In Vitro. Microorganisms. 2021;9:1151. doi: 10.3390/microorganisms9061151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fahsi N., Mahdi I., Mesfioui A., Biskri L., Allaoui A. Plant Growth-Promoting Rhizobacteria Isolated from the Jujube (Ziziphus lotus) Plant Enhance Wheat Growth, Zn Uptake, and Heavy Metal Tolerance. Agriculture. 2021;11:316. doi: 10.3390/agriculture11040316. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The DDBJ/ENA/GenBank accession numbers for the Whole Genome Shotgun project, 16S rRNA gene sequence obtained using the Sanger method, and 16S rRNA gene sequence obtained from the whole genome are as follows: Si-cT: JBFOHK000000000 (Whole Genome Shotgun), PP647361 (Sanger method 16S rRNA), and PP946760 (whole genome 16S rRNA). S2-gT: JBFOHL000000000 (Whole Genome Shotgun), PP647365 (Sanger method 16S rRNA), and PP946762 (whole genome 16S rRNA).