Abstract
Background:
Intestine epithelial hypoxia-inducible factor-1α (HIF-1α) plays a critical role in maintaining gut barrier function. The aim of this study was to determine whether pharmacological or genetic activation of intestinal HIF-1α ameliorates western diet–induced metabolic dysfunction–associated steatotic liver disease.
Methods:
Metabolic effects of pharmacological activation of HIF-1α by dimethyloxalylglycine were evaluated in HIF-α luciferase reporter (ODD-luc) mice. Male and/or female intestinal epithelial–specific Hif1α overexpression mice (Hif1αLSL/LSL;VilERcre) and wild-type littermates (Hif1αLSL/LSL) were fed with regular chow diet, high fructose (HFr) or high-fat (60% Kcal) high-fructose diet (HFHFr) for 8 weeks. Metabolic phenotypes were profiled.
Results:
Dimethyloxalylglycine treatment led to increased intestine HIF-α luciferase activity and decreased blood glucose levels in HFr diet–fed male ODD-luc mice. Male Hif1αLSL/LSL;VilERcre mice exhibited markedly improved glucose tolerance compared to Hif1αLSL/LSL mice in response to HFr diet. Eight weeks HFHFr feeding led to obesity in both Hif1αLSL/LSL;VilERcre and Hif1αLSL/LSL mice. However, male Hif1αLSL/LSL;VilERcre mice exhibited markedly attenuated hepatic steatosis along with reduced liver size and liver weight compared to male Hif1αLSL/LSL mice. Moreover, HFHFr-induced systemic inflammatory responses were mitigated in male Hif1αLSL/LSL;VilERcre mice compared to male Hif1αLSL/LSL mice, and those responses were not evident in female mice. Ileum RNA-seq analysis revealed that glycolysis/gluconeogenesis was up in male Hif1αLSL/LSL;VilERcre mice, accompanied by increased epithelial cell proliferation. Moreover, an in vitro study showed that HIF stabilization enhances glycolysis in intestine organoids.
Conclusions:
Our data provide evidence that pharmacological or genetic activation of intestinal HIF-1α markedly ameliorates western diet–induced metabolic dysfunction–associated steatotic liver disease in a sex-dependent manner. The underlying mechanism is likely attributed to HIF-1α activation–induced upregulation of glycolysis, which, in turn, leads to enhanced epithelial cell proliferation and augmented gut barrier function.
Keywords: DMOG, fructose, glucose tolerance, hepatic steatosis, RNA-seq
INTRODUCTION
Metabolic dysfunction–associated steatotic liver disease (MASLD) is the most common liver disease and affects more than 30% of the population in the United States and worldwide.1 Crosstalk between the gut microbiota and liver controls gastrointestinal and liver health.2 Emerging evidence suggests that impaired gut barrier function plays a critical role in the development of MASLD.3–5 Specific nutrients, including fat and fructose, have been shown to disrupt gut barrier function and facilitate the progression of obesity and MASLD through alterations of gut microbial activities.6,7 The impaired gut barrier integrity leads to bacteria translocation and systemic inflammation,8 which play a causal role in the development of metabolic syndrome and MASLD.9
The gut barrier is composed of several layers, including the mucus layer, the epithelial cell layer, and the vascular barrier.4 Stimulation of epithelial cell proliferation by activation of signaling pathways that control tissue regeneration has been shown to rescue gut barrier function in high-fructose diet–fed mice and, in turn, inhibit hepatic de novo lipogenesis and subsequent hepatic steatosis.7,10
HIF is a master transcriptional factor in the adaption to hypoxia and plays a critical role in the maintenance of gut barrier function.11 Physiological hypoxia is an important characteristic of a healthy gut and is maintained, in part, by the crosstalk between the host and the gut microbiota. The gut microbiota–generated short-chain fatty acid, butyrate, is an important energy source of colonocytes. Butyrate is metabolized through mitochondrial β-oxidation and consumes oxygen, thereby depleting oxygen, which consequently activates HIF-1 and its target genes. This, in turn, favors normal barrier function.11 Conversely, depletion of gut microbiota or butyrate-producing bacteria leads to metabolic reprogramming from β-oxidation to anaerobic glycolysis and consumes less oxygen, which disrupts intestine physiological hypoxia and results in pathogen colonization and impaired barrier function.11,12
HIF-1 activation protects against experimental colitis with improved gut epithelial barrier function,13 yet its role in MASLD remains elusive. The aim of this study is to test whether intestine HIF-1 activation is beneficial in the improvement of MASLD induced by fructose-containing western diet. Here, we report that pharmacological activation of HIF-1 and genetic overexpression of intestine epithelial HIF-1 markedly improves western diet–induced MASLD in murine models, likely through upregulation of glycolysis, leading to intestine epithelial cell proliferation and enhanced gut barrier function.
METHODS
A detailed description of the methodology is provided in the Supplemental Methods, http://links.lww.com/HC9/B80.
Statistical analysis
Data are expressed as mean ± SD and were analyzed using unpaired t test, Mann-Whitney test, 1-way or 2-way ANOVA followed by the Tukey multiple comparison test. Differences at p ≤ 0.05 were considered to be statistically significant.
RESULTS
Pharmacological activation of hypoxia-inducible factor-1α activity abrogated dietary high-fructose–induced hyperglycemia
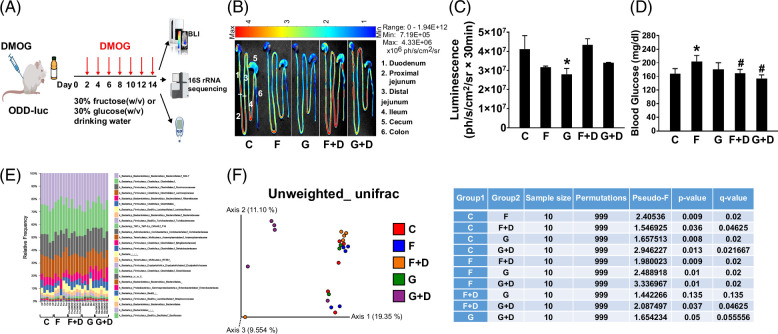
To test whether pharmacological activation of HIF-1 improves the dietary fructose–induced metabolic phenotype and gut microbiota dysbiosis, we treated male ODD-luc mice with the hydroxylase inhibitor, dimethyloxalylglycine (DMOG), through i.p. injection, a protocol that has been shown to cause HIF-1 activation in vivo.13 Meanwhile, the mice were ad libitum fed with 30% fructose or 30% glucose (w/v) in drinking water for 2 weeks (Figure 1A). DMOG-induced intestinal hypoxia-inducible factor-1α (HIF-1α) activation was validated by HIF-1α luciferase activity (Figures 1B, C). Notably, DMOG treatment abrogated high fructose (HFr)-induced hyperglycemia (Figure 1D). Moreover, DMOG treatment led to a remarkable alteration of gut microbiota composition in fructose-fed mice (p = 0.009) and a trend of alteration in glucose-fed mice (p = 0.05) as shown by β-diversity (unweighted_unifrac) and taxonomic composition (Figures 1E, F). These data suggest that systemic DMOG treatment leads to intestinal HIF-1 activation, which is associated with an improved metabolic phenotype. Therefore, the data provided evidence of therapeutic potential of DMOG in the treatment of fructose-induced metabolic disorders.
FIGURE 1.
Pharmacological activation of intestine HIF-1α improves blood glucose associated with altered gut microbiome in male ODD-luc mice. (A) Experimental design for pharmacological activation of intestine HIF-1α on the alteration of metabolic phenotypes (created with BioRender.com). (B) Representative pictures of BLI at 15 minutes after D-luciferin injection. (C) Quantification of BLI. (D) Blood glucose (nonfasted). (E) Cecum gut microbiota composition at the family level (16S rRNA sequencing results), and (F) β-diversity (unweighted-unifrac). Data represent means ± SD (n = 5), *, # p < 0.05, 1-way ANOVA. * versus C, # versus F. Abbreviations: BLI, bioluminescence imaging; C, control; D, DMOG; F, fructose; G, glucose; HIF-1α, hypoxia-inducible factor-1α.
Intestine-specific HIF-1α overexpression improves HFr-induced glucose intolerance
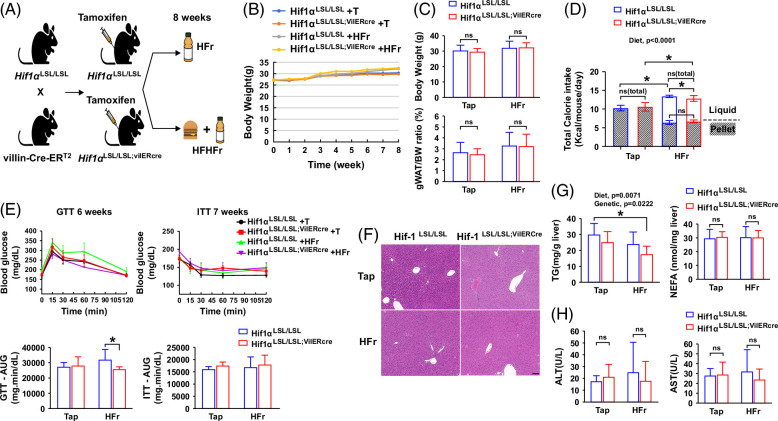
To validate the role of intestinal HIF-1 in the regulation of fructose-induced MASLD and metabolic phenotypes, male intestine–specific HIF-1α overexpression mice were chronically fed with a high-fructose diet (HFr) for 8 weeks (Figure 2A). Eight-week HFr feeding did not lead to obvious obesity in both Hif1αLSL/LSL and Hif1αLSL/LSL;VilERcre male mice (Figures 2B, C) despite increased calorie intake in Hif1αLSL/LSL;VilERcre mice (Figure 2D). To determine the effect of intestine-specific HIF-1α overexpression on glucose metabolism, we performed a glucose tolerance test and insulin tolerance test on mice exposed to the experimental diet for 5 and 7 weeks, respectively. Glucose tolerance was significantly improved in Hif1αLSL/LSL;VilERcre mice exposed to HFr compared to the control (Hif1αLSL/LSL) mice, while insulin action was not altered by intestine-specific HIF-1α overexpression as shown by glucose tolerance test and insulin tolerance test (Figure 2E). Eight-week HFr feeding did not induce obvious hepatic steatosis (Figures 2F, G) and liver injury as shown by plasma ALT and AST (Figure 2H), in both Hif1αLSL/LSL and Hif1αLSL/LSL;VilERcre mice. Nonetheless, hepatic triglyceride content was significantly lower in the Hif1αLSL/LSL;VilERcre mice exposed to HFr compared to the control mice (Figure 2G). Overall, these data suggest that intestine-specific HIF-1α overexpression is protective against HFr-induced metabolic phenotypes.
FIGURE 2.
Intestine-specific HIF-1α overexpression improves HFr diet–induced glucose intolerance in male mice. Male Hif1αLSL/LSL and Hif1αLSL/LSL;VilERcre mice were fed with HFr for 8 weeks. (A) Schematic overview of generation of intestinal epithelial–specific HIF-1α overexpression mice and diet-induced MASLD models (created with BioRender.com). (B) BW growth trajectory. (C) BW and gWAT/BW ratio. (D) Calorie intake (n = 8–9, male). (E) GTT, ITT, and calculated AUC (n = 4–5). (F) Representative photomicrographs of the H&E staining of the liver section. Scale bar, 100 μm. (G) Hepatic TG and NEFA (n = 8–9). (H) Plasma ALT and AST (n = 8–9). Data represent means ± SD. Statistical significance was set to *p < 0.05, 2-way ANOVA followed with Tukey’s multiple comparison test or Mann-Whitney test. Abbreviations: BW, body weight; GTT, glucose tolerance test; gWAT, gonadal white adipose tissue; H&E, hematoxylin and eosin; HFr, high-fructose; HIF-1α, hypoxia-inducible factor-1α; ITT, insulin tolerance test; NEFA, nonesterified fatty acid; TG, triglyceride.
Intestine-specific HIF-1α overexpression attenuates high-fat high-fructose-induced steatosis in male mice
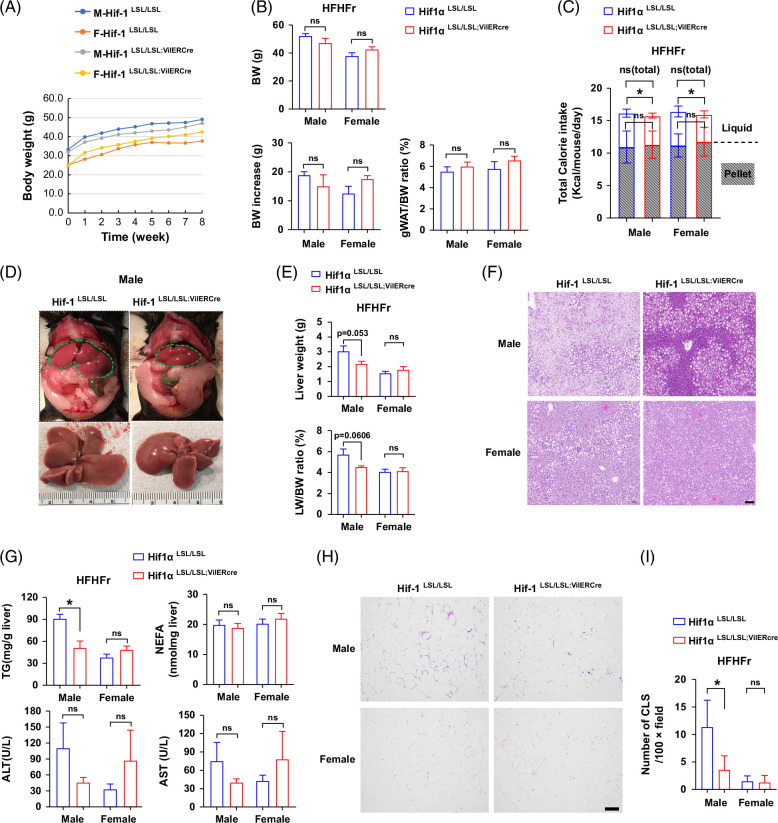
To further validate the beneficial role of intestine-specific HIF-1α overexpression on the improvement of metabolic phenotypes, we fed mice with HFr in the presence of a high-fat diet (60% Kcal fat) (HFHFr), which is known to induce obesity and MASLD.14 Eight-week HFHFr feeding led to obvious obesity in both Hif1αLSL/LSL and Hif1αLSL/LSL;VilERcre male mice (body weight [BW], 52.3 vs. 30.3 g, HFHFr-fed versus chow-fed male Hif1αLSL/LSL mice). However, intestine-specific HIF-1α overexpression did not lead to significant differences in BW, BW increase, and gonadal white adipose tissue/BW ratio in both male and female mice (Figures 3A, B). There were no significant differences in the total calorie intake between Hif1αLSL/LSL and Hif1αLSL/LSL;VilERcre mice in both males and females, despite a mild but significant increased calorie intake in terms of fructose in Hif1αLSL/LSL mice (Figure 3C). Liver size and liver weight trended lower in the male Hif1αLSL/LSL;VilERcre mice compared to the male Hif1αLSL/LSL mice. Female mice exhibited a lower liver weight and liver weight/BW ratio compared to the male mice, and no significant differences were observed between female Hif1αLSL/LSL and Hif1αLSL/LSL;VilERcre mice (Figures 3D, E). Eight-week HFHFr feeding led to severe hepatic steatosis in male Hif1αLSL/LSL mice, and that was attenuated in Hif1αLSL/LSL;VilERcre mice. Female mice developed moderate steatosis when exposed to the HFHFr diet. No significant differences were observed in hepatic steatosis between female Hif1αLSL/LSL and Hif1αLSL/LSL;VilERcre mice. Plasma ALT and AST trended to decrease in male Hif1αLSL/LSL;VilERcre mice. Female mice displayed opposite trends in terms of ALT and AST (Figures 3F, G). However, no statistical difference was reached. Of note, HFHFr feeding led to obvious adipose tissue inflammation in male Hif1αLSL/LSL mice as shown by pronounced crown-like structures and that was markedly reduced in male Hif1αLSL/LSL;VilERcre mice. No obvious adipose tissue inflammation was observed in either Hif1αLSL/LSL or Hif1αLSL/LSL;VilERcre female mice (Figures 3H, I). Collectively, these data further demonstrated the beneficial role of intestine-specific HIF-1 overexpression in protecting against western diet–induced MASLD.
FIGURE 3.
Intestine-specific HIF-1α overexpression attenuates HFHFr-induced steatosis in male mice. Male and female Hif1αLSL/LSL and Hif1αLSL/LSL;VilERcre mice were fed with HFHFr for 8 weeks. (A) BW growth trajectory. (B) BW, BW increase, and gWAT/BW ratio (n = 6–7, male; n = 5, female). (C) Calorie intake (n = 6–7, male; n = 5, female). (D) Representative gross morphology of liver in male Hif1αLSL/LSL and Hif1αLSL/LSL;VilERcre mice. The green dotted line defines the liver. (E) LW and LW/BW ratio (n = 6–7, male; n = 5, female). (F) Representative photomicrographs of H&E staining of liver section. Scale bar, 100 μm. (G) Liver TG, NEFA, plasma ALT, and AST (n = 6–7, male; n = 5, female). (H) Representative photomicrographs of the H&E staining of gWAT section. Scale bar, 100 μm. (I) Quantitation of CLSs in gWAT (5–10 field/mouse, n = 3). Data represent means ± SD. Statistical significance was set to *p < 0.05, Unpaired t test. Abbreviations: BW, body weight; CLS, crown-like structure; gWAT, gonadal white adipose tissue; H&E, hematoxylin and eosin; HFHFr, high-fat high-fructose; HIFα, hypoxia-inducible factor-1α; LW, liver weight; NEFA, nonesterified fatty acid; TG, triglyceride.
Intestine-specific HIF-1α overexpression attenuates HFHFr-induced systemic inflammatory response
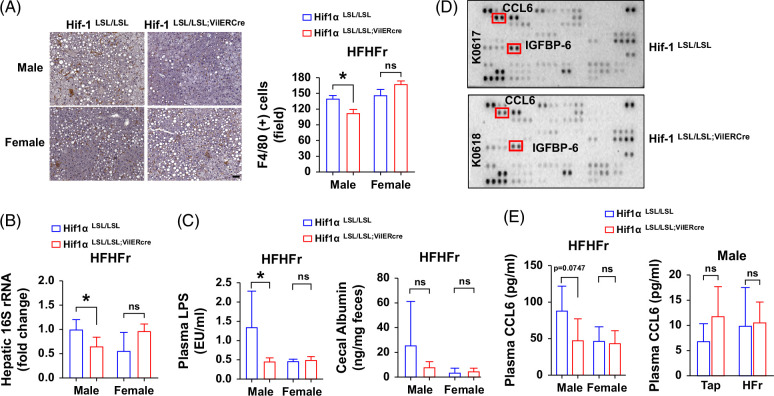
A major function of HIF-1 in the intestine is to maintain gut barrier function and integrity.11 Hence, we proposed that forced overexpression of intestine HIF-1α would enhance the gut barrier function. To this end, we sought to examine hepatic and systemic inflammatory responses and bacteria translocation. Our data show that the number of hepatic macrophages (F4/80 positive staining cell) was markedly reduced in male Hif1αLSL/LSL;VilERcre mice compared to Hif1αLSL/LSL mice when chronically fed with HFHFr. However, the difference was not obvious in female mice (Figure 4A). In line with this, real time quantitative PCR results showed that hepatic bacterial DNA copy number was significantly reduced in male Hif1αLSL/LSL;VilERcre mice compared to the male Hif1αLSL/LSL mice, suggesting reduced bacteria translocation and that effect was not evident in female mice (Figure 4B). Consistent with this, plasma lipopolysaccharide was significantly decreased in male Hif1αLSL/LSL;VilERcre mice compared to the male Hif1αLSL/LSL mice, and no differences were observed in female mice. Cecal albumin content, which is a reflection of intestinal permeability,15 was higher in male Hif1αLSL/LSL mice than in male Hif1αLSL/LSL;VilERcre mice, although the difference did not reach statistical significance. There was no obvious difference in female mice in terms of cecal albumin content (Figure 4C). Using a cytokine array, we identified that plasma chemokines, C-C motif chemokine ligand 6 (CCL6) and insulin-like growth factor binding protein 6, were markedly decreased in male Hif1αLSL/LSL;VilERcre mice compared to the male Hif1αLSL/LSL mice, and the changes of plasma CCL6 level were validated by ELISA. However, plasma CCL6 was detected at a very low level in chow-fed mice, and no significant differences were observed between Hif1αLSL/LSL and Hif1αLSL/LSL;VilERcre male mice in response to HFr feeding (Figures 4D, E). Collectively, these data suggest that intestine HIF-1α overexpression results in augmented gut barrier function, as shown by decreased hepatic and systemic inflammatory response and bacterial translocation when exposed to HFHFr.
FIGURE 4.
Intestine-specific HIF-1α overexpression attenuates HFHFr-induced systemic inflammatory response and bacteria translocation in male mice. Male and female Hif1αLSL/LSL and Hif1αLSL/LSL;VilERcre mice were fed with HFHFr for 8 weeks. (A) Representative photomicrographs (left panel) and quantitation (right panel) of IHC staining of F4/80 in the liver section (n = 6, male; n = 4–5, female). Scale bar, 50 μm. (B) Hepatic total 16S bacterial rRNAs normalized to host 18S rRNA (n = 5–6, male or female). (C) Plasma LPS and cecal albumin (n = 4–7, male or female); (D) Representative photomicrographs of cytokine array of plasma in male mice (n = 6/group, 1 dot represents the pulled results of 3 different plasma samples from the same group). (E) Plasma CCL6 level by ELISA (n = 4–6, male; n = 5, female). Left, HFHFr; Right, HFr. Data represent means ± SD. Statistical significance was set to *p < 0.05, Unpaired t test. Abbreviations: CCL6, C-C motif chemokine ligand 6; HFHFr, high-fat high-fructose; HIFα, hypoxia-inducible factor-1α; IGFBP6, insulin-like growth factor binding protein 6; IHC, immunohistochemical; LPS, lipopolysaccharide.
Intestine-specific HIF-1α overexpression leads to metabolic reprogramming toward glycolysis
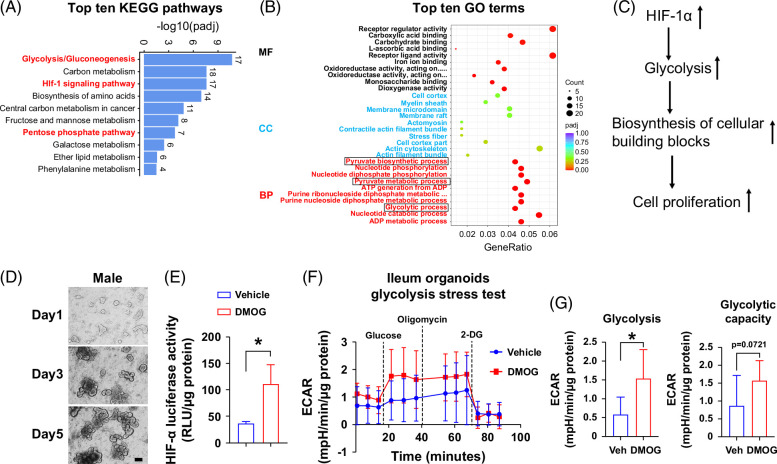
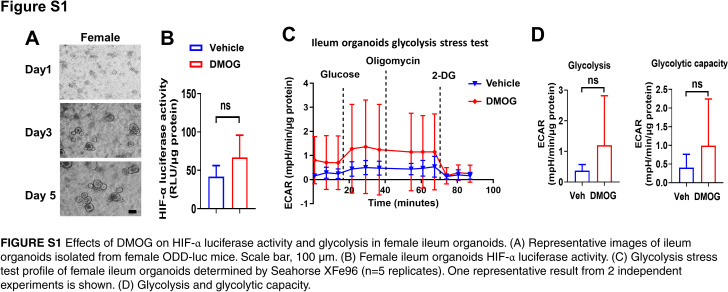
To understand the mechanisms of intestine HIF-1α overexpression on gut barrier function, we performed ileum bulk RNA-seq analysis in male Hif1αLSL/LSL and Hif1αLSL/LSL;VilERcre mice. Gene ontology and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed to identify the signaling pathways altered by intestine HIF-1α overexpression. Among the top 10 upregulated KEGG pathways by intestine HIF-1α overexpression, glycolysis was ranked number one. In addition, the pentose phosphate pathway was also upregulated. Intestine HIF-1α overexpression was validated by the upregulated HIF-1 signaling pathway in KEGG enrichment analysis (Figure 5A). In line with this, the glycolytic process and pyruvate metabolic process were also in the top gene ontology terms (Figure 5B). Given that an important biological function of glycolysis is to increase the synthesis of cellular building blocks and promote cell proliferation,16 this leads to the hypothesis that intestine HIF-1α overexpression rewires metabolic reprogramming toward glycolysis, which, in turn, promotes epithelial cell proliferation and subsequently enhances gut barrier function (Figure 5C). To test this hypothesis, we performed a Seahorse glycolysis stress test using isolated ileum organoids from male and female HIF-α reporter mice (Figure 5D). Pretreatment with DMOG led to significantly enhanced HIF-α luciferase activity and glycolysis in male ileum organoids (Figure 5E–G). However, these effects were not significant in female ileum organoids (Supplemental Figure S1, http://links.lww.com/HC9/B81), likely owing to the loss of estrogen effect in the vitro system.17
FIGURE 5.
Intestinespecific HIF-1α overexpression leads to metabolic reprogramming toward glycolysis. Ileum RNA-seq analysis revealed metabolic reprogramming toward glycolysis by intestine-specific HIF-1 overexpression in male mice. (A) Top 10 KEGG pathways. (B) Top 10 GO terms (n = 3/group, male). (C) Schematic of working hypothesis. (D) Representative images of ileum organoids isolated from male ODD-luc mice. Scale bar, 100 μm. (E) Male ileum organoids HIF-α luciferase activity treated with 1 mM DMOG for 24 hours. (F) Glycolysis stress test profile of ileum organoids determined by Seahorse XFe96 (n = 5 replicates). One representative result from 2 independent experiments is shown. (G) Glycolysis and glycolytic capacity. Statistical significance was set to *p<0.05, Unpaired t test. Abbreviations: DMOG, dimethyloxalylglycine; GO, gene ontology; HIFα, hypoxia-inducible factor-1α; KEGG, Kyoto Encyclopedia of Genes and Genomes.
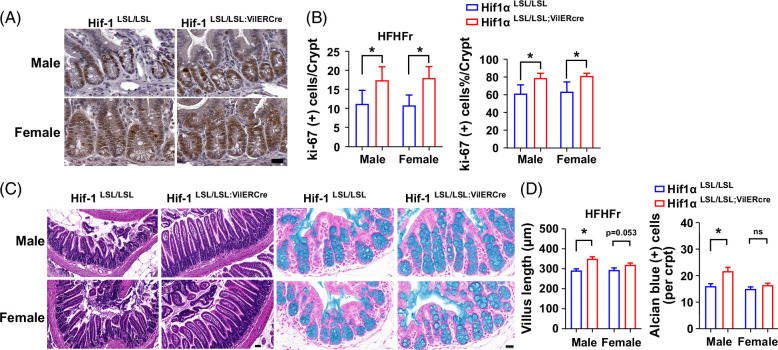
Intestine-specific HIF-1α overexpression promotes epithelial cell proliferation and improves intestinal morphology
To validate that intestine epithelial HIF-1α overexpression promotes epithelial cell proliferation, we performed Ki-67 immunohistochemical staining. The number and percentage of Ki-67–positive staining cells in the crypts of ileum were significantly increased by intestine epithelial HIF-1α overexpression in both males and females (Figures 6A, B). Consistent with this, the villus length of the ileum was markedly increased in the male Hif1αLSL/LSL;VilERcre mice accompanied with a more tidy alignment along the intestinal lumen compared to the male Hif1αLSL/LSL mice, as shown by H&E staining. Similar findings were present in female mice. Moreover, there were more abundant goblet cells in the colons of male Hif1αLSL/LSL;VilERcre mice compared to Hif1αLSL/LSL male mice, as shown by Alcian blue positive staining cells. Although the number of goblet cells was not significantly changed in female mice, the size of mucin-secreting granules was larger in Hif1αLSL/LSL;VilERcre mice than in Hif1αLSL/LSL mice (Figures 6C, D). Collectively, intestine epithelial HIF-1α overexpression led to a significantly increased epithelial cell proliferation and markedly improvement of intestinal morphology, implying enhanced gut barrier function.
FIGURE 6.
Intestinespecific HIF-1α overexpression promotes epithelial cell proliferation and improves intestinal morphology. (A) Representative photomicrographs of IHC staining of Ki-67 in the ileum section. Scale bar, 20 μm. (B) Quantitation of Ki-67–positive cell numbers and percentages in ileum crypts (30–50 crypts/10–15 field/mouse, n = 3). (C) Representative photomicrographs of H&E staining of the ileum (left panel, scale bar 50 μm) and Alcian blue staining of colon section (right panel, scale bar 20 μm) (n = 5–7, male; n = 5, female). (D) Villus length of ileum and goblet cell number quantification. Data represent means ± SD. Statistical significance was set to *p < 0.05, Unpaired t test. Abbreviations: H&E, hematoxylin and eosin; HIFα, hypoxia-inducible factor-1α; IHC, immunohistochemical
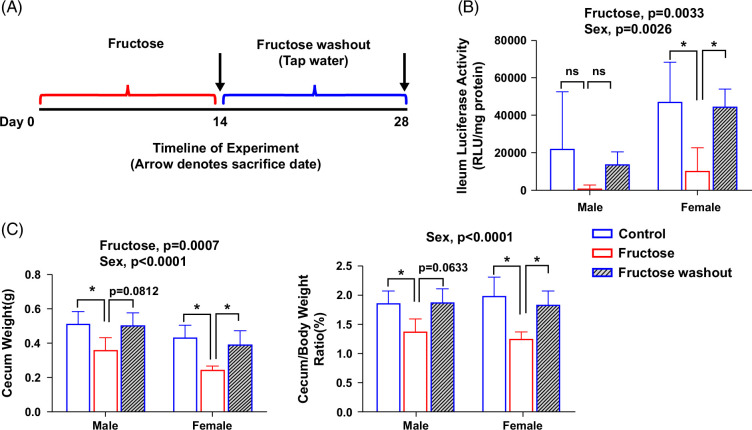
The effect of sex on intestinal HIF activity in response to dietary fructose
To examine the effect of sex on tissue-specific HIF activity, we measured the intestine HIF-α luciferase activity in both male and female ODD-luc mice in response to fructose feeding and fructose washout (Figure 7A). We found that basal HIF-α luciferase activity of ileum epithelium is higher in female mice than in male mice. Fructose feeding led to reduced HIF-α luciferase activity and that was restored with fructose washout. The changes in HIF-α luciferase activity were more pronounced in female mice (Figure 7B). Moreover, the alterations of ileum luciferase activity parallel the alterations of cecum weight and cecum/BW ratio (Figure 7C), which is an indicator of gut bacteria mass,18 suggesting a link between HIF-1 activity and gut microbiota.
FIGURE 7.
The effect of sex on intestinal HIF activity in response to dietary fructose. (A) Schematic timeline of the experiment. (B) Ileum HIF-α luciferase activity. (C) Cecum weight. (D) Cecum/body weight ratio. Data represent means ± SD (n = 5 except n = 3 in male fructose washout). *p < 0.05, 2-way ANOVA followed with the Tukey multiple comparison test. Abbreviation: HIFα, hypoxia-inducible factor-1α.
DISCUSSION
Accumulated evidence has shown that gut barrier dysfunction plays a causal role in the development of MASLD as well as in ALD through the gut-liver axis. A variety of strategies aimed at the improvement of gut barrier function have been shown to be promising in the attenuation of metabolic diseases. HIF-1 is known to regulate gut barrier function through a cascade of gene transcription that is directly involved in gut barrier protective function, including TFF3, CLDN1, ABCB1, MUC-3, LL-37, and β-defensin.19 Intestine epithelial Hif1α deficiency exacerbates alcohol-associated liver disease by worsening gut barrier function.20 Conversely, pharmacological activation of HIF-1 improves gut barrier function and protects against murine colitis.13 In the current study, we demonstrated that pharmacological activation of HIF-1 and genetic forced intestine HIF-1 overexpression protects against western diet–induced MASLD and was associated with reduced systemic inflammatory response and hepatic bacterial translocation. Our study suggests that intestine HIF-1 activation is a promising strategy for the treatment of MASLD. Of note, while both prolyl hydroxylase (PHD) and von Hipple Lindau inhibition result in HIF activation, PHD inhibitors or genetic disruption of PHDs have been shown to effectively activate HIF-1α and to improve gut barrier function in murine colitis models.13,21–23 However, disruption of von Hipple Lindau in the gut epithelium leads to stabilization of both HIF-1α and HIF-2α, with the proinflammatory effects of HIF-2α being dominant in the colitis model.23 In the current study, we demonstrated the beneficial effects of PHD inhibitors in the HFr-induced metabolic disorder model.
Under physiological hypoxia of the intestine lumen, HIF is stabilized through oxygen depletion owing to mitochondrial β-oxidation of butyrate, a metabolic process which consumes oxygen at a higher rate.11 Depletion of butyrate-producing bacteria with broad-spectrum or specific antibiotics reprograms metabolism toward glycolysis, diminishes physiologic hypoxia, and leads to reduced intestinal HIF stabilization and impaired gut barrier function that can be rescued by butyrate supplementation.11,24,25 These studies highlight the importance of metabolic regulation of intestine HIF activity under physiological conditions. However, our RNA-seq data show that forced intestine HIF-1 overexpression resulted in enhanced glycolysis in the ileum of transgenic mice. This is not surprising since it is well-known that a major role of HIF-1 is the regulation of glycolysis.26 Unlike in physiological conditions, this metabolic reprogramming led to a marked improvement in intestine morphology and reduced bacterial translocation and inflammatory responses, suggesting enhanced gut barrier function.
The biological significance of glycolysis in the regulation of proliferation is well documented.16 This leads to the hypothesis that upregulated glycolysis promotes cell proliferation, in turn enhancing gut barrier function. This is supported by increased Ki-67–positive staining cells and improved intestine morphology as a result of intestine HIF-1α overexpression (Figure 6). In line with this, stimulation of epithelial cell proliferation by targeting gp130 signaling confers protection of gut barrier function and improves fructose-induced steatosis.7,10 While most studies focus on the role of HIF-1 on the transcriptional regulation of tight junction, antimicrobial peptide, and mucosa protective genes, our study provides evidence that intestine HIF-1 overexpression–induced metabolic reprogramming toward glycolysis may lead to intestine epithelial cell proliferation and enhanced gut barrier function. Nonetheless, more detailed studies are needed to validate this concept.
While HIF-1α is ubiquitously expressed across normal tissues and cell types,27,28 its role appears to be cell type–specific and context-specific. Contrary to the role of HIF-1 in the intestine epithelial cells, hepatocyte HIF-1 deficiency protects against high-fat diet–induced MASLD.29 However, Ochiai et al30 reported that hepatocyte HIF-1 deficiency exacerbates insulin resistance and glucose intolerance in mice fed with a high-fat high-sucrose diet. Moreover, the debate also exists regarding the role of hepatocyte HIF-1 in alcohol-induced hepatic steatosis and liver injury,31,32 highlighting the complexity of HIF signaling in the context of metabolic liver diseases.
Similarly, the role of HIF-1 in the intestine is cell-type and disease-dependent. A previous study showed that endothelial-specific deletion of HIF-1α protects against radiation-induced enteritis, whereas epithelial-specific deletion of HIF-1α does not.33 Given that the primary lesion initiated by intestinal radiation damage is endothelial cell apoptosis, it is likely that HIF-1α plays a critical in the regulation of this process. In addition to epithelial and endothelial cells, the gut microenvironment is composed of a variety of cell types, including innate immune cells and adaptive immune cells. The communication between these cells is essential to maintain intestinal homeostasis and gut barrier function.34 Accumulated evidence suggests that HIF plays a central role in the maintenance of gut barrier function by integration of a variety of cell types.35 For instance, HIF-1α–dependent induction of FoxP3 enhanced anti-inflammatory effect of regulatory T cells and protected against T-cell–mediated colitis.36 Moreover, HIF-1 signaling in dendritic cells indirectly contributes to the activation of regulatory T cells.37 On the other hand, HIF-1–dependent IL-22 production in group 3 innate lymphoid cells contributes to the protection of C. rodentium–induced colitis.38 Deletion of HIF-1 in myeloid cells leads to unresolved inflammation in a DSS-induced colitis murine model.39 However, HIF-1 deficiency suppresses inflammation in murine models of arthritis and skin inflammation owing to metabolic reprogramming toward glycolysis.40 Given the cell-type–specific role of HIF-1 in gut barrier function, it will be interesting to examine the effects of gain- and loss of HIF-1α in the different cell types of intestine in the context of MASLD.
Sex difference in patients with MASLD has been noted, with women of reproductive age being protected from MASLD and having a 50% lower risk compared to men.41 In our study, the sex difference in HFHFr-induced MASLD was recapitulated. Male mice exhibited more severe hepatic steatosis compared to female mice, and that was markedly attenuated by intestine epithelial HIF-1α overexpression in male but not female mice, suggesting that the sex difference in MASLD is intestine epithelial HIF-1 dependent. A sex difference in HIF-1–mediated Treg response has been reported in the liver.42 Our data show a decreased bacterial translocation and inflammatory response in male mice, suggesting improved gut barrier function and/or altered gut microbial composition resulting from intestine epithelial HIF-1α overexpression. On the other hand, we noticed that hepatic macrophage numbers were comparable between female and male Hif1αLSL/LSL mice when fed with the HFHFr diet, despite reduced hepatic fat accumulation in female mice. However, the number of F4/80-positive macrophages was not reduced in female mice by intestine HIF-1 overexpression. These seemingly paradoxical phenotypes suggest that a functional difference in hepatic macrophages may exist between male and female mice.43 Whether or not the subpopulations of hepatic macrophages are sex-specific warrants further investigation.
In the current study, tamoxifen was injected to induce intestine epithelial–specific HIF-1α overexpression. Recent studies suggest that tamoxifen treatment results in off-target metabolic effects independent of Cre recombinase activation–induced gene expression in a sex-dependent manner.44–46 Thus, tamoxifen may be a confounding factor in the sex differential effects on the improvement of MASLD by intestine epithelial HIF-1α overexpression. Although both male and female flox control mice [Cre (−)] and Cre (+) mice received an equal dose of tamoxifen, whether tamoxifen induces off-target effects in a sex-dependent manner in the context of HFHFr and intestine HIF-1α overexpression remains elusive. Given that higher basal levels of HIF-1α protein were observed in female human pulmonary artery smooth muscle cells, which increases the risk of females developing pulmonary arterial hypertension,47 we examined the sex difference of HIF-α activity in the intestine in HIF-α reporter mice. Interestingly, we found a higher basal ileum HIF-α activity with more pronounced alterations in response to dietary fructose and washout in female mice, likely owing to the effect of estrogen.17 Given the critical role of HIF in the gut barrier function, a higher basal intestine HIF activity may explain, at least partially, why females are resistant to developing MASLD. On the other hand, it is conceivable that forced intestine epithelial HIF-1α overexpression has more prominent effects on the protection against MASLD in males than in females, likely due to the sex difference in the basal intestine HIF activity. Future studies investigating sex differences in cell-specific and tissue-specific HIF activities may help to understand sex as a biological variable in health and disease.
In summary, intestine HIF-1 activation is a promising therapeutic strategy for MASLD. However, the sex differential response to intestine HIF activation in the context of MASLD must be taken into consideration.
Supplementary Material
Acknowledgments
ACKNOWLEDGMENTS
The authors thank Ezanya Obeten-Nance from the UofL Health, Department of Pathology, for technical assistance in histology. Seahorse study was supported by the Center for Cardiometabolic Science Core P30GM127607. Sequencing was performed with assistance of the UofL Genomics Facility and Bioinformatics, which are supported by NIH/NIGMS Phase III COBRE P30GM106396 (Donald Miller), NIH/NIGMS KYINBRE P20GM103436 (Martha Bickford), the James Graham Brown Foundation.
FUNDING INFORMATION
This work was supported by the National Institutes of Health (NIH) grants R21CA290420 (Ming Song), U01AA026934, U01AA026936, P20GM113226, and P50AA024337 (Craig J. McClain); R01HL168198 (Bradford G. Hill), R01HL147844 (Bradford G. Hill), the Veterans Administration (I01BX002996) (Craig J. McClain); the American Heart Association (23TPA1141824 [Bradford G. Hill]); and the Jewish Heritage Fund for Excellence Pilot Grant Program at the University of Louisville School of Medicine (Ming Song). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DATA AVAILABILITY
The 16S rRNA raw data (Figure 1) are available in NCBI SRA (BioProject # PRJNA1177794). RNA sequencing data (Figure 2) are available in NCBI GEO (GSE280362).
CONFLICTS OF INTEREST
The authors have no conflicts to report.
Footnotes
Abbreviations: BLI, bioluminescent imaging; BW, body weight; CCL6, C-C motif chemokine ligand 6; DMOG, dimethyloxalylglycine; HIF-1α, hypoxia-inducible factor-1α; KEGG, Kyoto Encyclopedia of Genes and Genomes; MASLD, metabolic dysfunction–associated steatotic liver disease; PHD, prolyl hydroxylase; VHL, von Hipple Lindau.
Preliminary data in the abstract, entitled as “Intestine epithelial specific HIF-1α overexpression ameliorates western diet-induced NAFLD and metabolic phenotypes,” has been selected for oral presentation at The Liver Meeting 2023 (Boston, MA, USA) before the submission.
Present affiliation: Manman Xu, Department of the Liver Disease Center, Beijing Youan Hospital, Capital Medical University, Beijing 100069, China.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.hepcommjournal.com.
Contributor Information
Manman Xu, Email: xmm1903@ccmu.edu.cn.
Madison S. Taylor, Email: madison.taylor.5@louisville.edu.
Bradford G. Hill, Email: bradford.hill@louisville.edu.
Xiaohong Li, Email: xiaohong.li@louisville.edu.
Eric C. Rouchka, Email: eric.rouchka@louisville.edu.
Craig J. McClain, Email: craig.mcclain@louisville.edu.
Ming Song, Email: m0song03@louisville.edu.
REFERENCES
- 1.Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology. 2023;77:1335–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tilg H, Adolph TE, Trauner M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022;34:1700–1718. [DOI] [PubMed] [Google Scholar]
- 3.Ringseis R, Gessner DK, Eder K. The gut-liver axis in the control of energy metabolism and food intake in animals. Annu Rev Anim Biosci. 2020;8:295–319. [DOI] [PubMed] [Google Scholar]
- 4.Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558–577. [DOI] [PubMed] [Google Scholar]
- 5.Febbraio MA, Karin M. “Sweet death”: Fructose as a metabolic toxin that targets the gut-liver axis. Cell Metab. 2021;33:2316–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. [DOI] [PubMed] [Google Scholar]
- 7.Todoric J, Di Caro G, Reibe S, Henstridge DC, Green CR, Vrbanac A, et al. Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat Metab. 2020;2:1034–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut. 2016;65:2035–2044. [DOI] [PubMed] [Google Scholar]
- 9.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivera-Chávez F, Zhang LF, Faber F, Lopez CA, Byndloss MX, Olsan EE, et al. Depletion of butyrate-producing Clostridia from the gut microbiota drives an aerobic luminal expansion of Salmonella. Cell Host Microbe. 2016;19:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. [DOI] [PubMed] [Google Scholar]
- 14.Softic S, Gupta MK, Wang GX, Fujisaka S, O’Neill BT, Rao TN, et al. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J Clin Invest. 2017;127:4059–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartmann P, Chen P, Wang HJ, Wang L, McCole DF, Brandl K, et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013;58:108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, AlTahan A, Jones DT, Buffa FM, Bridges E, Interiano RB, et al. Estrogen receptor-α directly regulates the hypoxia-inducible factor 1 pathway associated with antiestrogen response in breast cancer. Proc Natl Acad Sci USA. 2015;112:15172–15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang C, Hui S, Lu W, Cowan AJ, Morscher RJ, Lee G, et al. The small intestine converts dietary fructose into glucose and organic acids. Cell Metab. 2018;27:351–361.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell EL, Colgan SP. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2019;16:106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao T, Zhao C, Li F, Gu Z, Liu L, Zhang L, et al. Intestinal HIF-1alpha deletion exacerbates alcoholic liver disease by inducing intestinal dysbiosis and barrier dysfunction. J Hepatol. 2018;69:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, et al. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. 2010;139:2093–2101. [DOI] [PubMed] [Google Scholar]
- 23.Xue X, Ramakrishnan S, Anderson E, Taylor M, Zimmermann EM, Spence JR, et al. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology. 2013;145:831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byndloss MX, Olsan EE, Rivera-Chávez F, Tiffany CR, Cevallos SA, Lokken KL, et al. Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science (New York, NY). 2017;357:570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarrinpar A, Chaix A, Xu ZZ, Chang MW, Marotz CA, Saghatelian A, et al. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat Commun. 2018;9:2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, et al. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schödel J, Ratcliffe PJ. Mechanisms of hypoxia signalling: New implications for nephrology. Nat Rev Nephrol. 2019;15:641–659. [DOI] [PubMed] [Google Scholar]
- 29.Mesarwi OA, Shin MK, Bevans-Fonti S, Schlesinger C, Shaw J, Polotsky VY. Hepatocyte hypoxia inducible factor-1 mediates the development of liver fibrosis in a mouse model of nonalcoholic fatty liver disease. PLoS One. 2016;11:e0168572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ochiai D, Goda N, Hishiki T, Kanai M, Senoo-Matsuda N, Soga T, et al. Disruption of HIF-1α in hepatocytes impairs glucose metabolism in diet-induced obesity mice. Biochem Biophys Res Commun. 2011;415:445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nath B, Levin I, Csak T, Petrasek J, Mueller C, Kodys K, et al. Hepatocyte-specific hypoxia-inducible factor-1alpha is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology. 2011;53:1526–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishiyama Y, Goda N, Kanai M, Niwa D, Osanai K, Yamamoto Y, et al. HIF-1alpha induction suppresses excessive lipid accumulation in alcoholic fatty liver in mice. J Hepatol. 2012;56:441–447. [DOI] [PubMed] [Google Scholar]
- 33.Toullec A, Buard V, Rannou E, Tarlet G, Guipaud O, Robine S, et al. HIF-1α deletion in the endothelium, but not in the epithelium, protects from radiation-induced enteritis. Cell Mol Gastroenterol Hepatol. 2018;5:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor CT, Colgan SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol. 2017;17:774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steiner CA, Cartwright IM, Taylor CT, Colgan SP. Hypoxia-inducible factor as a bridge between healthy barrier function, wound healing, and fibrosis. Am J Physiol Cell Physiol. 2022;323:C866–c878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci USA. 2012;109:E2784–E2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fluck K, Breves G, Fandrey J, Winning S. Hypoxia-inducible factor 1 in dendritic cells is crucial for the activation of protective regulatory T cells in murine colitis. Mucosal Immunol. 2016;9:379–390. [DOI] [PubMed] [Google Scholar]
- 38.Valle-Noguera A, Sancho-Temiño L, Castillo-González R, Villa-Gómez C, Gomez-Sánchez MJ, Ochoa-Ramos A, et al. IL-18-induced HIF-1α in ILC3s ameliorates the inflammation of C. rodentium-induced colitis. Cell Rep. 2023;42:113508. [DOI] [PubMed] [Google Scholar]
- 39.Lin N, Shay JES, Xie H, Lee DSM, Skuli N, Tang Q, et al. Myeloid cell hypoxia-inducible factors promote resolution of inflammation in experimental colitis. Front Immunol. 2018;9:2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, et al. Sex differences in nonalcoholic fatty liver disease: State of the art and identification of research gaps. Hepatology. 2019;70:1457–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groneberg M, Hoenow S, Marggraff C, Fehling H, Metwally NG, Hansen C, et al. HIF-1α modulates sex-specific Th17/Treg responses during hepatic amoebiasis. J Hepatol. 2022;76:160–173. [DOI] [PubMed] [Google Scholar]
- 43.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. [DOI] [PubMed] [Google Scholar]
- 44.Ceasrine AM, Ruiz-Otero N, Lin EE, Lumelsky DN, Boehm ED, Kuruvilla R. Tamoxifen improves glucose tolerance in a delivery-, sex-, and strain-dependent manner in mice. Endocrinology. 2019;160:782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dubner AM, Lu S, Jolly AJ, Noble T, Hinthorn T, Nemenoff RA, et al. Confounding effects of tamoxifen: Cautionary and practical considerations for the use of tamoxifen-inducible mouse models in atherosclerosis research—Brief report. Arterioscler Thromb Vasc Biol. 2023;43:2223–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blum KM, Roby LC, Zbinden JC, Chang YC, Mirhaidari GJM, Reinhardt JW, et al. Sex and Tamoxifen confound murine experimental studies in cardiovascular tissue engineering. Sci Rep. 2021;11:8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Docherty CK, Nilsen M, MacLean MR. Influence of 2-methoxyestradiol and sex on hypoxia-induced pulmonary hypertension and hypoxia-inducible factor-1-α. J Am Heart Assoc. 2019;8:e011628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The 16S rRNA raw data (Figure 1) are available in NCBI SRA (BioProject # PRJNA1177794). RNA sequencing data (Figure 2) are available in NCBI GEO (GSE280362).