Abstract
INTRODUCTION:
Splenic stiffness (SS) measurement (SSM) is an evolving noninvasive assessment to evaluate portal hypertension. Studies with respect to SSM in patients with alcohol use disorder are limited.
METHODS:
We studied patients seeking treatment for alcohol use disorder in an inpatient treatment protocol at the National Institutes of Health and parsed SSM into 3 groups based on degree of change.
RESULTS:
The improved SS group had statistically higher initial SSM and a nonstatistically increased liver stiffness measurement compared with others.
DISCUSSION:
SS is dynamic in a subset of patients immediately after alcohol cessation, and improved SS is associated with a normalization of platelet count.
KEYWORDS: splenic stiffness, liver stiffness, noninvasive liver disease assessment, alcohol, alcohol use disorder
INTRODUCTION
Splenic stiffness (SS) is a noninvasive liver disease assessment used to predict portal hypertension and complications (1). Studies are heterogenous and inconclusive over superiority of SS measurement (SSM) over liver stiffness measurement (LSM) (2). However, there are data that SSM by transient elastography may have a stronger correlation with hepatic venous pressure gradient than LSM in patients with portal hypertension (3,4).
Patients with alcohol use disorder (AUD) are at higher risk of developing alcohol-associated liver disease (ALD) (5). SS has been shown to be dynamic in response to treatment, with a study showing a significant decrease in SS after hepatitis C virus treatment and a nonsignificant decrease in patients with ALD in the immediate period after alcohol cessation (6). There is a gap in the literature in measuring SS in patients with AUD after acute cessation without established ALD.
METHODS
Patients seeking treatment for AUD from January 2017 to August 2022 were enrolled per protocol in a 4-week interdisciplinary inpatient treatment program at our institution. Inclusion criteria for this natural history protocol included adults aged 18 years or older, willingness to undergo blood testing, genetic testing, and radiology imaging over the study period. Laboratory values were collected on all patients at admission, weeks 2, and 4 per protocol.
Patients were evaluated by the hepatology service with serial vibration-controlled transient elastography (FibroScan, Echosens) performed for LSM and SSM at weeks 1, 2, and 4 with patients in a fasting state. Patients with a minimum of 2 SSMs at least 14 days apart were included in this analysis with the week 4 SSM as the primary comparator. Patients were categorized into 3 groups based on SS changes with a threshold of change in SSM of 15 or more kPA: (A) improved (<15 kPa), (B) static (within +/− 15 kPa range), and (C) worsened (>15 kPa).
For continuous variables, groups (A and B) and (B and C) were compared using analysis of variance with Dunnett's correction (SAS proc Generalized Linear Model). Group A (improved) was compared with the combined groups B and C by a t test with equal variances or unequal variances if the variances were different (SAS proc TTEST). The Fisher exact test was used for percentages (SAS proc FREQ).
RESULTS
One hundred twenty-four patients underwent SSMs during the study period, with 69 completing a minimum of 2 SSMs at least 14 days apart. Fifty-one patients had static SS, 9 had improved SS, and 9 had worsened SS during the study period based on an SSM threshold of 15 kPa (Table 1).
Table 1.
Characteristics of patients with improved, static, and worsened splenic stiffness
| Improved N = 9 (A) | Static N = 51 (B) | Worsened N = 9 (C) | C vs B P valuea |
A vs B P valuea |
A vs others P valueb |
|
| Demographicsc | ||||||
| Age at admission, yr | 55 (12) | 44 (12) | 45 (13) | 0.94 | 0.033* | 0.017* |
| Male sex, n (%) | 9 (100) | 32 (63) | 7 (78) | 0.049* | ||
| White race, n (%)d | 7 (77) | 33 (65) | 5 (56) | 0.48 | ||
| Black race, n (%) | 2 (22) | 11 (22) | 3 (33) | |||
| Other race, n (%) | 0 (0) | 7 (14) | 1 (11) | |||
| Initial laboratory valuesc | ||||||
| ALT | 31 (15) | 53 (47) | 61 (57) | 0.33 | 0.88 | 0.006* |
| AST | 48 (24) | 76 (88) | 77 (53) | 0.99 | 0.55 | 0.044* |
| Total bilirubin | 0.71 (0.31) | 0.73 (1.36) | 0.49 (0.19) | 0.82 | 0.99 | 0.91 |
| Alkaline phosphatase | 90 (22) | 85 (36) | 86 (38) | 0.99 | 0.92 | 0.72 |
| Platelets | 164 (54) | 235 (109) | 183 (66) | 0.29 | 0.10 | 0.011* |
| INR | 1.11 (0.15) | 1.01 (0.12) | 1.06 (0.06) | 0.45 | 0.056 | 0.040* |
| Albumin | 4.20 (0.37) | 4.49 (0.37) | 4.66 (0.46) | 0.41 | 0.081 | 0.026* |
| GGT | 103 (66) | 257 (482) | 228 (206) | 0.98 | 0.53 | 0.019* |
| Creatinine | 0.83 (0.18) | 0.73 (0.15) | 0.85 (0.39) | 0.20 | 0.34 | 0.28 |
| Glucose | 113 (21) | 100 (23) | 104 (23) | 0.83 | 0.23 | 0.14 |
| Ferritin | 455 (655) | 412 (707) | 695 (1,313) | 0.55 | 0.99 | 0.99 |
| CRP | 2.41 (1.70) | 4.31 (8.74) | 1.82 (1.84) | 0.60 | 0.74 | 0.21 |
| ESR | 11.0 (11.6) | 10.6 (10.0) | 14.0 (9.2) | 0.58 | 0.99 | 0.98 |
| Fib-4 score | 2.89 | 1.95 | 2.42 | |||
| Final laboratory valuesc | ||||||
| ALT | 33 (14) | 29 (18) | 42 (26) | 0.12 | 0.75 | 0.70 |
| AST | 31 (7) | 26 (14) | 33 (15) | 0.30 | 0.55 | 0.22 |
| Total bilirubin | 0.46 (0.21) | 0.49 (0.87) | 0.32 (0.13) | 0.79 | 0.99 | 0.93 |
| Alkaline phosphatase | 73 (21) | 71 (28) | 74 (35) | 0.93 | 0.98 | 0.90 |
| Platelets | 233 (85) | 268 (79) | 230 (82) | 0.35 | 0.40 | 0.31 |
| Albumin | 3.83 (0.36) | 4.08 (0.43) | 4.07 (0.47) | 0.99 | 0.20 | 0.11 |
| GGT | 60 (35) | 89 (118) | 110 (116) | 0.84 | 0.72 | 0.10 |
| Creatinine | 0.80 (0.18) | 0.78 (0.16) | 0.88 (0.36) | 0.31 | 0.95 | 0.94 |
| Glucose | 98 (8) | 99 (15) | 95 (6) | 0.71 | 0.99 | 0.99 |
| Ferritin | 172 (123) | 173 (185) | 234 (267) | 0.62 | 0.99 | 0.88 |
| CRP | 1.68 (1.27) | 3.95 (6.74) | 3.26 (2.25) | 0.94 | 0.50 | 0.021* |
| ESR | 11.9 (8.7) | 14.8 (11.2) | 24.1 (20.5) | 0.08 | 0.76 | 0.34 |
| Fib-4 score | 1.27 | 0.79 | 1.00 | |||
| Transient elastographyc | ||||||
| Interval first to last scan, d | 20.4 (1.3) | 19.7 (3.2) | 20.3 (0.9) | 0.78 | 0.71 | 0.28 |
| Week 1 | ||||||
| LSM, kPa | 19.1 (27.4) | 8.0 (10.2) | 9.1 (5.0) | 0.97 | 0.044 | 0.27 |
| Liver CAP | 258 (55) | 258 (58) | 276 (36) | 0.60 | 0.99 | 0.87 |
| SSM, kPa | 54.9 (16.7) | 18.7 (12.3) | 17.2 (6.5) | 0.93 | <0.001* | <0.001* |
| Week 4 | ||||||
| LSM, kPa | 16.3 (22.4) | 7.7 (10.1) | 8.1 (6.8) | 0.99 | 0.097 | 0.29 |
| Liver CAP | 227 (73) | 238 (55) | 232 (57) | 0.95 | 0.84 | 0.63 |
| SSM, kPa | 18.4 (13.4) | 18.9 (13.3) | 45.4 (15.2) | <0.001* | 0.99 | 0.44 |
ALT, alanine aminotransaminase; AST, aspartate aminotransferase; CAP, controlled attenuation parameter; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GGT, gamma-glutamyl transferase; INR, international normalized ratio; LSM, liver stiffness measurement; SSM, splenic stiffness measurement.
P value for worse vs static or better vs static adjusting for multiple comparisons using Dunnett's correction.
P value for better vs same or worse from t test with unequal variances or Fisher exact test.
Mean (SD) or n (%).
P value is for White race vs others.
P value <0.05 denotes statistical significance.
Patients with improved SS were significantly older (54.6 years) than patients who did not improve (54.6 vs. 44.3, P = 0.017). On initial laboratory evaluation, patients with improved SS had lower mean aspartate aminotransferase (48 vs. 76, P = 0.044), higher mean international normalized ratio (1.11 vs 1.01, P = 0.040), lower mean albumin (4.20 vs 4.51, P = 0.026), and lower mean platelets (164 vs 227, P = 0.011) compared with those who did not improve. Although platelets increased in patients with improved SS, with a mean change of 68.9 (SD 82) compared with those who did not improve (mean change 35 [SD 95]), this difference was not significant, P = 0.31. Final laboratory values at 4 weeks reported lower C-reactive protein (1.68 vs 3.85, P = 0.021) in patients with improved SS compared with those who did not.
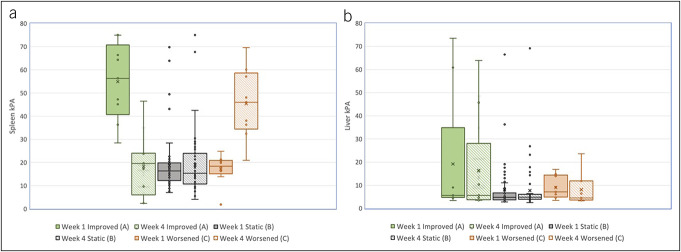
The improved SS group had statistically higher week 1 SS (54.9 kPa) compared with the static SS group (18.5 kPa, P < 0.001). As expected, patients who did not improve had higher SS kPa at week 4 (Figure 1). Improved SS patients also had increased LS at weeks 1 and 4, although this difference was not significant (P = 0.27 and 0.29, respectively).
Figure 1.
(a) SS at weeks 1 and 4 by improved, static, and worsened SS. (b) LS at weeks 1 and 4 by improved, static, and worsened SS. LS, liver stiffness; SS, splenic stiffness.
DISCUSSION
Our results suggest that in some patients, SS is dynamic in the immediate period after alcohol cessation. SS improves in a subset of patients in the period directly after alcohol cessation. Patients with improved SS had higher initial mean SS and LS, although the difference in LS was not statistically significant. Improvement in SS was associated with normalization of platelet count. The improvement in thrombocytopenia in the subgroup may be related to transient and improved portal hypertension as well as the direct bone marrow toxicity of alcohol. Patients with improved SS had significantly lower final C-reactive protein, suggesting that acute inflammation may play a role in improved SS.
Our results differ from a study of SS in patients with ALD. In this study, the mean follow-up interval between SS was 5.3 days compared with ours of 20.4 days. In this study, patients experienced a mean decrease of −4.5 kPa, with patients with ALD not demonstrating a significant decrease in SS after alcohol withdrawal (6).
Our data must be interpreted in the context of the study design. As most patients had static SS, we had a small sample size of those with dynamic SS. As such, differences in SS and LS were mostly insignificant, likely because of inadequate sample size. Another limitation of the study was intrinsic to our patient population with AUD because most patients did not have a diagnosis or manifestations of decompensated cirrhosis. Larger studies are needed to better understand the role of SS in AUD and early ALD. In conclusion, a subset of patients with AUD experience dynamic changes in SS in the period immediately after alcohol cessation.
CONFLICTS OF INTEREST
Guarantor of the article: Theo Heller, MD.
Specific author contributions: M.B.K., H.L.B., A.V., T.H.: conceptualization and design of the study and review. M.B.K., H.L.B.: manuscript preparation and editing. M.B.K., H.L.B., A.V., B.A.A., A.H.Y., N.K.: data collection. E.C.W.: statistics. D.G., Y.H., N.D.: natural history study design and patient recruitment. C.K., T.H.: data review and editing. All authors approved the final draft submitted.
Financial support: This work was supported by Intramural Research Programs of National Institute on Alcohol Abuse and Alcoholism and National Institute of Diabetes, and Digestive and Kidney Diseases.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Splenic stiffness noninvasively predicts portal hypertension in liver disease.
WHAT IS NEW HERE
✓ Splenic stiffness can fluctuate in some patients acutely after alcohol cessation.
✓ Improvement in splenic stiffness is associated with normalization of platelets.
Footnotes
Mian B. Khalid and Hanna L. Blaney contributed equally and shared the first authorship to this work.
Contributor Information
Hanna L. Blaney, Email: hannablaney@gmail.com.
Anusha Vittal, Email: anu.vit14@gmail.com.
Alexander H. Yang, Email: alex.hc.yang@gmail.com.
Bilal A. Asif, Email: bilal.a.asif@gmail.com.
Natasha Kamal, Email: kamal.natasha@gmail.com.
Elizabeth C. Wright, Email: elizabeth.wright@nih.gov.
Chris Koh, Email: christopher.koh@nih.gov.
David George, Email: david.george2@nih.gov.
David Goldman, Email: davidgoldman@mail.nih.gov.
Yvonne Horneffer, Email: horneffery@mail.nih.gov.
Nancy Diazgranados, Email: nancy.diazgranados@nih.gov.
Theo Heller, Email: theoh@intra.niddk.nih.gov.
REFERENCES
- 1.Dajti E, Marasco G, Ravaioli F, et al. The role of liver and spleen elastography in advanced chronic liver disease. Minerva Gastroenterol (Torino) 2021;67(2):151–63. [DOI] [PubMed] [Google Scholar]
- 2.Sterling RK Asrani SK Levine D, et al. AASLD Practice Guideline on non-invasive liver disease assessments of portal hypertension. Hepatology. 2004. [DOI] [PubMed] [Google Scholar]
- 3.Colecchia A, Montrone L, Scaioli E, et al. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology 2012;143(3):646–54. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan DE, Ripoll C, Thiele M, et al. AASLD Practice Guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology 2024;79(5):1180–211. [DOI] [PubMed] [Google Scholar]
- 5.Blaney HL, Khalid MB, Yang AH, et al. Hepatology consultation is associated with decreased early return to alcohol use after discharge from an inpatient alcohol use disorder treatment program. Hepatol Commun 2024;8(5):e0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elshaarawy O, Mueller J, Guha IN, et al. Spleen stiffness to liver stiffness ratio significantly differs between ALD and HCV and predicts disease-specific complications. JHEP Rep 2019;1(2):99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]