Abstract
Cu(II) and Mn(II) coordination polymers [Cu(ttpa)(sub)]n (Cuttpa or 1) and {[Mn2(ttpa)2(nip)2(H2O)2]·3H2O}n (Mnttpa or 2) (ttpa = tris(4-(1,2,4-triazol-1-yl)phenyl)amine, H2sub = suberic acid, nip = 5-nitroisophthalicate) were hydrothermally prepared and the structures were characterized. Cuttpa exhibited a 2D (4,4) network based on [Cu2(COO)4] dimers with upper and lower dangled ttpa ligands and a 2D → 3D polythreaded network. Mnttpa showed a 2D (4,4) network with dangled uncoordinated triazole rings from ttpa ligands and nitro groups from nip2− ligands and a 2D → 3D polythreaded network. Eg data of Cuttpa and Mnttpa were 1.88 eV and 2.11 eV. Cuttpa and Mnttpa exhibited good catalytic activity for the decomposition of methyl blue (MB) under visible light and supersound irradiation. The decomposition mechanism using Cuttpa was explored. The holes (h+) and •OH hydroxyl radicals played the main roles, and the •O2− superoxide radicals played certain auxiliary roles in the decomposition of MB within the Cuttpa catalyst.
Keywords: coordination polymer, polythreaded, photocatalysis, sonocatalysis, catalytic mechanism
1. Introduction
In recent years, more and more inorganic chemistry and materials scientists have become interested in coordination polymers, not only due to their greatly varying and interesting structures [1], but also due to their multifunctional materials for use in applications such as chemical sensors [2], gas storage and adsorption [3], adsorbents for the elimination of toxic chemicals and pollutants [4], catalysis [5], magnetism [6], luminescence [7] and biology [8]. Entanglement is an important topic related to coordination polymers which usually allows for improvement in their packing efficiency when one single network has large free voids [9]. In addition to interpenetration, polycatenation and polyknots, polythreaded systems represent one more intriguing subgroup of entanglement [9,10,11]. Polythreaded frameworks are usually constructed from low-dimensional networks such as 0D, 1D and 2D networks with dangled side arms which are frequency assembled by longer organic ligands [9,10,11]. Even now, polythreaded systems are still less commonly explored. Examples of 2D → 3D polythreaded networks are scarcely documented [12,13,14,15,16].
With the population increasing and social development, the increasing prevalence of organic wastewater is a serious environmental topic. In the past two decades, the effective treatment of organic wastewater has been an important topic. Compared with other methods, such as membrane technology, coagulation and flocculation, to remove dyes from dirty water, photocatalysis has its own unique advantages, such as simple manipulation procedures, low energy consumption, no secondary pollution and high degradation efficiency, and it can use photocatalysts to degrade organic dyes into non-toxic small molecules with solar energy [17]. Nevertheless, the traditional inorganic semiconductor photocatalysts such as TiO2 and ZnO with high band gaps (Eg) (3.2 eV for TiO2, 3.4 eV for ZnO) only could utilize a small amount of ultraviolet light (<4%) or visible light or sunlight from solar energy [18,19]. Sunlight contains approximately 45% visible light. Coordination polymer catalysts showing low band gaps could degrade organic dyes under visible light irradiation or sunlight with high efficiency [20,21,22,23,24,25]. This is one good example of utilizing solar energy. Meanwhile, the sonocatalytic degradation of organic dyes under ultrasound also is one good method of removing organic dyes [26].
The topologies and properties of coordination polymers are strongly dependent on central metal cations and ligands [27,28,29,30]. N-donor ligands (containing pyridine, triazole, imidazole) and O-donor carboxylate co-ligands are widely employed for constructing coordination polymers [27,28,29,30]. Tris(4-(1,2,4-triazol-1-yl)phenyl)amine (ttpa) contains three triazole rings around the triphenylamine center and can strongly coordinate to metal ions with its three four-position triazole N atoms [31,32,33]. Multicarboxylate ligands such as suberic acid (H2sub) and 5-nitroisophthalic acid (H2nip) containing two carboxylate groups can link metal ions with diverse coordination modes and can deprotonate to balance the charge of the coordination network [34,35]. Herein, copper(II) and manganese(II) coordination polymers, [Cu(ttpa)(sub)]n (Cuttpa or 1) and {[Mn2(ttpa)2(nip)2(H2O)2]·3H2O}n (Mnttpa or 2), were hydrothermally successfully prepared by employing the N-donor ligand ttpa and O-donor ligands H2sub and H2nip. Their structures were characterized. Cuttpa has a 2D (4,4) network with a Cu2(COO)4] unit and a 2D → 3D polythreaded framework. Mnttpa has a 2D (4,4)-network and a 2D → 3D polythreaded framework. The catalytic decomposition of methyl blue (MB) under visible light and supersound irradiation were observed. The decomposition mechanism using Cuttpa was studied.
2. Results and Discussion
2.1. Structures
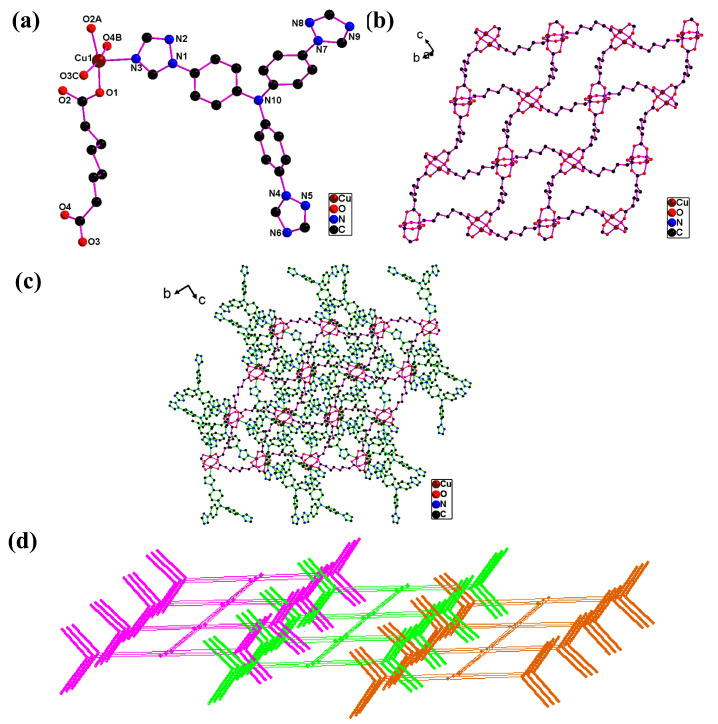
Cuttpa has a 2D (4,4)-network with a Cu2(COO)4] unit and a 2D → 3D polythreaded framework. The Cuttpa crystal was monoclinic and P21/c. The unsymmetric units were the Cu(II) (Cu(1)), the ttpa, as well as the sub2−. Cu(1) was in distorted square pyramidal coordination through four COO− O atoms (O(1), O(2A), O(3B), O(4C)) of four sub2− (Cu(1)-O(1) 1.962(3) Å; Cu(1)-O(2A) 1.984(3) Å; Cu(1)-O(3B) 1.969(2) Å; Cu(1)-O(4C) 1.965(2) Å) in the square plane and one triazole N atom (N(3)) of one ttpa (Cu(1)-N(3) 2.172(2) Å) at the axis (Figure 1a).
Figure 1.
(a) The coordination of Cu(II) in Cuttpa. (b) The 2D network [Cu(sub)]n in Cuttpa. (c) The 2D network [Cu(ttpa)(sub)]n in Cuttpa. (d) Scheme showing the 2D→3D polythreaded framework in Cuttpa.
Each oxygen atom of two carboxylates from one sub2− coordinates a copper(II) atom. A sub2− connects four copper(II) atomc, forms a [Cu2(COO)4] unit, and assembles a [Cu(sub)]n 2D (4,4) network with a [Cu2(COO)4] unit (Figure 1b). The point symbol for this 2D network is 44·62 [36].
The ttpa ligand contains three triazole rings based on the tris(phenyl)amine frame. But only a four-position triazole N (N3) of one ttpa ligand coordinates a copper(II) atom. Two other four-position triazole N atoms (N6, N9) do not coordinate. Therefore, uncoordinated triazole rings serve as dangling arms which are located above and below the [Cu(sub)]n 2D network (Figure 1c). The dangling arms thread into the windows of adjacent 2D networks and yield the 2D → 3D polythreaded framework (Figure 1d).
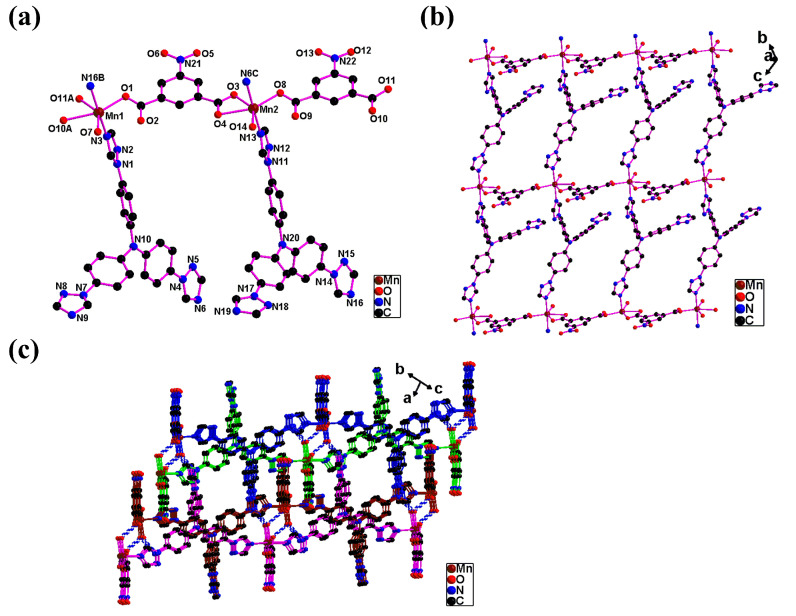
Mnttpa has a 2D (4,4)-network and a 2D → 3D polythreaded framework. Crystal Mnttpa is a triclinic crystal system and a space group. The unsymmetric unit is two Mn(II) atoms (Mn(1), Mn(2)), two ttpa ligands, two nip2− ligands, as well as two coordinated H2O atoms (O(7), O(14)). The Mn(1) is in the distorted octahedral coordination with three carboxylate O atoms (O(1), O(10A), O(11A)) of two nip2− ligands (Mn(1)-O(1) 2.1357(16) Å; Mn(1)-O(10A) 2.3936(17) Å; Mn(1)-O(11A) 2.2382(17) Å), one oxygen atom, O(7), of coordinated water (Mn(1)-O(7) 2.1469(17) Å), and two triazole N atoms (N(3), N(16B)) of two ttpa ligands (Mn(1)-N(3) 2.238(2) Å; Mn(1)-N(16B) 2.225(2) Å) (Figure 2a). The Mn(2) is in the distorted octahedral coordination with three COO− O atoms (O(3), O(4), O(8)) of two nip2− ligands (Mn(1)-O(3) 2.2362(17) Å; Mn(1)-O(4) 2.2340(17) Å; Mn(1)-O(8) 2.1537(17) Å), one oxygen atom, O(14), of coordinated water (Mn(1)-O(14) 2.1652(18) Å), and two triazole N atoms (N(6C), N(13)) of two ttpa ligands (Mn(1)-N(6C) 2.249(2) Å; Mn(1)-N(13) 2.247(2) Å) (Figure 2a).
Figure 2.
(a) The coordination Mn(II) atoms in Mnttpa. (b) The 2D network in Mnttpa. (c) The 2D→3D polythreaded framework in Mnttpa. The blue dashed lines present the hydrogen bonding interaction from adjacent 2D networks. The dashed lines show the hydrogen bonding interactions.
The carboxylate (O(1)O(2) or O(8)O(9)) of one nip2− ligand exhibits monodentate. The other carboxylate (O(3)O(4) or O(10)O(11)) of one nip2− ligand serves as a chelating coordinated mode (Figure 2a). Each nip2− ligand behaves as a bridge and links two Mn(II) atoms. The ttpa ligand contains three triazole rings based on the tris(phenyl)amine frame. But two four-position triazole N atoms (N(3)N(6) or N(13)N(16)) of one ttpa ligand serve bridging functions and link two Mn(II) atoms. One other four-position triazole N (N(9) or N(19)) was uncoordinated. Therefore, the Mn(II) atoms are linked by nip2− and ttpa bridging and assemble the 2D (4,4) network (Figure 2b). The point symbol of this 2D network is 44·62 [36].
Therefore, uncoordinated triazole rings from the ttpa ligands and nitro groups from the nip2− ligands serve as dangling arms which are located above and below the 2D network (Figure 2c). The dangling arms thread into the windows of adjacent 2D networks and yield a 2D → 3D polythreaded framework (Figure 2c). The hydrogen bond interactions between coordinated H2O molecules and carboxyl O and triazole N atoms from adjacent 2D networks (O(7)…O(2) (1 − X, 1 − Y, −Z) 2.701(3) Å; O(7)…N(19) (–X, 1 − Y, –Z) 2.788(3) Å; O(14) …O(9) (1 − X, 2 − Y, 1 − Z) 2.675(3) Å; O(14)…N(9) (–X, 1 − Y, –Z) 2.834(3) Å) have important roles in the stability of 3D polythreaded supramolecular architecture (Figure 2c). A polythreaded framework is an interesting topic within crystal engineering [9,10,11,12,13,14,15,16]. The 2D → 3D polythreaded frameworks are uncommon, and few have been documented [12,13,14,15,16]. This research gave two new examples of 2D → 3D polythreaded frameworks.
2.2. Syntheses, PXRDs, FT-IR, and Eg
Cuttpa and Mnttpa were successfully synthesized by using ttpa, H2suc, or H2nip and Cu(II) or Mn(II) with the hydrothermal method. The reaction conditions (solvent H2O/DMF, the reaction temperature, the ratio of reactants, and pH (the reactant NaOH)) were first employed according to our previous synthesis experience. The ratios of ttpa:H2suc:Cu(II) = 1:2:3 and ttpa:H2nip:Mn(II) = 1:2:3 were used due to the need to improve yield based on the expensive ttpa ligand. The reaction products from different reaction temperatures and different reaction times were observed. The different reaction temperatures and reaction times for syntheses of Cuttpa and Mnttpa were selected because of the high quality of single crystals suitable for single-crystal X-ray diffraction (91 °C and 3.1 days for the synthesis of Cuttpa; 101 °C and 2.2 days for the synthesis of Mnttpa) that were obtained for these reactions. Meanwhile, the synthesis of coordination polymers was not successful when using ttpa, H2nip, and Cu(II). No coordination polymer was obtained by using ttpa, H2suc, and Mn(II).
The powder X-ray diffraction (PXRD) patterns of Cuttpa and Mnttpa were recorded at room temperature (Figures S1 and S2). The peak positions of the simulated and experimental PXRD patterns are in agreement with each other, which confirms their phase purities.
The FT-IR spectra of Cuttpa and Mnttpa were recorded. For Cuttpa, the peaks at 1615, 1592, and 1495 cm−1 are attributed to the asymmetric and symmetric stretching vibrations of carboxylate. There are no absorptions in the 1690~1730 cm−1 region, indicating the complete deprotonation of H2suc [34]. The peaks at 1515 and 1275 cm−1 are attributed to the stretching vibration region of C=N bonds of the ttpa ligand. For Mnttpa, the broad absorption (3112 cm−1) is due to the O-H group stretching vibration from the water molecules. The peaks at 1604, 1376, and 1349 cm−1 are attributed to the asymmetric and symmetric stretching vibrations of carboxylate [34]. The peak at 1558 cm−1 is attributed to the nitro group from nip2−. The peaks at 1517 and 1274 cm−1 are attributed to the stretching vibration region of C=N bonds of the ttpa ligand.
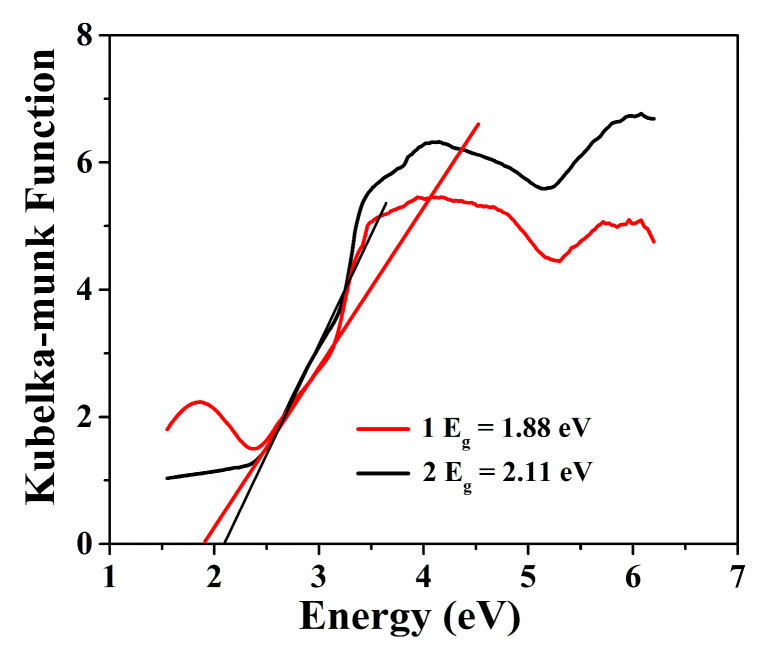
UV spectra for Cuttpa and Mnttpa showed that the Eg data of Cuttpa and Mnttpa were 1.88 and 2.11 eV, respectively (Figure 3).
Figure 3.
The UV spectra of Cuttpa (1) and Mnttpa (2).
2.3. Photocatalytic and Sonocatalytic Decomposition of MB
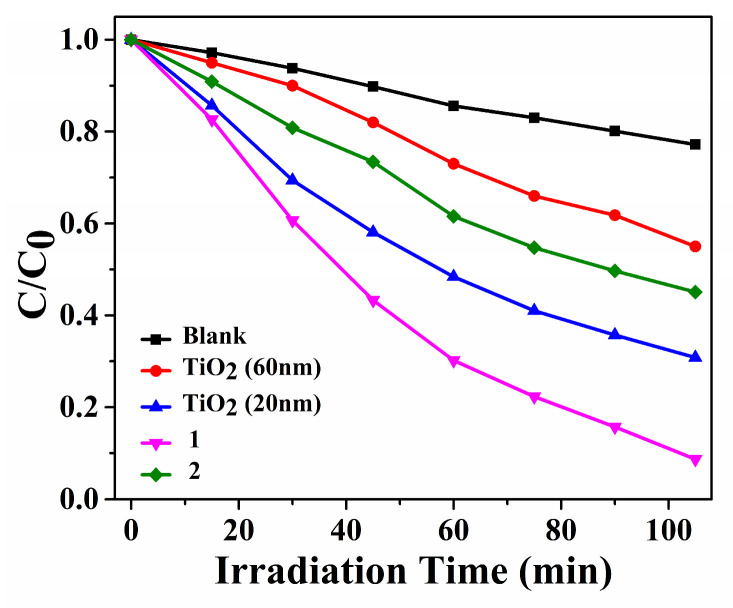
The photocatalytic and sonocatalytic decomposition of methyl blue (MB) was investigated within Cuttpa and Mnttpa catalysts under visible light and supersound irradiation. The decomposition efficiencies of trade TiO2 (60 nm) and TiO2 (20 nm) were also measured.
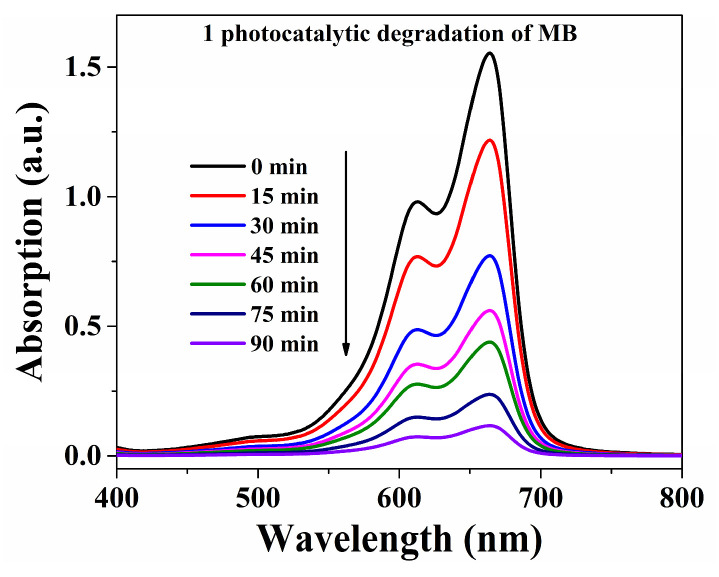
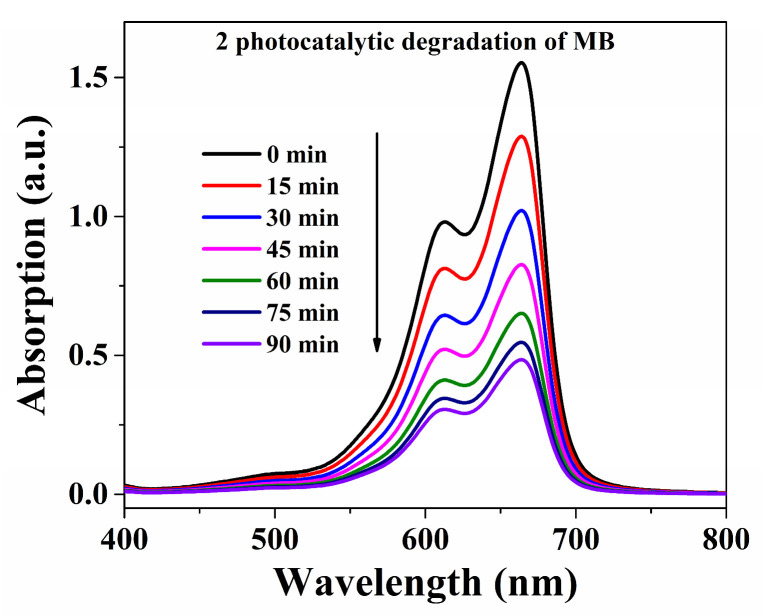
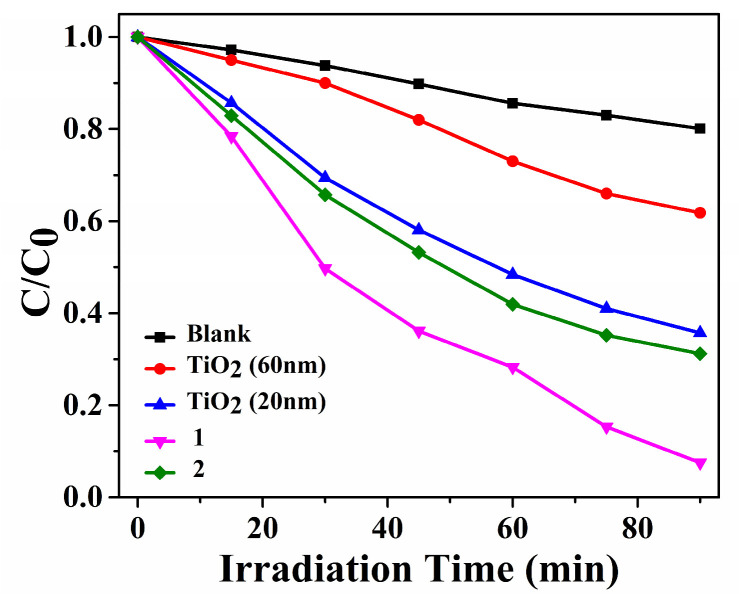
The decomposition efficiencies of MB were 92.5% within Cuttpa, 68.8% within Mnttpa, 38.2% within TiO2 (60 nm), 64.3% within TiO2 (20 nm), and 19.9% within blank H2O2 within visible light after 90 min (Figure 4, Figure 5 and Figure 6). After the photocatalytic decomposition experiments, Cuttpa and Mnttpa sustained their structures (Figures S1 and S2). The catalytic efficiency with decomposition MB was Cuttpa > Mnttpa > TiO2 (20 nm) > TiO2 (60 nm) within visible light. These results showed that Cuttpa is a good photocatalyst, and Mnttpa is an effective photocatalyst with the decomposition of MB.
Figure 4.
Absorption intensities for MB solution within photocatalytic process within Cuttpa (1) catalyst.
Figure 5.
Absorption intensities for MB solution within photocatalytic process within Mnttpa (2) catalyst.
Figure 6.
The decomposition efficiencies for MB within catalyst Cuttpa (1), Mnttpa (2), TiO2, and blank in the photocatalytic process.
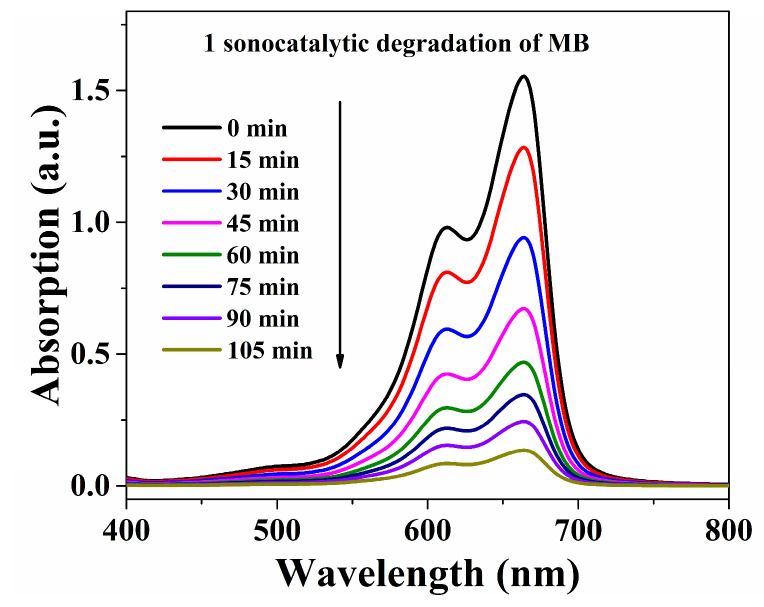
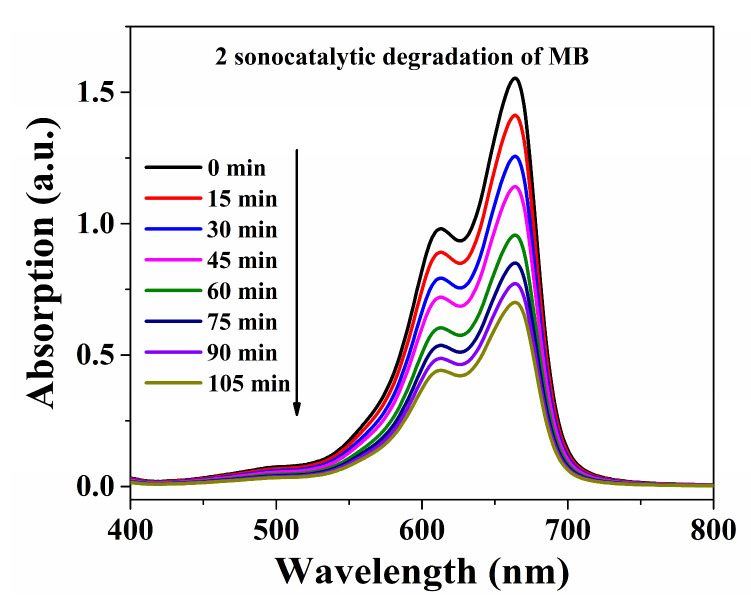
The decomposition efficiencies with MB were 91.3% within Cuttpa, 54.9% within Mnttpa, 45.0% within TiO2 (60 nm), 69.2% within TiO2 (20 nm), and 22.8% within blank H2O2 within ultrasound after 105 min (Figure 7, Figure 8 and Figure 9). After the sonocatalytic decomposition experiments, Cuttpa and Mnttpa sustained their structures (Figures S1 and S2). The catalytic efficiency with the decomposition of MB was Cuttpa > TiO2 (20 nm) > Mnttpa > TiO2 (60 nm) within ultrasound. These experimental results showed that Cuttpa is a good sonoocatalyst, and Mnttpa is an effective sonocatalyst for the decomposition of MB.
Figure 7.
Absorption intensities of MB solution within sonocatalytic decomposition process within Cuttpa (1) catalyst.
Figure 8.
Absorption intensities of MB solution within sonocatalytic decomposition process within Mnttpa (2) catalyst.
Figure 9.
The decomposition efficiencies for MB within Cuttpa (1) and Mnttpa (2) catalysts and TiO2 and blank in the sonocatalytic process.
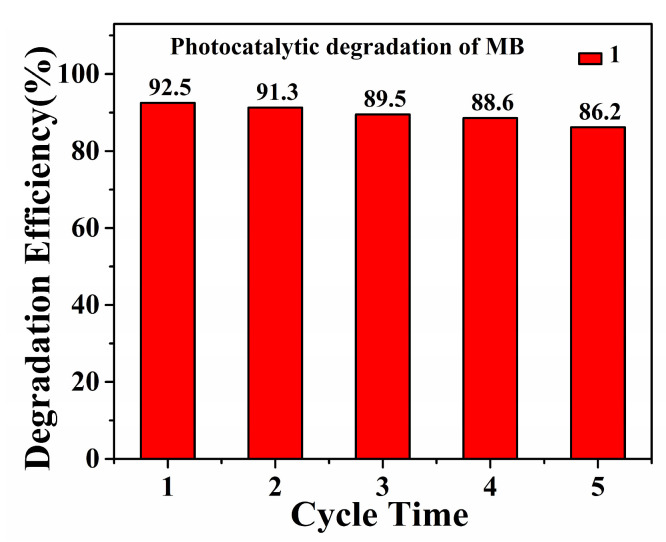
The stability of the catalyst could be observed in the recycling experiments. After each cycling experiment, the Cuttpa (1) catalyst was recovered by centrifugation, filtration, washing with deionized water and ethanol several times, and then drying under room temperature. Figure 10 is a graph of five cycles of the photocatalytic degradation of MB by Cuttpa (1) under visible light irradiation. It can be seen that the catalytic activity decreased slightly after five cycles, and the final degradation efficiency reached 86.2% after five cycles. The suspension after the last cycle of the degradation experiment was taken out, centrifuged, and filtrated to give a clear solution. The amount of copper species in the solution was measured by ICP. The concentration of the copper species in the solution was low (0.13 mg/L). The removal rate of Cu(II) ions from the Cuttpa (1) catalyst was 0.35%, indicating the stable and reusable nature of the Cuttpa (1) catalyst.
Figure 10.
Five cycles of photocatalytic degradation of MB by Cuttpa (1) under the visible light irradiation.
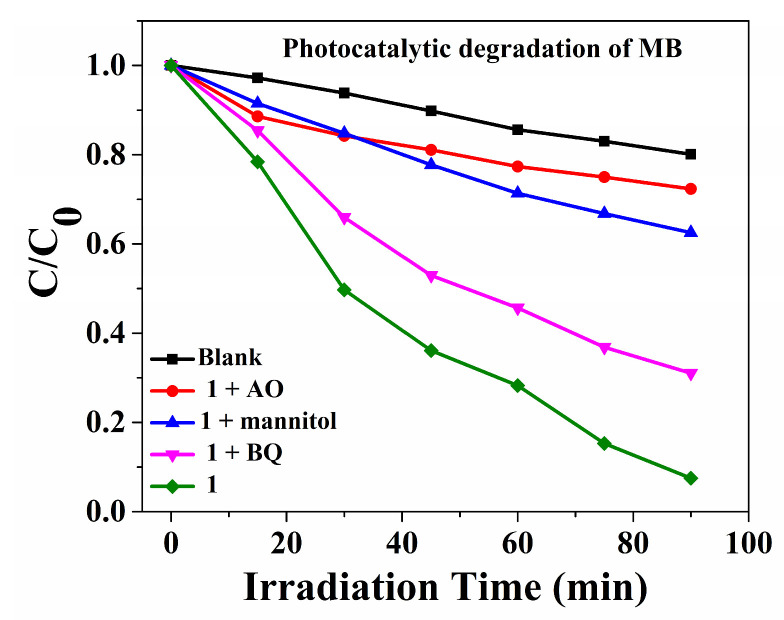
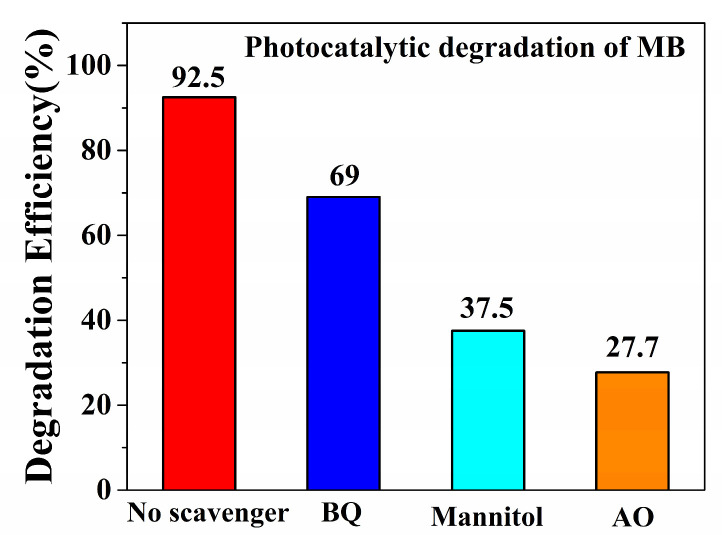
The photocatalytic and sonocatalytic mechanisms are similar [37]. The photocatalytic experiments within Cuttpa, scavenger mannitol, benzoquinone (BQ), and ammonium oxalate (AO) were carried out (Figure 11 and Figure 12). The decomposition efficiency of MB was 92.5% with Cuttpa. However, the decomposition efficiencies declined to 69.0% with BQ, 37.5% with mannitol, and 27.7% with AO. Here, the holes (h+) and •OH hydroxyl radicals played the main roles, and the •O2− superoxide radicals played certain auxiliary roles in the decomposition of MB within the Cuttpa catalyst.
Figure 11.
The decomposition efficiencies of MB with Cuttpa (1) and within the scavengers and blanks within the photocatalytic decomposition process.
Figure 12.
The decomposition efficiencies of MB with Cuttpa (1) and within the scavengers within the photocatalytic decomposition process.
The catalytic mechanism using Cuttpa and Mnttpa catalysts was supposed. When the Cuttpa or Mnttpa catalyst was exposed to visible radiation/supersound, the electrons of Cuttpa or Mnttpa could be emitted away from the valence band (VB) to the conduction band (CB), leading to the same amounts of holes (h+) in VB. The photoexcited electrons could react with H2O2 to result in the •OH hydroxyl radicals and react with oxygen and result in •O2− superoxide. The holes (h+), •OH hydroxyl, and •O2− superoxide radicals perhaps decompose MB to result in CO2, H2O, and other inorganic substances.
3. Experimental Section
3.1. Materials and Methods
All other reagents were commercial and used without further purification. The spectra were measured on a Varian 1000 FT-IR spectrometer (Varian, Inc., Palo Alto, CA, USA) in the 4000–400 cm−1 region. Powder X-ray diffractions (PXRDs) were performed using with a D2 Phaser (Bruker, Billerica, MA, USA) X-ray diffractometer with Cu-Kα radiation (λ = 1.5406 Å) was used at room temperature. C, H and N were carried out on a Perkin-Elmer 240C analyser (Perkin-Elmer, Waltham, MA, USA). The UV-vis spectra were collected using a Cary 500 spectrometer (Agilent, Santa Clara, CA, USA). The amount of copper was measured using the inductively coupled plasma (ICP) spectrometer Optima 2100 (Perkin-Elmer, Ningbo, China).
3.2. Preparation of [Cu(ttpa)(sub)]n (Cuttpa or 1)
The reactants of ttpa (0.10 mmol, 0.045 g), H2sub (0.20 mmol, 0.035 g), Cu(NO3)2·3H2O (0.30 mmol, 0.075 g), NaOH (0.037 mmol, 0.015 g), H2O (3.6 mL), and DMF (1.7 mL) were placed into one hard glass tube (8.0 mL). The mixed reactants were heated to 91 °C for 3.1 days, and then chilled to 19 °C. The blue crystals [Cu(ttpa)(sub)]n were obtained and weighed as 0.038 g with a yield of 56% (based on ttpa). Anal. Calc. for C32H30CuN10O4 (Cuttpa): C, 56.34%; H, 4.43%; N, 20.54%. Found: C, 56.23%; H, 4.37%; N, 20.45%. IR data (cm−1): 1615 m, 1592 m, 1515 m, 1419 m, 1399 w, 1326 w, 1308 w, 1275 s, 1177 w, 1052 w, 978 m, 954 w, 833 m, 709 w, 654 m, 628 w.
3.3. Preparation of {[Mn2(ttpa)2(nip)2(H2O)2]·3H2O}n (Mnttpa or 2)
The reactants of ttpa (0.10 mmol, 0.045 g), H2nip (0.20 mmol, 0.042 g), MnSO4.H2O (0.030 mmol, 0.051 g), NaOH (0.037 mmol, 0.015 g), H2O (3.6 mL), and DMF (1.7 mL) were placed into the hard glass tube (8.0 mL). The mixed reactants were heated to 101 °C for 2.2 days, and then chilled to 19 °C. The yellow crystals {[Mn2(ttpa)2(nip)2(H2O)2]·3H2O}n were obtained and weighed as 0.031 g with a yield of 41% (based on ttpa). Anal. Calc. for C64H52Mn2N22O17 (Mnttpa): C, 50.87%; H, 3.47%; N, 20.40%. Found: C, 50.72%; H, 3.41%; N, 20.29%. IR data (cm−1): 3112 m, 1604 m, 1558 m, 1517 s, 1451 w, 1434 w, 1376 m, 1349 m, 1274 m, 1145 w, 1113 m, 1081 w, 979 m, 832 w, 791 w, 733 m, 724 m, 673 m, 652 w.
3.4. X-Ray Crystallography
Crystal diffractions of [Cu(ttpa)(sub)]n (Cuttpa or 1) and {[Mn(ttpe)2(ttpa)2(nip)2(H2O)2]·3H2O}n (Mnttpa or 2) were explored with one Bruker APEX-II CCD. The structures were worked out and improved with the SHELXTL-2018 program [38]. The disordering lattice molecules of Mnttpa were omitted using PLATON (https://www.platonsoft.nl/xraysoft/, accessed on 6 November 2024). The crystallographic data are shown at Table 1. Important bond lengths and angles are presented in Table S1.
Table 1.
Crystallographic data for Cuttpa and Mnttpa.
| Cuttpa | Mnttpa | |
|---|---|---|
| Formula | C32H30CuN10O4 | C64H46Mn2N22O14 |
| Fw | 682.20 | 1457.11 |
| T/K | 188 (2) | 189 (2) |
| Crystal system | Monoclinic | Triclinic |
| Space group | P21/c | |
| a/Å | 14.7579 (10) | 11.5963 (8) |
| b/Å | 18.6867 (13) | 17.6243 (11) |
| c/Å | 11.5203 (9) | 19.6708 (12) |
| α (°) | 90 | 113.519 (2) |
| β (°) | 100.047 (2) | 94.839 (2) |
| γ (°) | 90 | 104.434 (2) |
| V /Å3 | 3128.3 (4) | 3492.6 (4) |
| F(000) | 1412 | 1492 |
| Z | 4 | 2 |
| ρcalcd (g cm−3) | 1.448 | 1.386 |
| µ(mm−1) | 0.753 | 0.440 |
| Reflections collected | 60030 | 68,021 |
| Unique reflections | 7209 [R(int) = 0.0894] | 15,953 [R(int) = 0.0951] |
| Parameter | 424 | 939 |
| Goodness of fit | 1.050 | 1.050 |
| R1 [I > 2σ(I)] | 0.0541 | 0.0591 |
| wR2 (all data) | 0.1519 | 0.1797 |
3.5. Photocatalytic and Sonocatalytic Decomposition
The photocatalytic experiment was carried out using a PCR-I multipurpose photoreactor (Beijing China Education Au-Light Company Limited, Beijing, China) equipped a CEL-HXF300 Xe lamp with a UV cut-off filter (providing visible light with λ > 400 nm). The amounts 40 mg of catalyst (Cuttpa or Mnttpa, or trade TiO2 (60 nm), or trade TiO2 (20 nm)) and 0.50 mL of 30% H2O2 were added into 100 mL of methylene blue (MB) solution (10 mg/L). The suspension solutions were stirred in dark conditions for about 30 min to ensure the complete equilibration of the adsorption/desorption of dyes on the photocatalyst surface. Then, the mixture was stirred continuously under visible light irradiation. At a given interval, aliquots of the reaction mixtures were periodically taken, purified by centrifugation, and analyzed with a UV-vis spectrophotometer at an absorption wavelength of 664 nm for MB. In order to evaluate the reusability of the catalyst Cuttpa for MB degradation, recycling experiments were carried out.
The sonocatalytic experiment was carried out in an ultrasonic bath (KQ-200VDE, Kunshan, China) which was operated at a frequency of 100 kHz and with an effective power output of 100 W. The amounts of 40 mg of catalyst (Cuttpa or Mnttpa, or trade TiO2 (60 nm), or trade TiO2 (20 nm)) and 0.50 mL of 30% H2O2 were added into 100 mL of methylene blue (MB) solution (10 mg/L). The suspension solutions were stirred in dark conditions for about 30 min to ensure the complete equilibration of the adsorption/desorption of dyes on the catalyst surface before ultrasonic irradiation was started. The entire catalytic sonication process took place at 26 ± 2 °C in the dark to prevent the influence of daily or ambient light. Then, the mixture was submitted for ultrasonic irradiation. At a given interval, aliquots of the reaction mixture were periodically taken, purified by centrifugation, and analyzed with a UV-vis spectrophotometer at an absorption wavelength of 664 nm for MB.
4. Conclusions
The copper(II) and manganese(II) coordination polymers were prepared and characterized. Cuttpa exhibited a 2D (4,4)-network with [Cu2(COO)4] and a 2D → 3D polythreaded network. Mnttpa showed a 2D (4,4)-network and a 2D → 3D polythreaded network. The catalytic decomposition of methyl blue (MB) by visible light and supersound irradiation were observed. The decomposition mechanism using Cuttpa was studied. The holes (h+) and •OH hydroxyl radicals played the main role, and the •O2− superoxide radicals played a certain auxiliary role in the decomposition of MB within the Cuttpa catalyst. Cuttpa and Mnttpa are good catalysts in the decomposition of MB within visible light and supersound.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules29225289/s1, Figure S1: PXRD patterns of the simulated and the measured of Cuttpa (1), and after photocatalytic and sonocatalytic degradation. Figure S2: PXRD patterns of the simulated and the measured of Mnttpa (2), and after photocatalytic and sonocatalytic degradation. Table S1: Selected bond lengths (Å) and angles (o) for Cuttpa and Mnttpa.
Author Contributions
C.Y.: Investigation, Data Curation, Writing—Original Draft. X.W.: Methodology. J.-G.D.: Conceptualization, Writing—Review. B.-L.L.: Supervision, Review and Editing, Funding Acquisition. B.W.: Instrument. C.-J.H.: Funding Acquisition. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
No applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The crystallographic data have been deposited in the Cambridge Crystallographic Data Center (CCDC) with CCDC numbers 2094503, 2094504 at July 2021. These data can be obtained free of charge, either from the CCDC via https://www.ccdc.cam.ac.uk/structures (accessed on 6 November 2024) or can be obtained from the corresponding authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China (22071169), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the State and Local Joint Engineering Laboratory for Functional Polymeric Materials, and the project of scientific and technological infrastructure of Suzhou (SZS201905).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lin Z.J., Lü J., Hong M., Cao R. Metal–organic frameworks based on flexible ligands (FL-MOFs): Structures and applications. Chem. Soc. Rev. 2014;43:5867–5895. doi: 10.1039/C3CS60483G. [DOI] [PubMed] [Google Scholar]
- 2.Wang D.K., Chen K.X., Wang M., You Y.J., Zhou X.H. A two-fold interpenetrated Zn-based coordination polymer for highly selective and sensitive detection of MnO4−. J. Mol. Struct. 2021;1239:130486. doi: 10.1016/j.molstruc.2021.130486. [DOI] [Google Scholar]
- 3.Hong W.H., Perera S.P., Burrows A.D. Manufacturing of metal-organic framework monoliths and their application in CO2 adsorption. Microporous Mesoporous Mater. 2015;214:149–155. doi: 10.1016/j.micromeso.2015.05.014. [DOI] [Google Scholar]
- 4.Ashouri V., Adib K., Nasrabadi M.R., Ghalkhani M. Preparation of the extruded UiO-66-based Metal-Organic Framework for the diazinon removal from the real samples. J. Mol. Struct. 2021;1240:130607. doi: 10.1016/j.molstruc.2021.130607. [DOI] [Google Scholar]
- 5.Hashemian S., Sedrpoushan A., Eshbala F.H. Co-Zeolite imidazolate frameworks (ZIF-9@Zeolite) as heterogeneous catalyst for alcohols oxidation. Catal. Lett. 2017;147:196–203. doi: 10.1007/s10562-016-1855-x. [DOI] [Google Scholar]
- 6.Wan Q.Y., Wakizaka M., Yamashita M. Single-ion magnetism behaviors in lanthanide(III) based coordination frameworks. Inorg. Chem. Front. 2023;10:5212–5224. doi: 10.1039/D3QI00925D. [DOI] [Google Scholar]
- 7.Butorlin O.S., Petrova A.S., Toikka Y.N., Kolesnikov I.E., Orlov S.N., Ryazantsev M.N., Bogachev N.A., Skripkin Y.M., Mereshchenko A.S. The Structure and Optical Properties of Luminescent Europium Terephthalate Antenna Metal–Organic Frameworks Doped by Yttrium, Gadolinium, and Lanthanum Ions. Molecules. 2024;29:3558. doi: 10.3390/molecules29153558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaur H., Mahanta G.C., Gupta V., Kukkar D., Tyagi S. Synthesis and characterization of ZIF-8 nanoparticles for controlled release of 6-mercaptopurine drug. J. Drug Del. Sci. Technol. 2017;41:106–112. doi: 10.1016/j.jddst.2017.07.004. [DOI] [Google Scholar]
- 9.Carlucci L., Ciani G., Proserpio D.M. Proserpio, polycatenation, polythreading and polyknotting in coordination network chemistry. Coord. Chem. Rev. 2003;246:247–289. doi: 10.1016/S0010-8545(03)00126-7. [DOI] [Google Scholar]
- 10.Jiang H.L., Makal T.A., Zhou H.C. Interpenetration control in metal–organic frameworks for functional applications. Coord. Chem. Rev. 2013;257:2232–2249. doi: 10.1016/j.ccr.2013.03.017. [DOI] [Google Scholar]
- 11.Cai S.L., Lu L., Shi C.C., Wang J., Sun Y.C. Effect of ligand on the assembly of two entangled coordination polymers: Structures and photocatalytic properties. Polyhedron. 2020;191:114804. doi: 10.1016/j.poly.2020.114804. [DOI] [Google Scholar]
- 12.Yang J., Yan S.W., Wang X., Xiao D.R., Zhang H.Y., Chi X.L., Zhang J.L., Wang E.B. An unusual polythreaded coordination network self-assembled from 2D motifs with two distinct lateral arms. Inorg. Chem. Commun. 2013;38:100–103. doi: 10.1016/j.inoche.2013.10.006. [DOI] [Google Scholar]
- 13.Zhang J.L., Yang J., Wang X., Zhang H.Y., Chi X.L., Yang Q., Chen Y., Xiao D.R. A series of polythreaded architectures based on a long flexible tetracarboxylate ligand and different N-donor ligands. Inorg. Chim. Acta. 2016;447:66–76. doi: 10.1016/j.ica.2016.03.037. [DOI] [Google Scholar]
- 14.Wang K.B., Wang X., Zhang D., Wang H.J., Wang Z.K., Zhao M.Y., Xi R., Wu H., Zheng M.B. Interpenetrated nano-MOFs for ultrahigh-performance supercapacitors and excellent dye adsorption performance. CrystEngComm. 2018;20:6940–6949. doi: 10.1039/C8CE01067F. [DOI] [Google Scholar]
- 15.Zhao F.H., Guo W.Y., Guo S.Y., Li S.Y., Li Z.L., Yan X.Q., Jia X.M., Huang L.W., You J.H. Two entangled photoluminescent MOFs of naphthalenedisulfonate and bis(benzimidazole) ligands for selective sensing of Fe3+ J. Solid State Chem. 2019;278:120926. doi: 10.1016/j.jssc.2019.120926. [DOI] [Google Scholar]
- 16.Liu J.D., Wang Z.K., Bi R., Mao F.F., Wang K.B., Wu H., Wang X. A polythreaded MnII-MOF and its superperformances for dye adsorption and supercapacitors. Inorg. Chem. Front. 2020;7:718–730. doi: 10.1039/C9QI01204D. [DOI] [Google Scholar]
- 17.Singh A., Singh A.K., Liu J.Q., Kumar A. Syntheses, design strategies, and photocatalytic charge dynamics of metal–organic frameworks (MOFs): A catalyzed photo-degradation approach towards organic dyes. Catal. Sci. Technol. 2021;11:3946–3989. doi: 10.1039/D0CY02275F. [DOI] [Google Scholar]
- 18.Zhang Y., Fan W., Du H.Q., Zhao Y.W. Study on photocatalytic performance of TiO2 and Fe3+/TiO2 coatings. Surf. Eng. 2017;33:849–856. doi: 10.1080/02670844.2017.1323434. [DOI] [Google Scholar]
- 19.Baradaran M., Ghodsi F.E., Bittencourt C., Llobet E. The role of Al concentration on improving the photocatalytic performance of nanostructured ZnO/ZnO:Al/ZnO multilayer thin films. J. Alloys Compd. 2019;788:289–301. doi: 10.1016/j.jallcom.2019.02.184. [DOI] [Google Scholar]
- 20.Alvaro M., Carbonell E., Ferrer B., Xamena F.X.L., Garcia H. Semiconductor behavior of a metal-organic framework (MOF) Chem. Eur. J. 2007;13:5106–5112. doi: 10.1002/chem.200601003. [DOI] [PubMed] [Google Scholar]
- 21.Lu L., Wang J., Shi C.C., Sun Y.C., Wu W.P., Pan Y., Muddassir M. Four structural diversity MOF-photocatalysts readily prepared for the degradation of the methyl violet dye under UV-visible light. New J. Chem. 2021;45:551–560. doi: 10.1039/D0NJ04478D. [DOI] [Google Scholar]
- 22.Wu Z.B., Yuan X.Z., Zhang J., Wang H., Jiang L.B., Zeng G.M. Photocatalytic decontamination of wastewater containing organic dyes by metal–organic frameworks and their derivatives. ChemCatChem. 2017;9:41–64. doi: 10.1002/cctc.201600808. [DOI] [Google Scholar]
- 23.Bala S., Bhattacharya S., Goswami A., Adhikary A., Konar S., Mondal R. Designing functional metal−organic frameworks by imparting a hexanuclear copper-based secondary building unit specific properties: Structural correlation with magnetic and photocatalytic activity. Cryst. Growth Des. 2014;14:6391–6398. doi: 10.1021/cg501226v. [DOI] [Google Scholar]
- 24.Dong J.P., Shi Z.Z., Li B., Wang L.Y. Synthesis of a novel 2D zinc(II) metal–organic framework for photocatalytic degradation of organic dyes in water. Dalton Trans. 2019;48:17626–17632. doi: 10.1039/C9DT03727F. [DOI] [PubMed] [Google Scholar]
- 25.Liu X.X., Lu L.P., Zhu M.L., Englert U. Design and synrhesis of three new copper coordination polymers: Efficient degradation of an organic dye at alkaline pH. Dalton Trans. 2021;50:13866–13876. doi: 10.1039/D1DT02463A. [DOI] [PubMed] [Google Scholar]
- 26.Mosleh S., Rahimi M.R., Ghaedi M., Dashtian K. Sonophotocatalytic degradation of trypan blue and vesuvine dyes in the presence of blue light active photocatalyst of Ag3PO4/Bi2S3-HKUST-1-MOF: Central composite optimization and synergistic effect study. Ultrason. Sonochem. 2016;32:387–397. doi: 10.1016/j.ultsonch.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Razavi S.A.A., Morsali A. Linker functionalized metal-organic frameworks. Coord. Chem. Rev. 2019;399:213023. doi: 10.1016/j.ccr.2019.213023. [DOI] [Google Scholar]
- 28.Zeng B.X., Zhang Y., Chen Y.H., Liu G.P., Li Y.Z., Chen L.J., Zhao J.W. 3-D Antimonotungstate framework based on 2,6-H2pdca-connecting iron-cerium heterometallic Krebs-type polyoxotungstates for detecting small biomolecules. Inorg. Chem. 2021;60:2663–2671. doi: 10.1021/acs.inorgchem.0c03556. [DOI] [PubMed] [Google Scholar]
- 29.Martín-García Y., Tapiador J., Orcajo G., Ayala J., Lago A.B. [BMIM][X] ionic liquids supported on a pillared-layered metal–organic framework: Synthesis, characterization, and adsorption properties. Molecules. 2024;29:3644. doi: 10.3390/molecules29153644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elenkova D., Dimitrova Y., Tsvetkov M., Morgenstern B., Milanova M., Todorovsky D., Zaharieva J. Investigation of the sensing properties of lanthanoid metal–organic frameworks (Ln-MOFs) with terephthalic acid. Molecules. 2024;29:3713. doi: 10.3390/molecules29153713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Z.Z., Pan Z.R., Jia H.L., Chen S.G., Qin L., Zheng H.G. Zn(II)/Cd(II) terephthalate coordination polymers incorporating bi-, tri-, and tetratopic phenylamine derivatives: Crystal structures and photoluminescent properties. Cryst. Growth Des. 2016;16:2747–2755. doi: 10.1021/acs.cgd.6b00056. [DOI] [Google Scholar]
- 32.Qian L.L., Blatov V.A., Wang Z.X., Ding J.G., Zhu L.M., Li K., Li B.L., Wu B. Sonochemical synthesis and characterization of four nanostructural nickel coordination polymers and photocatalytic degradation of methylene blue. Ultrason. Sonochem. 2019;56:213–228. doi: 10.1016/j.ultsonch.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Ngue C.M., Baskoro F., Wong H.Q., Yen H.J., Leung M.K. Co- and Ni-based electroactive metal−organic frameworks for stable lithium storage: Electrochemical and charge-storage behavior in response to different metal centers. Cryst. Growth Des. 2022;22:5872–5882. doi: 10.1021/acs.cgd.2c00354. [DOI] [Google Scholar]
- 34.Luo G.G., Wu D.L., Liu L., Wu S.H., Li D.X., Xiao Z.J., Dai J.C. A novel 1D T5(0)A(2) water tape incorporated in the channel of the first 3D silver-suberate framework. J. Mol. Struct. 2012;1014:92–96. doi: 10.1016/j.molstruc.2012.02.003. [DOI] [Google Scholar]
- 35.Dutta B., Ahmed F., Mir M.H. Coordination polymers: A promising candidate for photo-responsive electronic device application. Dalton Trans. 2023;52:17084–17098. doi: 10.1039/D3DT02768F. [DOI] [PubMed] [Google Scholar]
- 36.Blatov V.A., Shevchenko A.P., Proserpio D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des. 2014;14:3576–3586. [Google Scholar]
- 37.Siadatnasab F., Farhadi S., Khataee A. Sonocatalytic performance of magnetically separable CuS/CoFe2O4 nanohybrid for efficient degradation of organic deys. Ultrason. Sonochem. 2018;44:359–367. doi: 10.1016/j.ultsonch.2018.02.051. [DOI] [PubMed] [Google Scholar]
- 38.Sheldrick G.M. SHELXTL-2016, Program for the Refinement of Crystal Structures from Diffraction Data. University of Göttingen; Göttingen, Germany: 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The crystallographic data have been deposited in the Cambridge Crystallographic Data Center (CCDC) with CCDC numbers 2094503, 2094504 at July 2021. These data can be obtained free of charge, either from the CCDC via https://www.ccdc.cam.ac.uk/structures (accessed on 6 November 2024) or can be obtained from the corresponding authors upon request.