Abstract
LiFePO4 is a cathode material for lithium (Li)-ion batteries known for its excellent performance. However, compared with layered oxides and other ternary Li-ion battery materials, LiFePO4 cathode material exhibits low electronic conductivity due to its structural limitations. This limitation significantly impacts the charge/discharge rates and practical applications of LiFePO4. This paper reviews recent advancements in strategies aimed at enhancing the electronic conductivity of LiFePO4. Efficient strategies with a sound theoretical basis, such as in-situ carbon coating, the establishment of multi-dimensional conductive networks, and ion doping, are discussed. Theoretical frameworks underlying the conductivity enhancement post-modification are summarized and analyzed. Finally, future development trends and research directions in carbon coating and doping are anticipated.
Keywords: lithium iron phosphate, conductivity, carbon coating, doping
1. Introduction
Currently, the research and development of high-energy-density cathode materials is a crucial focus within battery material science, with a parallel emphasis on battery safety. Since its initial report in 1997, olivine-type LiFePO4 has emerged as a leading contender in power battery and energy storage applications due to its exceptional thermal stability and safety [1,2,3,4]. However, inherent structural limitations impede the free diffusion of electrons and Li ions within the LiFePO4 olivine framework. Notably, Li-ion movement along the c-axis is obstructed, restricting ions to a non-linear zigzag pathway along the b-axis. Because of the structural limitations, LiFePO4 exhibits low electronic conductivity and Li-ion diffusion coefficients, hampering its potential for achieving high energy density and rapid charge–discharge performance [5,6,7]. A key strategy to enhance the electrochemical performance of LiFePO4 is carbon coating, which involves enveloping LiFePO4 particles with a carbon layer and interconnecting them with a conductive carbon network. This approach significantly enhances the external conductive environment of the particles, thereby enhancing the electrochemical performance of the material. Additionally, the presence of carbon materials effectively inhibits the growth of particles, creating excellent conditions for particle nanoization. Different from surface coating, ion doping involves introducing metal or non-metal ions into various positions within the LiFePO4 structure to reduce band gap width, generate lattice defects, change semiconductor properties, broaden ion transport pathways, add ionic conductive materials, construct defects in carbon layers, etc. [8,9,10,11,12,13,14]. Thus, this review highlights recent advancements in enhancing the conductivity of LiFePO4 materials, encompassing strategies such as in situ carbon coating, the establishment of multi-dimensional conductive networks, and ion doping.
2. The Effectiveness of Modification and Ion Doping
In the stable internal spatial structure of lithium iron phosphate, electrons and lithium ions are difficult to diffuse and shuttle freely, the movement of ions in the c-axis direction is hindered, and they can only perform a non-linear sawtooth-like shuttle motion in the b-axis direction [1]. Furthermore, relevant theoretical calculations have shown that lithium iron phosphate is a semiconductor with low electronic conductivity [12]. The above shortcomings completely limit the large-scale application of lithium iron phosphate, and are also the theoretical basis for surface modification and internal doping of lithium iron phosphate. In power batteries and energy storage devices, it is also necessary to consider the kinetic characteristics and thermodynamic stability of the particle transport process [15,16,17]. Through thermodynamic and kinetic simulation analysis, materials can be purposefully designed. The strategy for modifying and doping materials requires the selection of efficient and effective implementation methods and characterization methods. Compared to traditional simple physical mixed calcination carbon coating, in situ nanoparticle growth carbon coating, the construction of a multi-dimensional conductive system, and ion doping will be emphasized in this review.
2.1. Strategy for Surface Modification
2.1.1. In Situ Carbon Coating
Carbon coating involves applying a layer of carbon material with excellent electrical conductivity onto the surface of LiFePO4 particles using various methods. This enhances the electronic conductivity between particles and stabilizes the coated cathode material during electrolyte and electrochemical reactions [18,19]. The carbon sources utilized include organic and inorganic carbon materials, carbon fibers, and carbon nanomaterials [20,21,22,23,24]. In addition to improving the electron conductivity, a uniform carbon layer on nanoparticles prevents uneven conduction due to material agglomeration.
The in-situ carbon coating method yields superior coating results by significantly enhancing particle-to-collector fluid contact, thus improving electron conductivity. The introduction of the carbon source before particle formation prevents particle growth during high-temperature sintering, controlling particle size and enhancing material electrochemical activity [25]. In situ carbon coating can be understood as two aspects: (1) the in-situ growth of LiFePO4 particles on the surface of carbon materials (such as graphene and carbon nanotubes) [26,27]; (2) the in-situ growth of carbon-containing materials on the surface of LiFePO4 [21]. Regardless of the selection of raw materials, the purpose of in situ coating is to achieve chemical bonding between carbon and LiFePO4, achieving good conductivity.
Graphene is a typical graphite carbon structure material which has a regular layered carbon structure and can construct an excellent 2D conductive carbon network and elastic structure [28]. The distinctive two-dimensional configuration, irregular surface topography, impurities from different atoms, enhanced contact between the electrode and the electrolyte, augmented spacing between layers, and heightened electrical conductivity all contribute to swift surface lithium-ion absorption and extremely rapid lithium-ion diffusion along with electron transfer [29,30].
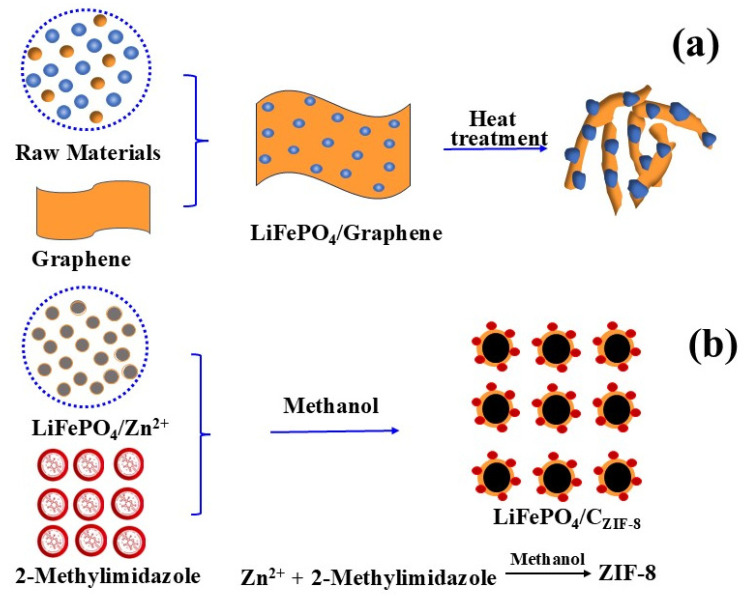
Yang [26] studied an in-situ growth method, growing LiFePO4 nanoparticles on monolayer graphene with excellent dispersion (Figure 1a). Monolayer graphene provides a high-quality three-dimensional (3D) conductive network, enabling each LiFePO4 particle to attach to the conductive layer (Figure 1a). This method substantially improves material electrical conductivity, leading to enhanced electrochemical properties. The initial discharge capacity reached 166.2 mAh g−1 (98% of theoretical value).
Figure 1.
(a) Schematic image of LiFePO4 growth on unfolded graphene [26]. Adapted from [26]. (b) Schematic image of CZIF-8 growth on the surface of LiFePO4 [31]. Adapted from [31].
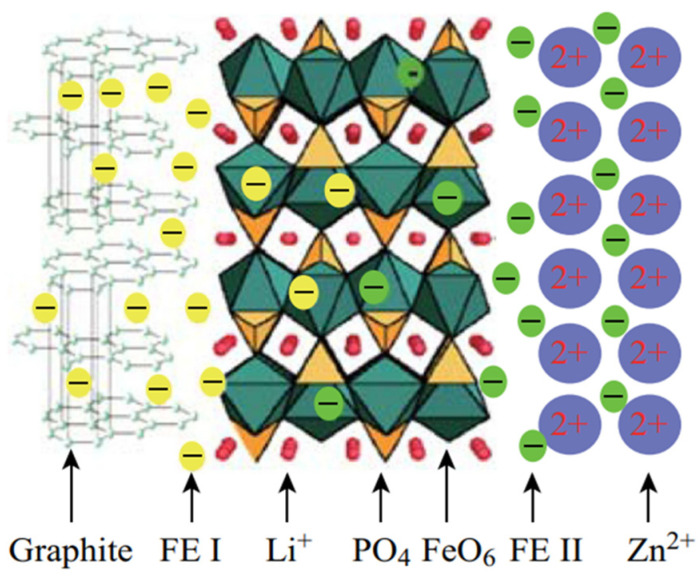
Xu [31] studied the in-situ coating of zeolite-imidazole ZIF-8 on commercial LiFePO4 material with a thickness of ~10 nm. The study analyzed coating structure and metal zinc (Zn) distribution on the LiFePO4 (LFP) surface (Figure 1b). Nucleation and crystal growth of ZIF-8 nanoparticles on LiFePO4 surfaces were followed by new graphite-like carbon appearance and generation post-calcination. The results indicated that the LFP/CZIF-8 material exhibited a heterogeneous electrical conductivity mechanism, and the graphitic carbon in the material exhibited exceptional electrical conductivity because of the ordered sp2 carbon and free electrons (yellow sphere, FE I in Figure 2). An optimal carbon coating material should maximize free electrons, facilitating inter-regional electron flow to enhance electrochemical material properties.
Figure 2.
Mechanism of conductivity improvement. FE I represents free electron in graphite; FE II is free electron in metal zinc [31]. Reprinted from [31].
The carbon coating process represents material surface modification. The carbon layer serves as an interface between the cathode material and electrolyte, facilitating electron and Li-ion transfer crucial to material performance. The binding force between C and LiFePO4 and the mechanism of enhanced conductivity have become a focus of attention after carbon coating.
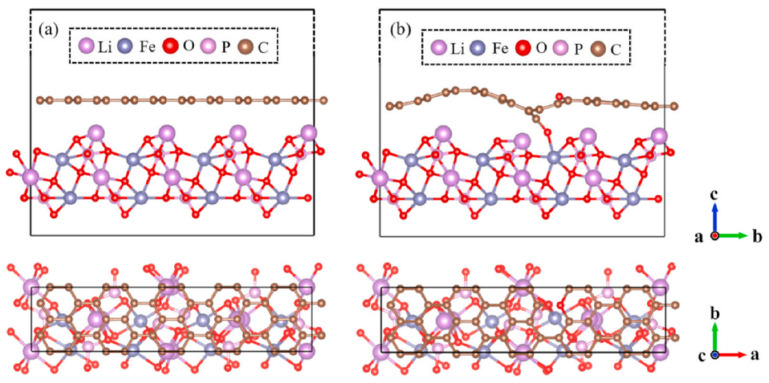
Recent studies have elucidated how defective graphene oxide (GO) coating enhanced LiFePO4 conductivity through theoretical calculations. Chen [32] investigated the electronic structure of GO parallel to the LiFePO4 surface using first-principles density functional theory calculations within the DFT+U framework. The results indicated that the emergence of bands in gap states originated from graphene coating. Furthermore, GO was attached to LiFePO4 (010) through C-O and Fe-O bonds, instead of the attraction of van der Waals forces. The chemical bonds (Fe-O-C) are shown in Figure 3. Thus, the LFP/GO interface facilitated the electronic conductivity of the interface.
Figure 3.
The relaxed atomic structures of (a) LFP/G and (b) LFP/GO [32]. Reprinted from [32].
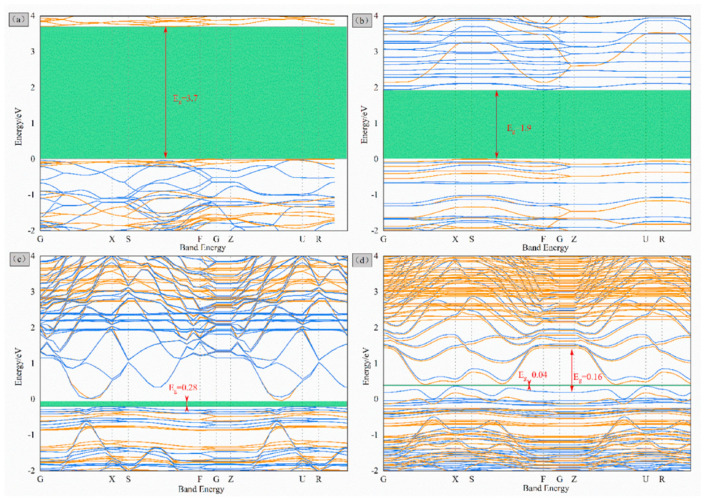
The electronic energy band calculations (Figure 4) indicate increased density in valence and conduction bands owing to GO interaction with LiFePO4 (010), indicating Fe-O-C bond existence. This review elucidates the principle and advantages of in situ carbon coating, enhancing the surface conductivity of LiFePO4 materials, and offering theoretical support for carbon coating modification of other similar materials.
Figure 4.
Band structure at the Fermi level for (a) LFP bulk, (b) LFP (010), (c) G on LFP (010), and (d) GO on LFP (010) [32]. Reprinted from [32].
Graphite carbon has excellent electrical conductivity, which is why researchers choose this type of material for carbon coating. In addition to graphene, other carbon materials can also be processed to achieve graphite carbon, and their conductivity can be optimized through improvements in the manufacturing process. Apart from graphene, other carbon materials can also be processed to obtain graphite carbon, and their electrical conductivity can be maximized through advancements in the manufacturing process. Raman analysis can be used to detect the degree of graphitization of the carbon layer coated on the surface of materials. The use of this technique makes the design of materials more superior [33,34].
In summary, the in-situ carbon coating strategy is an excellent method for enhancing the surface and conductivity of materials. However, before implementing carbon coating, a detailed analysis and explanation of the conductive mechanism should be conducted to enhance coating material design and structural optimization. In situ carbon coating holds promise for modifying surface conductivity in other insulators or semiconductor materials, achieving dual electronic conduction and material application effects.
2.1.2. Surface Carbon Layer Doping and LiFePO4 Modification
In research on some non-in situ carbon coating processes, researchers have devoted considerable effort to doping the carbon layer with non-metal atoms. The main non-metal elements used and their primary functions in carbon layer doping are listed in Table 1 below.
Table 1.
Elements used to dope the carbon layer and their primary functions.
| Materials | Doped Atoms |
Functions | Discharge Capacity (High Rate) |
Conductivity/ ×10−2 S cm−1 |
Ref. |
|---|---|---|---|---|---|
| Egg white (1 mL) | N | offer superior electronic transportation between LiFePO4 active particles | 120 mAh g−1 (LFP/C+N at 5 C) 113 mAh g−1 (LFP/C at 5 C) |
[35,36] | |
| Polyvinylidene fluoride (5% wt) | F | play a vital role in the improvement of electron transfer kinetics | 121.5 mAh g−1 (LFP@FC-II at 10 C) approaching 120.0 mAh g−1 (LFP at 0.1 C) |
[37] | |
| Sulfur-doped graphene sheet | S | promote the transportation of electrons and Li-ions; prevent volume change during the Li+ intercalation/deintercalation procedure | 130.5 mAh g−1 (LiFePO4@C/S-doped graphene at 10 C) 116.5 mAh g−1 LiFePO4@C |
[38,39] | |
| Oxalic acid and benzyl disulfide | S | promote the electronic conductivity and defect level of the carbon | 137 mAh g−1 (LiFePO4/SC at 5 C) 128.5 mAh g−1 (LiFePO4/C at 5 C) |
[40] | |
| Triphenylphosphine (0.2 g mL−1 was mixed with 4 g LiFePO4/C) | P | benefit the graphitization of the carbon; decrease transfer resistance | 124.0 mAh g−1 (LFP/C-P3 at 20 C) 105.4 mAh g−1 (LFP/C at 20 C) |
[41] | |
| Melamine, boric acid | N+B | electron-type and the hole-type carriers donated by nitrogen and boron atoms generate the synergistic effect to greatly elevate the high-rate capacity | 121.6 mAh g−1 (LFP/C-N+B at 20 C) 101.1 mAh g−1 (LFP/C at 20 C) |
13.6 (LFP/C-N+B) 2.56 (LFP/C) |
[42] |
| Methionine | S+N | good ionic and electronic conductivities | 103 mAh g−1 (NSC@LFP at 2 C) 63 mAh g−1 (pristine LFP at 2 C) |
[43] |
It was reported that additional electrons contributed by the N atom can provide electron carriers for the conduction band, which can contribute to the electrical conductivity of the material by introducing N into the carbon structures [35,36]. The F atom has a higher electronegativity than other anions, and F doping will accelerate the decrease in the interfacial resistance of the battery [37]. Coating lithium iron phosphate with sulfur-doped graphene nanosheets can create an electronic conductive network and can also promote the transportation of electrons and Li-ions [38,39,40]. Phosphorus-doped carbon layers can decrease transfer resistance and are good at the graphitization of the carbon [41]. The multi-element doping of carbon layers can achieve higher electronic conductivity and lower migration activation energy [42]. The main function of carbon coating and carbon layer doping is to enhance the electronic conductivity of the material. Investigations have demonstrated that the application of electrochemically active electron-conducting polymer coatings on LiFePO4 particles could potentially replicate the roles of carbon coatings, while also being applicable under less stringent conditions and offering the extra benefit of improved ionic conductivity within the active material [44,45].
Data from Table 1 demonstrate that the electrochemical performance and electronic conductivity of lithium iron phosphate (LFP) materials are significantly enhanced after doping the carbon layer with certain non-metal elements. By doping the carbon layer with heteroatoms such as nitrogen (N), sulfur (S), and boron (B), the conductivity of the carbon layer can be increased. For example, nitrogen doping can introduce additional electrons, thereby enhancing the electronic conductivity of the carbon layer. On the other hand, non-metal elements can assist in forming a conductive network within the carbon layer: doping with heteroatoms can promote the formation of a more comprehensive conductive network, thereby increasing the electron transfer rate of the electrode material. Furthermore, doping the carbon layer can improve the ion diffusion pathways: doping with heteroatoms can alter the surface structure of the material, providing lithium ions with shorter diffusion paths and thus enhancing the migration rate of ions. For instance, co-doping the carbon layer with nitrogen and boron can greatly enhance the electrochemical performance: at a rate of 20 C, the co-doped sample can increase the discharge capacity of LFP/C from 101.1 mAh g−1 to 121.6 mAh g−1 [42].
2.1.3. Ion Conductive Materials
Charging and discharging reactions of a battery are the result of the combined migration of ions and electrons. Especially during high-current charging and discharging, it is necessary to consider both the transport of electrons and the migration of ions. If the combined effects of both can be taken into account comprehensively, it will greatly enhance the electrochemical performance of the material. The main ways to improve the electrical conductivity include coating and modification with conductive carbon materials on the surface and doping with ions inside the material. To increase the migration rate of lithium ions, it is common to reduce the particle size of the material and construct special structures [46,47].
The ion conductive materials can enhance the electrochemical performances of LiFePO4 because of their high ionic conductivity and lithium ionic storage ability. Typically, graphene is often regarded as an excellent electronic conductor which can significantly enhance the electronic conductivity on the surface of LiFePO4. Most layered structures of graphene without defects would hinder the transition of Li+ [48]. The enhancement of ionic conductivity requires more attention. Incorporating GO (graphene oxide) contributes to the preservation of material stability and the augmentation of lithium ion diffusion coefficients since the lithium ions have lower insertion and extraction potentials along the [010] facet in LiFePO4 [32,49]. After being coated with graphene or GO, the average Fe-O bonds on the LiFePO4 (010) surface underwent significant changes, which led to the expansion of the Li+ channel, facilitating the insertion and extraction of migrating Li+. In addition to the lithium ions provided by LiFePO4 itself, incorporating lithium-ion conductive materials into the material can provide additional support for the intercalation and deintercalation of lithium ions, which will greatly enhance the material’s rate performance and cycling performance. Some ion conducting materials have a special three-dimensional structure that can facilitate rapid diffusion of Li+ [50]. Chien [51] designed a LiFePO4/Li3V2(PO4)3/C composite cathode material to help enhance the diffusion properties of lithium ions. Essentially, the enhancement of ionic conductivity of coated materials is contingent upon their unique post-modification structures. Materials that facilitate lithium-ion conduction possess both high ionic conductivity and lithium-ion storage capability, thereby significantly boosting the ionic conductivity of LiFePO4.
2.2. Strategy for Building a Multi-Dimensional Conductive Network
Nanoparticles of LiFePO4 are expected to be used as the cathode material of high-performance lithium-ion batteries [52]. The design and preparation of nanocomposites can effectively improve the low thermal stability and multiple surface side reactions of nanoparticles [53]. The design and the construction of conductive network structures have been the main focus of research in recent years [27,54,55,56,57,58,59,60]. Constructing a multi-dimensional conductive network is one of the effective means to enhance the electronic conductivity of LiFePO4. The construction of conductive networks generally uses one-dimensional materials [27] and two-dimensional materials [61] to provide a conductive skeleton in combination with two-dimensional methods. LiFePO4 is then used to gradually fill the surface and interior of the skeleton, achieving multi-dimensional conductive effects. A typical multi-dimensional conductive network is shown on Table 1. The main network structure could include a porous structure [54], a hierarchically porous structure [59], a highly meso-porous structure [60], a 3D conducting network [56], a distinctive loose and scaffolded structure [27], etc.
Typical conductive network images are shown in Table 2.
Table 2.
Characteristic, function, and network structure of multi-dimensional conductive network.
| Chemical Formula | Network Structure | Characteristic/ Function |
Electrical Conductivity |
Discharge Capacity (High Rate) |
Ref. |
|---|---|---|---|---|---|
| C@LFP/CNTs |
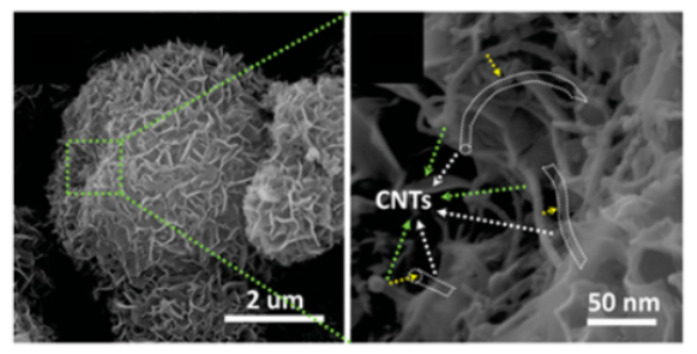
|
Porous structure/provides favorable kinetics for both electrons and Li+ | 7.71 × 10−2 S cm−1 (Conductivity of C@LFP/CNTs) 5.91 × 10−3 S cm−1 (Conductivity of C@LFP) |
102 mAh g−1 (C@LFP/CNTs at 20 C) 50 mAh g−1 (C@LFP at 20 C) |
[54] |
| LFP@C/G |
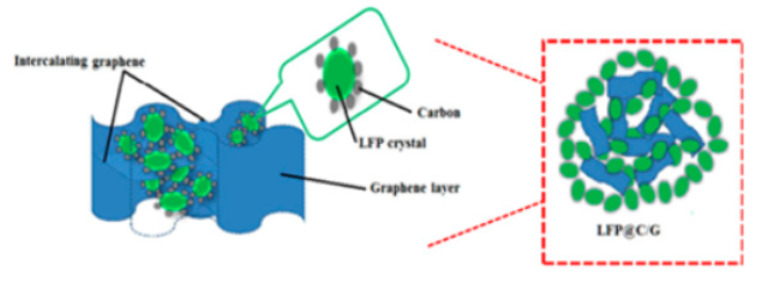
|
3D “sheets-in-pellets” and “pellets-on-sheets” conducting network structure/highly conductive and plentiful mesopores promote electronic and ionic transport | 28.4 Ω (Rct values for LFP@C/G) 75.5 Ω (Rct values for LFP@C) |
81.2 mAh g−1 (LFP@C/G at 20 C) |
[56] |
| CNT/LFP |

|
Distinctive loose and scaffolded composite structure/enhances the overall conductivity of the composite | 32.47 Ω (Rct values for LFP-CNT-G) 46.23 Ω (Rct values for LFP) |
143 mAh g−1 (LFP-CNT at 20 C) |
[27] |
| LFP-CNT-G |
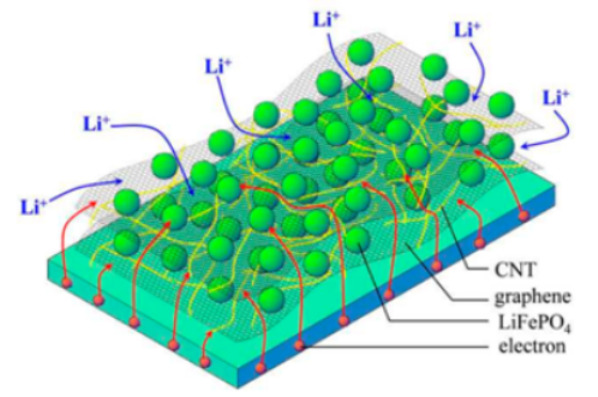
|
3D conducting networks/faster electron transfer and lower resistance during the Li-ions’ reversible reaction | 50.17 Ω (Rct values for LFP-CNT-G) 103.93 Ω (Rct values for LFP-CNT) |
115.8 mAh g−1 (LFP-CNT-G at 20 C) 99.4 mAh g−1 (LFP-CNT at 20 C) |
[58] |
| LFP@C/MXene |
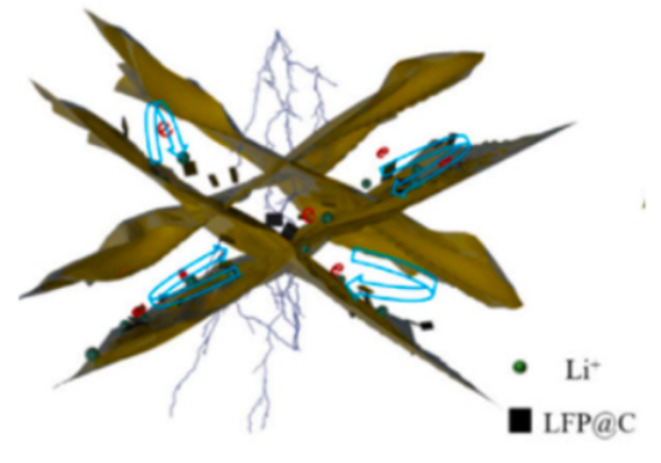
|
Hierarchically porous structure and “dot-to-surface” conductive network/fast ion and electron transfer for redox reactions | 17.26 Ω (Rct values for LFP@C/MX-3.0) 93.32 Ω (Rct values for LFP@C) |
140.3 mA h·g−1 (LFP@C/MX-3.0 at 20 C) 86.6 mA h·g−1 (LFP@C at 20 C) |
[59] |
| LFP/R-GO |
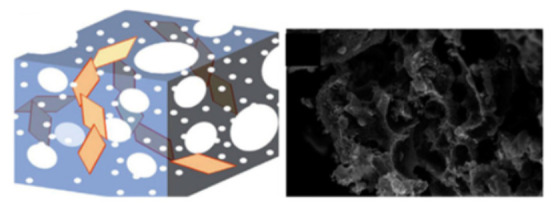
|
Highly meso-porous structure/good electronic conductivity and high electrolyte permeability | 25 Ω (Rct values for LiFePO4/R-GO) 50 Ω (Rct values for LiFePO4) |
135 mAh g−1 (LiFePO4/R-GO at 5 C) |
[60] |
| LFP/MXene/CNT/Cellulose |
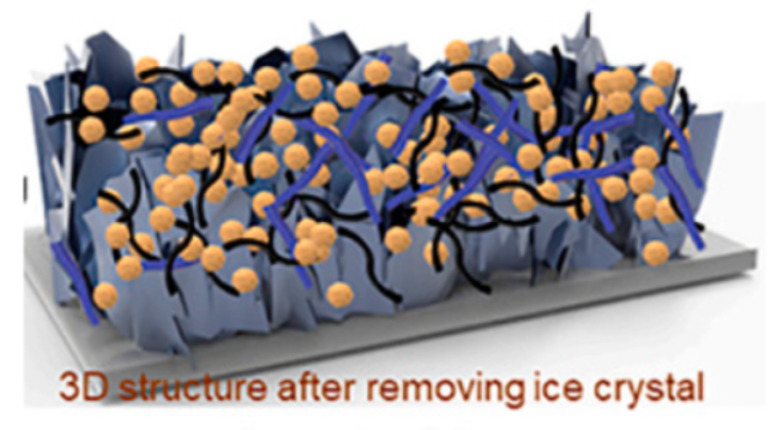
|
3-dimensional MXene-Carbon nanotubes-Cellulose-LiFePO4 (3D-MCC-LFP)/faster electronic/ionic transport | 26.6 Ω (Rct values for 3D-MCC-LFP10) 32.2 Ω (Rct values for Con-LFP10) |
159.5 mAh g−1
(3D-MCC-LFP120 at 1 mA cm−2) |
[61] |
| LFP/Ti3C2Tx/CNTs |
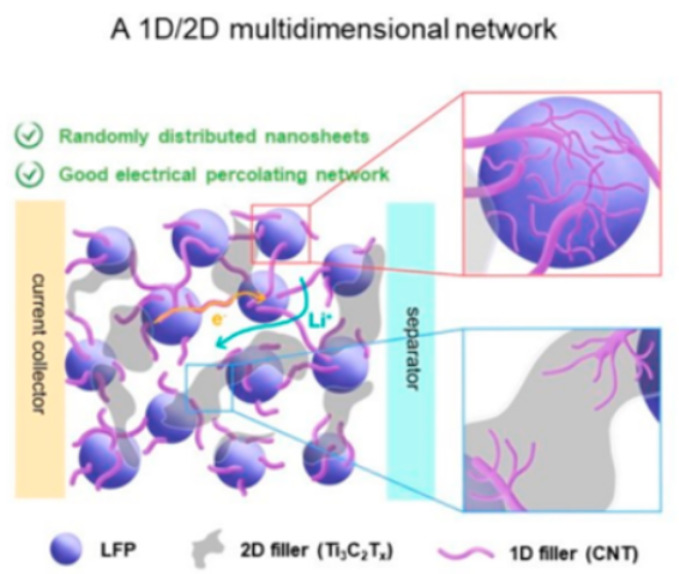
|
1D single-walled carbon nanotubes (CNTs)/bound together using 2D MXene (Ti3C2Tx) nanosheets/highlights the ability of multi-dimensional conductive fillers to realize simultaneously superior electrochemical and mechanical properties | 27.7 Ω (Rct values for LFP/CNT/Ti3C2Tx) 32.2 Ω (Rct values for LFP/Ti3C2Tx) |
[62] |
Data from Table 2 clearly indicate that the construction of a 3D conductive network significantly enhances the electrical conductivity and high-rate discharge capacity of lithium iron phosphate (LFP) materials. The excellent electrochemical performance is attributed to the following factors: (i) increased contact between particles, enhancing the efficiency of electron transport within the material; (ii) providing a larger surface area for electrochemical reactions; (iii) allowing more lithium ions to participate in the charging and discharging process. The following text provides a more detailed narrative.
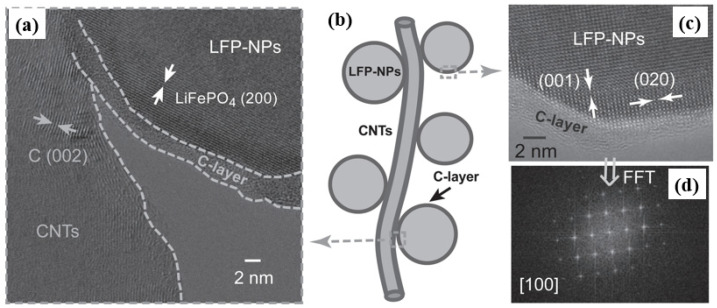
Wu [63] designed a new LiFePO4 nanoparticle, which exhibited two types of carbon complexes, including amorphous carbon coating and a graphitized conductive structure (Figure 5). Furthermore, compared with the original LFP@C and LFP/CNT coatings, the initial carbon layer evenly coated all nanoscale LiFePO4 particles due to the synergistic effect of amorphous carbon. This stabilized the interface of LiFePO4 nanoparticles, thereby enhancing electrical conductivity and Li diffusion.
Figure 5.
(a,c) HRTEM images and (b) a schematic illustration of the prepared LFP@C/CNT nanocomposite. (d) The corresponding FFT of the HRTEM in (c) [63]. Reprinted from [63].
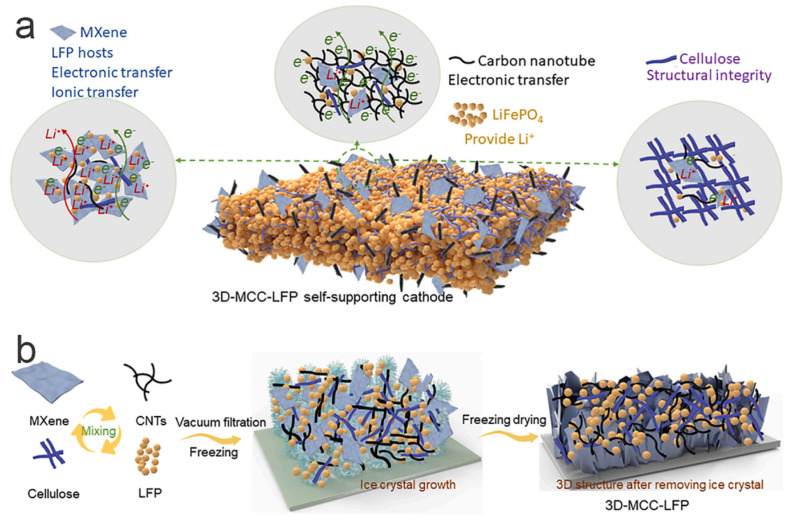
Dong and colleagues [61] designed a 3D-MCC-LFP material with a high load rate, exceptional mechanical properties, and excellent electrical conductivity using an assembly method (Figure 6). In this structure, 2D MXene served as a key component, providing sites for LiFePO4 particle loading, connecting materials, and facilitating simultaneous electron and ion transport. One-dimensional carbon nanotubes served as conductive agents, enabling full interconnectivity of the scaffold and thereby enhancing electronic conductivity. One-dimensional cellulose served as a reinforcing filler, preserving the mechanical properties and structural integrity of 3D-MCC-LFP while accommodating a large amount of LiFePO4 material.
Figure 6.
Schematic illustration of the structure (a) and fabrication process (b) of 3D-MCC-LFP cathode [61]. Reprinted from [61].
The author employed SnO2-NF as the negative electrode material to test the performance of the assembled battery, and due to its excellent conductivity and high loading capacity of LiFePO4, the electrochemical properties of 3D-MCC-LFP materials surpassed those prepared using traditional methods.
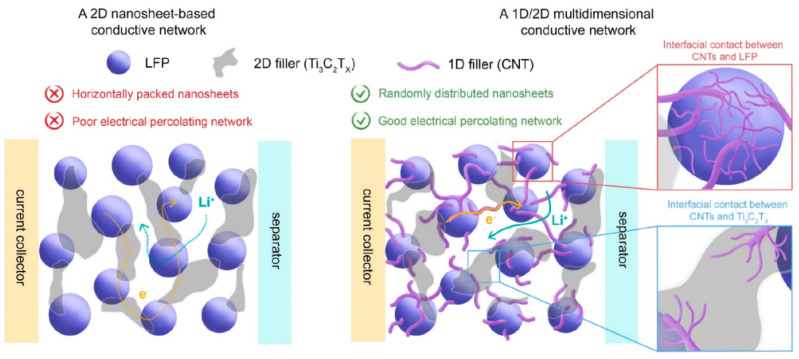
Similarly, Checko [62] designed a new multi-dimensional network to enhance local electrical transport across the LiFePO4 surface (Figure 7). This structure is consistent with single-walled carbon nanotubes (1D) and MXene nanosheet (2D) bound together. The CNTs facilitated local electron transport across the LiFePO4 surface, while Ti3C2Tx nanosheets provided conductive pathways through the bulk of the electrode. The electrochemical characterization supported by numerical simulation verified the charge transfer characteristics of this multi-dimensional conductive network.
Figure 7.
Schematic illustration depicting electrode characteristics in a 2D nanosheet-based conductive network (left) and a 1D/2D multi-dimensional conductive network (right) with inset schemes showing magnified views of the interfacial contacts between CNTs and LFP particles (top) and between CNTs and Ti3C2Tx (bottom) in the multi-dimensional conductive network [62]. Reprinted from [62].
Contrastingly, Luo [64] exploited N-doped graphene (NG)-modified LiFePO4 material with a 3D conductive network structure for Li-ion batteries. In this structure, NG effectively coated and connected LiFePO4 particles, and N doping reduced electrode polarization, enhancing electrochemical reaction reversibility. The special structure constructed with NG provided faster and more efficient 3D transport channels for Li+ and electrons.
The construction of a multi-dimensional conductive network is an efficient approach for rapid transmission on and between particle surfaces. The dual connected channel composed of a solid phase network channel and an internal cavity channel with a conductive network structure achieves rapid electron and ion transport, and also ensures uniform contact between the electrolyte and the positive electrode, thereby forming a good interface. The porous structure can achieve effective infiltration of electrolytes, which is beneficial for improving the lithium diffusion rate and reaction kinetics. However, critical factors to consider include the mechanical stability of the structure, electronic transmission efficiency, and compatibility between the conductive network and the material interface.
In addition to intrinsic material properties, achieving excellent carbon coating is essential to fully utilize electrochemical performance. A uniform and effective carbon skeleton conductive network should form between particles, emphasizing molecular-level mixing of carbon skeleton and LiFePO4. Conventional physical coating methods with simple processes and low costs may struggle to achieve accurate carbon coating requirements. Therefore, they have become a prospective technology to study new carbon coating methods with controlled nano-growth mechanisms.
2.3. Strategy for Ion Doping
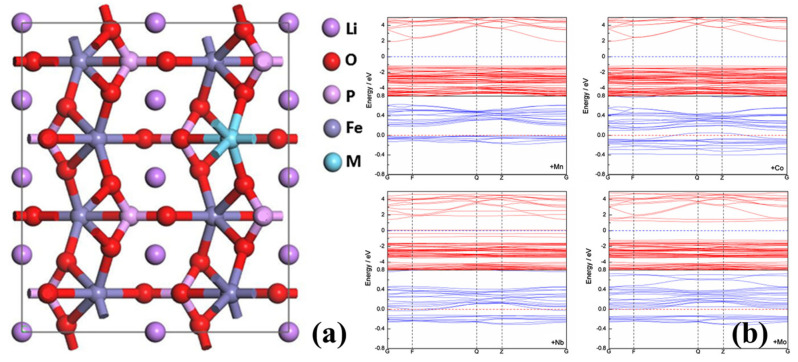
The purpose of in situ carbon coating and the construction of a multi-dimensional conductive network on particle surfaces is to enhance electrical conductivity both within and between particles, representing a physical modification of battery materials. However, ion doping constitutes a chemical modification aimed at enhancing intrinsic electrical conductivity [65,66]. Specific doping locations include iron (Fe), phosphorus (P), and Li sites, among which Fe-site doping is the most prevalent [66,67,68,69,70,71]. Additionally, various doping elements have been studied, including manganese (Mn), nickel (Ni), niobium (Nb), magnesium (Mg), cobalt (Co), vanadium (V), and others [72,73,74,75,76,77]. However, Fe-site doping serves a dual purpose. First, it narrows the band gap between the conduction and valence bands of semiconductor material (LiFePO4), thereby increasing material conductivity. Second, ion doping induces Li or Fe vacancies, forming charge compensation defects, and the conductivity of electrons is enhanced. Zhang [78] studied the electronic properties of LiFePO4 doped with Mn, Co, Nb, Mo, and other elements using first-principles calculations. The results (Figure 8) indicated reduced band gaps with the doping of these elements, facilitating electron transitions. Notably, Co and Nb doping exhibited obvious enhancement effects. Moreover, studies have shown that doped materials inhibit microcracks, prevent electrode polarization, and enhance overall material performance. Furthermore, doping with Mn, Nb, Mo, and Co also enhances the mechanical stability of LiFePO4, altering parameters such as material structure, M-O (metal–oxygen) bond energy, and band gap width, thereby enhancing both electrical and mechanical properties.
Figure 8.
(a) Bulk model of M-doped LiFePO4 (M = Mn, Co, Nb, Mo). (b) Band structures of LiFePO4. The red and blue lines represent majority spin states and minority spin states, respectively (for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article) [78]. Reprinted from [78].
Dou [12] examined the electronic structure of LiFexMn1−xPO4 doped with different Mn contents using first-principles density functional methods. The study found that LiFe0.75Mn0.25PO4 exhibited the smallest bandgap width with a Mn doping amount of x = 0.25. This was attributable to the Fe3d electron contributions dominating the density of states (DOS) near the Fermi plane of LiFePO4, while Mn3d electrons become predominant upon Mn incorporation, increasing the DOS near the Fermi level in LiFe0.75Mn0.25O2 and enhancing material conductivity. Conversely, introducing Mn lengthened Fe-O bonds and weakened Fe-O bond energy, widening Li-ion migration channels and facilitating ion diffusion.
Ban et al. [79] studied the co-doping of “donor-acceptor” charge compensation, combining theoretical calculations with experiments to significantly enhance material rate performance. It was observed that P and O sites co-doped with silicon (Si) and fluorine (F) altered the conduction band edge of LiFePO4, enhancing material conductivity by at least two to three orders of magnitude compared with pre-doping levels. This approach facilitated the positive magnification performance of LiFePO4.
In addition to theoretical studies, replacing and occupying the spatial structure of an element in LiFePO4 using doping elements to create lattice defects broadens ion transport channels, enhancing material intrinsic conductivity. Marnix [80] studied the doping of hypervalent ions in LiFePO4, observing that the ions that occupied Li positions maintained a positive bivalent state in Fe, thus contributing to improved material conductivity.
Recently, the doping of rare earth elements into positive electrode materials has also been widely studied [81,82]. The ionic radius of rare earth elements is larger than that of transition metal elements, resulting in an increased material cell volume and a reduced band gap after doping with rare earth elements. This increase in mobility and carrier concentration significantly enhances the electrical conductivity of the final material [83,84]. Studies have shown that doping Li-ion phosphate with rare earth element ions such as erbium (Er3+), yttrium (Y3+), and Nd3+ leads to Fe replacement by rare earth ions, resulting in increase in material conductivity of four orders of magnitude. This occurred because the electron-deficient rare earth element ions created holes that readily exited full electrons to the hole level, transforming the material from a N-type to a P-type semiconductor [85,86]. Qiu Peng [87] studied the effects of lanthanum (La), Nd, and Y doping on the structure and electrochemical properties of LiFePO4. The results indicated increased cell parameters and volume, smaller particle size, and uniform, tightly bonded pores in the doped material. In comparison with pure Li–Fe phosphate, the electrical conductivity of the doped material increased by four orders of magnitude, owing to enhanced Li-ion diffusion facilitated by the small particle size and internal lattice defects created by the doped elements. Studies have shown that rare earth element-doped materials exhibit smaller particle sizes [88,89,90,91,92,93].
Notably, enhancing the diffusion performance of Li-ions is also an important purpose of doping, and its double effect was achieved through the co-doping method [94,95]. Wang [96] successfully synthesized Y-F co-doped LiFePO4/C material using a high-temperature solid-phase method. The introduction of F enhanced the electron-cloud rearrangement of PO43−, significantly enhancing conductivity. Simultaneously, Y was introduced into Li+ vacancy, reducing spatial resistance to Li-ion diffusion and comprehensively enhancing material ionic conductivity. Additionally, X-ray diffraction analysis results revealed weakened Li-O bonds and widened Li-ion diffusion tunnels due to Y-F doping, leading to Li-ion diffusion rates. As a result, the material exhibited excellent electrochemical properties, that is, its specific discharge capacity reached a 179.3 mAh·g−1 capacity at a 0.1 C current density and a 135.5 mAh·g−1 capacity at 10 C.
The doping method theoretically optimized the structure of the crystal, and the conductivity of the material was fundamentally improved. However, the mechanism by which doping changes the electrochemical properties of materials remains unclear. The electronic conduction within the crystal of the material was very complicated, and whether the doped element fulfilled its designed role remains uncertain, with assessments largely based on macro-level performance. The particle size reduction following rare earth element doping lacks detailed analysis, indicating a partial absence of a relevant theoretical basis. Therefore, careful selection of doping elements and the acquisition of necessary theoretical support are essential prerequisites for material doping research.
3. New Carbon Coating Technologies and LiFePO4 Batteries
Some new carbon coating technologies are rapidly developing which not only apply to lithium iron phosphate materials but also provide new ideas for surface coating modification of other materials with poor conductivity.
Flash Joule heating (FJH): Using an ex situ carbon coating method, the precursor could rapidly decompose through flash Joule heating (FJH) technology. By depositing carbon heteroatom materials within a limited space in just 10 s, a uniform amorphous carbon layer can be obtained on LFP; at the same time, different heteroatoms can be introduced into the surface carbon layer. Solvent-free, versatile cathode surface modification is the highlight of this technology [97]. Supercritical CO2-enhanced surface modification: Employing supercritical CO2 (SCCO2) for a proficient ex situ carbon coating method on lithium iron phosphate (LFP) results in a superior carbon coating layer with a higher graphite carbon content and reduced oxygen-derived functional groups, significantly improving electron transfer efficiency [98]. Spray coating technology: Using spray coating technology to produce LiFePO4-coated carbon fiber (CF) as a structural battery cathode component can yield substantial electrochemical performance for the battery. The use of spray coating technology stands out as an innovative method for manufacturing electrodes in structural batteries, highlighting its capacity to enhance the efficiency of multifunctional energy storage systems [22]. Ultrafast nonequilibrium high-temperature shock technology: This technology introduces Li-Fe anti-site defects and controllable tensile strain into the LiFePO4 lattice. This design allows for research on the impact of strain fields on performance to extend from theoretical calculations to experimental perspectives [99]. Recycling and reuse technology for spent LiFePO4: This is a direct regeneration of LiFePO4 based on a doping strategy, a highly efficient additive for direct reactivation of waste LiFePO4, prilling, and a cocoating collaborative strategy [100,101,102].
4. Conclusions
The carbon layer structure significantly affects the conductivity of LiFePO4. To achieve optimal electrochemical performance, it is necessary to incorporate highly graphitized carbon (sp2 hybrid state) to enhance its conductivity. However, traditional organic or inorganic carbon struggles to achieve a high degree of graphitization at sintering temperatures of 500–800 °C. Therefore, while in situ growth of LiFePO4 on a single layer of graphene proves promising, the development and adoption of this technology must overcome challenges related to cost and synthesis methods. Furthermore, the quest for new carbon materials with enhanced graphitization remains imperative. Additionally, modifying crystal structures through cationic or anionic doping offers optimization potential. However, the mechanisms and effects of doping reactions on material properties require more comprehensive investigation. The analysis of substitution sites and doping quantities necessitates refined proof methods. Finally, future efforts concerning LiFePO4 and other types of cathode (anode) materials with poor conductivity must be focused on achieving high energy and vibration densities alongside stable cycling, excellent rate performance, and low-temperature capabilities. This trajectory underscores the imperative for ongoing advancements in material science and electrochemistry.
Author Contributions
L.W.: Writing (original draft, resources, investigation); H.C.: Conceptualization; Y.Z.: Writing (review and editing); J.L.: Conceptualization, Writing (review and editing); L.P.: Writing (review and editing). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the Science and Technology Project of Hebei Education Department (grant no. QN2022203) and the S&T Program of Chengde (grant no. 202201A062).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Padhi A.K., Nanjundaswamy K.S., Goodenough J.B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997;144:1188–1194. doi: 10.1149/1.1837571. [DOI] [Google Scholar]
- 2.Guyomard D., Tarascon J.M. Rocking-chair or Lithium-ion rechargeable Lithium batteries. Adv. Mater. 1994;6:408–412. doi: 10.1002/adma.19940060516. [DOI] [Google Scholar]
- 3.Zhang S.S., Allen J.L., Xu K., Jow T.R. Optimization of reaction condition for solid-state synthesis of LiFePO4-C composite cathodes. J. Power Sources. 2005;147:234–240. doi: 10.1016/j.jpowsour.2005.01.004. [DOI] [Google Scholar]
- 4.Cheng F.Q., Wan W., TAN Z., Huang Y.Y., Zhou H.H., Chen J.T., Zhang X.X. High power performance of nano-LiFePO4/C cathode material synthesized via lauric acid-assisted solid-state reaction. Electrochim. Acta. 2011;56:2999–3005. doi: 10.1016/j.electacta.2011.01.007. [DOI] [Google Scholar]
- 5.Liu W., Gao P., Mi Y.Y., Chen J.T., Zhou H.H., Zhang X.X. Fabrication of high tap density LiFe0.6Mn0.4PO4/C microspheres by a double carbon coating-spray drying method for high rate lithium ion batteries. J. Mater. Chem. A. 2013;1:2411–2417. doi: 10.1039/C2TA00939K. [DOI] [Google Scholar]
- 6.Li Y., Wang L., Zhang K.Y., Yao Y.C., Kong L.X. Optimized synthesis of LiFePO4 cathode material and its reaction mechanism during solvothermal. Adv. Powder Technol. 2021;32:2097–2105. doi: 10.1016/j.apt.2021.04.019. [DOI] [Google Scholar]
- 7.Xie Y., Yu H.T., Yi T.F., Zhu Y.R. Understanding the thermal and mechanical stabilities of olivine type LiMPO4 (M = Fe, Mn) as cathode materials for rechargeable lithium batteries from first principles. ACS Appl. Mater. Interfaces. 2014;6:4033–4042. doi: 10.1021/am4054833. [DOI] [PubMed] [Google Scholar]
- 8.Ramasubramanian B., Sundarrajan S., Chellappan V., Reddy M.V., Ramakrishna S., Zaghi K. Recent development in carbon-LiFePO4 cathodes for lithium-ion batteries: A mini review. Batteries. 2022;8:133. doi: 10.3390/batteries8100133. [DOI] [Google Scholar]
- 9.Chen S.P., Lv D., Chen J., Zhang Y.H., Shi F.N. Review on defects and modification methods of LiFePO4 cathode material for lithium-ion batteries. Energy Fuels. 2022;36:1232–1251. doi: 10.1021/acs.energyfuels.1c03757. [DOI] [Google Scholar]
- 10.Wang J.J., Sun X.L. Understanding and recent development of carbon coating on LiFePO4 cathode materials for lithium-ion batteries. Energy Environ. Sci. 2012;5:5163–5185. doi: 10.1039/C1EE01263K. [DOI] [Google Scholar]
- 11.Chung S.Y., Blocking J.T., Chiang Y.M. Electronically conductive phospho-olivines as lithium storage electrodes. Nat. Mater. 2002;1:123–128. doi: 10.1038/nmat732. [DOI] [PubMed] [Google Scholar]
- 12.Dou J.Q., Kang X.Y., Tuerdi W., Hua N., Han Y. The first principles and experimental study on Mn doped LiFePO4. Acta Phys. Sin. 2012;61:341–348. [Google Scholar]
- 13.Ruan Y.Y., Tang Z.Y., Guo H.Z. Effects on the structure and electrochemical performance of LiFePO4 by Mn2 + doping. J. Funct. Mater. 2008;39:747–750. [Google Scholar]
- 14.Li Y., Wang L., Liang F., Yao Y.C., Zhang K.Y. Enhancing high rate performance and cyclability of LiFePO4 cathode materials for lithium ion batteries by boron doping. J. Alloys Compd. 2021;880:160560. doi: 10.1016/j.jallcom.2021.160560. [DOI] [Google Scholar]
- 15.Xiao P.H., Henkelman G. Kinetic monte carlo study of Li intercalation in LiFePO4. ACS Nano. 2018;12:844–851. doi: 10.1021/acsnano.7b08278. [DOI] [PubMed] [Google Scholar]
- 16.Yang H., Ding Z., Li Y.T., Li S.Y., Wu P.K., Hou Q.H., Zheng Y., Gao B., Huo K.F., Du W.J., et al. Recent advances in kinetic and thermodynamic regulation of magnesium hydride for hydrogen storage. Rare Met. 2023;42:2906–2927. doi: 10.1007/s12598-023-02306-z. [DOI] [Google Scholar]
- 17.Ding Z., Li Y.T., Yang H., Lu Y.F., Tan J., Li J.B., Li Q., Chen Y.A., Shaw L.L., Pan F.S. Tailoring MgH2 for hydrogen storage through nanoengineering and catalysis. J. Magnes. Alloys. 2022;10:2946–2967. doi: 10.1016/j.jma.2022.09.028. [DOI] [Google Scholar]
- 18.Saikia D., Deka J.R., Chou C.J., Lin C.H., Yang Y.C., Kao H.M. Encapsulation of LiFePO4 Nanoparticles into 3D interpenetrating ordered mesoporous carbon as a high-performance cathode for lithium-ion batteries exceeding theoretical capacity. ACS Appl. Energy Mater. 2019;2:1121–1133. doi: 10.1021/acsaem.8b01682. [DOI] [Google Scholar]
- 19.Zhang Z., Wang M.M., Xu J.F., Shi F.C., Li M., Gao Y.M. Modification of lithium iron phosphate by carbon coating. Int. J. Electrochem. Sci. 2019;14:10622–10632. doi: 10.20964/2019.11.22. [DOI] [Google Scholar]
- 20.Varzi A., Bresser D., Zamory J.V., Müller F., Passerini S. ZnFe2O4-C/LiFePO4-CNT: A novel high-power lithium-ion battery with excellent cycling performance. Adv. Energy Mater. 2014;4:1400054. doi: 10.1002/aenm.201400054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao Y.C., Qu P.W., Gan X.K., Huang X.P., Zhao Q.F., Liang F. Preparation of porous-structured LiFePO4/C composite by vacuum sintering for lithium-ion battery. Ceram. Int. 2016;42:18303–18311. doi: 10.1016/j.ceramint.2016.08.158. [DOI] [Google Scholar]
- 22.Yücel Y.D., Zenkert D., Lindström R.W., Lindbergh G. LiFePO4-coated carbon fibers as positive electrodes in structural batteries: Insights from spray coating technique. Electrochem. Commun. 2024;160:107670. doi: 10.1016/j.elecom.2024.107670. [DOI] [Google Scholar]
- 23.Qin J.D., Zhang Y.B., Lowe S.E., Jiang L.X., Ling H.Y., Shi G., Liu P.R., Zhang S.Q., Zhong Y.L., Zhao H.J. Room temperature production of graphene oxide with thermally labile oxygen functional groups for improved lithium ion battery fabrication and performance. J. Mater. Chem. A. 2019;7:9646–9655. doi: 10.1039/C9TA02244A. [DOI] [Google Scholar]
- 24.Wang J.J., Sun X.L. Olivine LiFePO4: The remaining challenges for future energy storage. Energy Environ. Sci. 2015;8:1110–1138. doi: 10.1039/C4EE04016C. [DOI] [Google Scholar]
- 25.Wen L.Z., Guan Z.W., Wang L., Hu S.T., Lv D.H., Liu X.M., Duan T.T., Liang G.C. Effect of carbon-coating on internal resistance and performance of lithium iron phosphate batteries. J. Electrochem. Soc. 2022;169:050536. doi: 10.1149/1945-7111/ac716b. [DOI] [Google Scholar]
- 26.Yang J.L., Wang J.J., Tang Y.J., Wang D.N., Li X.F., Hu Y.H., Li R.Y., Liang G.X., Sham T.K., Sun X.L. LiFePO4-graphene as a superior cathode material for rechargeable lithium batteries: Impact of stacked graphene and unfolded graphene. Energy Environ. Sci. 2013;6:1521–1528. doi: 10.1039/c3ee24163g. [DOI] [Google Scholar]
- 27.Ren X.G., Li Y.J., He Z.J., Xi X.M., Shen X.J. In-situ growth of LiFePO4 with interconnected pores supported on carbon nanotubes via tavorite-olivine phase transition. Ceram. Int. 2023;49:40131–40139. doi: 10.1016/j.ceramint.2023.09.344. [DOI] [Google Scholar]
- 28.Stankovich S., Dikin D.A., Piner R.D., Kohlhaas K.A., Kleinhammes A., Jia Y., Wu Y., Nguyen S.T., Ruoff R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon. 2007;45:1558–1565. doi: 10.1016/j.carbon.2007.02.034. [DOI] [Google Scholar]
- 29.Dong Y.F., Wu Z.S., Ren W.C., Cheng H.M., Bao X.H. Graphene: A promising 2D material for electrochemical energy storage. Sci. Bull. 2017;62:724–740. doi: 10.1016/j.scib.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Geng J., Zhang S.C., Hu X.X., Ling W.Q., Peng X.X., Zhong S.L., Liang F.G., Zou Z.G. A review of graphene-decorated LiFePO4 cathode materials for lithium-ion batteries. Ionics. 2022;28:4899–4922. doi: 10.1007/s11581-022-04679-0. [DOI] [Google Scholar]
- 31.Xu X.L., Qi C.Y., Hao Z.D., Wang H., Jiu J.T., Liu J.B., Yan H., Suganuma K. The surface coating of commercial LiFePO4 by utilizing ZIF-8 for high electrochemical performance lithium-ion batteries. Nano-Micro Lett. 2018;10:1. doi: 10.1007/s40820-017-0154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z.X., Wang F.Z., Li T.B., Wang S.C., Yao C., Wu H. First-principles study of LiFePO4 modified by graphene and defective graphene oxide. J. Mol. Graph. Modell. 2024;129:108731. doi: 10.1016/j.jmgm.2024.108731. [DOI] [PubMed] [Google Scholar]
- 33.Wu S.Y., Luo E.M., Ouyang J., Lu Q., Zhang X.X., Wei D., Han W.K., Xu X., Wei L. Tuning the graphitization of the carbon coating layer on LiFePO4 enables superior properties. Int. J. Electrochem. Sci. 2024;19:100450. doi: 10.1016/j.ijoes.2023.100450. [DOI] [Google Scholar]
- 34.Choi J., Zabihi O., Ahmadi M., Naebe M. Advancing structural batteries: Cost-efficient high performance carbon fiber-coated LiFePO4 cathodes. RSC Adv. 2023;13:30633–30642. doi: 10.1039/D3RA05228A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ou J.K., Yang L., Jin F., Wu S.G., Wang J.Y. High performance of LiFePO4 with nitrogen-doped carbon layers for lithium-ion batteries. Adv. Powder Technol. 2020;31:1220–1228. doi: 10.1016/j.apt.2019.12.044. [DOI] [Google Scholar]
- 36.Wang Y.Y., Wang X.L., Jiang A., Liu G.X., Yu W.S., Dong X.T., Wang J.X. A versatile nitrogen-doped carbon coating strategy to improve the electrochemical performance of LiFePO4 cathodes for lithium-ion batteries. J. Alloys Compd. 2019;810:151889. doi: 10.1016/j.jallcom.2019.151889. [DOI] [Google Scholar]
- 37.Wang X.F., Feng Z.J., Hou X.L., Liu L.L., He M., He X.S., Huang J.T., Wen Z.H. Fluorine doped carbon coating of LiFePO4 as a cathode material for lithium-ion batteries. Chem. Eng. J. 2020;379:122371. doi: 10.1016/j.cej.2019.122371. [DOI] [Google Scholar]
- 38.Sun M.J., Han X.L., Chen S.G. NaTi (PO4)3@C nanoparticles embedded in 2D sulfur-doped graphene sheets as high-performance anode materials for sodium energy storage. Electrochim. Acta. 2018;289:131–138. doi: 10.1016/j.electacta.2018.08.061. [DOI] [Google Scholar]
- 39.Wang W., Tang M.Q., Yan Z.W. Superior Li-storage property of an advanced LiFePO4@C/S-doped graphene for lithium-ion batteries. Ceram. Int. 2020;46:22999–23005. doi: 10.1016/j.ceramint.2020.06.075. [DOI] [Google Scholar]
- 40.Xun D., Wang P.F., Shen B.W. Synthesis and characterization of sulfur-doped carbon decorated LiFePO4 nanocomposite as high performance cathode material for lithium-ion batteries. Ceram. Int. 2016;42:5331–5338. [Google Scholar]
- 41.Zhang J.L., Wang J., Liu Y.Y., Nie N., Gu J.J., Yu F., Li W. High-performance lithium iron phosphate with phosphorus-doped carbon layers for lithium-ion batteries. J. Mater. Chem. A. 2015;3:2043–2049. [Google Scholar]
- 42.Zhang J.L., Nie N., Liu Y.Y., Wang J., Yu F., Gu J.J., Li W. Boron and nitrogen co-doped carbon layers of LiFePO4 improve the high-rate electrochemical performance for lithium-ion batteries. ACS Appl. Mater. Interfaces. 2015;7:20134–20143. doi: 10.1021/acsami.5b05398. [DOI] [PubMed] [Google Scholar]
- 43.Nitheesha S.J., Feng J., Jae Y.S., Murugan N., Taehyung K., Byeong J.J., Soon P.J., Chang W.L. Heteroatoms-doped carbon effect on LiFePO4 cathode for Li-ion batteries. J. Energy Storage. 2023;72:108710. [Google Scholar]
- 44.Chepurnaya I., Smirnova E., Karushev M. Electrochemically active polymer components in next-generation LiFePO4 cathodes: Can small things make a big difference? Batteries. 2022;8:185. doi: 10.3390/batteries8100185. [DOI] [Google Scholar]
- 45.Rohland P., Schröter E., Nolte O., Newkome G.R., Hager M.D., Schubert U.S. Redox-active polymers: The magic key towards energy storage—A polymer design guideline progress in polymer science. Prog. Polym. Sci. 2022;125:101474. doi: 10.1016/j.progpolymsci.2021.101474. [DOI] [Google Scholar]
- 46.Yang Z.G., Dai Y., Wang S.P., Yu J.X. How to make lithium iron phosphate better: A review exploring classical modification approaches in-depth and proposing future optimizing methods. J. Mater. Chem. A. 2016;47:18193–18656. doi: 10.1039/C6TA05048D. [DOI] [Google Scholar]
- 47.Stenina I.A., Minakova P.V., Kulova T.L., Desyatov A.V., Yaroslavtsev A.B. LiFePO4/carbon nanomaterial composites for cathodes of high-power lithium-ion batteries. Inorg. Mater. 2021;57:620–628. doi: 10.1134/S0020168521060108. [DOI] [Google Scholar]
- 48.Li L., Wu L., Wu F., Song S.P., Zhang X.Q., Fu C., Yuan D.D., Xiang Y. Review-Recent research progress in surface modification of LiFePO4 cathode materials. J. Electrochem. Soc. 2017;164:A2138–A2150. doi: 10.1149/2.1571709jes. [DOI] [Google Scholar]
- 49.Hana N.H., Munasir Study of performance graphene oxide modification of LiFePO4/C material for the cathode of Li-ion batteries. J. Phys. Conf. Ser. 2023;2623:012014. doi: 10.1088/1742-6596/2623/1/012014. [DOI] [Google Scholar]
- 50.Zhong S.K., Wu L., Liu J.Q. Sol-gel synthesis and electrochemical properties of LiFePO4/Li3V2(PO4)3/C composite cathode material for lithium ion batteries. Electrochim. Acta. 2012;74:8–15. doi: 10.1016/j.electacta.2012.03.181. [DOI] [Google Scholar]
- 51.Chien W.C., Jhang J.S., Wu S.H., Wu Z.H., Yang C.C. Preparation of LiFePO4/Li3V2(PO4)3/C composite cathode materials and their electrochemical performance analysis. J. Alloys Compd. 2020;847:156447. doi: 10.1016/j.jallcom.2020.156447. [DOI] [Google Scholar]
- 52.Bruce P.G., Scrosati B., Tarascon J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008;47:2930–2946. doi: 10.1002/anie.200702505. [DOI] [PubMed] [Google Scholar]
- 53.Liu J.Y., Li X.X., Huang J.R., Li J.J., Zhou P., Liu J.H., Huang H.J. Three-dimensional graphene-based nanocomposites for high energy density Li-ion batteries. J. Mater. Chem. A. 2017;13:5977–5994. doi: 10.1039/C7TA00448F. [DOI] [Google Scholar]
- 54.Wang B., Liu T.F., Liu A.M., Liu J.G., Wang L., Gao T.T., Wang D.L., Zhao X.S. A hierarchical porous C@LiFePO4/carbon nanotubes microsphere composite for high-rate lithium-ion batteries: Combined experimental and theoretical study. Adv. Energy Mater. 2016;6:1600426. doi: 10.1002/aenm.201600426. [DOI] [Google Scholar]
- 55.Ren X.G., Li Y.G., He Z.J., Xi X.M., Shen X.J. In-situ growth of LiFePO4 on graphene through controlling phase transition for high-performance Li-ion battery. J. Energy Storage. 2023;74:109305. doi: 10.1016/j.est.2023.109305. [DOI] [Google Scholar]
- 56.Wang X.F., Feng Z.J., Huang J.T., Deng W., Li X.B., Zhang H.S., Wen Z.H. Graphene-decorated carbon-coated LiFePO4 nanospheres as a high-performance cathode material for lithium-ion batteries. Carbon. 2017;127:149–157. doi: 10.1016/j.carbon.2017.10.101. [DOI] [Google Scholar]
- 57.Wang F., Wang F.F., Hong R.Y., Lv X.S., Zheng Y. High-purity few-layer graphene from plasma pyrolysis of methane as conductive additive for LiFePO4 lithium ion battery. J. Mater. Res. Technol. 2020;5:10004–10015. doi: 10.1016/j.jmrt.2020.06.072. [DOI] [Google Scholar]
- 58.Lei X.L., Chen Y.M., Wang W.G., Ye Y.P., Zheng C.C., Deng P., Shi Z.C., Zhang H.Y. A three-dimensional LiFePO4/carbon nanotubes/graphene composite as a cathode material for lithium-ion batteries with superior high-rate performance. J. Alloys Compd. 2015;626:280–286. doi: 10.1016/j.jallcom.2014.09.169. [DOI] [Google Scholar]
- 59.Zhang H.W., Li J.Y., Luo L.Q., Zhao J., He J.Y., Zhao X.X., Liu H., Qin Y.B., Wang F.Y., Song J.J. Hierarchically porous MXene decorated carbon coated LiFePO4 as cathode material for high-performance lithium-ion batteries. J. Alloys Compd. 2021;876:160210. doi: 10.1016/j.jallcom.2021.160210. [DOI] [Google Scholar]
- 60.Mun J.Y., Ha H.W., Choi W.C. Nano LiFePO4 in reduced graphene oxide framework for efficient high-rate lithium storage. J. Power Sources. 2014;251:386–392. doi: 10.1016/j.jpowsour.2013.11.034. [DOI] [Google Scholar]
- 61.Dong G.H., Mao Y.Q., Li Y.Q., Huang P., Fu S.Y. M Xene-carbon nanotubes-cellulose-LiFePO4 based self-supporting cathode with ultrahigh-area-capacity for lithium-ion batteries. Electrochim. Acta. 2022;420:140464. doi: 10.1016/j.electacta.2022.140464. [DOI] [Google Scholar]
- 62.Checko S., Ju Z.Y., Zhang B.W., Zheng T.R., Takeuchi E.S., Marschilok A.C., Takeuchi K.J., Yu G.H. Fast-charging, binder-free lithium battery cathodes enabled via multidimensional conductive networks. Nano Lett. 2024;24:1695–1702. doi: 10.1021/acs.nanolett.3c04437. [DOI] [PubMed] [Google Scholar]
- 63.Wu X.L., Guo Y.G., Su J., Xiong J.W., Zhang Y.L., Wan L.J. Carbon-nanotube-decorated nano-LiFePO4@C cathode material with superior high-rate and low-temperature performances for lithium-ion batteries. Adv. Energy Mater. 2013;3:1155–1160. doi: 10.1002/aenm.201300159. [DOI] [Google Scholar]
- 64.Luo G.Y., Gu Y.G., Liu Y., Chen Z.L., Huo Y.L., Wu F.Z., Mai Y., Dai X.Y., Deng Y. Electrochemical performance of in situ LiFePO4 modified by N-doped graphene for Li-ion batteries. Ceram. Int. 2021;47:11332–11339. doi: 10.1016/j.ceramint.2020.12.259. [DOI] [Google Scholar]
- 65.Ren Z.G., Qu M.Z., Yu Z.L. Synthesis and electrochemical properties of LiFeP0.5B0.05O4-δ/C cathode materials. J. Inorg. Mater. 2010;25:230–234. doi: 10.3724/SP.J.1077.2010.00230. [DOI] [Google Scholar]
- 66.Islam M.S., Driscoll D.J., Fisher C.A.J., Slater P.R. Atomic-scale investigation of defects, dopants, and lithium transport in the LiFePO4 olivine-type battery material. Chem. Mater. 2005;17:5085–5092. doi: 10.1021/cm050999v. [DOI] [Google Scholar]
- 67.Trinh D.V., Nguyen M.T.T., Dang H.T.M., Dang D.T., Le H.T.T., Le H.T.N., Tran H.V., Huynh C.D. Hydrothermally synthesized nanostructured LiMnxFe1−xPO4 (x=0-0.3) cathode materials with enhanced properties for lithium-ion batteries. Nat. Portf. 2021;11:12280. doi: 10.1038/s41598-021-91881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nie X., Xiong J. Electrochemical properties of Mn-doped nanosphere LiFePO4. JOM. 2021;73:2525–2530. doi: 10.1007/s11837-021-04753-4. [DOI] [Google Scholar]
- 69.Sin B.C., Lee S.U., Jin B.S., Kim H.S., Kim J.S., Lee S.I., Noh J., Lee Y. Experimental and theoretical investigation of fluorine substituted LiFe0.4Mn0.6PO4 as cathode material for lithium rechargeable batteries. Solid State Ion. 2014;260:2–7. doi: 10.1016/j.ssi.2014.03.005. [DOI] [Google Scholar]
- 70.Pan X.X., Zhuang S.X., Sun Y.Q., Sun G.X., Ren Y., Jiang S.U. Research progress of modified-LiFePO4 as cathode materials for lithium-ion batteries. Inorg. Chem. Ind. 2023;55:18–26. [Google Scholar]
- 71.Zhang H.H., Zou Z.G., Zhang S.C., Liu J., Zhong S.L. A review of the doping modification of LiFePO4 as a cathode material for lithium-ion batteries. Int. J. Electrochem. Sci. 2020;15:12041–12067. doi: 10.20964/2020.12.71. [DOI] [Google Scholar]
- 72.Wu T., Liu J., Sun L., Cong L., Xie H., Ghany A.A., Mauger A., Julien C.M. V-insertion in Li(Fe, Mn)FePO4. J. Power Sources. 2018;383:133–143. doi: 10.1016/j.jpowsour.2018.01.086. [DOI] [Google Scholar]
- 73.Strobridge F.C., Liu H., Leskes M., Borkiewicz O.J., Wiaderek K.M., Chupas P.J., Chapman K.W., Grey C.P. Unraveling the complex delithiation mechanisms of olivine-type cathode materials, LiFexCo1−xPO4. Chem. Mater. 2016;28:3676–3690. doi: 10.1021/acs.chemmater.6b00319. [DOI] [Google Scholar]
- 74.Qing R., Yang M.C., Meng Y.S., Sigmund W. Synthesis of LiNixFe1−xPO4 solid solution as cathode materials for lithium ion batteries. Electrochim. Acta. 2013;108:827–832. doi: 10.1016/j.electacta.2013.07.032. [DOI] [Google Scholar]
- 75.Wang Y.M., Wang Y.J., Liu X.Y., Zhu B., Wang F. Solvothermal synthesis of LiFe1/3Mn1/3Co1/3PO4 solid solution as lithium storage cathode materials. RSC Adv. 2017;7:14354–14359. doi: 10.1039/C7RA01396E. [DOI] [Google Scholar]
- 76.Amin R., Lin C.T., Maier J. Aluminium-doped LiFePO4 single crystals. Part II. Ionic conductivity, diffusivity and defect model. Phys. Chem. Chem. Phys. 2008;10:3524–3529. doi: 10.1039/b801795f. [DOI] [PubMed] [Google Scholar]
- 77.Meethong N., Kao Y.H., Speakman S.A., Chiang Y.M. Aliovalent substitutions in olivine lithium iron phosphate and impact on structure and properties. Adv. Funct. Mater. 2009;19:1060–1070. doi: 10.1002/adfm.200801617. [DOI] [Google Scholar]
- 78.Zhang D.X., Wang J., Dong K.Z., Hao A. First principles investigation on the elastic and electronic properties of Mn, Co, Nb, Mo doped LiFePO4. Comput. Mater. Sci. 2018;155:410–415. doi: 10.1016/j.commatsci.2018.09.010. [DOI] [Google Scholar]
- 79.Ban C.M., Yin W.J., Tang H.W., Wei S.H., Yan Y.F., Dillon A.C. A novel codoping approach for enhancing the performance of LiFePO4 cathodes. Adv. Energy Mater. 2012;2:1028–1032. doi: 10.1002/aenm.201200085. [DOI] [Google Scholar]
- 80.Wagemaker M., Ellis B.L., Hecht D.L., Mulder F.M., Naza L.F. Proof of supervalent doping in olivine LiFePO4. Chem. Mater. 2008;20:6313–6315. doi: 10.1021/cm801781k. [DOI] [Google Scholar]
- 81.Altin S., Coban M., Altundag S., Altin E. Production of Eu-doped LiFePO4 by glass-ceramic technique and investigation of their thermal, structural, electrochemical performances. J. Mater. Sci. Mater. Electron. 2022;33:13720–13730. doi: 10.1007/s10854-022-08305-7. [DOI] [Google Scholar]
- 82.Zhang Q.Y., Zhou J., Zeng G.C., Ren S. Effect of lanthanum and yttrium doped LiFePO4 cathodes on electrochemical performance of lithium-ion battery. J. Mater. Sci. 2023;58:8463–8477. doi: 10.1007/s10853-023-08542-z. [DOI] [Google Scholar]
- 83.Hu J.Z., Zhao X.B., Yu H.M., Zhou X., Cao G.S., Tu J.P. The conductivity and electrochemical perform- ance of lanthanon doped lithium iron phosphate cathode material. J. Funct. Mater. 2007;38:1394–1397. [Google Scholar]
- 84.Liu Z.L., Zhang Z.Z., Zhu Y.M., Gao P. Progress in rare earth materials applied in cathode materials of Li-ion battery. Battery Bimon. 2019;49:520–523. [Google Scholar]
- 85.Chen H., Xiang K.X., Gong W.Q., Liu J.H. Effect of rare earth ions doping on the structure and performance of LiFePO4. Rare Met. Mater. Eng. 2011;40:1937–1940. [Google Scholar]
- 86.Bai Y.M., Qiu P., Han S.C. Synthesis and properties of Y-doped LiFePO4 as cathode material for lithium-ion batteries. Rare Met. Mater. Eng. 2011;40:917–920. [Google Scholar]
- 87.Qiu P., Han S.C., Bai Y.M. Structure and electrochemical properties of LiFePO4/C doped with La3+, Nd3+, Y3+ Chin. J. Power Sources. 2011;35:780–783. [Google Scholar]
- 88.Jiang A., Wang X.L., Gao M.S., Wang J.X., Liu G.X., Yu W.S., Zhang H.B., Dong X.T. Enhancement of electrochemical properties of niobium-doped LiFePO4/C synthesized by sol-gel method. J. Chin. Chem. Soc. 2018;65:977–981. doi: 10.1002/jccs.201700423. [DOI] [Google Scholar]
- 89.Zhao X., Tang X.Z., Zhang L., Zhao M.S., Zhai J. Effects of neodymium aliovalent substitution on the structure and electrochemical performance of LiFePO4. Electrochim. Acta. 2010;55:5899–5904. doi: 10.1016/j.electacta.2010.05.042. [DOI] [Google Scholar]
- 90.Zhang Q.M., Qiao Y.Q., Zhao M.S., Wang L.M. Structure and electrochemical properties of Sm-doped lithiumiron phosphate cathode materials. Chin. J. Inorg. Chem. 2012;28:67–73. doi: 10.1016/j.inoche.2012.06.009. [DOI] [Google Scholar]
- 91.Wang C.X., Xiang K.X., Gong W.Q., Chen H., Zeng J., Ji X.X. Synthesis and performance of cathode materials Li0.97Re0.01FePO4 for lithium-ion batteries. J. Hunan Univ. Technol. 2010;24:5–8. [Google Scholar]
- 92.Zhang Q.M., Qiao Y.Q., Zhao M.S., Wang L.M. Structure and electrochemical properties of Yb-doped lithium iron phosphate cathode materials. J. Chin. Soc. Rare Earths. 2012;30:78–85. [Google Scholar]
- 93.Liang M.M., Wang L.L., Wang J. Preparation and electrochemical performance test of rare earth gadolinium and yttrium doped LiFePO4 cathode materials. Appl. Chem. Ind. 2017;46:829–834. [Google Scholar]
- 94.Zhang B.F., Xu Y.L., Wang J., Lin J., Wang C., Chen Y.J. Lanthanum and cerium Co-doped LiFePO4: Morphology, electrochemical performance and kinetic study from −30–+50 °C. Electrochim. Acta. 2019;322:134686. doi: 10.1016/j.electacta.2019.134686. [DOI] [Google Scholar]
- 95.Cui Z.H., Guo X., Ren J.Q., Xue H.T., Tang F.L., La P.Q., Li H., Li J.C., Lu X.F. Enhanced electrochemical performance and storage mechanism of LiFePO4 doped by Co, Mn and S elements for lithium-ion batteries. Electrochim. Acta. 2021;388:138592. doi: 10.1016/j.electacta.2021.138592. [DOI] [Google Scholar]
- 96.Wang H.Q., Lai A.J., Huang D.Q., Chu Y.Q., Hu S.J., Pan Q.C., Liu Z.H., Zheng F.H., Huang Y.G., Li Q.Y. Y-F co-doping behavior of LiFePO4/C nanocomposites for high-rate lithium-ion batteries. New J. Chem. 2021;45:5695–5703. doi: 10.1039/D0NJ06081J. [DOI] [Google Scholar]
- 97.Chen J.H., Onah O., Cheng Y., Silva K., Choi C., Chen W.Y., Xu S.C., Eddy L., Han Y., Yakobson B., et al. Fast-charging lithium iron phosphate cathodes by flash carbon coating. ChemRxiv. 2024 doi: 10.26434/chemrxiv-2024-7325z. [DOI] [PubMed] [Google Scholar]
- 98.Chuang H.C., Teng J.W., Kuan W.F. Supercritical CO2-enhanced surface modification on LiFePO4 cathodes through ex-situ carbon coating for lithium-ion batteries. Colloids Surf. A. 2024;684:133110. doi: 10.1016/j.colsurfa.2023.133110. [DOI] [Google Scholar]
- 99.Luo J.W., Zhang J.C., Guo Z.X., Liu Z.D., Wang C.Y., Jiang H.R., Zhang J.F., Fan L.L., Zhu H., Xu Y.H., et al. Coupling antisite defect and lattice tensile stimulates facile isotropic Li-ion diffusion. Adv. Mater. 2024;36:2405956. doi: 10.1002/adma.202405956. [DOI] [PubMed] [Google Scholar]
- 100.Wang J.W., Ji S.J., Han Q.G., Wang F.Q., Sha W.X., Cheng D.P., Zhang W.X., Tang S., Cao Y.C., Cheng S.J. High performance of regenerated LiFePO4 from spent cathodes via an in situ coating and heteroatom-doping strategy using amino acids. J. Mater. Chem. A. 2024;12:15311–15320. doi: 10.1039/D4TA01098A. [DOI] [Google Scholar]
- 101.Gou Y.J., Zhang J.Y., Liu X., Zhou Z.H., Zhang M.D., Song L., Jin Y.C. A highly efficient additive for direct reactivation of waste LiFePO4 with practical electrochemical performance. Energy Fuels. 2024;38:6518–6527. doi: 10.1021/acs.energyfuels.4c00329. [DOI] [Google Scholar]
- 102.Li X.G., Wang M.Y., Zhou Q.B., Ge M., Zhang M.D., Liu W.F., Shi Z.P., Yue H.Y., Zhang H.S., Yin Y.H., et al. The prilling and cocoating collaborative strategy to construct high performance of regeneration LiFePO4 materials. ACS Mater. Lett. 2024;6:640–647. doi: 10.1021/acsmaterialslett.3c01161. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.