Abstract
Fibrinogen-dependent interactions of Staphylococcus aureus are believed to contribute to bacterial virulence by promoting bacterial attachment to fibrinogen-coated surfaces and inducing the formation of bacterial clumps that are likely resistant to phagocytosis. Although S. aureus produces several fibrinogen-binding proteins, the cell wall-associated protein clumping factor (encoded by clfA) appears to be most important in bacterial interactions with immobilized or soluble purified fibrinogen. We have compared bacterial clumping in several strains of S. aureus, including isogenic ClfA+ and ClfA− Newman strains, in the presence of purified rabbit fibrinogen, human plasma, and inflammatory fluid and examined the effect of clumping on bacterial sensitivity to mammalian group IIA phospholipase A2 (PLA2). This enzyme is the major extracellular bactericidal agent in inflammatory fluid active against S. aureus. Both ClfA-dependent and ClfA-independent bacterial clumping was observed, depending on the source and fibrinogen content of the biological fluid. In each case, clumping only partially reduced the antibacterial activity of PLA2, suggesting that this extracellular enzyme can substantially penetrate dense bacterial clumps. Bacterial clumps could be dispersed by added proteases, restoring full antibacterial activity to PLA2. Thus, the extracellular mobilization of group IIA PLA2 during inflammation may provide a mechanism by which the host can control the proliferation and survival of S. aureus even after bacterial clumping.
Staphylococcus aureus is an opportunistic pathogen that causes many different infections, including endocarditis, septicemia, abscesses, and catheter-related infections. A major characteristic of S. aureus is its ability to form bacterial aggregates (clumps) while adherent to host tissue or even in suspension. Bacteria are often found in clumps within abscesses and endocarditis vegetations (16, 17). Most clumping is mediated through fibrinogen-binding proteins that allow bacteria to bind to fibrinogen at these sites (4, 24, 25). S. aureus contains several fibrinogen-binding proteins, including coagulase (Coa) (25, 34), Map (26), Efb (2, 3), fibrinogen-binding protein (Fbp) (5), and clumping factors A and B (ClfA and ClfB) (24, 30). Data currently available suggest that, of the various surface-associated fibrinogen-binding proteins, ClfA has the highest affinity for fibrinogen (Kd = 9.9 × 10−9 [7, 13, 24]) and therefore may be expected to play a prominent role in fibrinogen-dependent clumping at low fibrinogen concentrations. Most clinical isolates of S. aureus are clumping factor positive (18, 23, 36, 37, 40, 41). Clumping may be advantageous to the bacteria by reducing susceptibility to host defense mechanisms such as phagocytosis by polymorphonuclear leukocytes (16). Because clumped bacteria are likely too large to be phagocytosed, the host may depend more on extracellular defenses to eliminate clumped bacteria.
Group IIA phospholipase A2 (PLA2) is an important component of extracellular defenses (11, 35, 47–49). PLA2s are ubiquitous enzymes that catalyze the hydrolysis of the sn-2 fatty acyl bonds of phospholipids, liberating free fatty acids and lysophospholipids. The mammalian secretory 14-kDa group IIA PLA2 is mobilized at sites of inflammation and exerts potent antibacterial activity against gram-positive bacteria, including S. aureus (47, 48), which is not exhibited by other, closely related PLA2s. The potent extracellular antibacterial activity of inflammatory fluid against S. aureus is accounted for almost entirely by the presence of group IIA PLA2 (100 to 1,000 ng/ml) (47, 48).
In this study, we have examined the clumping of S. aureus in the presence of purified fibrinogen, plasma, and a cell-free inflammatory fluid and the effects of clumping on bacterial susceptibility to extracellular group IIA PLA2. We show that clumped bacteria remain susceptible to the bactericidal action of PLA2, suggesting that PLA2 may aid in the destruction of clumped bacteria.
MATERIALS AND METHODS
Bacteria.
The S. aureus strains used in this study, Newman ClfA+ and Newman ClfA−, were obtained from Timothy Foster (Trinity College, Dublin, Ireland) and have been described elsewhere (8, 25). Strains 3, 5A, and 18 are clinical isolates from Tisch Hospital Clinical Microbiology Laboratory (New York, N.Y.). RN450 (8325-4) (31) was obtained from Barry Kreiswirth (Public Health Research Institute, New York, N.Y.). Bacteria were grown overnight at 37°C in Trypticase soy broth (TSB; Difco, Detroit, Mich.), washed in sterile physiological saline, and diluted to 1.5 × 107 bacteria/ml in fresh medium for subculture. Bacteria were subcultured to mid-log phase, washed, and resuspended in saline to a final concentration of 109/ml.
Materials.
Purified rabbit fibrinogen, proteinase K, plasmin, plasminogen, V8 protease, and pronase were obtained from Sigma Chemical Co. (St. Louis, Mo.). Sheep anti-rabbit fibrinogen was obtained from Enzyme Research Laboratories, Inc., South Bend, Ind. RPMI was obtained from BioWhittaker, Walkersville, Md. Blood was collected from healthy donors, with informed consent, into citrated, siliconized tubes and centrifuged to obtain human plasma. PLA2 was purified from the cell-free (ascitic) fluid (AF) of glycogen-elicited inflammatory rabbit peritoneal exudates as previously described (48). PLA2 and other cationic proteins were quantitatively removed by adsorption of AF to CM-Sephadex as previously described (48). The resulting “PLA2-depleted AF” contains >99% of the total AF protein. AF filtrate (<1% of the protein content of AF), which has the same electrolyte and small-molecule content as AF, was prepared by ultrafiltration of PLA2-depleted AF through Centriprep-10 filters (Amicon, Beverly, Mass.). Filtrate was supplemented with 1% albumin (wt/vol) before use to match the overall protein concentration of AF.
Assay of bacterial clumping.
The ability of certain fluids to produce bacterial clumping was assessed by microscopic examination and by assay of bacterial CFU. Assay mixtures typically contained 108 bacteria/ml and AF filtrate or RPMI supplemented with 1% albumin and 20 mM HEPES (pH 7.4). Various amounts of purified fibrinogen, PLA2-depleted AF, or human plasma were added to individual assay mixtures. Mixtures were incubated at 37°C. CFU was measured by diluting samples in sterile saline and plating in Trypticase soy agar (Difco). Bacterial colonies were enumerated after incubation at 37°C for 18 to 24 h.
Immunoelectrophoresis.
Laurell Rocket immunoelectrophoresis was performed as previously described (21). In brief, twofold serial dilutions of rabbit fibrinogen (starting concentration, 5 mg/ml) and PLA2-depleted AF in saline were electrophoresed overnight at 70 V in a 1% agarose gel containing 90 μg of sheep anti-rabbit fibrinogen/ml. The running buffer was 7 mM Tris-Tricine buffer (pH 8.6). The gel was washed twice in saline and once in distilled water. The gel was pressed, dried, and stained with Coomassie blue. The peak heights obtained with purified rabbit fibrinogen were used to create a standard curve, and the amount of fibrinogen in PLA2-depleted AF was determined by comparison to the standard curve. In agreement with results obtained by Hawiger et al. (12), the amount of fibrinogen in PLA2-depleted AF was determined to be ∼300 μg/ml.
Assay of effect of added protease(s) on clumped bacteria.
Bacteria (108/ml) were incubated with purified rabbit fibrinogen (12 μg/ml) in AF filtrate for 60 min to allow clumping to occur. Various concentrations (0 to 100 μg/ml) of different proteases (proteinase K, pronase, plasmin, and plasminogen) were added to clumped bacteria and incubated at 37°C for 15 min, followed by an assay of bacterial colony-forming capabilities as described above.
Radiolabeling of lipids of S. aureus.
S. aureus phospholipids were radiolabeled by growth in TSB supplemented with 1 μCi of 14C-oleate/ml and 0.1% albumin as previously described (48). In brief, washed bacteria were subcultured (1.5 × 107/ml) in 2 ml of TSB with 2 μCi of 14C-oleate for 2 1/2 h at 37°C. Bacteria were washed and resuspended in fresh TSB and further incubated for 20 min. Bacteria were then washed in TSB containing 0.5% albumin (to remove unesterified free fatty acids) and resuspended in saline to a final concentration of 109/ml.
Measurement of degradation of labeled phospholipids from S. aureus.
Labeled bacteria (Newman ClfA+ or ClfA−) were incubated at 37°C for 60 min in the typical assay mixtures, supplemented as indicated with purified rabbit fibrinogen or with PLA2-depleted AF containing fibrinogen (0 to 270 μg/ml) or 90% human plasma, to allow clumping to occur. Purified rabbit group IIA PLA2 was then added, and bacteria were further incubated at 37°C for 60 min. The action of PLA2 against S. aureus was assayed as the enzyme-triggered release of 14C-oleate-labeled material, which reflects the breakdown of membrane phospholipids and the capture of phospholipid breakdown products by extracellular albumin.
Visualization of clumped bacteria by light microscopy.
Aliquots of assay mixtures (10 μl) were routinely Gram stained (Bacto 3 step kit; Difco), and clumping was assessed microscopically.
Statistics.
One-tailed t tests were performed where indicated. P values of <0.05 were considered statistically significant.
RESULTS
Clumping of ClfA+ and ClfA− S. aureus in biological fluids: role of bacterial and fibrinogen concentrations.
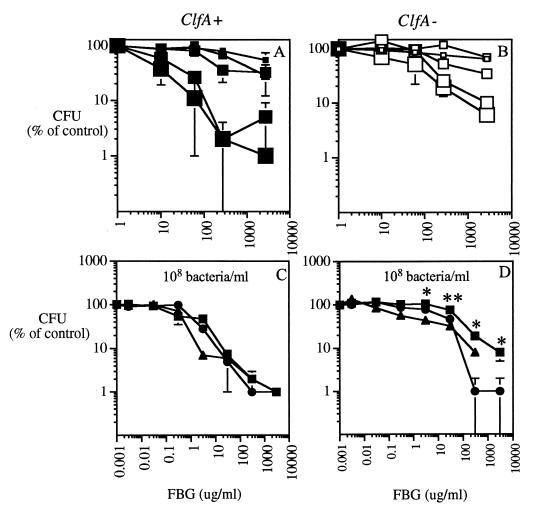
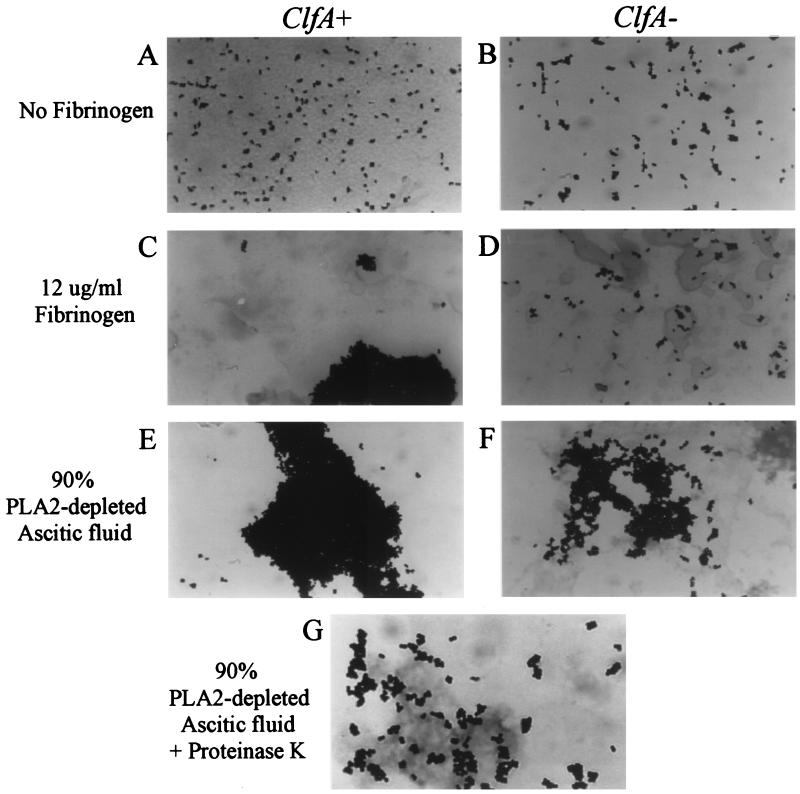
In previous studies, fibrinogen-S. aureus interactions were assessed by measuring bacterial adherence to fibrinogen-coated surfaces (1, 6, 25, 27, 43, 44), but few studies have analyzed bacterial clumping in solution. Therefore, to define experimental conditions in which clumping of S. aureus occurred, initial experiments were carried out in which the clumping of isogenic strains of ClfA+ and ClfA− S. aureus was measured in the presence of purified fibrinogen, PLA2-depleted inflammatory AF, and human plasma by using different starting bacterial concentrations. Because clumping reflects (fibrinogen-mediated) cross-linking of bacteria, it likely depends on bacterial as well as fibrinogen concentrations. Figure 1 shows the fibrinogen and bacterial concentration dependence of clumping of the Newman ClfA+ and ClfA− strains. Both the rate (data not shown) and the extent of bacterial clumping in the presence of purified rabbit fibrinogen or more complex biological fluids were greater at higher bacterial concentrations (greatest at 108/ml, intermediate at 107/ml, and least at 106/ml) and higher fibrinogen concentrations (Fig. 1). No clumping occurred in the absence of fibrinogen (i.e., in filtrate alone). Differences in clumping between the ClfA+ and ClfA− strains were most pronounced at lower fibrinogen concentrations. Visualization of the bacteria by light microscopy confirmed the differences in clumping between ClfA+ and ClfA− bacteria at low-intermediate concentrations of fibrinogen and revealed a remarkably dense three-dimensional network of aggregated bacteria at higher fibrinogen concentrations, again more pronounced in ClfA+ bacteria (Fig. 2).
FIG. 1.
Comparison of bacterial clumping in purified rabbit fibrinogen, PLA2-depleted AF, and human plasma. Bacteria (ClfA+ [A and C] or ClfA− [B and D]) were incubated at 37°C for 60 min with increasing doses of purified rabbit fibrinogen (FBG) in buffered AF filtrate (A and B) or with increasing doses of purified fibrinogen (squares), PLA2-depleted AF (triangles), or human plasma (circles) in buffered AF filtrate (C and D). (A and B) Sizes of squares, from smallest to largest, correspond to increasing bacterial concentrations of 106, 3 × 106, 107, 3 × 107, and 108/ml. (C and D) The concentration of bacteria was 108/ml. Doses of purified fibrinogen, PLA2-depleted AF, and plasma added are represented according to the amount of fibrinogen added. After incubation, bacterial CFU were measured as described in Materials and Methods; bacterial clumping is manifest as reduced CFU. Each result is expressed as the percentage of the CFU of bacteria incubated in AF filtrate without added fibrinogen and represents the mean ± standard error of the mean (SEM) of at least three independent determinations. Asterisks highlight experimental conditions in which clumping of the ClfA+ strain is significantly greater than that of the ClfA− strain (∗, P < 0.05; ∗∗, P < 0.0005).
FIG. 2.
Visual appraisal of clumping. Aliquots (10 μl) of assay mixtures were Gram stained and visualized by light microscopy as described in Materials and Methods. The Newman ClfA+ (A, C, and E) and ClfA− (B, D, and F) strains (108 bacteria/ml) are shown after 60 min of incubation in AF filtrate, AF filtrate plus 12 μg of fibrinogen/ml, or 90% PLA2-depleted AF, respectively. (G) Newman ClfA+ strain incubated for 60 min in 90% PLA2-depleted AF and then for 15 min with proteinase K (10 μg/ml).
Comparison of bacterial clumping induced by purified fibrinogen, PLA2-depleted AF, or plasma showed that bacterial clumping was similar in all three media when similar amounts of fibrinogen were present. Clumping of ClfA+ bacteria (as assessed by reduced bacterial CFU and visual inspection) was induced at concentrations of fibrinogen 25 to 100 times lower (Fig. 1) than those required for clumping of the ClfA− strain.
Clumping of other strains of S. aureus.
The Newman strain has been used in many studies of fibrinogen-dependent adherence and clumping of S. aureus because it expresses high levels of clumping factor (8). Therefore, we examined the clumping of a few other strains of S. aureus, including three randomly chosen clinical isolates (Table 1). The extent of bacterial clumping in media containing purified fibrinogen or in more complex biological fluids varied depending on the bacterial strain. One clinical strain (strain 18) exhibited clumping properties, in each of the media tested, similar to those of the ClfA+ Newman strain.
TABLE 1.
Clumping of several S. aureus strains in various mediaa
| Resultb in:
| ||||||
|---|---|---|---|---|---|---|
| Strain | Purified rabbit fibrinogen in AF filtrate at:
|
90% PLA2-depleted AF | 90% Human plasma | |||
| 0 μg/ml | 10 μg/ml | 270 μg/ml | 2.7 mg/ml | |||
| 3 | 156 ± 29 | 55 ± 12 | 31 ± 7 | 37 ± 31 | 51 ± 10 | 39 ± 13 |
| 5A | 202 ± 58 | 181 ± 36 | 82 ± 20 | 36 ± 12 | 38 ± 13 | 47 ± 19 |
| 18 | 257 ± 60 | 31 ± 3 | 16 ± 6 | 9 | 22 ± 2 | 2.5 |
| RN450 | 165 ± 9 | 141 ± 35 | 58 ± 24 | 18 | 24 ± 12 | 13.5 |
| Newman ClfA− | 173 ± 35 | 135 ± 27 | 17 ± 4 | 7 ± 2 | 34 ± 8 | 1 |
| Newman ClfA+ | 194 ± 54 | 27 ± 8 | 5 ± 2 | 2 ± 1 | 23 ± 7 | 0 |
Several strains of S. aureus at 108 bacteria/ml were incubated in the media indicated for 60 min, and CFU were assessed.
Expressed as the percentage of the CFU of the initial inoculum. Each result is the mean ± SEM of at least three independent determinations.
Susceptibility of clumped bacteria to PLA2.
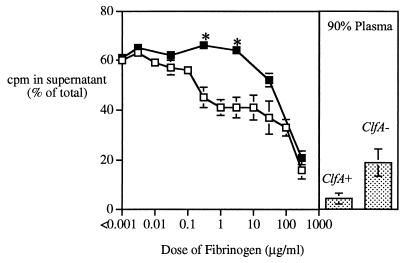
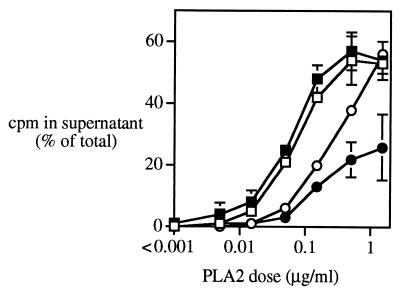
Since clumped bacteria are likely to be refractory to phagocytosis (16), we sought to test the susceptibility of clumped bacteria to group IIA PLA2, a potent member of the extracellular host defense arsenal. Figure 3 shows that fibrinogen produces dose-dependent inhibition of PLA2 activity against both ClfA+ and ClfA− Newman strains of S. aureus. At lower fibrinogen concentrations, PLA2 activity was reduced against the ClfA+ strain only (Fig. 3). However, at high concentrations of purified fibrinogen or in 90% human plasma (≥270 μg/ml), when clumping of both ClfA+ and ClfA− bacteria is similar (Fig. 1), the activity of PLA2 against both strains was significantly reduced. Little or no inhibitory effect of fibrinogen (or PLA2-depleted AF) on PLA2 activity was seen when incubations were carried out with bacterial concentrations too low for clumping (Fig. 4). Thus, the reduced sensitivity of bacteria to PLA2 reflects bacterial clumping and not simply the effect of fibrinogen binding to the bacterial surface. It should be noted that PLA2 retains appreciable activity even against densely clumped bacteria and that degradation of ≥50% of the membrane phospholipids of the entire bacterial population can be produced when higher concentrations of PLA2 are added (Fig. 4) (i.e., within the range of PLA2 concentrations mobilized at inflamed sites [19, 28, 29, 47, 48]).
FIG. 3.
Effect of bacterial clumping on susceptibility of S. aureus to rabbit group IIA PLA2. 14C-oleate-labeled bacteria (108 bacteria/ml; Newman ClfA+ [open squares] or ClfA− [solid squares]) were incubated in AF filtrate with increasing amounts of rabbit fibrinogen added in purified form or as part of PLA2-depleted AF (12.5 ng/ml to 270 μg/ml) or with 90% plasma for 60 min. PLA2 (500 ng/ml) was then added, and bacteria were further incubated for 60 min. The level of phospholipid degradation was assessed by measuring the release of labeled material into the supernatant after the bacteria had been pelleted. Results shown are means ± SEMs of at least three independent determinations. Results obtained in AF filtrate plus purified fibrinogen or PLA2-depleted AF were essentially the same and are therefore combined to simplify the presentation. Note that phospholipids represent about 70% of bacterial 14C-oleate-labeled material (48). Hence, release of ca. 60% of total counts per minute into the supernatant corresponds to nearly quantitative degradation of bacterial phospholipids. Asterisks highlight conditions under which phospholipid hydrolysis of the ClfA− strain is significantly greater than that of the ClfA+ strain (P < 0.007).
FIG. 4.
The Newman ClfA+ strain (14C-oleate labeled; 1 × 106 [squares] or 3 × 107 [circles] bacteria/ml) was incubated with AF filtrate alone (open symbols) or AF filtrate plus 270 μg of fibrinogen/ml (solid symbols) for 60 min. Increasing amounts of PLA2 were then added, and bacteria were further incubated for 60 min. The level of phospholipid degradation was measured as described in Materials and Methods and in the legend to Fig. 3. Results shown are means ± SEMs of at least three independent determinations. Note that fibrinogen caused extensive clumping of bacteria at the higher but not at the lower bacterial concentration (see Fig. 1).
Reversal of fibrinogen-induced bacterial clumping by the addition of proteases.
The ability of PLA2 added to the medium to degrade the membrane phospholipids of densely clumped S. aureus suggested that the fibrinogen attached to S. aureus could be susceptible to added proteases and hence that added proteases could reverse bacterial clumping. The addition of proteinase K, pronase, or plasmin to clumped bacteria promptly reversed bacterial clumping in a dose-dependent fashion (data not shown). Reversal of clumping was manifest by visual appraisal (Fig. 2G) and restoration of CFU (Table 2). Maximum effects occurred with ≥10 μg of protease/ml. Plasminogen at 10-fold-higher concentrations also reversed clumping. After protease treatment (Table 2; Fig. 5), the CFU of previously clumped bacterial suspensions was as great as that of bacteria not exposed to clumping conditions. Thus, bacterial growth continued at a normal rate during bacterial clumping. This was true even when bacteria were clumped for as long as 3 h (data not shown).
TABLE 2.
Reversal of clumping by protease treatmenta
| Additive (concn; incubation time) | Bacterial CFU (%)b |
|---|---|
| Filtrate (no fibrinogen; 75 min) | 246 ± 38 |
| Fibrinogen (12 μg/ml; 60 min) | 12 ± 4 |
| + pronase (10 μg/ml; 15 min) | 271 |
| + proteinase K (10 μg/ml; 15 min) | 281 ± 53 |
| + plasmin (10 μg/ml; 15 min) | 194 ± 20 |
| + plasminogen (100 μg/ml; 15 min) | 169 ± 64 |
Incubations were carried out with 108 bacteria (ClfA+ Newman strain)/ml. In the absence of fibrinogen, added proteases had no effect on bacterial CFU.
Data are expressed as percentages of the CFU of the initial inoculum and are means of at least two determinations.
FIG. 5.
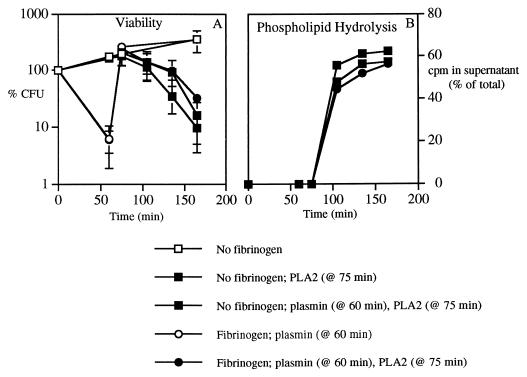
Effects of protease treatment on susceptibility to PLA2. Bacteria (ClfA+; 108 bacteria/ml) were incubated with AF filtrate or AF filtrate plus 270 μg of rabbit fibrinogen/ml for 60 min. Then plasmin (100 μg/ml) was added, and after further incubation for 15 min, PLA2 (1 μg/ml) was added and incubations were continued for an additional 30, 60, or 90 min. CFU and phospholipid hydrolysis were assessed as described in Materials and Methods. Results shown are means ± SEMs of at least three independent determinations. CFU are expressed as percentages of the CFU in the initial inoculum.
Reversal of bacterial clumping by plasmin restores sensitivity to PLA2.
Since the proteases were able to dissolve bacterial clumps, we tested whether protease treatment could also increase bacterial susceptibility to PLA2. As shown in Fig. 5, plasmin rendered previously clumped bacteria as susceptible to the bactericidal (Fig. 5A) and phospholipolytic (Fig. 5B) activities of PLA2 as bacteria not previously exposed to fibrinogen. Plasmin had no effect on PLA2 activity toward bacteria normally dispersed in suspension (Fig. 5B). Thus, the effect of plasmin on PLA2 activity apparently reflects its effect on bacterial clumping.
DISCUSSION
The principal goal of this study was to determine the effect of clumping on bacterial sensitivity to the antibacterial action of group IIA PLA2. This study was made possible by our ability to evaluate bacterial clumping in a semiquantitative fashion by measuring decreases in CFU and by microscopic analysis.
Clumping of S. aureus has long been studied as a potential virulence factor. Numerous studies have shown that S. aureus can bind to several extracellular matrix proteins (laminin, collagen, fibrinogen, and fibronectin) through microbial surface components recognizing adherent matrix molecules (MSCRAMMs) (9, 10, 14, 15, 33, 42, 46). Fibrinogen appears to be the predominant host matrix protein involved in clumping and adherence to surfaces such as catheters and prosthetic devices (e.g., heart valves) (1, 6, 25, 27, 43, 44). Clumping and adherence to surfaces containing bound fibrinogen are chiefly mediated by clumping factor (ClfA), apparently the highest-affinity fibrinogen-binding protein of S. aureus (7, 24, 25, 27, 44). Our results indicate an important role of fibrinogen and ClfA in clumping of the Newman strain in biological fluids. In isogenic strains differing only in ClfA, clumping of the ClfA+ strain was greater. These differences were observed under all conditions but especially at low fibrinogen concentrations (Fig. 1; Table 1), when the high-affinity fibrinogen-binding properties of ClfA are likely most important. In media containing higher levels of fibrinogen, such as undiluted biological fluids, extensive clumping of the ClfA− strain was also observed. Clumping of this strain was similar in medium containing added purified fibrinogen and in more complex biological fluids, provided that similar amounts of fibrinogen were added (Fig. 1; Table 1). These results suggest that ClfA-independent clumping of the Newman strain in biological fluids is also largely fibrinogen dependent and thus is likely mediated by ClfA-independent cell wall-associated fibrinogen-binding proteins such as ClfB (30), Fbp (5), and Map (26).
Examination of several other strains of S. aureus revealed a wide spectrum of clumping behaviors (Table 1). The molecular bases of these differences are unknown but presumably reflect variable expression of MSCRAMMs. In strains 5A and RN450, clumping was greater in biological fluids than in defined medium containing equivalent amounts of fibrinogen (Table 1), suggesting that clumping of these strains in biological fluids may include fibrinogen-independent interactions. Despite the presence of ClfA in strain RN450 (24), the clumping properties of this strain more closely resembled those of the ClfA− mutant derivative of the Newman strain (Table 1). Whether the more potent clumping properties of the wild-type Newman strain reflect higher levels of surface expression of ClfA or other surface properties of the Newman strain that act in concert with ClfA to increase bacterial clumping is unknown and requires further study.
The in vivo importance of adherence and clumping is still in question, but several studies have been performed to address this issue. Recent studies have shown that the lack of certain MSCRAMMs (Cna, FnbA and FnbB, Efb, and ClfA) decreases virulence in certain animal models (27, 32, 33, 38), implying that the presence of MSCRAMMs may increase virulence, perhaps in part by impairing the ability of host defenses to deal effectively with these strains. S. aureus bacteria adherent to polymethyl methacrylate coverslips are more resistant to phagocytosis than bacteria in suspension (45). Bacteria in large clumps also appear to be refractory to ingestion by phagocytes (16, 17). When phagocytosis is impaired, the action of extracellular host defenses may be more critical. Mammalian group IIA PLA2 appears to be the most prominent and potent component of the extracellular host defense arsenal against S. aureus (11, 35, 47, 48). PLA2 is present in inflammatory settings and accumulates in response to bacterial infection (19, 28, 29, 47, 48).
This is the first study to examine the susceptibility of clumped bacteria to an extracellular host defense (e.g., PLA2). Our findings demonstrate that the ability of group IIA PLA2 to cause bacterial phospholipid hydrolysis and killing is diminished by bacterial clumping. This is true both for ClfA-dependent and -independent clumping and when clumping occurs either with purified fibrinogen or in biological fluids (Fig. 3 and 4). Thus, whatever the molecular mechanism, clumping of S. aureus at sites of infection may promote bacterial survival and persistence by reducing the efficacy of both cellular (phagocytosis) and extracellular host defenses. Fibrinogen induced clumping of growing and nongrowing bacteria (Fig. 3 and 4; also data not shown), suggesting that clumping may have protective effects both early in abscess formation and during later stages of persistent infections.
It should be noted, however, that the greater resistance of bacterial clumps to PLA2 can be (partially) overcome when higher concentrations of enzyme are added. The ability of PLA2 to produce extensive bacterial phospholipid degradation under these conditions implies that even dense bacterial clumps are sufficiently porous to allow diffusion of PLA2 to many bacteria within the clump. The protective effects of PLA2 may extend beyond the direct cytotoxic effects of this enzyme. Extensive phospholipid degradation caused by PLA2 may activate autolysins (8a), disrupting the cell wall integrity needed to maintain bacteria-fibrinogen interactions and thereby increasing the likelihood of phagocytosis.
Group IIA PLA2 is not unique in its ability to penetrate bacterial clumps. Proteases gain access to bacterial cross-links and, in so doing, disperse bacterial clumps and render the bacteria more sensitive to extracellular PLA2 and perhaps also to phagocytes (Table 2; Fig. 5). Since proteases (e.g., plasmin) are mobilized during inflammation, they may synergize with PLA2 in this setting, enabling efficient killing and digestion of previously clumped bacteria. Mice lacking urokinase, a plasminogen activator, are more susceptible to local S. aureus infections, consistent with a possible role for plasmin in host defense against S. aureus (39). We demonstrate (Table 2) that, in vitro, bacterial clumps could also be dispersed by added plasminogen, suggesting that the bacteria are secreting a plasminogen activator, such as staphylokinase (20, 22). It would be advantageous to the host to produce plasmin locally, where it could be less susceptible to circulating host plasmin inhibitors. Conversely, the secretion of a plasminogen activator could be advantageous to the bacteria by providing a mechanism for bacterial dissemination after host defenses, mobilized during inflammation, have waned.
In summary, the outcome of S. aureus infection is the result of a race between the rate and extent of bacterial multiplication and the rate of mobilization and potency of host antibacterial defenses. Clumping reduces the efficiency of action of some host defenses, and ClfA accelerates the onset of clumping by reducing fibrinogen and bacterial concentration requirements. Conversely, mobilization of extracellular defenses, including PLA2 together with systems that reduce clumping (e.g., plasmin), permits host antibacterial action even when phagocytes may be less effective. It should be noted that interactions among bacteria are likely superimposed on interactions between bacteria and the host (tissue, matrix, foreign bodies). The effects of such interactions on extracellularly mobilized host defenses will require further study.
ACKNOWLEDGMENTS
We thank Barry Kreiswirth (Public Health Research Institute), Timothy Foster (Trinity College), and Philip Tierno and Ken Inglima (Department of Clinical Microbiology, Tisch Hospital) for making bacterial strains available. We also acknowledge Michael Nardi and Joan Hadzi-Nesic (Department of Pediatrics, Bellevue Hospital, New York, N.Y.) for their help with Laurell Rocket immunoelectrophoresis. We are grateful to Peter Elsbach for his critical review and assistance with the manuscript.
This work was supported by U.S. Public Health Service grant AI 18571.
REFERENCES
- 1.Baumgartner J, Cooper S L. Influence of thrombus components in mediating Staphylococcus aureus adhesion to polyurethane surfaces. J Biomed Mater Res. 1998;40:660–670. doi: 10.1002/(sici)1097-4636(19980615)40:4<660::aid-jbm18>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 2.Boden M K, Flock J I. Cloning and characterization of a gene for a 19-kDa fibrinogen-binding protein from Staphylococcus aureus. Mol Microbiol. 1994;12:599–606. doi: 10.1111/j.1365-2958.1994.tb01046.x. [DOI] [PubMed] [Google Scholar]
- 3.Boden M K, Flock J I. Evidence for three different fibrinogen-binding proteins with unique properties from Staphylococcus aureus strain Newman. Microb Pathog. 1992;12:289–298. doi: 10.1016/0882-4010(92)90047-r. [DOI] [PubMed] [Google Scholar]
- 4.Bodén M K, Flock J I. Fibrinogen-binding protein/clumping factor from Staphylococcus aureus. Infect Immun. 1989;57:2358–2363. doi: 10.1128/iai.57.8.2358-2363.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung A I, Projan S J, Edelstein R E, Fischetti V A. Cloning, expression, and nucleotide sequence of a Staphylococcus aureus gene (fbpA) encoding a fibrinogen-binding protein. Infect Immun. 1995;63:1914–1920. doi: 10.1128/iai.63.5.1914-1920.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung A L, Fischetti V A. The role of fibrinogen in staphylococcal adherence to catheters in vitro. J Infect Dis. 1990;161:1177–1186. doi: 10.1093/infdis/161.6.1177. [DOI] [PubMed] [Google Scholar]
- 7.Dickinson R B, Nagel J A, McDevitt D, Foster T J, Proctor R A, Cooper S L. Quantitative comparison of clumping factor- and coagulase-mediated Staphylococcus aureus adhesion to surface-bound fibrinogen under flow. Infect Immun. 1995;63:3143–3150. doi: 10.1128/iai.63.8.3143-3150.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duthie E S, Lorenz L L. Staphylococcal coagulase: mode of action and antigenicity. J Gen Microbiol. 1952;6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 8a.Foreman A, Weinrauch Y, Elsbach P, Weiss J. Cell-wall determinants of the bactericidal action of group IIA phospholipase A2 against Gram-positive bacteria. J Clin Investig. 1999;103:715–721. doi: 10.1172/JCI5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillaspy A F, Lee C Y, Sau S, Cheung A L, Smeltzer M S. Factors affecting the collagen binding capacity of Staphylococcus aureus. Infect Immun. 1998;66:3170–3178. doi: 10.1128/iai.66.7.3170-3178.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greene C, McDevitt D, Francois P, Vaudaux P E, Lew D P, Foster T J. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnb genes. Mol Microbiol. 1995;17:1143–1152. doi: 10.1111/j.1365-2958.1995.mmi_17061143.x. [DOI] [PubMed] [Google Scholar]
- 11.Harwig S S, Tan L, Qu X D, Cho Y, Eisenhauer P B, Lehrer R I. Bactericidal properties of murine intestinal phospholipase A2. J Clin Investig. 1995;95:603–610. doi: 10.1172/JCI117704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawiger J, Hawiger A, Koenig M G. Staphylococcal clumping and fibrinogen and fibrin degradation products in inflammatory exudate. Proc Soc Exp Biol Med. 1971;136:132–136. doi: 10.3181/00379727-136-35211. [DOI] [PubMed] [Google Scholar]
- 13.Hawiger J, Kloczewiak M, Timmons S, Strong D, Doolittle R F. Interaction of fibrinogen with staphylococcal clumping factor and with platelets. Ann N Y Acad Sci. 1983;408:521–535. doi: 10.1111/j.1749-6632.1983.tb23270.x. [DOI] [PubMed] [Google Scholar]
- 14.Herrmann M, Vaudaux P E, Pittet D, Auckenthaler R, Lew P D, Schumacher-Perdreau F, Peters G, Waldvogel F A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988;158:693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- 15.Hienz S A, Schennings T, Heimdahl A, Flock J I. Collagen binding of Staphylococcus aureus is a virulence factor in experimental endocarditis. J Infect Dis. 1996;174:83–88. doi: 10.1093/infdis/174.1.83. [DOI] [PubMed] [Google Scholar]
- 16.Kapral F A. Clumping of Staphylococcus aureus in the peritoneal cavity of mice. J Bacteriol. 1966;92:1188–1195. doi: 10.1128/jb.92.4.1188-1195.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapral F A, Godwin J R, Dye E S. Formation of intraperitoneal abscesses by Staphylococcus aureus. Infect Immun. 1980;30:204–211. doi: 10.1128/iai.30.1.204-211.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerr S, Kerr G E, Mackintosh C A, Marples R R. A survey of methicillin-resistant Staphylococcus aureus affecting patients in England and Wales. J Hosp Infect. 1990;16:35–48. doi: 10.1016/0195-6701(90)90047-r. [DOI] [PubMed] [Google Scholar]
- 19.Kortekangas P, Aro H T, Nevalainen T J. Group II phospholipase A2 in synovial fluid and serum in acute arthritis. Scand J Rheumatol. 1994;23:68–72. doi: 10.3109/03009749409103030. [DOI] [PubMed] [Google Scholar]
- 20.Lack C. Staphylokinase: an activator of plasma protease. Nature. 1948;161:559–560. doi: 10.1038/161559b0. [DOI] [PubMed] [Google Scholar]
- 21.Laurell C B, McKay E J. Electroimmunoassay. Methods Enzymol. 1981;73:339–369. [Google Scholar]
- 22.Lewis J, Ferguson J H. A proteolytic enzyme system of the blood. III. Activation of dog serum profibrinolysin by staphylokinase. Am J Physiol. 1951;166:594–603. doi: 10.1152/ajplegacy.1951.166.3.594. [DOI] [PubMed] [Google Scholar]
- 23.Matsunaga T, Kamata S, Kakiichi N, Uchida K. Characteristics of Staphylococcus aureus isolated from peracute, acute and chronic bovine mastitis. J Vet Med Sci. 1993;55:297–300. doi: 10.1292/jvms.55.297. [DOI] [PubMed] [Google Scholar]
- 24.McDevitt D, Francois P, Vaudaux P, Foster T J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 25.McDevitt D, Vaudaux P, Foster T J. Genetic evidence that bound coagulase of Staphylococcus aureus is not clumping factor. Infect Immun. 1992;60:1514–1523. doi: 10.1128/iai.60.4.1514-1523.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGavin M H, Krajewska-Pietrasik D, Ryden C, Hook M. Identification of a Staphylococcus aureus extracellular matrix-binding protein with broad specificity. Infect Immun. 1993;61:2479–2485. doi: 10.1128/iai.61.6.2479-2485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreillon P, Entenza J M, Francioli P, McDevitt D, Foster T J, Francois P, Vaudaux P. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect Immun. 1995;63:4738–4743. doi: 10.1128/iai.63.12.4738-4743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nevalainen T J. Serum phospholipases A2 in inflammatory diseases. Clin Chem. 1993;39:2453–2459. [PubMed] [Google Scholar]
- 29.Nevalainen T J, Gronroos J M, Kortesuo P T. Pancreatic and synovial type phospholipases A2 in serum samples from patients with severe acute pancreatitis. Gut. 1993;34:1133–1136. doi: 10.1136/gut.34.8.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni Eidhin D, Perkins S, Francois P, Vaudaux P, Hook M, Foster T J. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol Microbiol. 1998;30:245–257. doi: 10.1046/j.1365-2958.1998.01050.x. [DOI] [PubMed] [Google Scholar]
- 31.Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 32.Palma M, Nozohoor S, Schennings T, Heimdahl A, Flock J I. Lack of the extracellular 19-kilodalton fibrinogen-binding protein from Staphylococcus aureus decreases virulence in experimental wound infection. Infect Immun. 1996;64:5284–5289. doi: 10.1128/iai.64.12.5284-5289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patti J M, Bremell T, Krajewska-Pietrasik D, Abdelnour A, Tarkowski A, Ryden C, Hook M. The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis. Infect Immun. 1994;62:152–161. doi: 10.1128/iai.62.1.152-161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phonimdaeng P, O’Reilly M, Nowlan P, Bramley A J, Foster T J. The coagulase of Staphylococcus aureus 8325-4. Sequence analysis and virulence of site-specific coagulase-deficient mutants. Mol Microbiol. 1990;4:393–404. doi: 10.1111/j.1365-2958.1990.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 35.Qu X-D, Lehrer R I. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infect Immun. 1998;66:2791–2797. doi: 10.1128/iai.66.6.2791-2797.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruzickova V. Characteristics of strains of Staphylococcus aureus isolated at dairy farms. Vet Med (Prague) 1994;39:37–44. [PubMed] [Google Scholar]
- 37.Sanford B A, Thomas V L, Ramsay M A, Jones T O. Characterization of clinical strains of Staphylococcus aureus associated with pneumonia. J Clin Microbiol. 1986;24:131–136. doi: 10.1128/jcm.24.1.131-136.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheld W M, Strunk R W, Balian G, Calderone R A. Microbial adhesion to fibronectin in vitro correlates with production of endocarditis in rabbits. Proc Soc Exp Biol Med. 1985;180:474–482. doi: 10.3181/00379727-180-42205. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro R L, Duquette J G, Nunes I, Roses D F, Harris M N, Wilson E L, Rifkin D B. Urokinase-type plasminogen activator-deficient mice are predisposed to staphylococcal botryomycosis, pleuritis, and effacement of lymphoid follicles. Am J Pathol. 1997;150:359–369. [PMC free article] [PubMed] [Google Scholar]
- 40.Slobodnikova L, Kotulova D, Zahradnikova I. Staphylococcus aureus in chronic and recurrent infections. Folia Microbiol. 1995;40:655–658. doi: 10.1007/BF02818525. [DOI] [PubMed] [Google Scholar]
- 41.Smeltzer M S, Gillaspy A F, Pratt F, Jr, Thames M D, Iandolo J J. Prevalence and chromosomal map location of Staphylococcus aureus adhesin genes. Gene. 1997;196:249–259. doi: 10.1016/s0378-1119(97)00237-0. [DOI] [PubMed] [Google Scholar]
- 42.Valentin-Weigand P, Timmis K N, Chhatwal G S. Role of fibronectin in staphylococcal colonisation of fibrin thrombi and plastic surfaces. J Med Microbiol. 1993;38:90–95. doi: 10.1099/00222615-38-2-90. [DOI] [PubMed] [Google Scholar]
- 43.Vaudaux P, Pittet D, Haeberli A, Huggler E, Nydegger U E, Lew D P, Waldvogel F A. Host factors selectively increase staphylococcal adherence on inserted catheters: a role for fibronectin and fibrinogen or fibrin. J Infect Dis. 1989;160:865–875. doi: 10.1093/infdis/160.5.865. [DOI] [PubMed] [Google Scholar]
- 44.Vaudaux P E, Francois P, Proctor R A, McDevitt D, Foster T J, Albrecht R M, Lew D P, Wabers H, Cooper S L. Use of adhesion-defective mutants of Staphylococcus aureus to define the role of specific plasma proteins in promoting bacterial adhesion to canine arteriovenous shunts. Infect Immun. 1995;63:585–590. doi: 10.1128/iai.63.2.585-590.1995. . (Erratum, 63:3239.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaudaux P E, Zulian G, Huggler E, Waldvogel F A. Attachment of Staphylococcus aureus to polymethylmethacrylate increases its resistance to phagocytosis in foreign body infection. Infect Immun. 1985;50:472–477. doi: 10.1128/iai.50.2.472-477.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vercellotti G M, McCarthy J B, Lindholm P, Peterson P K, Jacob H S, Furcht L T. Extracellular matrix proteins (fibronectin, laminin, and type IV collagen) bind and aggregate bacteria. Am J Pathol. 1985;120:13–21. [PMC free article] [PubMed] [Google Scholar]
- 47.Weinrauch Y, Abad C, Liang N S, Lowry S F, Weiss J. Mobilization of potent plasma bactericidal activity during systemic bacterial challenge. Role of group IIA phospholipase A2. J Clin Investig. 1998;102:633–638. doi: 10.1172/JCI3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinrauch Y, Elsbach P, Madsen L M, Foreman A, Weiss J. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kD phospholipase A2. J Clin Investig. 1996;97:250–257. doi: 10.1172/JCI118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright G W, Ooi C E, Weiss J, Elsbach P. Purification of a cellular (granulocyte) and an extracellular (serum) phospholipase A2 that participate in the destruction of Escherichia coli in a rabbit inflammatory exudate. J Biol Chem. 1990;265:6675–6681. [PubMed] [Google Scholar]