Abstract
Systemic lupus erythematosus (SLE) is characterised by generalised immune dysfunction, including infection susceptibility. Infection-associated flares (IAFs) are common and might rapidly self-resolve, paralleling infection resolution, but their specific clinical phenotype is poorly understood. Therefore, we screened 2039 consecutive visits and identified 134 flares, defined as a loss of the lupus low disease activity state (LLDAS), from 1089 visits at risk spanning over multiple follow-up years, yielding an average yearly LLDAS deterioration rate of 17%. Thirty-eight IAFs were isolated from the total flares and were mostly related to bacterial and herpesvirus infections. When compared to other flares (OFs; n = 98), IAFs showed no milder patterns of organ involvement and similar rates of long-term damage accrual, as estimated by conventional clinimetrics. Arthritis in IAFs was more severe than that in OFs [median (interquartile range) DAS-28 2.6 (2.3–4.1) vs. 2.0 (1.6–2.7); p = 0.02]. Viral IAFs were characterised by atypically lower levels of anti-DNA antibodies (p < 0.001) and possibly abnormally high complement levels when compared to flares of different origin. These data suggest that IAFs are of comparable or even higher severity than OFs and may subtend distinct pathophysiological mechanisms that are poorly tackled by current treatments. Further research is needed to confirm these data.
Keywords: lupus, infections, flare, herpesvirus, LLDAS, remission
1. Introduction
Systemic lupus erythematosus (SLE) is a multi-organ disease with a wide spectrum of clinical presentations, sustained by generalised immune dysfunction [1,2]. In recent years, mechanistic and clinical studies have significantly improved our understanding of the pathophysiology of the disease, paving the way to innovative treatment strategies. The aberrant processing of self-antigens, favoured by permissive human leukocyte antigen repertoires and unsolicited antiviral-like interferon (IFN)-driven mechanisms, has been identified as the core pathogenic event, leading to non-physiological antibody responses and eventual organ damage [3,4,5,6,7,8]. Treatments aiming down- and up-stream of these events have progressively been introduced into clinical practice [9,10,11,12,13]. Nonetheless, patients with SLE still face a significant risk of morbidity and early mortality due to chronic and acute events secondary to disease activity and drug-related side-effects [14,15]. With infections, cardiovascular complications and organ failure due to active disease being the most significant causes of mortality [16], disease exacerbations (or flares) constitute a major driver of organ damage and a prominent therapeutic target for secondary prevention [15,17,18,19,20].
Prolonged remission has progressively emerged as a key clinical predictor of relapse-free survival in the long term [20,21], prompting the definition of accurate criteria to establish its attainment after treatment. In the setting of clinimetrics certifying remission, attainment of the Definitions Of Remission In SLE (DORIS) parameters [22] is currently regarded as the ideal target in the treatment of SLE. The lupus low disease activity state (LLDAS) [23] identifies a slightly broader set of patients with SLE enjoying a condition of limited active inflammation and relatively low treatment burden. Thanks to these characteristics, it may be more sensitive than the DORIS definition in intercepting a significant loss of disease stability in a wider fraction of patients with SLE, including those who have not achieved a complete remission state. Accordingly, it has been successfully validated to assess the occurrence of flares and predict damage accrual [24,25].
Beyond the generic association between failure to achieve remission and the risk of eventual flares, limited information is available about the determinants of lupus exacerbations and about potentially distinct flare profiles. Rising levels of anti-DNA antibodies (ADNAs) are generally associated with disease activity and may herald an incumbent flare in at least a fraction of patients with SLE [20,26]. However, other clinical and serological predictors may also correlate with eventual disease exacerbations, suggesting the existence of multiple pathogenic ways that can lead to the same detrimental phenotypic outcome [27,28,29].
Besides being immediate determinants of morbidity and a potential cause of death in patients with SLE [30,31], infections are also associated with an enhanced risk of disease exacerbation [32]. On the other hand, incomplete disease control and/or suboptimal treatment are also associated with a higher infectious risk in patients with SLE [32,33,34]. In fact, patients with SLE are charged with a disproportionately high susceptibility to infections [35,36], especially those due to airborne pathogens, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [32,36,37,38]. Influenzavirus is a major driver of disease flares due to its widespread diffusion [32]. Parvovirus B19 infection can also cause disease exacerbation, besides constituting a known mimicker of SLE [39,40]. In addition, the reactivation of latent viral agents, including Epstein–Barr virus (EBV), cytomegalovirus (CMV), Varicella Zoster Virus (VZV) and other Herpesviridae, along with endogenous retroviral elements, is also a pathogenic hallmark of SLE [41,42,43]. In particular, EBV has emerged as a major trigger of autoimmune phenomena within and beyond SLE, possibly also due to the molecular mimicry between key autoantigens and EBV components [44,45,46]. All these pathogens might boost SLE-related aberrations in the immune response in multiple ways, including para-physiological stimulation of innate immune mechanisms (such as IFN pathways), molecular mimicry with key endogenous antigens [45] and heterologous T-cell immunity [8,47,48]. Vaccination represents the cornerstone of prevention for patients with SLE. Most local and international guidelines recommend active immunisation of patients with SLE against a broad set of viral and bacterial pathogens in addition to standard vaccinations. Specifically, seasonal viral pathogens (such as influenza and, in more recent times, possibly SARS-CoV-2), along with pneumococcus and human papillomavirus, constitute the core target of vaccination in patients with SLE [49,50]. VZV immunisation with the recent recombinant adjuvanted vaccine is also part of the international recommendations for patients with SLE [50]. Extended immunisation against other capsulate bacteria such as meningococcus and Haemophilus is usually recommended in case of immune suppression, especially with B-cell-depleting strategies [51]. Although the response rates to vaccination can be lower in patients with SLE, due to disease-related immune dysfunction and/or immune suppression, safety and efficacy data support their extensive use to prevent clinically relevant complications [52,53,54,55,56,57]. Nonetheless, vaccination coverage is often disappointingly lower than the targets set by international guidelines [58,59,60]. This contributes to infection susceptibility and eventually to flare proneness in patients with SLE.
Little is known about potential clinical divergences between SLE-related events associated with infectious triggers and fluctuations in disease activity putatively related to other factors. Specifically, very limited information is available on the clinical characteristics of infection-associated flares (IAFs) in comparison with other SLE flares (OFs).
To address this issue, we set up an observational study on a relatively large cohort of patients with SLE longitudinally followed up with the aim of evaluating potential clinical and laboratory differences between IAFs and OFs.
2. Materials and Methods
2.1. Patient and Visit Selection
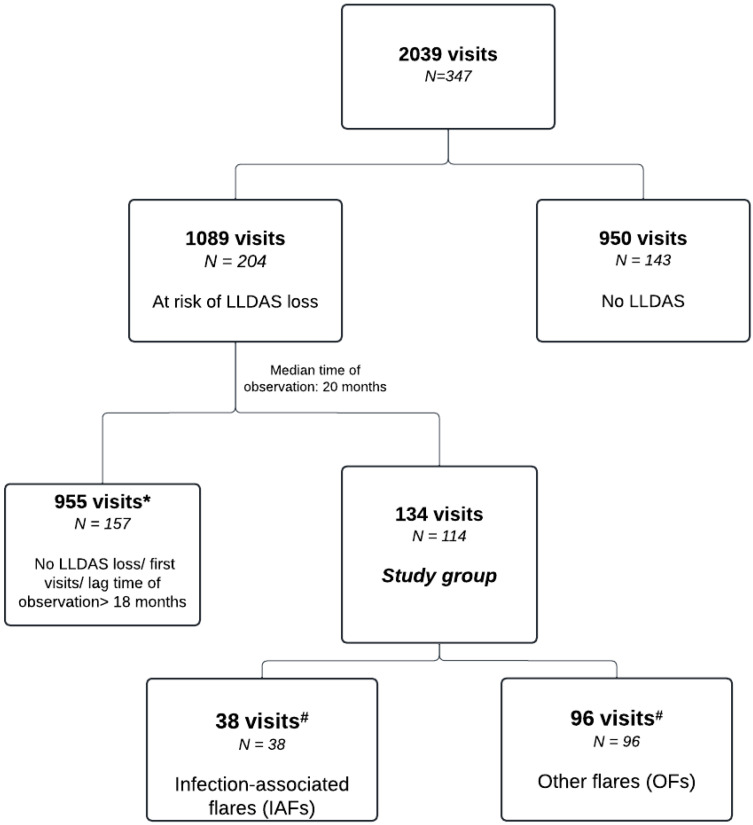
We retrospectively analysed a set of prospectively collected data from 347 patients with SLE who fulfilled the 2019 EULAR/ACR Classification Criteria for Systemic Lupus Erythematous [61] and, upon giving their written informed consent, were consecutively enrolled in the PanImmuno Research Protocol, which conformed to the Declaration of Helsinki and was approved by San Raffaele Institutional Review Board (approval number 22/INT/2018). Specifically, all consecutive visits recorded in the period between July 2015 and December 2023 at San Raffaele University Hospital Lupus Clinic were scrutinised. Longitudinal data were collected through dedicated in-house software built in a Microsoft Excel® 2019 environment [25]. Among the whole set of visits (Figure 1), we identified visit couples consisting of a visit performed at the time of flare and a pre-flare visit, setting the maximum lag time between the two visits at 18 months.
Figure 1.
Study flowchart. *: patients with visits fitting the study criteria were included into this and the “study group”, #: 20 patients had both IAF and OF records.
We defined a disease flare as a loss of the LLDAS [23] across two consecutive outpatient visits. First visits (patients at disease onset or newly referred to the Centre) were excluded. We then dichotomised flares into two groups: flares associated with a recent infection (IAFs), defined as an infectious event occurring between visits and requiring antimicrobial treatment and/or absence from work/school, and flares without any clear evidence of recent infection (OFs). Infections were attributed to a bacterial aetiology in case of proper isolation of non-contaminating bacteria in culture tests, the involvement of typical sites such as the urinary tract, characteristic clinical presentations (such as respiratory tract infections with purulent secretions), increased acute-phase reactants including C-reactive protein (CRP), and/or a response to antibiotics after no response to other treatments. Viral infections were defined as events without the abovementioned bacterial-like features and without evidence of active SLE and/or with serological or genomic evidence of active viral replication. Fungal infections were defined by the presence of typical yeast-related lesions or secretions in typical mycotic sites or positive culture tests. No data were homogeneously available in terms of antimicrobial treatments, since the treatment of infectious complications is usually managed in primary care. Immunosuppressant discontinuation during antimicrobial treatment was advised to all patients.
2.2. Clinical and Laboratory Data
The collected clinical data encompassed demographics, past and current SLE-related manifestations, comorbidities and treatments. SLE severity was measured by using the SLE Disease Activity Index 2000 (SLEDAI-2K) [45] (in terms of the absolute score and variation in the score (delta) across pre-flare and flare visits), the British Isles Lupus Assessment Group (BILAG) 2004 index [46] and a 0.0–3.0 physician global assessment scale (PGA) [62]. Patients’ impressions about their global health status were also quantitated through a 0–10 numerical rating scale (NRS), with 10 corresponding to the highest perceived degree of wellbeing. Chronic damage was measured by employing the SLE International Collaborating Clinics/American College of Rheumatology Damage index (SDI) [47]. Joint disease activity was estimated with the DAS-28 score.
We also collected data on patient vaccination status at the time of flare. Since the recent COVID-19 pandemic has caused major perturbations in health policies, including the introduction of a new set of vaccines, we categorised study flares into flares occurring before or after the pandemic. We considered 1 January 2020 as the starting date of the pandemic.
We used laboratory data acquired in the framework of standard clinical practice (CRP, erythrocyte sedimentation rate—ESR, creatinine, 24 h proteinuria). Due to the high variability among laboratories, complement consumption was coded dichotomously into low vs. normal C3 and/or C4. Anti-double-stranded DNA antibody (ADNA) titres were classified using a 0–4 discrete arbitrary scale to homogenise the results from different laboratory ranges of normality.
2.3. Statistical Analyses
We analysed the intra- and interindividual changes accompanying disease flares among the groups. The data for descriptive statistics are expressed as the median (interquartile range) or percentage unless otherwise specified. Continuous variables were tested for normality by using the Shapiro–Wilk test. Non-normally distributed continuous variables were compared between two groups by using the Mann–Whitney U-test. Intraindividual comparisons were performed by using the Wilcoxon matched-pairs signed rank test. Associations between categorical variables were assessed by performing Chi-square tests. Time-dependent differential outcomes among groups were assessed through Cox regression analysis. All data are presented as the median (interquartile range, IQR) or percentage unless otherwise specified. Data that are presented as stratified results among groups were all tested to identify statistically significant differences. p-values of <0.05 were considered significant and reported, unless otherwise specified. Data were analysed with JASP version 0.19.0 and Statacorp STATA version 15.0.
3. Results
3.1. Demographics and General Clinical Characteristics
Out of 2039 consecutive visits by 347 patients, we identified 1089 visits by patients with previous LLDAS attainment and at risk for deterioration. Over a median time of observation of 20 (9–38) months, 134/1089 (12%) visits from 114 patients met the criteria for an IAF (n = 38 flares) or OF (n = 96 flares; Figure 1). Eighty-four flares occurred before the onset of the COVID-19 pandemic: 35% (n = 29) were IAFs and 65% (n = 55) were OFs.
The average yearly loss rate of the LLDAS was 17% among patients at risk, with higher flare rates during the first years of observations (Supplementary Materials Table S1). Most patients with available visits were women (n = 100/114). The median age of the cohort was 44 (35–52) years. The median follow-up time at the index flare was 30 (13–62) months. The median lag time between the pre-flare and flare visits was 6 (4–8) months. The most frequent clinical manifestations in the patients’ history involved the haematological (82%), mucocutaneous (81%) and musculoskeletal (80%) domains. Eighty-five patients had a history of positive ADNA (79% of IAF patients and 74% of OF patients), while sixty-two patients had at least one positive antiphospholipid antibody in their history (Table 1).
Table 1.
Demographic, comorbidity and SLE disease characteristics and therapies of patients.
| All Patients (n = 114) | OFs (n = 96) | IAFs (n = 38) | |
|---|---|---|---|
| Demographics | |||
| Women: n (%) | 100 (88) | 85 (88) | 33 (87) |
| Age at disease onset (years): median (IQR) | 28 (20–36) | 29 (20–37) | 26 (20–37) |
| Age at time of flare (years): median (IQR) | 44 (35–52) | 44 (35–52) | 32 (34–54) |
| Follow-up duration at time of flare (months): median (IQR) | 30 (13–62) | 34 (15–71) | 22(12–43) |
| General clinical characteristics (history): n (%) | |||
| Musculoskeletal involvement | 93 (80) | 78 (81) | 30 (79) |
| Mucocutaneous involvement | 82 (81) | 70 (72) | 26 (68) |
| Renal involvement | 47 (40) | 38 (39) | 17 (44) |
| NPSLE | 28 (24) | 22 (22) | 10 (26) |
| Cardiopulmonary involvement | 14 (12) | 9 (9) | 6 (15) |
| Haematological manifestations | 94 (82) | 78 (81) | 30 (79) |
| Constitutional symptoms | 46 (40) | 38 (39) | 17 (45) |
| Gastrointestinal manifestations | 1 (1) | 1 (1) | 0 (0) |
| Anti-phospholipid syndrome | 11 (10) | 9 (9) | 6 (16) |
| Serology (history): n (%) | |||
| Anti-dsDNA | 85 (74) | 71 (74) | 30 (79) |
| Anti-Sm | 30 (26) | 24 (25) | 10 (26) |
| Low complement (C3 and/or C4) | 31 (27) | 27 (28) | 11 (29) |
| Antiphospholipid antibodies | |||
|
35 (30) | 29 (30) | 13 (34) |
|
18 (16) | 18 (18) | 7 (18) |
|
23 (20) | 20 (1) | 8 (21) |
NPSLE: neuropsychiatric systemic lupus erythematosus; LAC: lupus anticoagulant.
3.2. Pre-Flare Disease Status
At the time of the last LLDAS before a flare, the patient NRS was 7 (6–8). Accordingly, the total BILAG score was 1 (0–1), indicating an absence of/low disease activity. Modest alterations were only found in the haematological and mucocutaneous domains. In all, 109 patients (31/38 in the IAF group and 78/96 in the OF group) had serological activity at pre-flare. The differences between the two groups were not statistically significant (Table 2). At the pre-flare visit, 82 patients were under at least one immunosuppressant (42% of IAFs, 69% of OFs), while 120/134 were taking hydroxychloroquine (82% of all IAFs and 84% of all OFs). Due to persistent disease stability, corticosteroids were tapered in 21 cases (11/16 patients on steroids in the IAF group and 10/41 in the OF group; p = 0.005) and discontinued in 13 cases (4/16 IAFs and 9/41 OFs; p > 0.999) at the end of the visit. No patient discontinued immunosuppressants.
Table 2.
Clinical and treatment features at the last visit before flare.
| All Flares (n = 134) | OFs (n = 96) | IAFs (n = 38) | Viral IAFs (n = 13) | Bacterial IAFs (n = 23) | |
|---|---|---|---|---|---|
| Disease activity measures: median (IQR) | |||||
| SLEDAI-2K | 2(0–4) | 2 (0–4) | 2 (2–4) | 2 (0–2) | 2 (2–4) |
| PGA | 0 (0–1) | 0 (0–1) | 0 (0–0) | 0 (0–1) | 0 (0–1) |
| Patient-reported NRS | 7 (6–8) | 8 (7–8) | 8 (7–9) | 7 (7–8) | 8 (7–9) |
| Serology: n (%) | |||||
| Anti-dsDNA | 87 (65) | 56 (58) | 18 (47) | 5 (38) | 10 (43) |
| Low complement (C3 and/or C4) | 71 (52) | 43 (44) | 16 (42) | 4 (31) | 13 (57) |
| Treatment status: n (%) | |||||
| Hydroxychloroquine | 120 (90) | 88 (92) | 32 (84) | 11 (85) | 19 (83) |
| Immunosuppressants | |||||
| MTX | 12 (9) | 7 (7) | 1 (2) | 0 (0) | 1 (4) |
| AZA | 24 (18) | 18 (18) | 7 (18) | 2 (15) | 5 (22) |
| MMF | 44 (32) | 38 (39) | 7 (18) | 2 (15) | 5 (22) |
| CyA | 4(3) | 3 (3) | 1 (3) | 0 (0) | 1 (4) |
| Belimumab | 21 (15) | 16 (16) | 3 (8) | 1 (8) | 2 (9) |
| Treatment changes: n (%) | |||||
| Corticosteroid tapering | 21 (16) | 10 (10) | 11 (29) * | 4 (31) | 6 (26) |
| Corticosteroid discontinuation | 13 (10) | 9 (9) | 4 (10) | 2 (5) | 2 (9) |
| Immunosuppressant discontinuation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index 2000; PGA: physician global assessment scale; NRS: numerical rating scale; MTX: methotrexate; AZA: Azathioprine; MMF: mycophenolate mofetil; CyA: cyclosporine A. *: p < 0.05 compared to OFs.
3.3. Flare Profiles by Groups
3.3.1. Characteristics of Infection-Associated Flares
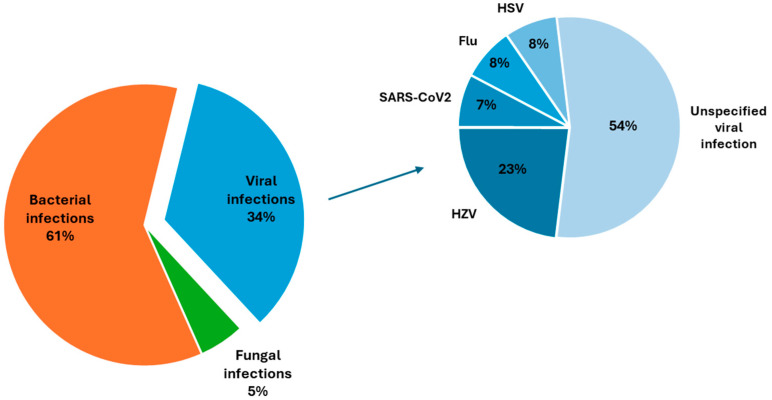
Putative bacterial infections were linked to 23/38 (61%) IAF cases. Thirteen cases (34%) were associated with a viral infection/reactivation. Two flares (5%) followed a fungal infection. Among the bacterial IAFs, 13 were associated with a respiratory tract infection, including 1 case of pneumonia and 12 cases of upper airway infections. Eight patients had a bacterial urinary tract infection preceding an IAF. One case of bacterial conjunctivitis and one case of gastroenteritis were also recorded. In the viral subgroup, Herpesviridae were responsible of four events (30%). Three cases were attributed to Varicella Zoster Virus (VZV) and one case to Herpes Simplex Virus (HSV). One case of viral IAF was associated with a recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Eight cases were classified as flu-like respiratory syndrome of potential viral aetiology, but no specific data were available about the insulting agent. In the IAF cohort, none of the patients with Herpes Zoster infection/reactivation were under steroids, although two out of four were on immunosuppressants. Both cases of fungal infection were sustained by Candida spp. (Figure 2).
Figure 2.
Pie charts showing the differential pathogenic distribution of infections in IAF patients (left) and in detail in the viral subgroup (right).
At the time of flare (for both IAFs and OFs), the vaccine coverage rates were 68% (n = 91) for the anti-influenza vaccine, 59% (n = 79) for the anti-pneumococcal disease vaccine, 16% (n = 22) for the tetravalent meningococcal vaccine, 11% (n = 15) for the B meningococcal vaccine, 5% (n = 7) for the anti-Haemophilus influenzae vaccine and 11% (n = 15) for the recombinant HZV disease vaccine. Fifty flares (nine IAFs and forty-one OFs) occurred during the COVID-19 pandemic. Twenty-eight of them occurred when anti-COVID-19 vaccines were available. Twenty patients out of these twenty-eight (71%) were vaccinated at the time of flare. No statistical differences were found when the vaccination profiles of IAF and OF patients at the time of flare were compared.
3.3.2. Clinical and Laboratory Features
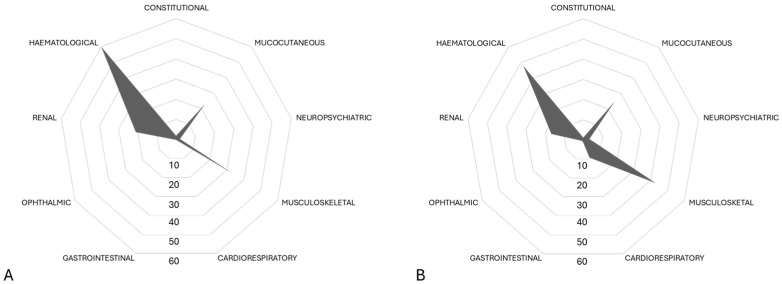
The differential clinical profile of patients with IAFs and OFs was evaluated by comparing the number and type of active BILAG domains. In patients with IAFs, one or two BILAG domains were active in 80% of cases. Haematological (60%), musculoskeletal (34%), mucocutaneous (23%) and renal (21%) domains were most frequently involved. Cardiopulmonary manifestations were numerically less frequent in IAFs than OFs (0/38 vs. 9/96; p = 0.060; Figure 3). No other differences were observed with regard to active BILAG domains among the OF and IAF groups. Viral and bacterial IAFs also showed similar patterns of BILAG domain involvement at the time of flare.
Figure 3.
(A,B); Radar plots showing the differential distribution of active BILAG domains among patients with OFs (A) and IAFs (B). Data are expressed as percentages. In both cases, the most represented BILAG domains were the haematological, musculoskeletal and mucocutaneus domains. BILAG: British Isles Lupus Assessment Group.
In terms of biochemical parameters at the time of flare, there were no statistically significant differences in the frequency of low C3 or C4 complement levels or in ADNA titres when patients with all types of IAF and patients with OFs were compared. However, positive ADNA was represented more among patients with bacterial IAFs (17/23) than in patients with viral IAFs (5/13; p = 0.036). Consistently, patients with bacterial IAFs more frequently had a history of positive ADNA than did patients with viral IAFs (96% vs. 46%; p < 0.001). Low complement was numerically more frequent among bacterial IAFs than among viral IAFs (13/23 vs. 3/13, respectively; p = 0.052). Similar trends were observed when viral IAFs were compared to OFs. No differences were found when biochemical parameters of the bacterial subgroup and OFs were compared. Platelet counts were relatively lower in patients with viral IAFs than in patients with OFs (p = 0.037), although the majority of observations fell within the range of normality. In addition, no significant differences were found in comparisons of the clinical features or biochemical profiles of OFs and IAFs stratified by onset before vs. after the COVID-19 pandemic. All clinical and laboratory features of the patients are summarised in Table 3.
Table 3.
Clinical and laboratory features at time of flare.
| All Flares (n = 134) | OFs (n = 96) | IAFs (n = 38) | Viral IAFs (n = 13) | Bacterial IAFs (n = 23) | |
|---|---|---|---|---|---|
| Disease activity measures: median (IQR) | |||||
| SLEDAI-2K | 5 (4–6) | 5 (4–6) | 5 (3–6) | 3 (0–6) | 6 (4–6) |
| Delta SLEDAI-2K | 2 (0–4) | 2 (0–4) | 2 (0–5) | 1 (0–4) | 4 (2–5) |
| Total BILAG score | 1 (1–2) | 1 (0–1) | 1 (1–2) | 1 (0–2) | 2 (1–2) |
| PGA | 1 (0–1) | 1 (0–1) | 1 (0–1) | 1 (0–1) | 1 (1–1) |
| Patient-reported NRS | 7 (6–8) | 7 (6–8) | 7 (5–8) | 6 (4–7) | 7 (5–8) |
| Serology: n(%) | |||||
| Anti-dsDNA | 87 (65) | 64 (67) | 23 (61) | 5 (38) * | 16 (74) |
| Low complement (C3 and/or C4) | 71 (53) | 53 (55) | 18 (47) | 3 (23) | 12 (57) |
| Other laboratory features: median (IQR) | |||||
| Hb (g/dL) | 12.8 (12–14) | 1.8 (11.8–14) | 12.6 (11.6–13.7) | 13 (11–13) | 12.5 (12–14) |
| Platelets × 103/microlitre | 227 (182–269) | 239(189–277) | 210 (169–262) | 177 (162–236) * | 215 (177–262) |
| WBCs/microlitre | 5000 (3600–6675) | 5375 (3875–6825) | 4510 (3100–5547) | 4630 (2880–6720) | 4400 (3100–5050) |
| Neutrophils (%) | 61 (53–67) | 61 (55–66) | 60 (47–68) | 62 (49–71) | 59 (42–66) |
| Lymphocytes (%) | 26 (53–67) | 26 (19–32) | 27 (19–40) | 25 (18–35) | 29 (20–41) |
| Monocytes (%) | 9 (7–12) | 9 (7–12) | 10 (8–12) | 9 (8–11) | 11 (9–12) |
| Eosinophils (%) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 3 (1–4) |
| Basophils (%) | 1 (0–1) | 0 (0–1) | 1 (0–1) | 1 (0–1) | 1 (0–1) |
| Serum creatinine (mg/dL) | 0.8 (0.6–0.9) | 0.8 (0.6–0.9) | 0.8 (0.6–1) | 0.8 (0.8–1.1) | 0.7 (0.7–1) |
| AST (U/L) | 22 (17–26) | 21 (17–25) | 23 (17–27) | 19 (13–28) | 24 (20–29) |
| ALT (U/L) | 17 (13–23) | 17 (13–22) | 19 (14–29) | 19 (15–31) | 20 (14–28) |
SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index 2000; BILAG: British Isles Lupus Assessment Group; PGA: physician global assessment scale; NRS: numerical rating scale; Hb: haemoglobin; WBCs: white blood cells; ALT: alanine aminotransferase; AST: aspartate aminotransferase. *: p < 0.05 compared to OFs.
3.3.3. Flare Severity
Flare severity was studied by analysing the SLEDAI-2K absolute scores at the time of flare and variations (delta) between pre-flare and flare visits. There were no significant differences between IAFs and OFs in terms of absolute SLEDAI-2K. The degree of SLEDAI-2K deterioration from pre-flare to flare status was also comparable among the groups. The total BILAG and PGA scores did not differ between OFs and IAFs, nor among IAF subgroups. The patient-reported NRS was also comparable among the groups. Despite a comparable frequency of musculoskeletal manifestations among patients with IAFs and OFs, higher DAS-28 scores [2.6 (2.3–4.1), n = 12] were found in IAFs than in OFs [2.0 (1.6–2.7), n = 47; p = 0.024]. Accordingly, when DAS-28 scores at the time of flare were compared intraindividually among patients who experienced both an IAF and an OF, higher DAS-28 scores were found during IAFs than during OFs (10/11 vs. 1/11; p = 0.004). The flare treatment strategies were similar among the groups.
3.4. Long-Term Disease Course
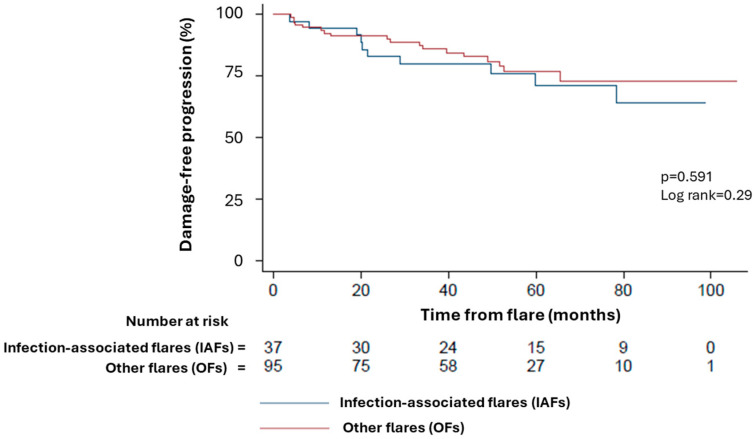
Patients experiencing IAFs (whether of viral, bacterial or fungal origin) had a similar likelihood of chronic damage progression over time when compared to those experiencing OFs (Log-rank = 0.29; hazard ratio 0.80, 95% CI: 0.37–1.75; p = 0.591; Figure 4). Furthermore, the monthly SDI item accrual rates were also comparable among OFs (0 (0–1)/100 months), IAFs (0 (0–0) items/100 months) and IAF subgroups.
Figure 4.
Kaplan–Meier survival curves showing the rate of damage accrual in patients with IAFs (blue line) and OFs (red line) over time.
4. Discussion
In this study, we found that patients with SLE in an LLDAS are exposed to a 17% annual risk of clinical deterioration, with more than one-quarter of flare cases being associated with a recent infection, most frequently of bacterial origin and affecting the upper respiratory tract. While IAFs and OFs were both characterised by prominent haematological and musculoskeletal manifestations, IAFs presented with arthritis of higher severity and showed converging damage accrual trends in the long term when compared to OFs.
Despite the availability of novel treatments and of more rational uses of existing therapeutic weapons, paving the way to ambitious targets, disease relapses are still common in the majority of patients with SLE. Consistent with our observations, previous studies have shown that more than 10% of patients with SLE lose a previously attained LLDAS each year [63]. This phenomenon tends to attenuate at later stages of the disease and be more prominent soon after LLDAS attainment [64] or closer to disease onset [65], with no clear risk-free conditions other than persistent LLDAS itself [20]. Infections have long been regarded as a major cause of morbidity (and potentially of mortality) in patients with SLE. Ever-growing evidence also supports a role of infections in favouring disease exacerbations. In contrast to the characteristic self-resolving course of SLE-related symptoms following microbial antigen stimulation in the setting of vaccines [66,67], wild-type infections in SLE are typically more severe, of longer course and associated with full-blown disease flares [32,68,69,70], possibly indicating that SLE pathogenic events potentially triggered by infections are unlikely to self-resolve and might be more resistant to treatments aiming at classical aseptic inflammatory mechanisms [71]. In line with this argument, intraindividual (besides interindividual) comparisons of arthritis severity at the time of flares in our cohort revealed a more severe joint involvement following infections.
In line with previous reports [33,36,38,72], bacterial infections were more frequent than viral infections in patients classified within the IAF group and were mainly localised to the respiratory tract. Inborn or acquired deficits of the complement system coexist in patients with SLE and contribute to the hallmark finding of low serum complement levels in patients with active disease [73]. In addition, they may concur with SLE-related susceptibility to bacterial infections [70,71]. Accordingly, a trend towards lower levels of complement was observed in patients with bacterial IAFs in this study.
Patients with viral IAFs constituted a standalone subgroup in the context of IAFs and were characterised by a lower frequency of typical SLE serological alterations (that is, positive ADNA and possibly low complement). The most frequent viral IAFs were sustained by VZV and other herpesviruses and occurred in patients who were off corticosteroids, in line with SLE’s intrinsic susceptibility to herpesviruses [41]. Regarding this, recent works have also shown that a fraction of patients with SLE might harbour natural anti-IFN neutralising antibodies among their serological repertoire. These antibodies might interfere with IFN-dependent mechanisms of inflammation, reducing the likelihood of SLE flares at the price of a higher susceptibility to infections [74,75]. Adding to previous evidence, we found that patients susceptible to disease flares after infection/viral reactivation have, however, no milder clinical presentation when compared to patients with flares without clear associations with infectious triggers, and they accrue similar amounts of chronic damage in the long term. Taken together, these data further support the hypothesis of unconventional mechanisms of inflammation occurring in IAFs in contrast to OFs, which might only be partially tackled by standard treatment approaches, currently chosen according to the clinical phenotype only. Ultimately, this can have a detrimental impact on patient prognosis and possibly account for the significant burden of damage accrual observed in patients with IAFs, similar to that in OFs. Consistent with this hypothesis, recent studies indicate an association between more complex alterations of multiple branches of the immune response and enhanced flare risk in patients with SLE [76].
Another potential consequence of the existence of atypical disease mechanisms in all or some patients with higher susceptibility to infections and to IAFs might be that some clinical manifestations of morbidity in these patients are not correctly captured by current clinimetrics. Indeed, the recent severe acute respiratory syndrome coronavirus 2 health crisis has highlighted the detrimental impact of difficult-to-diagnose/-treat post-infectious long-term syndromes on patient quality of life [77]. Consistent with this, post-infection-like symptoms such as fatigue, chronic pain or low-grade mood disorders are well-known features of SLE and major causes of disability, despite their relatively low weight in current SLE clinimetrics [78,79,80,81,82,83]. Patients with IAFs might therefore only apparently present with a comparable burden of disease compared to OFs due to the limitations of current clinical assessment tools. Unfortunately, the data from our research are insufficient to test this challenging hypothesis, warranting the acquisition of further data from ad hoc studies. The potential existence of a clinical/pathophysiological discrepancy between patients with IAFs and other SLE patients might, however, have clinical implications. For example, disease stability might be overestimated in patients at risk for IAFs. Consistent with this, steroid tapering but not final discontinuation pre-flare was associated with eventual IAFs, possibly indicating that enhanced monitoring during corticosteroid tapering might be advised in patients with atypical SLE features [84,85,86]. Vaccination constitutes the mainstay of prevention in patients with SLE and might constitute the key solution to uncoupling enhanced disease instability due to treatment tapering and infectious risk. Notably, despite being consistent with data from the literature, the vaccine coverage rates in our cohort were disappointingly lower than those indicated by local and international guidelines [58,59,60]. This evidence highlights the importance of vaccination and patient education on this topic as a major modifiable unmet need for patients with SLE, especially in the context of growing vaccine hesitancy due to mistrust in public institutions and science [87,88]. In line with this view, the impact of the COVID-19 pandemic in patients with SLE (in terms of both infections and disease flares) was proportional to the extent of public health measures, including vaccination [56,89].
Additional studies are required to overcome other potential areas of uncertainty unaddressed by this study due to its limitations. In particular, due to the absence of data on variations in biohumoral parameters among the pre-flare, post-infection and overt flare status, our mechanistic hypotheses to interpret the study clinical findings remain speculative. Along the same lines, insufficient direct microbiological evidence of pre-flare infections might have introduced potential biases in classifying IAF patients. Furthermore, as a large fraction of infectious events were managed in primary care, we were not able to take into account potential biases related to the types of antimicrobial treatment and concomitant medications in patients eventually developing IAFs. Despite the relatively significant number of patients in the starting cohort, the number of analysed subjects and events was also quite low, preventing an accurate dissection of less frequent features potentially differentiating IAFs and OFs. Our retrospective design also contributes to this limitation.
Notwithstanding the impact of these considerations on the strength of our findings, this study potentially opens new perspectives on a lesser-known aspect of SLE clinical/pathophysiological heterogeneity, that is, disease exacerbation in the setting of combined autoimmunity and infection susceptibility. A deeper understanding of SLE dynamics in the subset of patients with these characteristics might improve patient characterisation and selection for treatment in the setting of both clinical trials and routine practice and improve current management strategies towards more personalised approaches.
5. Conclusions
In conclusion, this study confirms that patients with SLE are exposed to a persistent risk of relapse, which can be associated with recent infections in more than one-quarter of cases. SLE flares following a recent infection present with similar clinical manifestations compared to other disease exacerbations but might cause more severe arthritis and show atypical serological features in association with recent viral infections. Further studies are required to investigate the existence of potentially unconventional mechanisms sustaining SLE flares in infection-susceptible subjects and possibly design tailored treatment strategies to tackle them.
Acknowledgments
We thank the medical and nursing staff of the Unit of Immunology, Allergy and Rare Diseases at San Raffaele University Hospital, Milan, Italy, for their support in caring for the patients involved in this research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens13110934/s1, Table S1: LLDAS retention over time.
Author Contributions
Conceptualisation, G.A.R., L.D. and M.M.-C.; methodology, software, G.A.R.; validation, L.M., E.P.B., L.D. and M.M.-C.; formal analysis, G.A.R., C.C. and M.S.; investigation, G.A.R., C.C., M.S., G.D.G. and G.B.; resources, L.D.; data curation, G.A.R., C.C., M.S., L.D. and M.M.-C.; writing—original draft preparation, C.C., M.S. and G.A.R.; writing—review and editing, G.A.R., E.P.B., L.M., L.D. and M.M.-C.; supervision, G.A.R., L.M., E.P.B., L.D. and M.M.-C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Data supporting this research were collected in the framework of the Panimmuno research protocol, conforming to the Declaration of Helsinki and approved by the San Raffaele Hospital Institutional Review Board (approval number 22/INT/2018).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting this research can be shared upon reasonable request to the corresponding author.
Conflicts of Interest
G.A.R. and L.M. have received honoraria for invited talks and advisory boards from Amgen, Astrazeneca, G.S.K. and Vifor.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Siegel C.H., Sammaritano L.R. Systemic Lupus Erythematosus: A Review. JAMA. 2024;331:1480–1491. doi: 10.1001/jama.2024.2315. [DOI] [PubMed] [Google Scholar]
- 2.Arnaud L., Tektonidou M.G. Long-term outcomes in systemic lupus erythematosus: Trends over time and major contributors. Rheumatology. 2020;59((Suppl. S5)):v29–v38. doi: 10.1093/rheumatology/keaa382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Romo G.S., Caielli S., Vega B., Connolly J., Allantaz F., Xu Z., Punaro M., Baisch J., Guiducci C., Coffman R.L., et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yap D.Y.H., Chan T.M. B Cell Abnormalities in Systemic Lupus Erythematosus and Lupus Nephritis-Role in Pathogenesis and Effect of Immunosuppressive Treatments. Int. J. Mol. Sci. 2019;20:6231. doi: 10.3390/ijms20246231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parodis I., Gatto M., Sjowall C. B cells in systemic lupus erythematosus: Targets of new therapies and surveillance tools. Front. Med. 2022;9:952304. doi: 10.3389/fmed.2022.952304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz-Gallo L.M., Oke V., Lundstrom E., Elvin K., Ling Wu Y., Eketjall S., Zickert A., Gustafsson J.T., Jonsen A., Leonard D., et al. Four Systemic Lupus Erythematosus Subgroups, Defined by Autoantibodies Status, Differ Regarding HLA-DRB1 Genotype Associations and Immunological and Clinical Manifestations. ACR Open Rheumatol. 2021;4:27–39. doi: 10.1002/acr2.11343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramirez G.A., Tassi E., Noviello M., Mazzi B.A., Moroni L., Citterio L., Zagato L., Tombetti E., Doglio M., Baldissera E.M., et al. Histone-Specific CD4(+) T Cell Plasticity in Active and Quiescent Systemic Lupus Erythematosus. Arthritis Rheumatol. 2024;76:739–750. doi: 10.1002/art.42778. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Z., Ren J., Dai C., Kannapell C.C., Wang H., Gaskin F., Fu S.M. Nature of T cell epitopes in lupus antigens and HLA-DR determines autoantibody initiation and diversification. Ann. Rheum. Dis. 2019;78:380–390. doi: 10.1136/annrheumdis-2018-214125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tani C., Cardelli C., Zen M., Moroni L., Piga M., Ceccarelli F., Fasano S., De Marchi G., Coladonato L., Emmi G., et al. Anifrolumab in Refractory Systemic Lupus Erythematosus: A Real-World, Multicenter Study. J. Rheumatol. 2024;51:jrheum.2024-0053. doi: 10.3899/jrheum.2024-0053. [DOI] [PubMed] [Google Scholar]
- 10.Gatto M., Saccon F., Andreoli L., Bartoloni E., Benvenuti F., Bortoluzzi A., Bozzolo E., Brunetta E., Canti V., Cardinaletti P., et al. Durable renal response and safety with add-on belimumab in patients with lupus nephritis in real-life setting (BeRLiSS-LN). Results from a large, nationwide, multicentric cohort. J. Autoimmun. 2021;124:102729. doi: 10.1016/j.jaut.2021.102729. [DOI] [PubMed] [Google Scholar]
- 11.Beheshti S.A., Shamsasenjan K., Ahmadi M., Abbasi B. CAR Treg: A new approach in the treatment of autoimmune diseases. Int. Immunopharmacol. 2022;102:108409. doi: 10.1016/j.intimp.2021.108409. [DOI] [PubMed] [Google Scholar]
- 12.Jin X., Han Y., Wang J.Q., Lu L. CAR-T cell therapy: New hope for systemic lupus erythematosus patients. Cell. Mol. Immunol. 2021;18:2581–2582. doi: 10.1038/s41423-021-00787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morand E.F., Furie R., Tanaka Y., Bruce I.N., Askanase A.D., Richez C., Bae S.C., Brohawn P.Z., Pineda L., Berglind A., et al. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N. Engl. J. Med. 2020;382:211–221. doi: 10.1056/NEJMoa1912196. [DOI] [PubMed] [Google Scholar]
- 14.Scofield L., Reinlib L., Alarcon G.S., Cooper G.S. Employment and disability issues in systemic lupus erythematosus: A review. Arthritis Rheum. 2008;59:1475–1479. doi: 10.1002/art.24113. [DOI] [PubMed] [Google Scholar]
- 15.Piga M., Arnaud L. The Main Challenges in Systemic Lupus Erythematosus: Where Do We Stand? J. Clin. Med. 2021;10:243. doi: 10.3390/jcm10020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ocampo-Piraquive V., Nieto-Aristizabal I., Canas C.A., Tobon G.J. Mortality in systemic lupus erythematosus: Causes, predictors and interventions. Expert Rev. Clin. Immunol. 2018;14:1043–1053. doi: 10.1080/1744666X.2018.1538789. [DOI] [PubMed] [Google Scholar]
- 17.Ugarte-Gil M.F., Acevedo-Vasquez E., Alarcon G.S., Pastor-Asurza C.A., Alfaro-Lozano J.L., Cucho-Venegas J.M., Segami M.I., Wojdyla D., Soriano E.R., Drenkard C., et al. The number of flares patients experience impacts on damage accrual in systemic lupus erythematosus: Data from a multiethnic Latin American cohort. Ann. Rheum. Dis. 2015;74:1019–1023. doi: 10.1136/annrheumdis-2013-204620. [DOI] [PubMed] [Google Scholar]
- 18.Stoll T., Sutcliffe N., Mach J., Klaghofer R., Isenberg D.A. Analysis of the relationship between disease activity and damage in patients with systemic lupus erythematosus--a 5-yr prospective study. Rheumatology. 2004;43:1039–1044. doi: 10.1093/rheumatology/keh238. [DOI] [PubMed] [Google Scholar]
- 19.Baragetti A., Ramirez G.A., Magnoni M., Garlaschelli K., Grigore L., Berteotti M., Scotti I., Bozzolo E., Berti A., Camici P.G., et al. Disease trends over time and CD4+CCR5+ T-cells expansion predict carotid atherosclerosis development in patients with systemic lupus erythematosus. Nutr. Metab. Cardiovasc. Dis. 2018;28:53–63. doi: 10.1016/j.numecd.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Gerosa M., Beretta L., Ramirez G.A., Bozzolo E., Cornalba M., Bellocchi C., Argolini L.M., Moroni L., Farina N., Segatto G., et al. Long-Term Clinical Outcome in Systemic Lupus Erythematosus Patients Followed for More Than 20 Years: The Milan Systemic Lupus Erythematosus Consortium (SMiLE) Cohort. J. Clin. Med. 2022;11:3587. doi: 10.3390/jcm11133587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zen M., Saccon F., Gatto M., Montesso G., Larosa M., Benvenuti F., Iaccarino L., Doria A. Prevalence and predictors of flare after immunosuppressant discontinuation in patients with systemic lupus erythematosus in remission. Rheumatology. 2020;59:1591–1598. doi: 10.1093/rheumatology/kez422. [DOI] [PubMed] [Google Scholar]
- 22.van Vollenhoven R.F., Bertsias G., Doria A., Isenberg D., Morand E., Petri M.A., Pons-Estel B.A., Rahman A., Ugarte-Gil M.F., Voskuyl A., et al. 2021 DORIS definition of remission in SLE: Final recommendations from an international task force. Lupus Sci. Med. 2021;8:e000538. doi: 10.1136/lupus-2021-000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franklyn K., Lau C.S., Navarra S.V., Louthrenoo W., Lateef A., Hamijoyo L., Wahono C.S., Chen S.L., Jin O., Morton S., et al. Definition and initial validation of a Lupus Low Disease Activity State (LLDAS) Ann. Rheum. Dis. 2016;75:1615–1621. doi: 10.1136/annrheumdis-2015-207726. [DOI] [PubMed] [Google Scholar]
- 24.Golder V., Kandane-Rathnayake R., Huq M., Nim H.T., Louthrenoo W., Luo S.F., Wu Y.-J.J., Lateef A., Sockalingam S., Navarra S.V., et al. Lupus low disease activity state as a treatment endpoint for systemic lupus erythematosus: A prospective validation study. Lancet Rheumatol. 2019;1:e95–e102. doi: 10.1016/S2665-9913(19)30037-2. [DOI] [PubMed] [Google Scholar]
- 25.Ramirez G.A., Canti V., Moiola L., Magnoni M., Rovere-Querini P., Coletto L.A., Dagna L., Manfredi A.A., Bozzolo E.P. Performance of SLE responder index and lupus low disease activity state in real life: A prospective cohort study. Int. J. Rheum. Dis. 2019;22:1752–1761. doi: 10.1111/1756-185X.13663. [DOI] [PubMed] [Google Scholar]
- 26.Floris A., Piga M., Cauli A., Mathieu A. Predictors of flares in Systemic Lupus Erythematosus: Preventive therapeutic intervention based on serial anti-dsDNA antibodies assessment. Analysis of a monocentric cohort and literature review. Autoimmun. Rev. 2016;15:656–663. doi: 10.1016/j.autrev.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Cunha R.N., Saraiva L., Jesus D., Doria A., da Silva J.P., Ines L.S. Predictors of flare in SLE patients fulfilling lupus low disease activity state: A cohort study of 292 patients with 36-month follow-up. Rheumatology. 2023;62:3627–3635. doi: 10.1093/rheumatology/kead097. [DOI] [PubMed] [Google Scholar]
- 28.Wu S.S., Perry A., Zimmerman N.M., Bryant G. Predictors of flare-related inpatient or emergency department stay in systemic lupus erythematosus: A real-world analysis of Medicaid claims in the United States. J. Manag. Care Spec. Pharm. 2024;30:61–70. doi: 10.18553/jmcp.2024.30.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato S., Yoshida S., Sumichika Y., Saito K., Matsumoto H., Temmoku J., Fujita Y., Matsuoka N., Asano T., Migita K. Clinical features of flare in Japanese patients with new-onset SLE and risk factors for SLE flare in daily clinical practice: A single-center cohort study. Immunol. Med. 2024:1–8. doi: 10.1080/25785826.2024.2360664. [DOI] [PubMed] [Google Scholar]
- 30.Goldblatt F., Chambers S., Rahman A., Isenberg D.A. Serious infections in British patients with systemic lupus erythematosus: Hospitalisations and mortality. Lupus. 2009;18:682–689. doi: 10.1177/0961203308101019. [DOI] [PubMed] [Google Scholar]
- 31.Navarro-Zarza J.E., Alvarez-Hernandez E., Casasola-Vargas J.C., Estrada-Castro E., Burgos-Vargas R. Prevalence of community-acquired and nosocomial infections in hospitalized patients with systemic lupus erythematosus. Lupus. 2010;19:43–48. doi: 10.1177/0961203309345776. [DOI] [PubMed] [Google Scholar]
- 32.Joo Y.B., Kim K.J., Park K.S., Park Y.J. Influenza infection as a trigger for systemic lupus erythematosus flares resulting in hospitalization. Sci. Rep. 2021;11:4630. doi: 10.1038/s41598-021-84153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruiz-Irastorza G., Olivares N., Ruiz-Arruza I., Martinez-Berriotxoa A., Egurbide M.V., Aguirre C. Predictors of major infections in systemic lupus erythematosus. Arthritis Res. Ther. 2009;11:R109. doi: 10.1186/ar2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez-Echavarri C., Capdevila O., Espinosa G., Suarez S., Marin-Ballve A., Gonzalez-Leon R., Rodriguez-Carballeira M., Fonseca-Aizpuru E., Pinilla B., Pallares L., et al. Infections in newly diagnosed Spanish patients with systemic lupus erythematosus: Data from the RELES cohort. Lupus. 2018;27:2253–2261. doi: 10.1177/0961203318811598. [DOI] [PubMed] [Google Scholar]
- 35.Sawada T., Fujimori D., Yamamoto Y. Systemic lupus erythematosus and immunodeficiency. Immunol. Med. 2019;42:1–9. doi: 10.1080/25785826.2019.1628466. [DOI] [PubMed] [Google Scholar]
- 36.Pego-Reigosa J.M., Nicholson L., Pooley N., Langham S., Embleton N., Marjenberg Z., Barut V., Desta B., Wang X., Langham J., et al. The risk of infections in adult patients with systemic lupus erythematosus: Systematic review and meta-analysis. Rheumatology. 2021;60:60–72. doi: 10.1093/rheumatology/keaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kroon F.P.B., Najm A., Alunno A., Schoones J.W., Landewe R.B.M., Machado P.M., Navarro-Compan V. Risk and prognosis of SARS-CoV-2 infection and vaccination against SARS-CoV-2 in rheumatic and musculoskeletal diseases: A systematic literature review to inform EULAR recommendations. Ann. Rheum. Dis. 2022;81:422–432. doi: 10.1136/annrheumdis-2021-221575. [DOI] [PubMed] [Google Scholar]
- 38.Danza A., Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: Susceptibility factors and preventive strategies. Lupus. 2013;22:1286–1294. doi: 10.1177/0961203313493032. [DOI] [PubMed] [Google Scholar]
- 39.D’Onofrio B., Virelli G., Pedrollo E., Caprioli M., Riva M., Renna D., Tonutti A., Luciano N., Ceribelli A., Gremese E., et al. High risk of misclassification of acute Parvovirus B19 infection into a systemic rheumatic disease. Rheumatol. Adv. Pract. 2024;8:rkae105. doi: 10.1093/rap/rkae105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diaz F., Collazos J., Mendoza F., De La Viuda J.M., Cazallas J., Urkijo J.C., Flores M. Systemic lupus erythematosus associated with acute parvovirus B19 infection. Clin. Microbiol. Infect. 2002;8:115–117. doi: 10.1046/j.1198-743x.2001.00361.x. [DOI] [PubMed] [Google Scholar]
- 41.Chakravarty E.F., Michaud K., Katz R., Wolfe F. Increased incidence of herpes zoster among patients with systemic lupus erythematosus. Lupus. 2013;22:238–244. doi: 10.1177/0961203312470186. [DOI] [PubMed] [Google Scholar]
- 42.Draborg A.H., Jacobsen S., Westergaard M., Mortensen S., Larsen J.L., Houen G., Duus K. Reduced response to Epstein-Barr virus antigens by T-cells in systemic lupus erythematosus patients. Lupus Sci. Med. 2014;1:e000015. doi: 10.1136/lupus-2014-000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perl A., Colombo E., Dai H., Agarwal R., Mark K.A., Banki K., Poiesz B.J., Phillips P.E., Hoch S.O., Reveille J.D., et al. Antibody reactivity to the HRES-1 endogenous retroviral element identifies a subset of patients with systemic lupus erythematosus and overlap syndromes. Correlation with antinuclear antibodies and HLA class II alleles. Arthritis Rheum. 1995;38:1660–1671. doi: 10.1002/art.1780381119. [DOI] [PubMed] [Google Scholar]
- 44.Draborg A.H., Sandhu N., Larsen N., Lisander Larsen J., Jacobsen S., Houen G. Impaired Cytokine Responses to Epstein-Barr Virus Antigens in Systemic Lupus Erythematosus Patients. J. Immunol. Res. 2016;2016:6473204. doi: 10.1155/2016/6473204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monneaux F., Muller S. Epitope spreading in systemic lupus erythematosus: Identification of triggering peptide sequences. Arthritis Rheum. 2002;46:1430–1438. doi: 10.1002/art.10263. [DOI] [PubMed] [Google Scholar]
- 46.Kang I., Quan T., Nolasco H., Park S.H., Hong M.S., Crouch J., Pamer E.G., Howe J.G., Craft J. Defective control of latent Epstein-Barr virus infection in systemic lupus erythematosus. J. Immunol. 2004;172:1287–1294. doi: 10.4049/jimmunol.172.2.1287. [DOI] [PubMed] [Google Scholar]
- 47.Silva J.M., Alves C.E.C., Pontes G.S. Epstein-Barr virus: The mastermind of immune chaos. Front. Immunol. 2024;15:1297994. doi: 10.3389/fimmu.2024.1297994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smatti M.K., Cyprian F.S., Nasrallah G.K., Al Thani A.A., Almishal R.O., Yassine H.M. Viruses and Autoimmunity: A Review on the Potential Interaction and Molecular Mechanisms. Viruses. 2019;11:762. doi: 10.3390/v11080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furer V., Rondaan C., Heijstek M.W., Agmon-Levin N., van Assen S., Bijl M., Breedveld F.C., D’Amelio R., Dougados M., Kapetanovic M.C., et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2020;79:39–52. doi: 10.1136/annrheumdis-2019-215882. [DOI] [PubMed] [Google Scholar]
- 50.Fanouriakis A., Kostopoulou M., Andersen J., Aringer M., Arnaud L., Bae S.C., Boletis J., Bruce I.N., Cervera R., Doria A., et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann. Rheum. Dis. 2024;83:15–29. doi: 10.1136/ard-2023-224762. [DOI] [PubMed] [Google Scholar]
- 51.Italian Ministry of Health Piano Nazionale Prevenzione Vaccinale 2017–2019. National Vaccine Prevention Plan 2017–2019. [(accessed on 21 October 2021)];2017 Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf.
- 52.Touma Z., Gladman D.D., Urowitz M.B. Vaccination and auto-immune rheumatic diseases: Lessons learnt from the 2009 H1N1 influenza virus vaccination campaign. Curr. Opin. Rheumatol. 2013;25:164–170. doi: 10.1097/BOR.0b013e32835d2b7b. [DOI] [PubMed] [Google Scholar]
- 53.Urowitz M.B., Anton A., Ibanez D., Gladman D.D. Autoantibody response to adjuvant and nonadjuvant H1N1 vaccination in systemic lupus erythematosus. Arthritis Care Res. 2011;63:1517–1520. doi: 10.1002/acr.20599. [DOI] [PubMed] [Google Scholar]
- 54.Sattui S.E., Liew J.W., Kennedy K., Sirotich E., Putman M., Moni T.T., Akpabio A., Alpizar-Rodriguez D., Berenbaum F., Bulina I., et al. Early experience of COVID-19 vaccination in adults with systemic rheumatic diseases: Results from the COVID-19 Global Rheumatology Alliance Vaccine Survey. RMD Open. 2021;7:e001814. doi: 10.1136/rmdopen-2021-001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furer V., Eviatar T., Zisman D., Peleg H., Paran D., Levartovsky D., Zisapel M., Elalouf O., Kaufman I., Meidan R., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: A multicentre study. Ann. Rheum. Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 56.Gerosa M., Schioppo T., Argolini L.M., Sciascia S., Ramirez G.A., Moroni G., Sinico R.A., Bonelli G., Alberici F., Mescia F., et al. The Impact of Anti-SARS-CoV-2 Vaccine in Patients with Systemic Lupus Erythematosus: A Multicentre Cohort Study. Vaccines. 2022;10:663. doi: 10.3390/vaccines10050663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramirez G.A., Batani V., Moroni L., De Luca G., Pizzetti G., Sala S., Peretto G., Campochiaro C., Della-Torre E., Bozzolo E.P., et al. Cardiac Safety of mRNA-Based Vaccines in Patients with Systemic Lupus Erythematosus and Lupus-like Disorders with a History of Myocarditis. Pathogens. 2022;11:1001. doi: 10.3390/pathogens11091001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chevallard M., Adinolfi A., Belloli L., Casu C., Di Cicco M., Destefani C., Di Rosa B., Gentile M.G., Filippini D.A., Luisi A., et al. Active vaccination campaign to increase seasonal influenza vaccination coverage: A monocenter experience in a cohort of Italian patients with systemic autoimmune diseases. Clin. Rheumatol. 2023;42:923–928. doi: 10.1007/s10067-022-06380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y., Chen B., Shen X., Zhou A., Liang Y., Wang Y., Chen H. A survey of systemic lupus erythematosus patients’ attitudes toward influenza and pneumococcal vaccination in Southwest China. Front. Public Health. 2022;10:1018899. doi: 10.3389/fpubh.2022.1018899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vieira de Rezende R., Mattos G., de Mello Leal Augusto R., Machado Gayer C., Mendes Klumb E. Predictors for seasonal influenza vaccination and reasons for inadequate vaccination coverage against a broad spectrum of vaccine-preventable diseases: A cross-sectional study among a Brazilian cohort of adult patients with systemic lupus erythematosus. Lupus. 2019;28:794–796. doi: 10.1177/0961203319846383. [DOI] [PubMed] [Google Scholar]
- 61.Aringer M., Costenbader K., Daikh D., Brinks R., Mosca M., Ramsey-Goldman R., Smolen J.S., Wofsy D., Boumpas D.T., Kamen D.L., et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann. Rheum. Dis. 2019;78:1151–1159. doi: 10.1136/annrheumdis-2018-214819. [DOI] [PubMed] [Google Scholar]
- 62.Petri M., Hellmann D., Hochberg M. Validity and reliability of lupus activity measures in the routine clinic setting. J. Rheumatol. 1992;19:53–59. [PubMed] [Google Scholar]
- 63.Tani C., Vagelli R., Stagnaro C., Carli L., Mosca M. Remission and low disease activity in systemic lupus erythematosus: An achievable goal even with fewer steroids? Real-life data from a monocentric cohort. Lupus Sci. Med. 2018;5:e000234. doi: 10.1136/lupus-2017-000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cho J., Shen L., Huq M., Kandane-Rathnayake R., Golder V., Louthrenoo W., Chen Y.H., Hamijoyo L., Luo S.F., Wu Y.J., et al. Impact of low disease activity, remission, and complete remission on flares following tapering of corticosteroids and immunosuppressive therapy in patients with systemic lupus erythematous: A multinational cohort study. Lancet Rheumatol. 2023;5:e584–e593. doi: 10.1016/S2665-9913(23)00209-6. [DOI] [PubMed] [Google Scholar]
- 65.Piga M., Floris A., Cappellazzo G., Chessa E., Congia M., Mathieu A., Cauli A. Failure to achieve lupus low disease activity state (LLDAS) six months after diagnosis is associated with early damage accrual in Caucasian patients with systemic lupus erythematosus. Arthritis Res. Ther. 2017;19:247. doi: 10.1186/s13075-017-1451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Felten R., Kawka L., Dubois M., Ugarte-Gil M.F., Fuentes-Silva Y., Piga M., Arnaud L. Tolerance of COVID-19 vaccination in patients with systemic lupus erythematosus: The international VACOLUP study. Lancet Rheumatol. 2021;3:e613–e615. doi: 10.1016/S2665-9913(21)00221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramirez G.A., Asperti C., Cucca V., Yacoub M.R. Challenges to Vaccination against SARS-CoV-2 in Patients with Immune-Mediated Diseases. Vaccines. 2021;9:1147. doi: 10.3390/vaccines9101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strangfeld A., Schafer M., Gianfrancesco M.A., Lawson-Tovey S., Liew J.W., Ljung L., Mateus E.F., Richez C., Santos M.J., Schmajuk G., et al. Factors associated with COVID-19-related death in people with rheumatic diseases: Results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramirez G.A., Gerosa M., Bellocchi C., Arroyo-Sánchez D., Asperti C., Argolini L.M., Gallina G., Cornalba M., Scotti I., Suardi I., et al. Efficacy and Safety of Anti-SARS-CoV-2 Antiviral Agents and Monoclonal Antibodies in Patients with SLE: A Case-Control Study. Biomolecules. 2023;13:1273. doi: 10.3390/biom13091273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rao M., Mikdashi J. A Framework to Overcome Challenges in the Management of Infections in Patients with Systemic Lupus Erythematosus. Open Access Rheumatol. 2023;15:125–137. doi: 10.2147/OARRR.S295036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Torres-Ruiz J., Barrera-Vargas A., Ortiz-Hernandez R., Alcocer-Varela J., Ponce-de-Leon A., Gomez-Martin D. Microbiological and immunological profile of patients with severe lupus flares related to bloodstream infections: A retrospective cohort study. Lupus. 2018;27:312–318. doi: 10.1177/0961203317720527. [DOI] [PubMed] [Google Scholar]
- 72.Zonana-Nacach A., Camargo-Coronel A., Yanez P., Sanchez L., Jimenez-Balderas F.J., Fraga A. Infections in outpatients with systemic lupus erythematosus: A prospective study. Lupus. 2001;10:505–510. doi: 10.1191/096120301678416088. [DOI] [PubMed] [Google Scholar]
- 73.Gandino I.J., Scolnik M., Bertiller E., Scaglioni V., Catoggio L.J., Soriano E.R. Complement levels and risk of organ involvement in patients with systemic lupus erythematosus. Lupus Sci. Med. 2017;4:e000209. doi: 10.1136/lupus-2017-000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mathian A., Breillat P., Dorgham K., Bastard P., Charre C., Lhote R., Quentric P., Moyon Q., Mariaggi A.A., Mouries-Martin S., et al. Lower disease activity but higher risk of severe COVID-19 and herpes zoster in patients with systemic lupus erythematosus with pre-existing autoantibodies neutralising IFN-alpha. Ann. Rheum. Dis. 2022;81:1695–1703. doi: 10.1136/ard-2022-222549. [DOI] [PubMed] [Google Scholar]
- 75.Chen L., Chi H., Teng J., Meng J., Zhang H., Su Y., Liu H., Ye J., Shi H., Hu Q., et al. Neutralizing anti-IFN-gamma IgG was increased in patients with systemic lupus erythematosus and associated with susceptibility to infection. Clin. Rheumatol. 2024;43:189–198. doi: 10.1007/s10067-023-06758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu R., Guthridge J.M., Chen H., Bourn R.L., Kamp S., Munroe M.E., Macwana S.R., Bean K., Sridharan S., Merrill J.T., et al. Immunologic findings precede rapid lupus flare after transient steroid therapy. Sci. Rep. 2019;9:8590. doi: 10.1038/s41598-019-54275-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Al-Aly Z., Davis H., McCorkell L., Soares L., Wulf-Hanson S., Iwasaki A., Topol E.J. Long COVID science, research and policy. Nat. Med. 2024;30:2148–2164. doi: 10.1038/s41591-024-03173-6. [DOI] [PubMed] [Google Scholar]
- 78.Bakshi J., Segura B.T., Wincup C., Rahman A. Unmet Needs in the Pathogenesis and Treatment of Systemic Lupus Erythematosus. Clin. Rev. Allergy Immunol. 2017;55:352–357. doi: 10.1007/s12016-017-8640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Petri M., Kawata A.K., Fernandes A.W., Gajria K., Greth W., Hareendran A., Ethgen D. Impaired health status and the effect of pain and fatigue on functioning in clinical trial patients with systemic lupus erythematosus. J. Rheumatol. 2013;40:1865–1874. doi: 10.3899/jrheum.130046. [DOI] [PubMed] [Google Scholar]
- 80.Wiseman S.J., Bastin M.E., Hamilton I.F., Hunt D., Ritchie S.J., Amft E.N., Thomson S., Belch J.F., Ralston S.H., Wardlaw J.M. Fatigue and cognitive function in systemic lupus erythematosus: Associations with white matter microstructural damage. A diffusion tensor MRI study and meta-analysis. Lupus. 2017;26:588–597. doi: 10.1177/0961203316668417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moroni L., Mazzetti M., Ramirez G.A., Farina N., Bozzolo E.P., Guerrieri S., Moiola L., Filippi M., Di Mattei V., Dagna L. Beyond Neuropsychiatric Manifestations of Systemic Lupus Erythematosus: Focus on Post-traumatic Stress Disorder and Alexithymia. Curr. Rheumatol. Rep. 2021;23:52. doi: 10.1007/s11926-021-01019-5. [DOI] [PubMed] [Google Scholar]
- 82.Arnaud L., Mertz P., Amoura Z., Voll R.E., Schwarting A., Maurier F., Blaison G., Bonnotte B., Poindron V., Fiehn C., et al. Patterns of fatigue and association with disease activity and clinical manifestations in systemic lupus erythematosus. Rheumatology. 2021;60:2672–2677. doi: 10.1093/rheumatology/keaa671. [DOI] [PubMed] [Google Scholar]
- 83.Piga M., Congia M., Gabba A., Figus F., Floris A., Mathieu A., Cauli A. Musculoskeletal manifestations as determinants of quality of life impairment in patients with systemic lupus erythematosus. Lupus. 2018;27:190–198. doi: 10.1177/0961203317716319. [DOI] [PubMed] [Google Scholar]
- 84.Floris A., Chessa E., Sebastiani G.D., Prevete I., Iannone F., Coladonato L., Govoni M., Bortoluzzi A., Mosca M., Tani C., et al. Glucocorticoid tapering and associated outcome in patients with newly diagnosed systemic lupus erythematosus: The real-world GULP prospective observational study. RMD Open. 2022;8:e002701. doi: 10.1136/rmdopen-2022-002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mathian A., Arnaud L., Ruiz-Irastorza G. Is it safe to withdraw low-dose glucocorticoids in SLE patients in remission? Autoimmun. Rev. 2024;23:103446. doi: 10.1016/j.autrev.2023.103446. [DOI] [PubMed] [Google Scholar]
- 86.Tselios K., Gladman D.D., Su J., Urowitz M.B. Gradual Glucocorticosteroid Withdrawal Is Safe in Clinically Quiescent Systemic Lupus Erythematosus. ACR Open Rheumatol. 2021;3:550–557. doi: 10.1002/acr2.11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miyachi T., Takita M., Senoo Y., Yamamoto K. Lower trust in national government links to no history of vaccination. Lancet. 2020;395:31–32. doi: 10.1016/S0140-6736(19)32686-8. [DOI] [PubMed] [Google Scholar]
- 88.Hammam N., Tharwat S., Shereef R.R.E., Elsaman A.M., Khalil N.M., Fathi H.M., Salem M.N., El-Saadany H.M., Samy N., El-Bahnasawy A.S., et al. Rheumatology university faculty opinion on coronavirus disease-19 (COVID-19) vaccines: The vaXurvey study from Egypt. Rheumatol. Int. 2021;41:1607–1616. doi: 10.1007/s00296-021-04941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ramirez G.A., Argolini L.M., Bellocchi C., Moroni L., Della-Torre E., Farina N., Caporali R.F., Beretta L., Gerosa M., Bozzolo E.P., et al. Impact of the COVID-19 pandemic in patients with systemic lupus erythematosus throughout one year. Clin. Immunol. 2021;231:108845. doi: 10.1016/j.clim.2021.108845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting this research can be shared upon reasonable request to the corresponding author.