Abstract
Staphylococcal enterotoxins have marked effects on the properties of T cells and monocytes and have recently been reported to affect neutrophil function. In this study, we investigated the abilities of staphylococcal enterotoxins A and B and toxic shock syndrome toxin 1 to affect respiratory burst activity and to delay apoptosis in human neutrophils. When cultures containing approximately 97% neutrophils were tested, the toxins all delayed neutrophil apoptosis in a dose-dependent manner and induced the expression of FcγRI on the neutrophil cell surface. These effects on apoptosis and expression of FcγRI were largely abrogated by the addition of a neutralizing anti-gamma interferon antibody. Similarly, the effects of these toxins on phorbol ester-induced chemiluminescence were decreased after neutralization of gamma interferon. These effects on neutrophil function were mimicked by the addition of conditioned medium from peripheral blood mononuclear cells incubated with the toxins, and again, neutralizing anti-gamma interferon antibodies largely negated the effects. However, when highly purified neutrophils prepared by immunodepletion of T cells and major histocompatibility complex class II-expressing cells were analyzed, the toxins were without effect on apoptosis and FcγRI expression, but granulocyte-macrophage colony-stimulating factor and gamma interferon could still delay apoptosis. These data indicate that these toxins have no direct effect on neutrophil apoptosis but can act indirectly via the production of T-cell-derived and monocyte-derived cytokines. It is noteworthy that such effects are detected in neutrophil suspensions containing only 3% contamination with T cells and other mononuclear cells.
Staphylococcal enterotoxins cause food poisoning and toxic shock and may lead to multiple organ failure resulting from massive activation of the immune system (23). There is also evidence that suggests a critical role for staphylococcal enterotoxins in the pathogenesis of rheumatoid arthritis (27). These toxins are superantigens which activate a subset of T cells (around 20% of T cells have the appropriate Vβ region) by binding to major histocompatibility complex (MHC) class II molecules on antigen-presenting cells and cross-linking to the T-cell receptor (16, 24). There is a large production of proinflammatory cytokines from both T cells and monocytes following superantigen activation: T cells produce interleukin-1β (IL-1β), gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and IL-2 (17, 20, 32), while monocytes produce both IL-1β and IFN-γ (31, 32, 35).
The effects of superantigens on T cells and monocytes have been well studied, but the effects of superantigens on other cells of the immune system have been relatively neglected. The indirect activation of these other immune cells may be expected in vivo, as cytokine secretion by T cells and monocytes will, in turn, activate other immune cells. There are a few reports of other immune cells being affected apparently without the involvement of T cells or monocytes. For example, CD56-positive natural killer (NK) cells have been shown to be directly stimulated by staphylococcal enterotoxin B (SEB) (4). This action on NK cells is not totally unexpected, as a subset of these cells is known to express MHC class II. There have also been reports of neutrophil responses to toxic shock syndrome toxin 1 (TSST-1), such as expression of heat shock proteins (HSPs) within 30 min of stimulation (15), and changes in surface protein expression and leukotriene B4 synthesis which occur within 10 min of stimulation (14). Such direct effects of TSST-1 on neutrophils are difficult to rationalize, as no surface structures that can act as targets for these superantigens have been identified on freshly isolated neutrophils (14). Recently, neutrophils have been shown to express MHC class II molecules, but only after culture for a day or more with IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), or IL-3 (12, 33), but this does not explain the rapid (10 min) effects of TSST-1 on freshly isolated neutrophils.
Neutrophils possess a very short half-life in the circulation because they constitutively undergo apoptosis (10, 25, 34). Cytokines, such as GM-CSF, IFN-γ, IL-1β, and IL-2, which are produced by activated T cells and monocytes, can delay neutrophil apoptosis and extend the functional life span of neutrophils to several days (1, 3, 21, 28). The effects of superantigens on neutrophil apoptosis have not been studied, although it may be predicted that neutrophil apoptosis in vivo may be delayed either directly or indirectly via the production of cytokines by other immune cells. The work of Hensler and colleagues (14, 15) suggests a direct effect of superantigens on neutrophils, including enhanced production of leukotriene B4. This leukotriene is able to delay neutrophil apoptosis (13, 26), and this could provide an indirect mechanism for superantigens to affect neutrophil apoptosis. The aim of this study was to determine if bacterial superantigens have any ability to alter neutrophil survival. We show that these agents have no direct effects on neutrophil apoptosis but can perform this function indirectly, via the production of cytokines by the very low levels of contaminating monocytes and T cells often present in neutrophil preparations.
MATERIALS AND METHODS
Materials.
Neutrophil isolation medium (NIM) was purchased from Cardinal Associates (Sante Fe, N.Mex.), RPMI 1640 medium was from ICN Biomedicals (Thame, Oxford, United Kingdom), and fetal calf serum (FCS) and l-glutamine were from GIBCO-BRL (Paisley, United Kingdom). Superantigens SEA, SEB, and TSST-1 were purchased from Sigma. IFN-γ was from Boehringer Mannheim (Lewes, United Kingdom). Monoclonal antibody 32.2 (anti-FcγRI) was purchased from Medarex Inc., and monoclonal antibody Leu11a (anti-FcγRIII), a fluorescein isothiocyanate-conjugated second-layer antibody, and immunoglobulin G controls were from Becton Dickinson (Cowley, United Kingdom). Polyclonal sheep (no. 90) anti-IFN-γ serum was from the National Institute for Biological Standards and Control. Pan T Dynabeads and anti-MHC class II Dynabeads (antibody-coated magnetic beads) were purchased from Dynal. All of the other specialist reagents used were from Sigma and were of the highest quality available.
Neutrophil isolation and purification.
Neutrophils were isolated from heparinized venous blood from healthy volunteers by centrifugation in NIM as described previously (8). After hypotonic lysis to remove contaminating erythrocytes, they were suspended in RPMI medium supplemented with FCS at 5% (vol/vol) and 2-mmol/liter l-glutamine. The purity (Wright’s stain) and viability (trypan blue exclusion) of freshly isolated cells were routinely ≥97% and 95%, respectively.
Depletion of T cells and MHC class II-expressing cells.
Neutrophil preparations were depleted of contaminating T cells, MHC class II-expressing cells, or both by using Dynabeads. The appropriate beads (after being washed three times in RPMI 1640 medium) were added to neutrophil preparations at 2 × 107 beads/ml and incubated at 4°C for 30 min with rotation by following the manufacturer’s instructions (which report removal of 99% of target cells under these conditions). Incubation was at 4°C to minimize phagocytosis of the beads by neutrophils. The beads were then magnetically removed from the cell suspensions six times. The resulting neutrophil preparations (T cell and MHC class II-expressing cell depleted) contained no remaining beads, and examination of >1,500 cells (with Wright’s stain) showed no contaminating mononuclear cells. Depletion of T cells alone resulted in just two lymphocytes in 1,500 cells. Final neutrophil preparations were >95% viable (as assessed by trypan blue exclusion) after depletion of T cells and MHC class II-expressing cells and were adjusted to 5 × 106 cells/ml in RPMI 1640 medium supplemented with 5% (vol/vol) FCS and 2-mmol/liter l-glutamine.
PBMC isolation.
Heparinized venous blood from healthy volunteers used for the preparation of neutrophils was centrifuged through NIM as described above with removal of peripheral blood mononuclear cells (PBMC) at the first step of neutrophil isolation (8). PBMC were then washed with RPMI medium, counted, and resuspended in RPMI 1640 medium supplemented with 5% (vol/vol) FCS and 2-mmol/liter l-glutamine.
PBMC-conditioned medium.
PBMC were isolated as described above and resuspended at 2 × 107/ml in RPMI 1640 medium supplemented with 5% (vol/vol) FCS and 2-mmol/liter l-glutamine. Incubations were done with conical polypropylene tubes at 37°C with gentle agitation in the absence (control) or presence of SEA (100 ng/ml), SEB (100 ng/ml), or TSST-1 (5 μg/ml). Following 22 h in culture, supernatants were collected and frozen for future use. The supernatants were added to neutrophil suspensions to mimic 5% contamination of PBMC. Therefore, 12.5 μl of conditioned medium was added per ml of neutrophil suspension. The concentration of carryover superantigen in these neutrophil suspensions was therefore less than 1.25 ng/ml for SEA and SEB and less than 62.5 ng/ml for TSST-1.
Neutrophil culture.
Neutrophils were suspended at 5 × 106 cells/ml in RPMI 1640 medium supplemented with 5% (vol/vol) FCS. Medium with no further additions served as a control, while SEA, SEB, and TSST-1 were added at 0.01, 0.05, 0.1, 0.5, 1.0, or 5.0 μg/ml; GM-CSF was added at 50 U/ml; and IFN-γ was added at 100 U/ml. PBMC-conditioned medium was added at 12.5 μl/ml. IFN-γ-neutralizing antiserum was added to incubations at a 1:1,000 dilution.
NADPH oxidase activity.
Chemiluminescence was assayed after supplementing neutrophil suspensions with 10-μmol/liter luminol. After addition of 0.1-μg/ml phorbol myristate acetate (PMA) or 1 μM formyl-methionyl-leucyl-phenylalanine, (fMLP), photon emission was measured by using an LKB 1251 luminometer in a final volume of 1.0 ml (7, 8).
Morphological assessment of apoptosis.
Apoptosis was assessed by morphology as described previously (10, 25). Essentially, cytocentrifuge preparations of 105 cells, made up to a volume of 200 μl with sterile phosphate-buffered saline, were prepared by using a Shandon cytospin centrifuge. Cells were then stained by using May-Grünwald-Giemsa stain and assessed for morphological changes characteristic of apoptosis (nuclear condensation, vacuolation) by using a 40× objective (30). At least 500 cells per slide were counted.
Surface expression of FcγRI (CD64) and FcγRIIIb (CD16).
Surface expression of FcγRIIIb was measured as described previously (9), using a standard indirect immunofluorescence technique. Cells were incubated with Leu11a as the first layer and then with a fluorescein isothiocyanate-labelled goat anti-mouse antibody as the second-layer antibody. FcγRI expression was measured by using monoclonal antibody 32.2 as the first layer. Fluorescence was measured by using an Ortho Diagnostics Cytron analyzer, and fluorescence distributions represent a total of 5,000 gated events.
Heat shock and de novo protein synthesis.
Neutrophils at 107/ml were preincubated with 60-μCi/ml [35S]methionine for 15 min at 37°C. Cells were then cultured for either 1 h at 37°C, 30 min at 42°C, 1 h at 42°C, or 1 h at 37°C with TSST-1 added at 1.0 and 5.0 μg/ml. Proteins (from 106 cells) were then solubilized in (boiling) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and subjected to SDS-PAGE by the method of Laemmli (19). Radioactivity in gels was detected by using a GS-363 Molecular Imager (Bio-Rad) with a GS-250 imaging screen-CS.
Statistical analysis.
All experimental data are expressed as means ± the standard deviations (SD). Where appropriate, samples were analyzed by using the Student t test, and statistical significance was defined as P ≤ 0.05 or P ≤ 0.01.
RESULTS
Effects of superantigens on neutrophil suspensions.
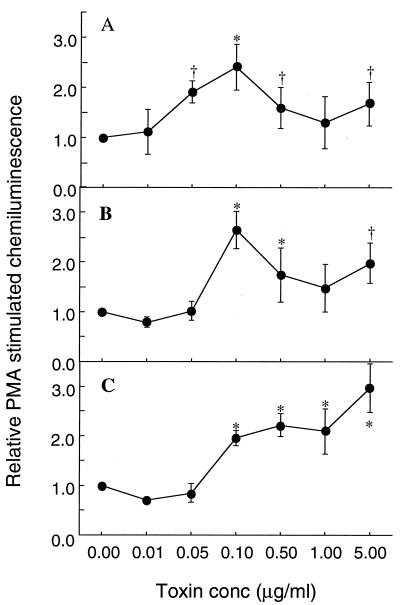
Delayed neutrophil apoptosis is associated with the preservation of functions such as the ability to produce reactive oxygen species in response to PMA or fMLP (10, 25, 34). The chemiluminescence of neutrophil suspensions cultured for 20 h and treated with a range of concentrations of SEA, SEB, or TSST-1 relative to that of untreated control cultures is shown in Fig. 1. All three toxins gave significant improvement in chemiluminescence responses after 20 h of culture at concentrations above 0.1 μg/ml and up to 5 μg/ml, which was the highest concentration tested. The maximally effective concentration of both SEA and SEB was 0.1 μg/ml, with increased concentrations of toxin showing a decline in effect up to 1.0 μg/ml. TSST-1 showed an increased effect at each concentration above 0.1 μg/ml, with maximal effectiveness at 5.0 μg/ml. The toxins were therefore added to neutrophil suspensions at 0.1 μg/ml for SEA and SEB and at 1.0 and 5.0 μg/ml for TSST-1 in further experiments.
FIG. 1.
Effects of bacterial superantigens on neutrophil respiratory burst activity. Following isolation, neutrophils were cultured in medium alone or supplemented with SEA, SEB, or TSST-1 at the concentrations (conc) shown. After 20 h of culture, aliquots of equal numbers of neutrophils were processed for assessment of oxidative-burst activity in response to stimulation with PMA (0.1 μg/ml). The results shown are means ±SD of three separate experiments. Significant difference from control incubations is indicated by the symbol † (P ≤ 0.05) or ∗ (P ≤ 0.01). A, effects of SEA; B, effects of SEB; C, effects of TSST-1.
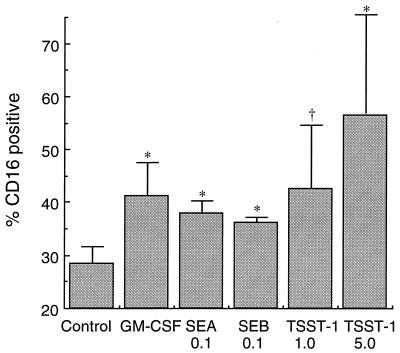
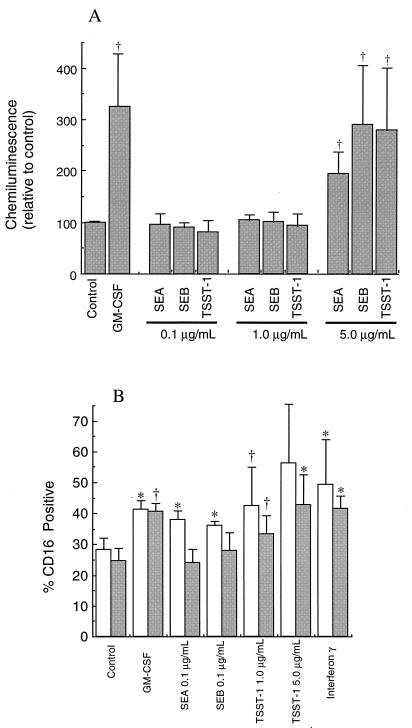
Assessment of protection from apoptosis was determined by measuring CD16 surface expression in neutrophils cultured for 20 h as shown in Fig. 2. Previous work has shown that surface levels of this receptor correlate well with other indicators of neutrophil apoptosis (5, 10). Each toxin gave significant protection from apoptosis as measured by this assay, with the protection from apoptosis comparable to that found with GM-CSF treatments. TSST-1 gave the best protection from apoptosis, with the higher TSST-1 concentration being the most potent in delaying apoptosis. These results were also confirmed by morphological assessment of apoptosis (data not shown), with the same pattern of results obtained by both measurements.
FIG. 2.
Effects of bacterial superantigens on neutrophil CD16 expression. Following isolation, neutrophils were cultured in medium alone (control) or supplemented with the agents shown at the indicated concentrations (micrograms per milliliter). After 20 h of culture, aliquots of neutrophils were processed for measurement of surface CD16 by flow cytometry. The data presented are means ± SD of three separate experiments. Significant difference from control incubations is indicated by the symbol † (P ≤ 0.05) or ∗ (P ≤ 0.01).
Role of PBMC-derived cytokines in superantigen-mediated effects.
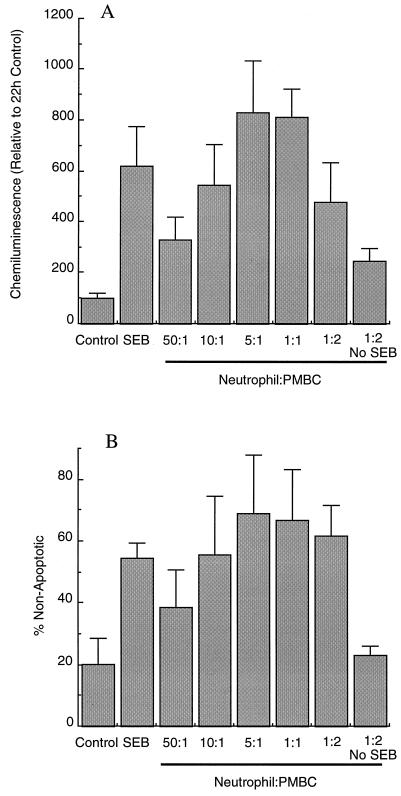
The isolation of neutrophils routinely gave >97% polymorphonuclear cells or <3% contamination with PBMC. However, the activation of PBMC by superantigen yields vast quantities of cytokines, such that contamination of neutrophil preparations with less than 3% PBMC could produce sufficient cytokines to delay neutrophil apoptosis. Figure 3 shows the results of experiments done to determine if the proportion of PBMC in a neutrophil preparation correlated with the delayed apoptosis observed following superantigen treatment. SEB added to a normal neutrophil preparation delayed apoptosis, as assessed by both morphology and preservation of the ability to produce reactive oxygen species in response to PMA. Addition of extra PBMC at a ratio of 50:1 (neutrophils to PBMC) gave no further delay in apoptosis in SEB-treated neutrophils, but as more PBMC were added to the culture, there was clear enhancement of the ability of SEB to delay the apoptosis of neutrophils. The effect was greatest at neutrophil-to-PBMC ratios between 5:1 and 1:1, which is similar to the ratio of neutrophils to PBMC in the circulation. The addition of extra PBMC to neutrophil suspensions at the highest ratio tested (one neutrophil to two PBMC) showed a delay in neutrophil apoptosis without SEB treatment, but this effect was much less than that found when SEB was included. It therefore seemed likely that cytokine production from PBMC activated by superantigen in normal neutrophil preparations could be responsible for the delayed apoptosis observed.
FIG. 3.
Effects of bacterial superantigens on cocultures of neutrophils and PBMC. Isolated neutrophils were cultured for 20 h in medium alone (control) or supplemented with SEB at 0.1 μg/ml (SEB). PBMC were added to isolated neutrophils at the ratios indicated without altering the neutrophil concentration before addition of SEB or with no SEB added. In panel A, the oxidative burst stimulated by PMA is shown relative to the 22-h control culture value, which was taken as 100%. In panel B, apoptosis was assessed morphologically and is expressed relative to that of the control suspension at time zero (which was 100% nonapoptotic).
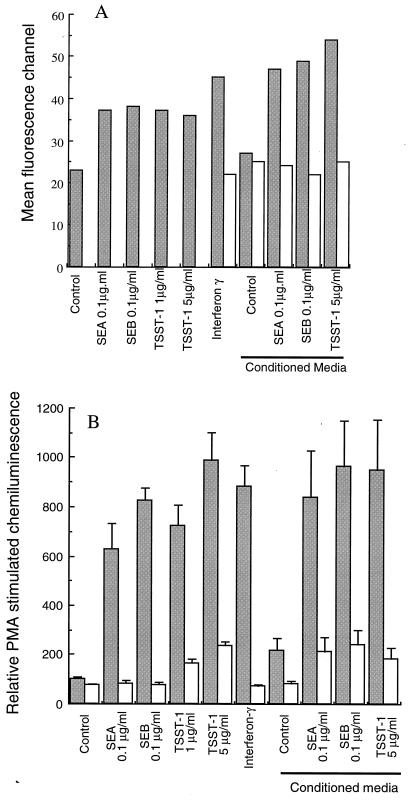
Of the cytokines produced by superantigen-activated PBMC, IFN-γ is perhaps the simplest to assay for, as it is known to induce surface expression of FcγRI on neutrophils (2, 29). Overnight culture with each of the superantigens (SEA or SEB at 0.1 μg/ml or TSST-1 at 5 μg/ml) clearly induced FcγRI expression determined by flow cytometry, as shown in Fig. 4A. At these concentrations, the toxins were almost as effective as IFN-γ used at 100 U/ml. The preparation of conditioned medium from PBMC incubated in the absence (control) or presence of each superantigen for 20 h provided confirmation that FcγRI expression could be induced by a PBMC-derived product. Untreated (control) conditioned medium from PBMC gave no induction of FcγRI on neutrophils, but conditioned medium from PBMC treated with any of the superantigens gave a marked induction of FcγRI. The addition of IFN-γ-neutralizing antiserum prevented induction of FcγRI by IFN-γ (mean channel fluorescence: IFN-γ-treated cells, 40.7 ± 12.6 [n = 3]; cells treated with IFN-γ plus neutralizing antibody, 20.8 ± 7.5 [n = 3]). Neutralization of IFN-γ with this antibody also decreased FcγRI expression stimulated by superantigen-activated PBMC-conditioned medium, indicating that the induction of FcγRI on neutrophils was almost entirely due to IFN-γ secreted from the contaminating PBMC found in neutrophil preparations.
FIG. 4.
Effects of bacterial superantigens, IFN-γ, and PBMC-conditioned medium on neutrophil functions. Neutrophils were cultured with medium alone (control) or supplemented with the agents shown. After 20 h of culture, aliquots were processed for analysis of surface FcγRI expression (A) and PMA-stimulated luminol chemiluminescence (B). Panel A shows mean channel fluorescence values for 20-h cultured neutrophils (from one of two experiments) ( ); panel B shows respiratory burst activity in response to 0.1-μg/ml PMA (
); panel B shows respiratory burst activity in response to 0.1-μg/ml PMA ( ). As indicated, antiserum to IFN-γ was included in some incubations to neutralize its action (□). Conditioned medium was prepared by incubating PBMC with each of the superantigens for 20 h as described in Materials and Methods. Results are means ± SD of three separate experiments.
). As indicated, antiserum to IFN-γ was included in some incubations to neutralize its action (□). Conditioned medium was prepared by incubating PBMC with each of the superantigens for 20 h as described in Materials and Methods. Results are means ± SD of three separate experiments.
Figure 4B shows the results of experiments done to determine if the effects of superantigen treatment on the preservation of the ability of neutrophils to generate a respiratory burst was also mediated by IFN-γ. Treatment of neutrophil cultures with 100-U/ml IFN-γ gave preservation of the oxidative burst (and protection from apoptosis) similar to that provided by treatment with all of the superantigens or superantigen-activated PBMC-conditioned medium. The inclusion of IFN-γ-neutralizing antiserum in superantigen-treated neutrophil suspensions prevented most of the effects of these agents on neutrophil function, but some effects remained at higher toxin concentrations. This may be due to inefficient neutralization of IFN-γ after time in culture. Therefore, the neutralizing antibody was added to conditioned medium 20 min before the addition of conditioned medium to neutrophils. This pretreatment completely abolished the effects of 100-U/ml IFN-γ. The superantigen-treated conditioned medium was, however, still slightly effective after neutralization of IFN-γ. The effects of superantigen were therefore mediated largely by IFN-γ. The remaining effect may be mediated by other cytokines produced by the PBMC or may be due to a direct effect of the toxins on neutrophils themselves. Similarly, the effects of superantigens on delayed neutrophil apoptosis (as assessed by morphology or surface CD16 expression) were not seen after neutralization of IFN-γ (data not shown).
Short-term effects of superantigens on neutrophils.
Experiments using fluo-3-loaded neutrophils (11, 37) indicated that addition of superantigens did not result in detectable increases in intracellular Ca2+ levels, thus excluding the possibility that these toxins stimulate receptor-mediated pathways leading to Ca2+ mobilization (data not shown). Also, we could not detect any changes in the levels of phosphorylation of neutrophil proteins on tyrosine residues (22, 36) after treatment with superantigens (data not shown). Likewise, the superantigens could neither prime nor activate the respiratory burst themselves (data not shown).
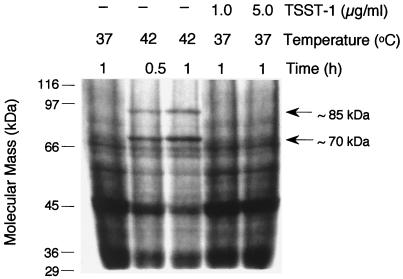
TSST-1 is reported to induce HSP expression in neutrophils (15). Figure 5 shows the results of an experiment investigating induction of HSPs following treatment of neutrophils for 1 h with TSST-1. No induction of HSPs was found following TSST-1 treatment, while parallel suspensions clearly showed induction of HSPs following heat shock at 42°C for 30 min or 1 h.
FIG. 5.
Effects of heat shock and TSST-1 on neutrophil protein biosynthesis. Neutrophils were incubated in medium containing [35S]methionine at the temperatures and times indicated. Neutrophils in identical medium were also treated with TSST-1 at 1.0 or 5.0 μg/ml for 1 h. Total proteins were then separated by SDS-PAGE, and radioactivity was detected as described in Materials and Methods.
Delayed apoptosis in superantigen-treated neutrophil suspensions depleted of T cells.
The above-described experiments indicate that most, if not all, of the effects of superantigens on neutrophil responses are due to the effects of cytokines (especially IFN-γ) generated by PBMC present in the neutrophil suspensions. The production of cytokines by PBMC is reported to require the presence of both T cells and antigen-presenting cells (32), so depletion of contaminating T cells from neutrophil preparations should prevent this cytokine production. T-cell-depleted neutrophils were rescued from apoptosis by GM-CSF, as assessed by PMA-induced chemiluminescence after 20 h of culture (Fig. 6A). Treatment with superantigens at both 0.1 and 1.0 μg/ml (which effectively delayed apoptosis in normal neutrophil suspensions) had no effect on these T-cell-depleted suspensions. However, at the highest concentration of superantigen used (5.0 μg/ml), SEA, SEB, and TSST-1 were still effective at preserving neutrophil function after 20 h of culture.
FIG. 6.
Effects of bacterial superantigens on apoptosis of T-cell-depleted neutrophil suspensions. Neutrophils were isolated and depleted of T cells as described in Materials and Methods. Following culture for 20 h under the conditions indicated, the oxidative burst in response to PMA was measured by luminol-dependent chemiluminescence (A) or apoptosis was assessed by determining levels of surface CD16 expression (B). The values shown are means ± SD of four separate experiments. Significant difference from the control is indicated by the symbol † (P ≤ 0.05) or ∗ (P ≤ 0.01). □, normal neutrophils; ■, T-cell-depleted neutrophils.
Figure 6B shows apoptosis of neutrophils following 20 h of culture as assessed by the percentage of cells retaining high levels of surface CD16. It is clear that the depletion of T cells had no adverse effect on the ability of exogenously added GM-CSF or IFN-γ to delay apoptosis (Fig. 6B). Superantigen treatment of T-cell-depleted neutrophils at 0.1 μg/ml showed a normal rate of apoptosis. However, at higher concentrations of superantigen, the entry into apoptosis was still delayed by a slight but significant extent at 1.0 μg/ml and more markedly at 5.0 μg/ml. The protection from apoptosis afforded by the higher concentrations of superantigen was not as great in T-cell-depleted neutrophils as it was in normal neutrophil preparations, suggesting that part of the protection from apoptosis is T cell dependent.
Apoptosis in superantigen-treated neutrophil suspensions depleted of both T cells and MHC class II-expressing cells.
The protection from apoptosis provided by high concentrations of superantigens in T-cell-depleted neutrophil suspensions suggested either (i) a direct effect of these superantigen treatments on neutrophil apoptosis or (ii) an effect mediated by cytokines from other, contaminating cells. For example, MHC class II-expressing cells may be able to produce cytokines when stimulated with high concentrations of superantigen, even in the absence of T cells. It was therefore necessary to deplete neutrophil suspensions of both T cells and MHC class II-expressing cells to confirm if the superantigens had a direct effect on neutrophil apoptosis.
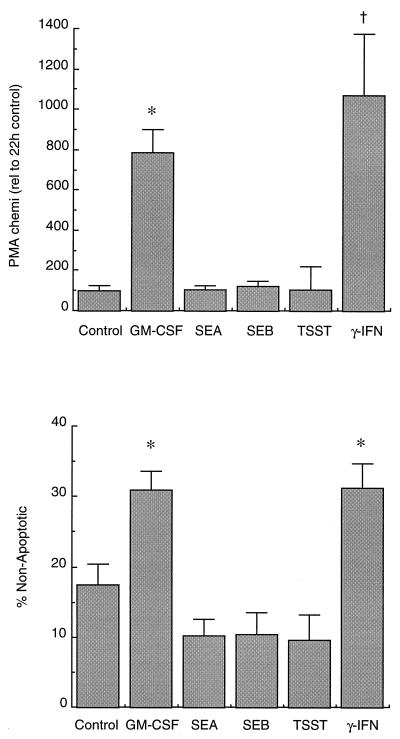
Figure 7A and B shows PMA-stimulated chemiluminescence and apoptosis results of neutrophil preparations that had been depleted of both T cells and MHC class II-expressing cells and then cultured for 20 h. Both GM-CSF and IFN-γ were effective at preserving the ability of neutrophils to generate a respiratory burst (Fig. 7A) and protected against apoptosis in these highly purified preparations. However, none of the superantigens (used at 5 μg/ml) could preserve function or protect against apoptosis in these purified neutrophils.
FIG. 7.
Effects of bacterial superantigens on apoptosis of highly purified neutrophil suspensions. Neutrophil suspensions depleted of both T cells and MHC class II-expressing cells were incubated for 20 h with the agents indicated. Cells were analyzed for the ability to generate a respiratory burst (A), or apoptosis was determined by morphology (B). The values shown are means ± SD of three separate experiments, and a value significantly different from the control is indicated by the symbol † (P ≤ 0.05) or ∗ (P ≤ 0.01).
DISCUSSION
In this report, we have shown that neutrophil apoptosis is delayed by superantigen treatment but that this delay in apoptosis is dependent upon the presence of a small number of contaminating PBMC in the suspensions. The levels of cytokines produced by contaminating PBMC in normal neutrophil preparations are sufficiently high to delay neutrophil apoptosis. IFN-γ accounts for almost all of the delayed apoptosis observed, as neutralization of IFN-γ in superantigen-treated neutrophil suspensions abolished around 80% of the action of superantigens on neutrophil apoptosis (Fig. 4).
From previously published reports, 3% contamination of PBMC could result in the production of cytokines to the following levels: IFN-γ, 65 to 80 U/ml (17, 32); IL-2, 3 to 250 U/ml (17, 32); IL-1β, ∼4 U/ml (20, 31); TNF-α, 1 to 2.5 U/ml (17, 20). These cytokine levels are sufficient to delay neutrophil apoptosis, as IFN-γ will delay apoptosis at concentrations between 50 and 500 U/ml (3). IL-1β is able to delay neutrophil apoptosis at concentrations as low as 2 U/ml but is more effective at concentrations between 20 and 200 U/ml (3). IL-2 can delay neutrophil apoptosis, but the concentrations produced by 3% contamination with PBMC is unlikely to be sufficient to delay apoptosis, as concentrations of around 1,000 U/ml are required (28). Neutralization of IFN-γ still leaves the other cytokines present, which could explain the slight effects that remain after IFN-γ neutralization (Fig. 4). IL-1β, which was present at a concentration sufficient to delay neutrophil apoptosis slightly, could be the cytokine present after IFN-γ neutralization that resulted in delayed apoptosis.
The depletion of T cells from the neutrophil suspensions did abolish the delayed apoptosis observed with superantigen concentrations below 5.0 μg/ml, but there was still a delay in apoptosis when they were used at this higher concentration. This may be explained by the observation of Jupin et al. (17), who reported that increasing concentrations of superantigen gave a corresponding increase in IFN-γ production. Therefore, the depletion of T cells, if not complete, would still allow the production of IFN-γ at levels sufficient to delay neutrophil apoptosis. Alternatively, the work of Jupin et al. (17) suggests that monocytes are able to produce cytokines (TNF-α and IL-1β) without the presence of T cells. Therefore, the removal of T cells alone may not prevent all cytokine production, with cytokines derived from the remaining PBMC being responsible for the delayed neutrophil apoptosis.
The depletion of both T cells and MHC class II-expressing cells from neutrophil preparations allowed the action of superantigens on neutrophil apoptosis to be assessed without any contribution from other superantigen-stimulated cells. This clearly demonstrated that superantigens had no direct action on neutrophils that we could measure in our experiments (Fig. 7). Apart from an inability to delay apoptosis or preserve function, the superantigens did not affect levels of phosphorylation of neutrophil proteins on tyrosine residues, did not stimulate intracellular Ca2+ transients, and neither activated nor primed the respiratory burst (data not shown).
The possibility remains that superantigens do have other direct actions on neutrophils, such as the ability of TSST-1 to modulate fMLP receptor expression (14), and induce HSP expression (15). We have examined the induction of HSP expression by TSST-1 and found no induction under conditions similar to those used by Hensler et al. (15). This difference in results may be explained by the purity of the neutrophils used. Hensler et al. (15) reported neutrophil purity of 95%, which indicates approximately twofold higher contamination with PBMC than in the preparations used in this report. Therefore, cytokine production would be considerably higher in Hensler’s cultures, and cytokines such as IL-1 and TNF-α are able to induce HSP expression rapidly in neutrophils (18).
ACKNOWLEDGMENTS
We thank the North West Cancer Research Fund for financial support.
REFERENCES
- 1.Brach M A, deVos S, Gruss H-J, Hermann F. Prolongation of survival of human polymorphonuclear neutrophils by granulocyte-macrophage colony stimulating factor is caused by inhibition of programmed cell death. Blood. 1992;80:2920–2924. [PubMed] [Google Scholar]
- 2.Buckle A M, Hogg N. The effect of IFN-γ and colony stimulating factors on the expression of neutrophil cell membrane receptors. J Immunol. 1989;143:2295–2301. [PubMed] [Google Scholar]
- 3.Colotta F, Re F, Polentarutti N, Sozanni S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- 4.D’Orazio J A, Burke G W, Stein-Streileiln J. Staphylococcal enterotoxin B activates purified NK cells to secrete IFN-γ but requires T lymphocytes to augment NK cytotoxicity. J Immunol. 1995;154:1014–1023. [PubMed] [Google Scholar]
- 5.Dransfield I, Buckle A, Savill J S, McDowall A, Haslett C, Hogg N. Neutrophil apoptosis is associated with a reduction in CD16 (FcγRIII) expression. J Immunol. 1994;153:1254–1263. [PubMed] [Google Scholar]
- 6.Dreissen C, Hiru K, Weelinghausen N, Kircher H, Rink L. Influence of serum on zinc, toxic shock syndrome toxin-1, and lipopolysaccharide-induced production of IFN-γ and IL-1β by human mononuclear cells. J Leukocyte Biol. 1995;57:904–908. doi: 10.1002/jlb.57.6.904. [DOI] [PubMed] [Google Scholar]
- 7.Edwards S W. Luminol- and lucigenin-dependent chemiluminescence of neutrophils: role of degranulation. J Clin Lab Immunol. 1987;22:35–39. [PubMed] [Google Scholar]
- 8.Edwards S W. The O2-generating NADPH oxidase of phagocytes. Structure and methods of detection. Methods a companion Methods. Enzymol. 1997;9:563–577. doi: 10.1006/meth.1996.0064. [DOI] [PubMed] [Google Scholar]
- 9.Edwards S W, Watson F, MacLeod R, Davies J M. Receptor expression and oxidase activity in human neutrophils: regulation by granulocyte macrophage-colony stimulating factor and dependence upon protein biosynthesis. Biosci Rep. 1990;10:393–401. doi: 10.1007/BF01117239. [DOI] [PubMed] [Google Scholar]
- 10.Gasmi L, McLennan A G, Edwards S W. The diadenosine polyphosphates Ap3A and Ap4A and adenosine triphosphate interact with granulocyte-macrophage colony-stimulating factor to delay neutrophil apoptosis: implications for neutrophil-platelet interactions during inflammation. Blood. 1996;87:3442–3449. [PubMed] [Google Scholar]
- 11.Gasmi L, McLennan A G, Edwards S W. Diadenosine polyphosphates induce intracellular Ca2+ mobilization in human neutrophils via a pertussis toxin sensitive G-protein. Immunology. 1997;90:154–159. doi: 10.1046/j.1365-2567.1997.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gosselin E J, Wardwell K, Rigby W F C, Guyre P M. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-γ, and IL-3. J Immunol. 1993;151:1482–1490. [PubMed] [Google Scholar]
- 13.Hébert M-J, Takano T, Holthöfer H, Brady H R. Sequential morphologic events during apoptosis of human neutrophils: modulation by lipoxygenase derived eicosanoids. J Immunol. 1996;157:3105–3115. [PubMed] [Google Scholar]
- 14.Hensler T, Köller M, Geoffroy C, Alouf J E, König W. Staphylococcus aureus toxic shock syndrome toxin 1 and Streptococcus pyogenes erythrogenic toxin A modulate inflammatory mediator release from human neutrophils. Infect Immun. 1993;61:1055–1061. doi: 10.1128/iai.61.3.1055-1061.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hensler T, Köller N, Alouf J E, König W. Bacterial toxins produce heat shock proteins in human neutrophils. Biochem Biophys Res Commun. 1991;179:872–879. doi: 10.1016/0006-291x(91)91899-n. [DOI] [PubMed] [Google Scholar]
- 16.Herman A, Kappler J W, Marrack P, Pullen A M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- 17.Jupin C, Anderson S, Damais C, Alouf J E, Parant M. Toxic shock syndrome toxin-1 as an inducer of human tumour necrosis factors and γ interferon. J Exp Med. 1988;167:752–761. doi: 10.1084/jem.167.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konig W, Koller M, Brom J. Priming mechanisms and induction of heat-shock proteins in human polymorphonuclear granulocytes induced by eicosanoids and cytokines. Eicosanoids. 1992;5:S39–S41. [PubMed] [Google Scholar]
- 19.Laemelli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Lagoo A S, Lagoo-Deenadayalan S, Lorenz H-M, Byrne J, Barber W H, Hardy K J. IL-2, IL-4, and IFN-γ gene expression versus secretion in superantigen-activated T cells. J Immunol. 1994;152:1641–1652. [PubMed] [Google Scholar]
- 21.Lee A, Whyte M K B, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukocyte Biol. 1993;54:283–289. [PubMed] [Google Scholar]
- 22.Lowe G M, Slupsky J R, Galvani D W, Edwards S W. GTPγS-stimulated phospholipase D activation in human neutrophils occurs by protein kinase C-dependent and -independent pathways, but not via tyrosine kinases. Biochem Biophys Res Commun. 1996;220:484–490. doi: 10.1006/bbrc.1996.0431. [DOI] [PubMed] [Google Scholar]
- 23.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 24.Misfeldt M L. Microbial “superantigens.”. Infect Immun. 1990;58:2409–2413. doi: 10.1128/iai.58.8.2409-2413.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moulding D A, Quayle J A, Hart C A, Edwards S W. Mcl-1 expression in human neutrophils: regulation by cytokines and correlation with cell survival. Blood. 1998;92:2495–2502. [PubMed] [Google Scholar]
- 26.Murray J, Barbara J A J, Dunkley S A, Lopez A F, Van Stade X, Condliffe A M, Dransfield I, Haslett C, Chilvers E R. Regulation of neutrophil apoptosis by tumor necrosis factor-alpha: requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood. 1997;90:2772–2783. [PubMed] [Google Scholar]
- 27.Origuchi T, Eguchi K, Kawabe Y, Yamashita I, Mizokami A, Ida H, Nagataki S. Increased levels of serum IgM antibody to staphylococcal enterotoxin B in patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54:713–720. doi: 10.1136/ard.54.9.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pericle F, Liu J H, Diaz J I, Blanchard D K, Wei S, Forni G, Djeu J Y. Interleukin-2 prevention of apoptosis in human neutrophils. Eur J Immunol. 1994;24:440–444. doi: 10.1002/eji.1830240226. [DOI] [PubMed] [Google Scholar]
- 29.Petroni K C, Shen I, Guyre P M. Modulation of human polymorphonuclear leukocytes, IgG Fc receptors, and Fc receptor-mediated functions by IFN-γ and glucocorticoids. J Immunol. 1988;140:3467–3472. [PubMed] [Google Scholar]
- 30.Savill J S, Wyllie A H, Henson J E, Walport M J, Henson P M, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. J Clin Investig. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.See R H, Chow A W. Role of the adhesion molecule lymphocyte function associated antigen 1 in toxic shock syndrome toxin 1-induced tumor necrosis factor alpha and interleukin-1β secretion by human monocytes. Infect Immun. 1992;60:4957–4960. doi: 10.1128/iai.60.11.4957-4960.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.See R H, Kum W W S, Chang A H, Goh S H, Chow A W. Induction of tumor necrosis factor and interleukin-1 by purified staphylococcal toxic shock syndrome toxin 1 requires the presence of both monocytes and T lymphocytes. Infect Immun. 1992;60:2612–2618. doi: 10.1128/iai.60.7.2612-2618.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith W B, Guida L, Sun Q, Korpelainen E I, van den Hoeval C, Gillis D, Harrylowicz C M, Vadas M A, Lopez A F. Neutrophils activated by GM-CSF express receptors for IL-3 which mediate class II expression. Blood. 1995;86:3938–3944. [PubMed] [Google Scholar]
- 34.Stringer R E, Hart C A, Edwards S W. Sodium butyrate delays neutrophil apoptosis: role of protein biosynthesis in neutrophil survival. Br J Hematol. 1996;92:169–175. doi: 10.1046/j.1365-2141.1996.00307.x. [DOI] [PubMed] [Google Scholar]
- 35.Trede N S, Moris T, Scholl P R, Geha R S, Chatila T. Early activation events induced by the staphylococcal superantigen toxic shock syndrome toxin-1 in human peripheral blood monocytes. Clin Immunol Immunopathol. 1994;70:137–144. doi: 10.1006/clin.1994.1021. [DOI] [PubMed] [Google Scholar]
- 36.Watson F, Edwards S W. Stimulation of primed neutrophils by soluble immune complexes. Priming leads to enhanced intracellular Ca2+ elevations, activation of phospholipase D and activation of the NADPH oxidase. Biochem Biophys Res Commun. 1998;247:819–826. doi: 10.1006/bbrc.1998.8524. [DOI] [PubMed] [Google Scholar]
- 37.Watson F, Gasmi L, Edwards S W. Stimulation of intracellular Ca2+ levels in human neutrophils by soluble immune complexes: functional activation of FcγRIIIb during priming. J Biol Chem. 1997;272:17944–17951. doi: 10.1074/jbc.272.29.17944. [DOI] [PubMed] [Google Scholar]