Abstract
Neutrophil collagenase (matrix metalloproteinase 8 [MMP-8]) is an important mediator of tissue destruction in inflammatory diseases. Studies of anaerobic periodontal infections have shown that active MMP-8 in gingival crevicular fluid is associated with the degradation of periodontal tissues in progressive periodontitis whereas the latent enzyme is predominant in gingivitis. Since the activation of MMP-8 appears to be a crucial step in periodontitis, we have examined the activation of MMP-8 in gingival crevicular fluid samples by using a soluble biotinylated collagen substrate. Analysis of gingival crevicular fluid in periodontitis, gingivitis, and controls revealed sixfold (P < 0.001)-higher levels of active collagenase in periodontitis (n = 12) samples compared to gingivitis (n = 17) samples, which exhibited low levels of activity, while controls (n = 25) showed no activity. After gingival crevicular fluid was collected, no further activation of latent collagenase occurred in vitro. Although both MMP-1 and MMP-8, but not MMP-13, could be detected by immunoblots, blocking antibodies to MMP-1 showed that collagenase activity was largely contributed by MMP-8, which was localized to the matrix of diseased tissues. The MMP-8 in gingival crevicular fluid migrated primarily as a 60-kDa form with smaller amounts of a 78-kDa species, whereas MMP-8 isolated from peripheral neutrophils migrated at 70 and 89 kDa, corresponding to active and latent forms of the enzyme, respectively. Most of the MMP-8 in the 60- and 70-kDa bands selectively bound to tissue inhibitor of metalloproteinase 2 and collagen, indicating that most, but not all, of the enzyme in these bands was in an activated form. However, the amounts of the 78- and 60-kDa forms from gingival crevicular fluid in different samples did not correlate (r2 = 0.028) with the latent and active enzyme measured by collagenase assay. Collectively, these studies have identified distinct forms of latent and active MMP-8 in gingival crevicular fluid that appear to result from a unique activation mechanism that occurs in periodontitis. The complexity of MMP-8 activation is further indicated by the presence of latent, activated, and superactivated forms of MMP-8 in the 60- and 70-kDa bands obtained from gingival crevicular fluid and neutrophil samples, respectively.
In chronic infections of connective tissues, adhesion and invasion of host tissues by bacterial pathogens can initiate destruction of structural proteins including collagens. The degradation of collagen can be mediated directly by bacterial proteases or, if there is significant inflammation, more extensively by enzymes secreted by polymorphonuclear leukocytes (PMNs). For the study of collagen degradation mechanisms in chronic, gram-negative anaerobic bacterial infections, periodontitis is widely used as a model in which connective tissue destruction is mediated in part by host cell collagenases (17). Vertebrate collagenases are members of the matrix metalloproteinase (MMP) family of proteolytic enzymes that are involved in extracellular matrix degradation and remodelling during the course of periodontal diseases. Resident fibroblasts, epithelial cells, and macrophages synthesize MMP-1 (collagenase 1) while infiltrating neutrophils release MMP-8 (collagenase 2 [1]). A third collagenase (MMP-13) with wide substrate specificity (5) has recently been shown to be produced by both epithelial and mesenchymal cells in inflamed and remodelling connective tissues (7, 28, 31) and in healing sites (32). All three enzymes could contribute to the collagenolytic activity in diseased periodontal tissues.
Collagenases cleave collagen under physiological conditions of pH, temperature, and osmolarity (23). Consequently, their regulation is of considerable importance in connective tissue homeostasis. Collagenase regulation is a complex process involving enzyme synthesis, secretion, activation, and inhibition. Various cytokines and growth factors can regulate the local expression of interstitial collagenases (MMP-1 and MMP-8) and their inhibitors, such as the tissue inhibitor(s) of metalloproteinase (TIMP[s]) during growth, development, remodelling, and repair processes (1). In contrast, MMP-8 synthesis by neutrophils is completed during granulocyte precursor cell differentiation in the bone marrow (1). When neutrophils are recruited to a site of inflammation, they release large quantities of MMP-8 stored in specific granules, and the inflammatory response is sustained by recruitment of new cells rather than by the local synthesis of MMP-8.
As collagenases are secreted in a latent form, their activation is a rate-limiting step in the initiation of collagen breakdown and connective tissue destruction. Although the mechanism of activation of MMPs in vivo is not completely understood, in vitro studies have demonstrated that collagenases can be activated by a diverse range of agents that remove or modify the conformation of the prodomain (1, 11, 20). Removal of the prodomain by enzymes of host or bacterial origin (3, 4, 27, 29) results in a reduction of the molecular mass of the MMPs. Different proteolytic enzymes cleave at different sites in the proenzyme domain, generating different sizes of active enzyme with different levels of enzyme activity (12). Notably, the correct folding of the activated MMP-8 is critical for enzyme activity and stability (2). Full activation, or superactivation, occurs only when the correct amino-terminal amino acid is generated following proteolytic cleavage by intermolecular or complex autocatalytic reactions. Thus, amino-terminal Phe-99 forms a salt bridge network with an α-helix at the active site which is precluded by spatial constraints when cleavage occurs at other sites in the catalytic domain (14).
In adult periodontitis, the levels of neutrophil collagenase (MMP-8) increase in the gingival crevicular fluid (GCF) exudate (22) and consequently in samples of whole saliva (30). Moreover, active MMP-8, but not the latent enzyme, is associated with periods of active connective tissue destruction and a clinical diagnosis of periodontitis (17). However, little is known about the nature of the activation process of MMP-8 in periodontitis or the relationship between different molecular forms of MMP-8 and their activity in GCF. The aim of this study was, therefore, to characterize the active and latent forms of MMP-8 in GCF of patients with adult periodontitis and to provide insights into the process of MMP-8 activation that leads to destruction of periodontal tissues.
MATERIALS AND METHODS
Reagents.
Anti-MMP-8 mouse monoclonal antibody (immunoglobulin G1 [IgG1]) was obtained from Calbiochem (Cambridge, Mass.). A rabbit anti-human MMP-1 blocking antibody, used to neutralize MMP-1 activity in samples and for Western blots, was kindly donated by B. and H. Birkedal-Hansen (National Institute of Dental Research, National Institutes of Health, Bethesda, Md.). Rabbit anti-human antibody to MMP-13 was from Chemicon (Temecula, Calif.). Horseradish peroxidase (HRP)-labeled anti-mouse IgG1 and anti-rabbit IgG were from Caltag Laboratories (Burlingame, Calif.). Prestained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) low-range molecular weight standards were from Bio-Rad Laboratories (Hercules, Calif.). Purified human TIMP-2 was from Chemicon, and activated CH-Sepharose 4B, Percoll and Dextran T-500, and protein G-Sepharose were from Pharmacia (Uppsala, Sweden). p-Aminophenylmercuric acetate (APMA), phorbol myristate acetate (PMA), and sodium azide were from Sigma (St. Louis, Mo.). Immuno Pure N-hydroxysuccinimide–LC-biotin for collagen labeling was from Pierce (Rockford, Ill.). ECL reagents and HRP-labeled streptavidin were from Amersham (Little Chalfont, Buckinghamshire, England). Vitrogen 100 for collagen binding experiments was from Celtrix (Palo Alto, Calif.).
GCF samples.
Human subjects were recruited from the periodontal graduate clinic, Faculty of Dentistry, University of Toronto. Three different groups of subjects were involved in the study: (i) periodontitis (exhibited radiographic bone loss and >5-mm loss of connective tissue attachment) (n = 12), (ii) gingivitis (no radiographic bone loss but marked gingival inflammation) (n = 17), and (iii) control subjects without clinically detectable gingival inflammation (n = 25). Informed consent was obtained from each subject as directed in the study protocol approved by the Human Subject Experimentation Committee, University of Toronto. GCF was obtained in a mouth rinse as described in detail previously (6). Briefly, patients first rinsed thoroughly with tap water for 10 s and after 2 min rinsed again with 3 ml of sterile saline (0.15 M NaCl). The 3-ml rinse was centrifuged at 500 × g for 20 min, and the supernatants were either analyzed immediately or stored at −20°C.
Neutrophil preparations.
Approximately 40 to 80 ml of blood drawn from healthy male donors was placed in a sterile, polypropylene centrifuge tube containing 4.4 ml of 3.8% citrate as anticoagulant for each 40 ml of blood and centrifuged for 20 min at 175 × g at rooom temperature (RT) (22°C). The pelleted cells were sedimented in 90% Percoll and 5 ml of 6% Dextran T-500 (containing 0.9% NaCl). The platelets were removed from the plasma, and a leukocyte-enriched supernatant was obtained. This supernatant was centrifuged, resuspended in platelet-poor plasma, and layered on plasma-Percoll gradients by a series of centrifugation steps. The upper monocyte layer was removed with a sterile transfer pipette, and the lower PMN layer was washed. The final preparation was resuspended in an appropriate volume of buffer containing dextrose phosphate, KCl, NaCl, and magnesium to yield final concentrations of 8 × 106 cells/ml with a purity of PMNs of >98%. The buffer maintained cell viability for 4 to 5 h at this concentration. Immediately after isolation, PMNs were used for assays. The cells were first concentrated 10-fold (∼8 × 107 cells/ml) by resuspension following centrifugation and stimulated with 0.1 μM PMA in the presence or absence of 1 mM sodium azide (a myeloperoxidase inhibitor) for 15 to 30 min at 37°C in a water bath. In total, 13 neutrophil samples were prepared in the absence, and 20 samples were prepared in the presence, of sodium azide. Cells were pelleted, and the supernatant was used to analyze samples for the presence of the neutrophil MMP-8.
Collagenase activation.
GCF samples and preparations of MMP-1 (from human gingival explants) and MMP-8 were activated with 1 mM APMA for 1 h to assay for total collagenase activity and to obtain indirect estimations of latent enzyme. To examine the pattern of natural MMP-8 activation by released oxidants from PMNs, resuspended cells (8 × 106 cells/ml) were stimulated with 0.1 μM PMA at 37°C for 15 min and incubated at RT in the absence or presence of 1 mM sodium azide for 0, 2, 4, and 19 h. The PMN preparations were centrifuged, the supernatant containing naturally activated MMP-8 was assayed for collagenolytic activity by soluble biotinylated collagen assay (SBA), and the MMP-8 molecular species was assayed by Western blotting and compared to results obtained at time zero.
Measurement of collagenolytic activity.
Collagenase activity was measured by SBA, which is described in detail elsewhere (18). Briefly, samples (50 μl) in collagenase assay buffer (0.05 M Tris-HCl [pH 7.4], containing 0.2 M NaCl, 5 mM CaCl2, 0.5 μl of Brij 35 per ml, and 0.2 μg of NaN3 per ml) were incubated with 0.5 μg of biotinylated collagen substrate for 18 h at RT. Digestion was terminated by adding 25 μl of SB/DTT (0.34 M Tris-HCl [pH 6.8] containing 6 M urea, 1.5% [wt/vol] SDS, 6% [vol/vol] bromophenol blue, and 0.18 M dithiothreitol) and then heating the mixture at 100°C for 5 min. Collagen fragments were separated on 7.5% polyacrylamide cross-linked SDS-PAGE gels and transferred to a nitrocellulose membrane with the PHAST system (Pharmacia). After blocking of nonspecific binding sites with BLOTTO (5% [wt/vol] Carnation milk in Tris-buffered saline [TBS]–Tween), the membranes were rinsed with TBS-Tween (0.02 M Tris-HCl, 0.14 M NaCl [pH 7.6], containing 0.1% Tween 20) and incubated with HRP-labeled streptavidin diluted 1/1,500 in TBS-Tween. After a second wash, ECL reagents were used for detection of biotinylated collagen fragments by chemiluminescence. Autoradiographs were scanned, and full-length α and three-fourths α chains were quantified by computer analysis with the IP Lab Gel Scientific Image Processing program (Signal Analytics, Vienna, Va.). Collagenase activity was measured from densitometric analysis of the amount of biotinylated collagen (for standard assay, 0.5 μg/50-μl sample) converted to three-fourths α chains and expressed as nanounits (note that 1 nU = 1 pg of biotinylated collagen degraded/min at 22°C).
Analysis of MMP-1 and MMP-13 activity in GCF samples.
The presence of MMP-1 activity in GCF samples was determined by analyzing the change in collagenase activity measured by SBA before and after incubation with 0.1 μg of rabbit anti-MMP-1 antibodies, an amount which completely inhibits 100 nU of MMP-1 activity. For assessment of MMP-13 activity, 100 μg of a rabbit polyclonal antibody against MMP-13 was bound to protein G-Sepharose (300 μl of a packed gel). An aliquot (200 μl) of a mouth rinse sample was passed through a microcolumn of 100 μl of packed resin bound with antibody or protein G-Sepharose alone. The SBA activities before and after the column absorption were compared.
Western blots.
Enzyme samples in SB/DTT were electrophoresed in SDS-PAGE 10% cross-linked polyacrylamide minigels for 1 h at 120 V. Proteins were electrophoretically transferred onto nitrocellulose membranes overnight at 25 mA/gel. After blocking of nonspecific binding sites with 5% (wt/vol) BLOTTO, membranes were incubated with primary antibody at RT for 1 h. Mouse monoclonal anti-MMP-8 was used at 5 μg/ml, while anti-MMP-1 antibody was used at 2 μg/ml and anti-MMP-13 was used at 2 μg/ml. An anti-mouse IgG1-HRP conjugate was used at a 1/4,000 dilution to detect MMP-8, and an anti-rabbit IgG-HRP conjugate was used at a 1/2,000 dilution for MMP-1 and MMP-13 blots. Proteins were visualized with ECL reagents, and the molecular weights of immunoreactive bands for MMP-1, MMP-8, and MMP-13 were determined by comparison with prestained molecular weight standards. Under the conditions used, no immunoreactivity was observed in the absence of primary antibody.
TIMP-2 inhibition studies.
The efficacy of MMP inhibition by human TIMP-2 was first determined by adding 25 and 75 ng of the inhibitor to 100 nU of APMA-activated neutrophil collagenase and incubating the mixture for 1 h at 4°C before assaying the residual enzyme activity. To capture enzyme, 5 μg of TIMP-2 that had been dialyzed against 0.1 M NaHCO3 buffer (pH 8.0) containing 0.5 M NaCl was coupled to ∼55 mg of activated CH-Sepharose and incubated for 4 h at 4°C. Ethanolamine (1 M) in 0.1 M Tris-HCl buffer (pH 8.0) was used to block remaining active groups on the resin, and alternating washes with basic (0.1 M Tris-HCl [pH 8.0], containing 0.5 M NaCl) and acidic (0.1 M acetate [pH 4.0], containing 0.5 M NaCl) buffers were employed to remove noncovalently bound protein. Control resin was prepared by following the same steps in the absence of TIMP-2. Enzyme samples containing 200 nU of collagenase were incubated with TIMP-2 resin (20 μl), or the control resin, on a rotator at 4°C for 1 h. Following centrifugation of the resin, supernatants were tested by Western blotting and SBA and compared to equivalent amounts of the original, untreated samples. Enzyme complexed to TIMP-2 or control resins was eluted by boiling the resin in SB/DTT for 5 min, and the eluates were tested by Western blotting.
Collagen binding.
Collagen films were made in 96-well tissue culture, or on Reacti-Bind maleic anhydride-activated plates (Pierce) with 60 μl of bovine type I collagen (Vitrogen 100) per well diluted to 1 mg/ml in phosphate-buffered saline (PBS), pH 7.4. To promote fibril formation, the plates were incubated at 37°C for 1 h before drying them overnight at RT in a laminar flow hood. Control wells were incubated with PBS and treated in the same manner. Films were washed three times for 1 h with TBS to rehydrate the collagen and to remove loosely bound collagen. Other plates were coated with the same amount of monomeric collagen or heat-denatured collagen (gelatin) dried down from 60 μl of 0.5 M acetic acid. Samples of MMP-8 from peripheral blood neutrophils (n = 52) or from mouth rinse samples (n = 16), with or without APMA activation, were applied in 50-μl volumes to individual wells and incubated for 2 h at 4°C to minimize degradation of the collagen film and autocatalytic degradation of the collagenase. After incubation, enzyme bound to the substrate was removed with SB/DTT and analyzed on Western blots. Loss of enzyme activity in the supernatant was determined by SBA. Approximately 20% of collagen and gelatin was lost from the plates during the incubations as determined by Bio-Rad protein assay.
Immunolocalization studies.
Biopsy samples of inflamed gingival tissue (n = 8) were obtained from periodontitis sites at the time of extraction, while control samples (n = 6) were taken from clinically healthy gingiva during the removal of excess but normal tissue at the time of periodontal surgery. All specimens were frozen in liquid nitrogen within a few minutes of excision and stored at −70°C before being mounted on cryostat specimen holders with O.C.T. compound (Miles, Inc., Elkhart, Ind.). Frozen sections, 8 μm thick, were fixed for 10 min at RT with 2% paraformaldehyde. All subsequent procedures were performed at RT. Sections were washed with PBS and permeabilized with 3% Triton in Ca2+- Mg2+-free PBS. Nonspecific binding sites were blocked with 1% normal mouse serum and 1% normal goat serum in PBS. After being washed once with PBS, the sections were incubated with mouse monoclonal anti-MMP-8 antibody (100 μg of IgG/ml of PBS) for 1 h and washed three times for 1 h with PBS. Fluorescein isothiocyanate-labeled goat anti-mouse IgG was incubated for 1 h in the dark at a 1/64 dilution in PBS. The sections were washed 3 times for 1 h with PBS, and nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole; 1 μg/ml in 0.01% Nonidet and PBS) for 2 min and washed once with PBS. Sections were covered with Immunofluor, mounted, and examined immediately by epifluorescence microscopy. Fluorescence micrographs were made on Kodak high-speed TMX P3200 film. With each series of tissue sections studied for MMP-8 immunolocalization, peripheral blood neutrophils or GCF samples were examined as positive controls. Negative controls were cytospins of peripheral blood lymphocytes or neutrophils treated without primary antibody. Prior to immunostaining, adjacent sections from all tissue specimens were stained with hematoxylin and eosin for light microscopy and photography to assess the orientation of the sample.
Statistical analysis.
The Pearson correlation was used to determine the linearity of relationships between assays. Means and standard errors of the means were computed for groups when appropriate. Student’s t test was used to assess the significance of the differences between SBA activities in control and diseased populations in which single comparisons were made.
RESULTS
Collagenase activity in GCF.
The relative amounts of active and latent enzyme were examined in 50-μl samples of GCF from patients with a clinically healthy periodontium, with gingivitis, or with periodontitis. In assays conducted without APMA activation, collagenase activity was not detected in GCF from control patients (n = 25) whereas activity in subjects with gingival inflammation but without bone destruction was 23.6 ± 7.9 nU (mean ± standard error of the mean; n = 17). Periodontitis patients (n = 12) showed nearly sixfold-higher levels of activity (138.4 ± 29.2 nU; P < 0.001), and approximately 80% of the total collagenolytic activity was in an active form as shown by APMA activation of the latent enzyme. In contrast, there was a fivefold increase of activity in samples from gingivitis subjects after APMA activation (i.e., only 20% of the enzyme was active before APMA treatment).
Identification of collagenases in GCF.
The type of collagenase in GCF samples was determined by using rabbit antibodies to inhibit MMP-1 and MMP-13 activities in GCF. Whereas the MMP-1 antibody completely inhibited active collagenase (463 nU) obtained from the culture medium of human gingival explants, it had no effect on active MMP-8 from PMN preparations. The MMP-13 antibody bound to protein G-Sepharose beads also showed no inhibitory effect on collagenase activity in GCF as measured by the SBA. Since the MMP-1 and MMP-13 antibodies did not affect collagenase activity in GCF samples (n = 9; mean = 145 nU), the collagenolytic activity detected in GCF samples most likely originated from neutrophils (Fig. 1). Western blots of GCF samples from healthy, gingivitis, and periodontitis patients probed with anti-MMP-13, anti-MMP-8, and anti-MMP-1 antibodies revealed no immunoreactivity for MMP-13 and negligible amounts of immunoreactive MMP-1 in periodontitis patients. In contrast, there were significant amounts of MMP-1, migrating at 66 and 63 kDa, present in GCF of patients with gingivitis and healthy controls (Fig. 2). Further, healthy patients had no detectable MMP-8 in GCF, whereas GCF from periodontitis patients contained significant amounts of MMP-8 migrating at 78 and 60 kDa (Fig. 2). Therefore, MMP-8 appeared to be the enzyme responsible for most of the collagenolytic activity in GCF of periodontitis patients.
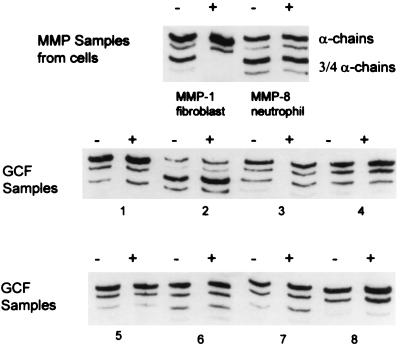
FIG. 1.
Absence of MMP-1 activity in GCF samples. APMA-activated MMP-8 from neutrophil preparations, MMP-1 from cultured fibroblasts, and GCF samples from eight different periodontitis patients were incubated with blocking antibody to MMP-1 (+) or distilled water (−) for 1 h and then tested by SBA. Digestion patterns are shown above. Note that, in the MMP-1 sample, activity was completely inhibited by the neutralizing antibody while the activity in neutrophils (MMP-8) and in GCF samples remained unchanged.
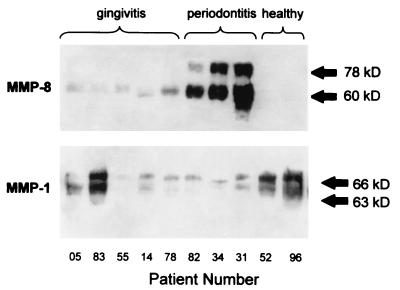
FIG. 2.
Immunoreactive MMP-1 and MMP-8 in GCF. Samples (10 μl) of GCF from periodontitis and gingivitis patients without attachment loss were tested by Western blotting for immunoreactive MMP-1 and MMP-8. In periodontitis patients, MMP-8 predominates and is mostly present in a 60-kDa form whereas MMP-1 is present in approximately equal amounts of 66- and 63-kDa forms in gingivitis and healthy patients.
Stability of MMP-8 in GCF.
To determine whether the collagenolytic activity in the GCF sample was altered during the assay or during storage at 4°C, freshly prepared samples of GCF and PMN supernatants were incubated at 4 and 22°C over a 24-h period and aliquots were assessed for collagenolytic activity (Fig. 3). In both preparations, the enzyme activity remained relatively stable over the first 4 h, especially in the PMN samples. However, in both the GCF and PMN samples, between 4 and 24 h approximately 75% of the enzyme activity was lost. Analysis of the samples by Western blotting over this period did not reveal any changes in the relative densities of the low- and high-molecular-mass species of immunoreactive MMP-8.
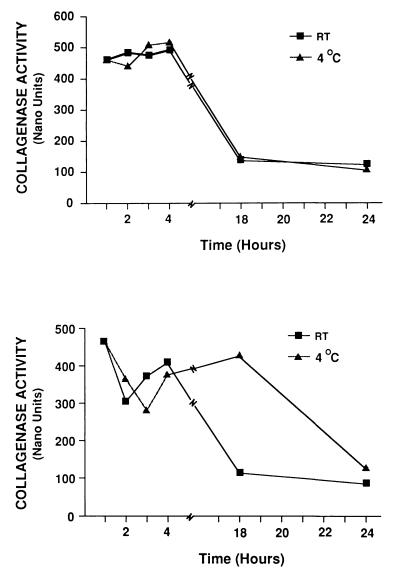
FIG. 3.
Stability of neutrophil and GCF MMP-8. MMP-8 from a neutrophil preparation (top) and that from a GCF sample (bottom) were incubated at 22 or 4°C for various periods in collagenase assay buffer. Collagenolytic activity was assayed by SBA after incubation. The figure shows one example of neutrophil preparation and one of GCF that were typical of the total of 19 separate samples that were tested. In each case, the enzyme was initially stable in the first few hours and then rapidly lost activity.
Comparison of MMP-8 from GCF and that from neutrophils.
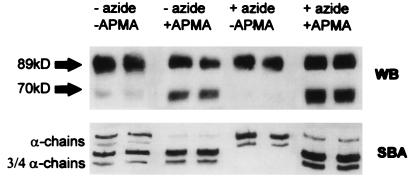
To characterize the molecular forms of MMP-8 in GCF, MMP-8 in the GCF of periodontitis patients was compared with MMP-8 in PMN preparations on Western blots (Fig. 4). As routinely observed for the GCF samples, antibodies to MMP-8 revealed prominent bands at 78 and 60 kDa. In comparison, 89- and 70-kDa bands were present in the PMN supernatant, with no bands corresponding to the major bands detected in GCF. To determine the relationship between the MMP-8 bands in the PMN preparations and the activation of the latent enzyme released by the cells, the relative amounts of active and latent collagenase activities were first determined in supernatants of PMNs that had been previously stimulated with phorbol ester (PMA) in the presence or absence of sodium azide, an inhibitor of endogenous myeloperoxidase activity (35). The stimulation with PMA was performed immediately after the addition of sodium azide. In the absence of sodium azide, collagenase activity was increased by 5 to 10%, and in a few cases by over 50%, as detected by SBA (data not shown). Pretreatment with APMA converted the latent enzyme to an active form and increased collagenase activity twofold with a conversion of ∼50% of the 89-kDa form of MMP-8 to a 70-kDa form (Fig. 5). PMN preparations incubated at RT in the presence or absence of sodium azide for 2, 4, and 19 h before stimulation with PMA showed that after 4 h, enzyme activity in the presence of sodium azide was undetectable (Fig. 5) whereas treatment with APMA and sodium azide pretreatment produced a fourfold increase of activity (from 100 to 400 nU). The increase in collagenolytic activity was again consistent with the appearance of the 70-kDa immunoreactive band on Western blots. However, after incubation of preparations for 19 h in sodium azide, much of the original enzyme activity (without APMA activation) was lost, as observed in the stability experiments.
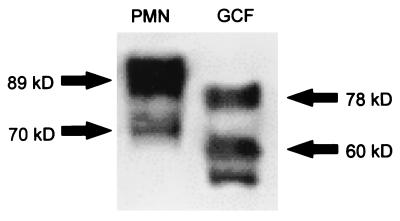
FIG. 4.
MMP-8 molecular mass in neutrophils and GCF. Naturally activated collagenase obtained from a neutrophil preparation and a GCF sample from a periodontitis patient were electrophoresed on 10% SDS gels. The blots were probed with a mouse monoclonal anti-MMP-8 antibody. Note that MMP-8 species in GCF migrate faster on SDS gels than does MMP-8 in neutrophil preparations. The 89- and 78-kDa bands represent latent MMP-8, whereas the 70- and 60-kDa bands represent active MMP-8. In the GCF, further fragmentation of the 60-kDa species is evident with the appearance of a 55-kDa immunoreactive band.
FIG. 5.
Natural activation of MMP-8 by oxidants released from neutrophils. Neutrophils were stimulated with PMA in the presence of sodium azide. Supernatants containing MMP-8 were treated with 1 mM APMA or the same volume of water, and samples were assayed by SBA and Western blotting (WB). There was no active MMP-8 in supernatants of neutrophils stimulated in the presence of sodium azide. In the absence of sodium azide, oxidant-activated MMP-8 was released and was associated with the appearance of a lower-mass (70-kDa) fragment. APMA activation further increased the enzyme activity and converted the 89-kDa form to the 70-kDa form.
TIMP-2 capture of active MMP-8.
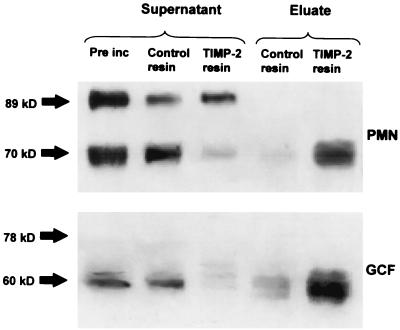
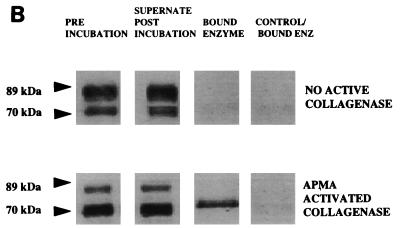
Although the generation of the 70-kDa band in PMN preparations activated with PMA or APMA was consistent with the appearance of active enzyme, neutrophils from different donors exhibited wide variations in both collagenolytic activity and the relative amounts of the 89- and 70-kDa forms analyzed by Western blotting (data not shown). Also, despite similar densities of the 60-kDa MMP-8 between different GCF samples, there was a wide variability in collagenolytic activity. Indeed, the collagenolytic activity of GCF samples did not correlate with densitometric measurements of the immunoreactive 60-kDa MMP-8 band (Pearson correlation, r2 = 0.028; n = 53), presumed to be the activated form of the enzyme. In view of these findings, we further investigated the active forms of the enzymes in the PMN and GCF samples by using TIMP-2 to identify the active species. A TIMP-2 capture experiment in which the active MMP-8 enzyme was bound to TIMP-2 that had been immobilized onto Sepharose beads was performed. Preliminary experiments revealed that addition of the TIMP-2 beads effectively blocked collagenase activity in both PMN and GCF samples, whereas control resin was without effect, indicating that the immobilized TIMP could remove active enzyme quantitatively from the samples. Western blot analysis of the supernatants remaining after removal of the TIMP-2 beads from the samples by centrifugation showed a dramatic and selective reduction in the 70- and 60-kDa bands from the PMN and GCF samples, respectively, although the proteins were not completely removed (Fig. 6). Further, we demonstrated that TIMP-2 selectively removed the 70- and 60-kDa bands by Western blot analysis of the material bound to the resin (Fig. 6).
FIG. 6.
TIMP-2 capture of active MMP-8 fragments. A neutrophil preparation and a GCF sample from a periodontitis patient were incubated with 20 μl of TIMP-2 resin or control resin at 4°C for 1 h. Supernatants were electrophoresed on SDS–10% PAGE gels, and blots were probed with a mouse monoclonal antibody to MMP-8. Pre inc, sample before incubation with TIMP-2 resin. The low-mass (70-kDa and 60-kDa) forms were removed by the resin and later eluted from the resin by being boiled with SB/DTT, showing that only the low-mass fragment was captured by the TIMP-2 resin.
Immunolocalization of MMP-8 in diseased tissues.
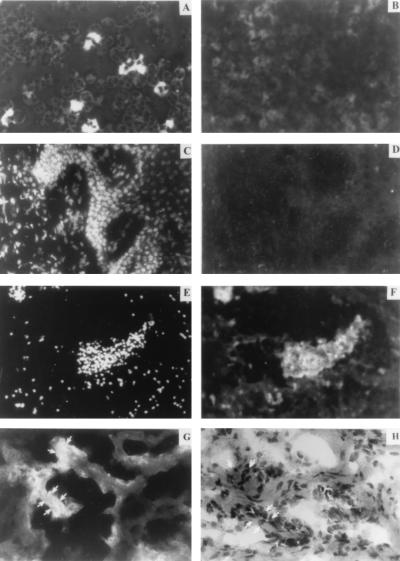
To determine whether the collagenase detected in GCF of periodontitis patients was related to the collagenase in the diseased tissues where destruction occurs, immunolocalization studies for MMP-8 were carried out. In cytospin preparations stained with anti-MMP-8 antibodies, neutrophils were strongly stained intracellularly, but no significant immunoreactivity was observed for lymphocytes or neutrophils treated with secondary antibody alone (Fig. 7A and B). In specimens taken from healthy sites that did not exhibit clinical signs of inflammation, there were only sparse numbers of inflammatory cells, and these did not stain for MMP-8 (Fig. 7C and D). In contrast, all sections of severely inflamed gingival tissue obtained from around teeth with advanced periodontitis demonstrated a large number of inflammatory cells with strong immunoreactivity to the MMP-8 antibody (Fig. 7E and F). Although the spatial distribution of the staining varied between clinical samples, the immunoreactive MMP-8 frequently exhibited two patterns. In one, the enzyme was spatially associated with dense clusters of inflammatory cells (Fig. 7E), whereas in the other pattern the enzyme was localized throughout the tissue and appeared to be associated with the connective tissue matrix (Fig. 7G) visualized by hematoxylin and eosin staining (Fig. 7H). Since the antibody recognizes both the latent and active forms of MMP-8, both forms of the enzyme could be present in the tissues.
FIG. 7.
Localization of immunoreactive MMP-8 in connective tissue. (A) Cytospin preparation of peripheral blood neutrophils showing immunoreactive collagenase. (B) Cytospin preparation of lymphocytes showing no significant immunoreactivity. (C) Healthy tissue section stained with DAPI. (D) Same field as shown in panel C stained with anti-MMP-8 and fluorescein isothiocyanate-conjugated secondary antibody. (E) Severely inflamed tissue section stained with DAPI. (F) Same field as shown in panel E stained with anti-MMP-8. (G) Inflamed tissue specimen from a different subject stained with anti-MMP-8. (H) Comparable field in an adjacent section stained with hematoxylin and eosin shows the location of collagen matrix.
Collagen binding.
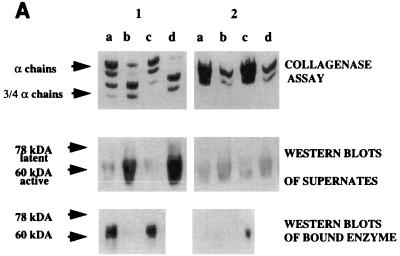
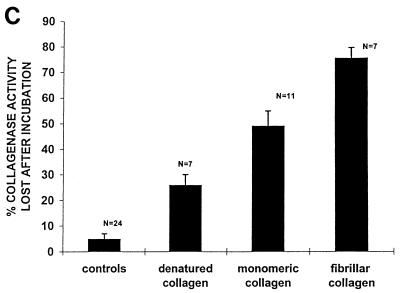
Since the immunohistochemical results demonstrated that MMP-8 was localized throughout the connective tissue matrix of diseased tissues, we studied the binding of MMP-8 to collagen, which could influence the amount of the MMP-8 that was detectable in the GCF. Binding of both APMA-treated PMN samples and GCF samples to plates incubated with buffer alone was undetectable. However, Western blot analysis showed that when GCF containing either active or latent enzyme was added to each well of a plastic plate coated with type I fibrillar collagen, only the low (60-kDa) active form of collagenase bound to the collagen (Fig. 8A). Similarly, when samples of latent and APMA-activated MMP-8 from PMNs were applied to wells coated with type I fibrillar collagen, only the low (70-kDa) active form of collagenase bound to collagen (Fig. 8B). Incubation of enzyme on collagen plates decreased collagenase activity in the supernatants by approximately 50% after the first incubation; complete loss of enzyme activity was noted only after four consecutive incubations on fresh collagen-coated wells, suggesting that the binding was weak. Enzyme binding to collagen fibrils was ∼1.5-fold higher than that with monomeric collagen and 3-fold higher than that with denatured collagen (Fig. 8C). These differences in binding were not due to loss of collagen or gelatin from the plates, which was minimal in each case, and appear to reflect the affinity of the collagenase for the different forms of collagen.
FIG. 8.
(A) Analysis of active and latent forms of MMP-8 from GCF that bind to collagen. GCF from a patient containing ∼460 nU of enzyme activity (panel 1) and GCF from a patient containing no active enzyme (panel 2) were analyzed. Aliquots (50 μl; neat) of GCF from both patients were applied to each well of a Reacti-Bind plate coated with monomeric collagen (lanes a) or Tris (control; lanes b) or a plastic plate incubated with type I fibrillar collagen (lanes c) and allowed to bind for 2 h at 4°C. Lanes d show initial collagenase activity prior to incubation. MMP-8 activity remaining in the supernatants was measured by SBA, and the molecular mass of the MMP-8 species which was bound to the collagen film as well as of that which remained in the supernatant was examined by Western blotting. There is abundant binding of active MMP-8 preferentially to fibrillar collagen and only limited binding of latent enzyme. (B) Analysis of active and latent forms of neutrophil collagenase binding to collagen. MMP-8 from purified neutrophils containing no natural activity was APMA activated. Aliquots (50 μl) of both the latent and activated enzyme were applied to each well of a plastic plate coated with either type I fibrillar collagen or Tris (control) and allowed to bind for 2 h at 4°C. The molecular mass of the MMP-8 species which was bound to the collagen film as well as of that which remained in the supernatant was examined by Western blotting. Note that the low (70-kDa) active form of collagenase but not the latent form binds to collagen. (C) Binding of collagen to different collagen substrates. Active MMP-8 from peripheral blood neutrophils was applied in 50-μl volumes to wells of plastic plates coated with either Tris (controls), denatured collagen (0.8 μg/μl), or type I collagen (monomers or fibrils; 0.8 μg/μl). Collagenase was incubated for 2 h at 4°C. MMP-8 activity in the supernatants was measured by SBA, and the percentage of initial enzyme activity lost after incubation is shown.
DISCUSSION
Consistent with previous studies, collagenase activity was positively associated with the severity of periodontal disease (16, 17), and MMP-8 accounted for most of the collagenase activity in adult periodontitis patients (22, 30). While latent MMP-1, in addition to latent MMP-8, was found in the mouth rinses of gingivitis and clinically healthy patients on immunoblots, MMP-13 could not be detected. Activation of MMP-8 most likely occurs in the tissues or GCF, as no activation was observed on storage of mouth rinse samples or during enzyme activity assays. Active MMP-8 was bound preferentially to collagen fibrils and likely represents the enzyme immunolocalized in the matrix of diseased tissue. The predominant form of active MMP-8 in GCF migrated on SDS-PAGE with a size of 60 kDa and appears to be generated from a 78-kDa latent MMP-8, contrasting with the 70-kDa active and 89-kDa latent forms of MMP-8 obtained from freshly isolated and activated PMNs. The variability in the specific activity of the enzyme in the putative active bands from GCF and PMNs is indicative of multiple forms of activated and latent MMP-8 within these bands.
Multiple collagenases in GCF.
A critical finding for the validity of the observations made in our study was that MMP-8 is the major collagenase in GCF of periodontitis patients. Based on the SBA data, the collagenolytic activity in GCF samples could be due to at least three human collagenases: MMP-1, MMP-8, and MMP-13. Since some MMP-1, in addition to MMP-8, was evident in samples from gingivitis and healthy patients when analyzed by immunoblotting, we used a neutralizing antibody to block MMP-1 activity in SBAs. While the antibody effectively blocked MMP-1 activity in conditioned medium from human gingival explants, it did not affect activities in GCF samples. The possibility that MMP-13, a recently characterized collagenase (5), contributes to the collagenolytic activity in GCF was also considered, since MMP-13 is expressed in inflamed periodontal tissues, where it is primarily associated with basal epithelial cells and suprabasal epithelial cells juxtaposed to the gingival sulcus (31), and also appears in sulcal fluid (7). However, we could not detect this enzyme by immunoblotting in mouth rinse samples, indicating that the levels of MMP-13 in GCF must be very low relative to those of MMP-1, and especially those of MMP-8. Further, immunoadsorption of MMP-13 did not affect collagenolytic activities in SBA analyses (results not shown). Although we did not attempt to block the MMP-8 with an inhibitory antibody, the data presented here and previous reports (6, 8, 17, 22, 30) indicate that most of the collagenolytic activity in the GCF of adult periodontitis patients is derived from MMP-8. Notably, there is the possibility that cells other than neutrophils may produce MMP-8 (9), and it is conceivable that endothelial cells could, for example, contribute to a small part of the enzyme activity that was measured. Finally, it is possible that gelatinase A (MMP-2) could contribute to the collagenase activity reported here, but our previous data (22) indicate that MMP-2 is a very minor component of GCF and that its activity against native collagen is limited, suggesting that the relative contribution of MMP-2 toward the total collagenase activity was small.
The predominance of MMP-8 in GCF of periodontitis patients is consistent with the increased number of PMNs recruited to the periodontal tissues as part of the inflammatory response and suggests that neutrophil hyperresponsiveness in some subjects may contribute to tissue destruction in periodontal disease similar to the destruction that occurs in systemic disorders such as emphysema (34). In comparison, MMP-1 and MMP-13 appear to be associated with tissue remodelling in chronic wounds (13, 28, 32). These collagenases are, therefore, potential markers of repair in diseased periodontal tissues. Thus, while MMP-1 concentrations in periodontitis patients analyzed by Western blotting were negligible, immunoreactive MMP-1 was detected in healthy patients who exhibited no collagenolytic activity as analyzed by SBA. These findings agree with previous data in the literature which show immunolocalization of MMP-1 in gingival tissues of healthy patients (19) but not in diseased tissues (10). In comparison, our own immunolocalization studies showed abundant MMP-8 in gingival tissues of patients with periodontitis and its absence in healthy tissues, as observed in previous studies (10, 37).
Collagen binding.
Based on the pattern of distribution of MMP-8 in diseased tissue and our knowledge that active enzyme is present in GCF during progressive periodontitis, we examined the relationship between binding and availability of the MMP-8 in the GCF and its activity. Our results showed that the major activated species of the PMN (70 kDa) and GCF-derived MMP-8 (60 kDa) can preferentially bind to fibrillar collagen. Selective binding of active MMP-8 to collagen affinity columns has been observed earlier by Knauper et al. (14), who also postulated that the binding site of neutrophil collagenase for triple-helical collagen consists of parts of the catalytic and C-terminal domains (15). While the preferential binding suggests that the active enzyme would be retained in the diseased tissue matrix where it can initiate collagenolysis, it is evident from the incomplete binding of MMP-8 in the presence of a vast excess of collagen that the binding affinity is not strong. Further, the presence of glycoproteins such as fibronectin that coat collagen fibrils in vivo may alter the affinity of MMP-8 binding, and the use of collagen films as models for MMP-8 binding in inflamed tissues is imperfect. However, it is likely that in inflamed tissues collagen-coating proteins would be removed prior to degradation, a requirement for degradation to occur. Thus, in inflamed tissues, MMP-8 binding to collagen is likely to occur at some point in the inflammatory process. Saturation of collagenolytic active MMP-8 in diseased tissues would likely be reached at low levels of enzyme, consistent with its appearance as overflow in the GCF. In this regard, the lack of any detectable activation of MMP-8 following the collection of mouth rinse samples indicates that activation takes place within the tissues or in the GCF.
MMP-8 species.
The finding that the sizes of MMP-8 species in GCF were smaller than those of the latent and APMA-activated forms of MMP-8 obtained directly from PMNs indicates that additional proteolytic processing of MMP-8 occurs in diseased periodontal tissue. A residual 60-kDa band detected on immunoblots indicates that some latent or inactive MMP-8 is also present in this band. Notably, the appearance of the band was not due to saturation of TIMP-2, which binds to active MMP-8 with a 1:1 stoichiometry (36), since virtually all of the MMP-8 activity had been captured. Moreover, the presence of latent inactive enzyme in the 60-kDa bands is unlikely to be the result of complexing of active enzyme to TIMP in GCF since the levels of TIMP-1 and TIMP-2, measured by immunoblotting, were <1% of that required to inhibit the MMP-8 in GCF samples. The presence of latent MMP-8, together with variation in the specific activity of active 60-kDa MMP-8, due to small differences in the length of the MMP-8, can account for the poor correlation between the amount of the 60-kDa form observed on Western blotting and enzyme activity measured by SBA (14). The proteolytic processing generating the multiple MMP-8 species can be attributed to the activity of factors present in GCF, since there was a lack of enzyme processing or enzyme activation during storage or assay of the GCF samples. The existence of these processed forms of MMP-8 in GCF from periodontitis patients could be of significant biological importance, since it would appear that these smaller MMP-8 species can be more efficiently activated. Also, superactivated species of MMP-8 in GCF may be characteristic of more severe cases of periodontitis in which there is extensive degradation of periodontal tissues.
Although there is a large discrepancy in the sizes of active and latent MMP-8 described in the literature, with values ranging from 85 kDa (12) to 58 kDa (33), the MMP-8 species observed by immunoblotting in our study were within this range. Notably, the difference in molecular weight between MMP-8 from PMN preparations and that in GCF samples that we observed has not been reported previously. Indeed, Western blot analysis of MMP-8 species obtained from PMNs and from GCF samples, collected with filter paper strips, revealed similar sizes for both the PMN and GCF MMP-8 (24, 25). However, while the MMP-8 present in GCF and from purified neutrophils had a molecular mass of ∼80 kDa, it was shown in a subsequent study (26) that the MMP-8 present in dental plaque had a molecular mass of 58 kDa, a size comparable to the 60-kDa MMP-8 identified in GCF samples in our study. The smaller size of MMP-8 in dental plaque may be due to proteolytic fragmentation by bacterial enzymes.
Notably, some of the 58-kDa MMP-8 observed in dental plaque was suggested to be in a latent form (26), and the 58-kDa form of latent MMP-8 has been identified by Van Wart (33). Van Wart (33) also showed that after activation of both a 58-kDa and a 75-kDa MMP-8 species by the same reagents, the 58-kDa form yielded fivefold-higher collagenolytic activity than a 75-kDa form of latent MMP-8 isolated from freshly harvested neutrophils. These data indicate that partially processed, smaller species of latent MMP-8 may be more efficiently activated. Conceivably, superactivated species of MMP-8 are present in GCF samples from patients with periodontitis, especially from those who demonstrated extremely high collagenolytic activity. While there is evidence that bacterial enzymes can also activate MMPs (4, 21, 27), it has also been shown that Porphyromonas gingivalis can catalyze the superactivation of MMP-8 by stromelysin (3). Notably, stromelysin can superactivate an ∼70-kDa MMP-8 intermediate, generated by truncation of the amino terminus by trypsin cleavage, producing a 64-kDa superactivated form of MMP-8 (15). This provides a potentially important link between periodontopathic bacteria such as Treponema denticola and P. gingivalis and their ability to activate MMPs. Thus, both host-cell-derived and microbial proteases have the potential to participate in the in vivo activation cascades that lead to periodontal tissue destruction.
Based on the results of the studies described above, the 60-kDa band that we identified in GCF samples may be composed of latent, active, or superactivated species of MMP-8. As C-terminal deletions result in loss of activity, the variations in specific activity of the 60-kDa enzyme in different GCF samples are anticipated to arise from N-terminal cleavages. While it would be necessary to separate the 60-kDa species to provide definitive proof, the rapid activation of the partially processed 78-kDa latent enzyme by bacterial or host enzymes could explain the preponderance of the 60-kDa MMP-8 in GCF samples.
ACKNOWLEDGMENTS
R. Romanelli, S. Mancini, and C. Laschinger contributed equally to this work.
REFERENCES
- 1.Birkedal-Hansen H, Moore W G, Bodden M K, Windsor L J, Birkedal-Hansen B, DeCarlo A, Engler J A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 2.Blaser J, Knauper V, Osthues A, Reinke H, Tschesche H. Mercurial activation of human polymorphonuclear leucocyte procollagenase. Eur J Biochem. 1991;202:1223–1230. doi: 10.1111/j.1432-1033.1991.tb16494.x. [DOI] [PubMed] [Google Scholar]
- 3.De Carlo A A, Jr, Windsor L J, Bodden M K, Harber G J, Birkedal-Hansen B, Birkedal-Hansen H. Activation and novel processing of matrix metalloproteinases by a thiol-proteinase from the oral anaerobe Porphyromonas gingivalis. J Dent Res. 1997;76:1260–1270. doi: 10.1177/00220345970760060501. [DOI] [PubMed] [Google Scholar]
- 4.Ding Y, Uitto V J, Firth J, Salo T, Haapasalo M, Konttinen Y T, Sorsa T. Modulation of host matrix metalloproteinases by bacterial virulence factors relevant in human periodontal diseases. Oral Dis. 1995;1:279–286. doi: 10.1111/j.1601-0825.1995.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 5.Freije J M, Diez-Itza I, Balbin M, Sanchez L M, Blasco R, Tolivia J, Lopez-Otin C. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem. 1994;269:16766–16773. [PubMed] [Google Scholar]
- 6.Gangbar S, Overall C M, McCulloch C A, Sodek J. Identification of polymorphonuclear leukocyte collagenase and gelatinase activities in mouthrinse samples: correlation with periodontal disease activity in adult and juvenile periodontitis. J Periodontal Res. 1990;25:257–267. doi: 10.1111/j.1600-0765.1990.tb00914.x. [DOI] [PubMed] [Google Scholar]
- 7.Golub L M, Lee H M, Greenwald R A, Ryan M E, Sorsa T, Salo T, Giannobile W V. A matrix metalloproteinase inhibitor reduces bone-type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflamm Res. 1997;46:310–319. doi: 10.1007/s000110050193. [DOI] [PubMed] [Google Scholar]
- 8.Haerian A, Adonogianaki E, Mooney J, Docherty J P, Kinane D F. Gingival crevicular stromelysin, collagenase and tissue inhibitor of metalloproteinase levels in healthy and diseased sites. J Clin Periodontol. 1995;22:505–509. doi: 10.1111/j.1600-051x.1995.tb00797.x. [DOI] [PubMed] [Google Scholar]
- 9.Hanemaaijer R, Sorsa T, Konttinen Y T, Ding Y, Sutinen M, Visser H, van Hinsbergh V W, Helaakoski T, Kainulainen T, Ronka H, Tschesche H, Salo T. Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor-alpha and doxycycline. J Biol Chem. 1997;272:31504–31509. doi: 10.1074/jbc.272.50.31504. [DOI] [PubMed] [Google Scholar]
- 10.Ingman T, Sorsa T, Michaelis J, Konttinen Y T. Immunohistochemical study of neutrophil- and fibroblast-type collagenases and stromelysin-1 in adult periodontitis. Scand J Dent Res. 1994;102:342–349. doi: 10.1111/j.1600-0722.1994.tb01481.x. [DOI] [PubMed] [Google Scholar]
- 11.Kleiner D E, Stetler-Stevenson W G. Structural biochemistry and activation of matrix metalloproteinases. Curr Opin Cell Biol. 1993;5:891–897. doi: 10.1016/0955-0674(93)90040-w. [DOI] [PubMed] [Google Scholar]
- 12.Knauper V, Kramer S, Reinke H, Tschesche H. Partial amino-acid sequence of human PMN leukocyte procollagenase. Biol Chem Hoppe-Seyler. 1990;371(Suppl.):295–304. [PubMed] [Google Scholar]
- 13.Knauper V, Lopez-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- 14.Knauper V, Osthues A, DeClerck Y A, Langley K E, Blaser J, Tschesche H. Fragmentation of human polymorphonuclear-leucocyte collagenase. Biochem J. 1993;291:847–854. doi: 10.1042/bj2910847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knauper V, Wilhelm S M, Seperack P K, DeClerck Y A, Langley K E, Osthues A, Tschesche H. Direct activation of human neutrophil procollagenase by recombinant stromelysin. Biochem J. 1993;295:581–586. doi: 10.1042/bj2950581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kryshtalskyj E, Sodek J, Ferrier J M. Correlation of collagenolytic enzymes and inhibitors in gingival crevicular fluid with clinical and microscopic changes in experimental periodontitis in the dog. Arch Oral Biol. 1986;31:21–31. doi: 10.1016/0003-9969(86)90109-3. [DOI] [PubMed] [Google Scholar]
- 17.Lee W, Aitken S, Sodek J, McCulloch C A. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: role of active enzyme in human periodontitis. J Periodontal Res. 1995;30:23–33. doi: 10.1111/j.1600-0765.1995.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 18.Mancini, S., R. Romanelli, C. A. Laschinger, C. M. Overall, J. Sodek, and C. A. McCulloch. Assessment of a novel screening test for neutrophil collagenase activity in the diagnosis of periodontal disease. Submitted for publication. [DOI] [PubMed]
- 19.Meikle M C, Hembry R M, Holley J, Horton C, McFarlane C G, Reynolds J J. Immunolocalization of matrix metalloproteinases and TIMP-1 (tissue inhibitor of metalloproteinases) in human gingival tissues from periodontitis patients. J Periodontal Res. 1994;29:118–126. doi: 10.1111/j.1600-0765.1994.tb01100.x. [DOI] [PubMed] [Google Scholar]
- 20.Nagase H, Suzuki K, Enghild J J, Salvesen G. Stepwise activation mechanisms of the precursors of matrix metalloproteinases 1 (tissue collagenase) and 3 (stromelysin) Biomed Biochim Acta. 1991;50:749–754. [PubMed] [Google Scholar]
- 21.Okamoto T, Akaike A, Suga M, Tanase S, Horie H, Miyajima S, Ando M, Ichinase Y, Maeda H. Activation of human matrix metalloproteinases by various bacterial proteinases. J Biol Chem. 1997;272:6059–6066. doi: 10.1074/jbc.272.9.6059. [DOI] [PubMed] [Google Scholar]
- 22.Overall C M, Sodek J, McCulloch C A, Birek P. Evidence for polymorphonuclear leukocyte collagenase and 92-kilodalton gelatinase in gingival crevicular fluid. Infect Immun. 1991;59:4687–4692. doi: 10.1128/iai.59.12.4687-4692.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sellers A, Murphy G. Collagenolytic enzymes and their naturally occurring inhibitors. Int Rev Connect Tissue Res. 1981;9:151–190. doi: 10.1016/b978-0-12-363709-3.50010-3. [DOI] [PubMed] [Google Scholar]
- 24.Sirkka T, Sorsa T, Ingman T, Salo T, Konttinen Y T. Characterization of matrix metalloproteinase (MMP-8 and MMP-9) activities in the saliva and gingival crevicular fluid of children with Down’s syndrome. J Periodontol. 1996;67:748–754. doi: 10.1902/jop.1996.67.8.748. [DOI] [PubMed] [Google Scholar]
- 25.Sorsa T, Ding Y, Salo T, Lauhio A, Teronen O, Ingman T, Ohtani H, Andoh N, Takeha S, Konttinen Y T. Effects of tetracyclines on neutrophil, gingival, and salivary collagenases. A functional and western-blot assessment with special reference to their cellular sources in periodontal diseases. Ann N Y Acad Sci. 1994;732:112–131. doi: 10.1111/j.1749-6632.1994.tb24729.x. [DOI] [PubMed] [Google Scholar]
- 26.Sorsa T, Ding Y L, Ingman T, Salo T, Westerlund U, Haapasalo M, Tschesche H, Konttinen Y T. Cellular source, activation and inhibition of dental plaque collagenase. J Clin Periodontol. 1995;22:709–717. doi: 10.1111/j.1600-051x.1995.tb00831.x. [DOI] [PubMed] [Google Scholar]
- 27.Sorsa T, Ingman T, Suomalainen K, Haapasalo M, Konttinen Y T, Lindy O, Saari H, Uitto V J. Identification of proteases from periodontopathogenic bacteria as activators of latent human neutrophil and fibroblast-type interstitial collagenases. Infect Immun. 1992;60:4491–4495. doi: 10.1128/iai.60.11.4491-4495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stahle-Backhadl M, Sandstedt B, Bruce K, Lindhal A, Jimenez M G, Vega J A, Lopez-Otin C. Collagenase-3 (MMP-13) is expressed during human fetal ossification and re-expressed in postnatal bone remodelling and in rheumatoid arthritis. Lab Investig. 1997;76:717–728. [PubMed] [Google Scholar]
- 29.Uitto V J, Raeste A M. Activation of latent collagenase of human leukocytes and gingival fluid by bacterial plaque. J Dent Res. 1978;57:844–851. doi: 10.1177/00220345780570071401. [DOI] [PubMed] [Google Scholar]
- 30.Uitto V J, Suomalainen K, Sorsa T. Salivary collagenase. Origin, characteristics and relationship to periodontal health. J Periodontal Res. 1990;25:135–142. doi: 10.1111/j.1600-0765.1990.tb01035.x. [DOI] [PubMed] [Google Scholar]
- 31.Uitto V J, Airola K, Vaalamo M, Johansson N, Putnis E E, Firth J D, Salonen J, Saarialhokere U, Kahari V M. Collagenase-3 (matrix metalloproteinase-13) expression is induced in oral mucosal epithelium during chronic inflammation. Am J Pathol. 1998;152:1489–1499. [PMC free article] [PubMed] [Google Scholar]
- 32.Vaalamo M, Mattila L, Johansson N, Kariniemi A, Karjalainen-Lindsberg M, Kahari V M, Saarialho-Kere U. Distinct population of stromal cells express collagenase-3 (MMP-13) and collagenase-1 (MMP-1) in chronic ulcers but not in normally healing wounds. J Investig Dermatol. 1997;109:96–101. doi: 10.1111/1523-1747.ep12276722. [DOI] [PubMed] [Google Scholar]
- 33.Van Wart H E. Human neutrophil collagenase. Matrix. 1992;1(Suppl.):31–36. [PubMed] [Google Scholar]
- 34.Weiss S J. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 35.Weiss S J, Peppin G, Ortiz X, Ragsdale C, Test S T. Oxidative autoactivation of latent collagenase by human neutrophils. Science. 1985;227:747–749. doi: 10.1126/science.2982211. [DOI] [PubMed] [Google Scholar]
- 36.Welgus H G, Jeffrey J J, Eisen A Z, Roswit W T, Stricklin G P. Human skin fibroblast collagenase: interaction with substrate and inhibitor. Collagen Relat Res. 1985;5:167–179. doi: 10.1016/s0174-173x(85)80038-8. [DOI] [PubMed] [Google Scholar]
- 37.Woolley D E, Davies R M. Immunolocalization of collagenase in periodontal disease. J Periodontal Res. 1981;16:292–297. doi: 10.1111/j.1600-0765.1981.tb00977.x. [DOI] [PubMed] [Google Scholar]