Abstract
Background: β-carotene is an essential antioxidant, providing protection against type 2 diabetes mellitus, cardiovascular illnesses, obesity, and metabolic syndrome. This study investigates the impact of β-carotene on biochemical parameters and pancreatic insulin expression in mice exposed to ethanol. Methods: Thirty-six C57BL/6 mice (Mus musculus) were divided into six groups: 1. C (control), 2. LA (3% alcohol dose), 3. MA (7% alcohol dose), 4. B (0.52 mg/kg body weight/day β-carotene), 5. LA+B (3% alcohol dose + 0.52 mg/kg body weight/day β-carotene), and 6. MA+B (7% alcohol dose plus 0.52 mg/kg body weight/day β-carotene). After 28 days, the animals were euthanized for serum and pancreatic tissue collection. Biochemical analysis and pancreatic insulin expression were performed. One-way ANOVA was used. Results: The B, LA+B, and MA+B groups improved insulin levels and decreased HOMA-β versus the C group, with the LA+B and MA+B groups also showing lower ADH and ALDH levels than their nonsupplemented counterparts (p < 0.05). The B, LA+B, and MA+B groups showed a greater β-cell mass area compared to the unsupplemented groups. Additionally, the LA+B and MA+B groups demonstrated significantly increased β-cell area and integrated optical density compared to the LA and MA groups, respectively (p < 0.001). Conclusions: In mice, β-cell loss led to increased glucose release due to decreased insulin levels. β-carotene appeared to mitigate ethanol’s impact on these cells, resulting in reduced insulin degradation when integrated optical density was used. These findings suggest that antioxidant supplementation may be beneficial in treating ethanol-induced type 2 diabetes in animal models.
Keywords: alcohol intake, insulin, antioxidant treatment, chronic alcohol consumption
1. Introduction
Alcohol consumption poses a significant public health concern, with an estimated annual cost of EUR 155 billion attributed to related issues [1]. It is widely recognized as a major risk factor for both acute and chronic pancreatitis, contributing to approximately 50% to 80% of all documented cases [2]. Recent research highlights alcohol as the predominant factor responsible for pancreatitis in the United States [3].
However, studies indicate that less than 5% of individuals who chronically consume large amounts of alcohol are at risk of developing pancreatitis [4]. This discrepancy suggests that the development of alcohol-induced pancreatitis may require additional risk factors, influenced by genetic or environmental variables [5,6].
The complex and multifaceted effects of alcohol on the pancreas contribute to an incomplete understanding of the etiology of alcohol-induced pancreatitis. Alcohol is believed to damage pancreatic acinar cells, ductal epithelium, and stellate cells, potentially promoting pancreatic fibrosis [7,8]. Alcohol-induced pancreatitis is likely to develop only when compensatory mechanisms are exhausted or when other genetic or environmental stresses increase pancreatic vulnerability. Findings from animal models support this hypothesis, suggesting that the pancreas can mitigate alcohol-induced damage by initiating an adaptive stress response [9,10].
Pancreatic acinar cells metabolize alcohol through both oxidative and nonoxidative pathways [11,12,13,14,15]. The metabolic breakdown of alcohol in these cells generates toxic byproducts, including fatty acid ethyl esters (FAEEs), acetaldehyde, and reactive oxygen species (ROS), which contribute to cellular damage [11,16,17]. This oxidative stress destabilizes zymogen granules and lysosomes, as well as disrupts other organelle functions within the cell [4,11,18].
Chronic pancreatitis is marked by recurring abdominal pain and is frequently accompanied by symptoms such as nausea and weight loss [19]. Persistent pancreatic damage reduces the secretion of enzymes essential for digestion and fat absorption, leading to a progressive decline in digestive function. Additionally, the destruction of pancreatic β-cells, which produce, store, and release insulin, increases the risk of diabetes development [20].
Diabetes mellitus (DM) is a common chronic metabolic disorder characterized by elevated blood glucose levels, arising from either insufficient insulin production or impairments in insulin signaling [21,22]. As a growing global public health challenge, diabetes incidence is anticipated to rise significantly in the coming decades, driven in part by aging populations worldwide [23].
Both type 1 and type 2 diabetes (T2DM) involve pancreatic dysfunction, impairing the β-cells’ capacity to meet increased insulin demands [24]. A family history of diabetes is widely recognized as a significant risk factor for developing T2DM and pre-diabetes [25,26,27].
Evidence suggests that β-carotene supplementation enhances GSH concentration by stimulating the activity of GSH synthetase. Previous research has shown that adding β-carotene can stop the loss of GSH caused by ethanol by raising the amount of GSH inside cells [28,29]. These studies indicate that it may be due to the stimulation of GSH synthetase activity caused by β-carotene. Therefore, β-carotene supplementation may serve as an effective approach to mitigate liver damage resulting from excessive alcohol consumption and to prevent the progression of alcoholic disease to more severe conditions [14].
Previous studies have shown that ethanol exposure induces oxidative stress and apoptosis, leading to tissue damage, while β-carotene supplementation (0.52 mg/kg BW/day) can alleviate ethanol-induced injury by reducing oxidative stress and inhibiting apoptosis in tissues [30]. Additional findings also report an increase in serum total amylase levels and a decrease in lipase levels in groups receiving 7% alcohol plus β-carotene supplementation compared to those without it [15]. Histological analysis further revealed that perilobular parenchyma, intralobular parenchyma, and fibrosis scores were lower in the 7% alcohol plus β-carotene group compared to the groups given 3% alcohol, 7% alcohol, or 3% alcohol plus β-carotene [15]. These findings suggest that antioxidant therapy could be beneficial in addressing the effects of ethanol exposure in animal models.
β-carotene, a member of the carotene family, is among the most prevalent carotenoids in food sources and is also present in the human body. About 17–45% of the consumed β-carotene remains intact in organisms, indicating its significant bioavailability, which refers to its effective absorption and utilization capacity [31]. Research indicates that β-carotene serves as a safeguard against several conditions, including type 2 diabetes mellitus (T2DM), cardiovascular disease, obesity, and metabolic syndrome (MetS) [32,33,34]. β-carotene is noted for its ability to enhance expression and secretion, which in turn improves insulin sensitivity [35].
The consumption of β-carotene is linked to several outcomes, including a decrease in the size of adipocytes and overall body adipose tissue; a decline in proinflammatory markers, low-density lipoprotein cholesterol (LDL-c), and very-low-density lipoprotein cholesterol (VLDL-c); and an increase in high-density lipoprotein cholesterol (HDL-c) [36,37,38,39]. Furthermore, they have the potential to enhance insulin resistance and maintain insulin receptors [37,39]. These actions take place to manage oxidative stress, which plays a role in all of the diseases mentioned [40].
β-carotene has the ability to modulate lipid and carbohydrate metabolism, thereby enhancing the function of pancreatic cells and improving hyperglycemic conditions. The regulation of β-pancreatic cell functions stimulates insulin secretion, regulates lipid metabolism, and alleviates oxidative and inflammatory stress [39,41,42].
The American Diabetes Association defines diabetes as a condition marked by the degeneration of insulin- and glucagon-producing cells. Histological examination reveals fibrosis, the shrinkage of pancreatic acini, chronic inflammation, the deformation of pancreatic ducts with narrowed regions, and damage to ß- and α-cells [14,15,43].
Thus, a comprehensive histological evaluation is essential. In particular, assessing insulin activity requires a multifaceted approach involving the analysis of serum biochemical markers alongside the measurement of insulin production in pancreatic islets [15]. This assessment may include evaluating the dimensions of the pancreatic islets or calculating their integrated optical density (IOD), with average values typically presented per cell [44,45]. The final evaluation involves staining the pancreatic islets with specific markers, such as immunohistochemistry staining, followed by image capture with a light microscope equipped with specialized optical filters. The IOD quantifies the light absorption across the entire pancreatic islet as captured in the images.
The cellular IOD serves as a quantitative metric. Previous research utilizing immunohistochemistry and Sirius Red staining on liver tissue observed significant increases in IOD levels following moderate alcohol consumption and carotene supplementation [14]. Additionally, IOD can be instrumental in identifying morphologically and functionally distinct cell subpopulations by maintaining consistent light contrast in imaging [14,45]. Consequently, the IOD analysis of cell dynamics may correlate with various pathophysiological states, offering valuable insights into disease processes.
Previous research has documented the effects of alcohol on the pancreas; however, the potential benefits of antioxidant supplementation during chronic ethanol exposure remain insufficiently understood. This study aimed to evaluate glucose and insulin serum levels, as well as insulin expression within the endocrine pancreas, in C57BL/6 mice subjected to ethanol exposure and/or β-carotene supplementation.
2. Results
2.1. Biochemistry
Table 1 presents the biochemical analyses of insulin, glucose, ADH, ALDH, HOMA-β, and HOMA-IR indices. The LA+B group showed significantly lower HOMA-β levels than the C, LA, MA, and B groups, while the MA+B group displayed reduced insulin and HOMA-β levels but higher glucose levels relative to the C group (p < 0.05). β-carotene supplementation improved the insulin levels and decreased HOMA-β versus the C group, with the LA+B and MA+B groups also showing lower ADH and ALDH levels than their nonsupplemented counterparts (p < 0.05).
Table 1.
Biochemical evaluation of male C57BL/6 mice following ethanol consumption and β-carotene supplementation.
| Media ± SD | |||||||
|---|---|---|---|---|---|---|---|
| C (n = 6) | LA (n = 6) | MA (n = 6) | B (n = 6) | LA+B (n = 6) | MA+B (n = 6) | p | |
| Insulin (μU/mL) | 28.60 ± 5.45 | 16.44 ± 7.27 a | 14.96 ± 5.01 a | 24.27 ± 9.58 abc | 11.34 ± 3.91 ad | 9.46 ± 1.55 acd | <0.001 |
| Glucose (mmol/L) | 9.03 ± 0.53 | 13.45 ± 1.27 a | 15.11 ± 0.45 ab | 14.63 ± 0.84 ab | 13.46 ± 0.43 acd | 14.31 ± 0.86 a | <0.001 |
| ADH (nmol/min/mL) | 20.42 ± 2.52 | 33.03 ± 3.42 a | 36.15 ± 2.74 a | 23.22 ± 5.58 bc | 26.09 ± 3.16 abc | 29.83 ± 3.51 acd | <0.001 |
| ALDH (pmol/min/mL) | 11.81 ± 1.82 | 15.81 ± 1.62 a | 17.04 ± 1.78 a | 11.21 ± 1.30 bc | 14.25 ± 1.67 acd | 15.81 ± 1.77 ad | <0.001 |
| HOMA-β | 104.41 ± 21.30 | 33.34 ± 15.51 a | 25.77 ± 6.77 a | 43.53 ± 15.51 a | 22.77 ± 7.38 a | 17.61 ± 3.19 ad | <0.001 |
| HOMA-IR | 11.44 ± 1.49 | 9.83 ± 4.59 | 10.06 ± 2.76 | 15.84 ± 6.04 | 6.79 ± 2.21 d | 6.01 ± 0.85 d | <0.001 |
a significant differences (p < 0.05) with the C group. b significant differences (p < 0.05) with the LA group. c significant differences (p < 0.05) with the MA group. d significant differences (p < 0.05) with the B group. Differences were analyzed by one-way ANOVA.
2.2. Immunohistochemistry
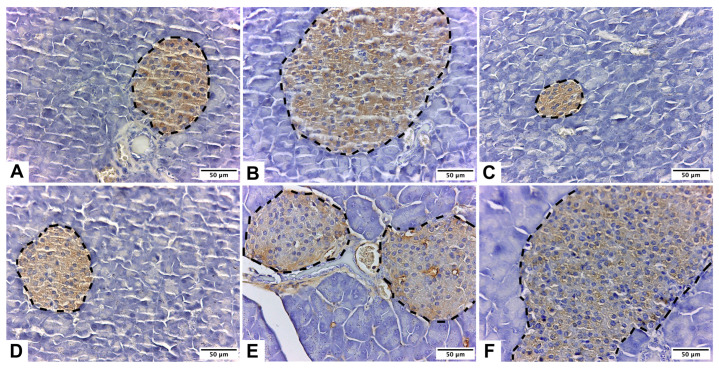
The immunohistochemical localization of insulin was observed in the endocrine pancreas across all the experimental groups (Figure 1). The histological analysis revealed variations in immunolabeling patterns and intensity among the groups. The C group exhibited a normal morphology of the beta-pancreatic islets, with the cells showing strong positive immunostaining (Figure 1A).
Figure 1.
Pancreas of male C57BL/6 mice stained with anti-insulin IgG Guinea pig primary antibody. Insulin expression in the endocrine pancreas was observed in groups: control (A); low-dose alcohol (B); moderate-dose alcohol (C); β-carotene (D); low-dose alcohol + β-carotene (E); and moderate-dose alcohol + β-carotene (F). Pancreatic islets are in the segmented lines.
Figure 1D–F illustrate an association between β-carotene supplementation and an increase in both the number and size of the pancreatic islets. Notably, no insulin marker immunoreactivity was detected outside the beta-pancreatic islets or the endocrine pancreas. Among the groups, the MA+B group displayed the highest immunostaining intensity (Figure 1D).
2.3. Area
The groups supplemented with β-carotene (B, LA+B, and MA+B) exhibited at least a 30% increase in the β-cell mass area compared to those without β-carotene supplementation (C, LA, and MA) (Table 2). In ethanol-consuming mice (LA and MA vs. LA+B and MA+B, respectively), β-carotene supplementation led to a 30–50% restoration of the median β-cell mass relative to the C and MA+B groups (p < 0.05).
Table 2.
Analysis of area and integrated optical density properties of β-cell in pancreatic islets of C57BL/6 mice.
| Media ± SD | |||||||
|---|---|---|---|---|---|---|---|
| C (n = 6) | LA (n = 6) | MA (n = 6) | B (n = 6) | LA+B (n = 6) | MA+B (n = 6) | p | |
| Area (μm2) | 194.78 ± 38.30 | 270.44 ± 107.99 a | 69.24 ± 22.61 ab | 429.50 ± 246.77 ac | 330.79 ± 117.64 ac | 1028.27 ± 356.19 ac | <0.001 |
| Integrated optical density (lum/μm2) | 26,006.67 ± 5063.97 | 35,661.82 ± 13,921.15 a | 9722.07 ± 3049.34 ab | 51,611.28 ± 29,343.24 ac | 43,537.44 ± 15,480.09 ac | 54,324.13 ± 29,600.55 ac | <0.001 |
a significant differences (p < 0.05) with the C group. b significant differences (p < 0.05) with the LA group. c significant differences (p < 0.05) with the MA group. A total of 144 pancreatic islets were evaluated, 24 pancreatic islets by group. Differences were analyzed by one-way ANOVA.
2.4. Integrated Optical Density Analysis
The immunohistochemistry analysis demonstrated the immunolabeling of β-cells throughout different regions of the beta-pancreatic islets in all the experimental groups. The concentration and intensity of insulin within the islets were higher in the groups supplemented with β-carotene (Table 2).
3. Discussion
3.1. Summary of Key Findings and Interpretation
Studies have shown a positive correlation between higher alcohol consumption and an increased incidence of T2DM [46]. In contrast, research also suggests that moderate alcohol intake may be linked to a reduced risk of T2DM [47]. Animal model studies investigating insulin production after ethanol exposure indicate that antioxidants can be effective in managing elevated blood glucose levels [14,48].
Although the specific mechanisms through which alcohol influences insulin remain unclear, numerous studies have identified a U-shaped or J-shaped relationship between alcohol consumption and insulin sensitivity or plasma insulin concentrations [49,50,51,52]. In individuals with T2DM, insulin production continues during the early stages of the disease; however, the body becomes resistant to insulin’s effects, as reflected in our results. Initially, the pancreas compensates for this resistance by increasing insulin synthesis, but eventually, it reaches a threshold where it can no longer produce adequate insulin.
3.2. Biochemistry
The HOMA-IR is considered a straightforward, cost-effective, and trustworthy indicator of insulin resistance, whereas the HOMA-β index has been identified as an effective measure of β-cell function [53]. Our results show that the groups that regularly consumed low and moderate amounts of ethanol had a lower HOMA-IR index and better insulin sensitivity (Table 1), which is in line with what other researchers have found. Nonetheless, the HOMA-β index in the mice subjected to prolonged ethanol consumption varies from what has been previously stated.
Chronic alcoholics experience an increase in alcohol metabolism [54]. This is often regarded as a significant factor contributing to alcohol-induced injury [55]. The primary enzymes involved in alcohol metabolism include alcohol dehydrogenase (ADH), mitochondrial aldehyde dehydrogenase (ALDH), and cytochrome P450 2E1 (CYP2E1). The alcohol is broken down into acetaldehyde by ADH and then further converted into acetate by ALDH. Therefore, significant toxicity caused by ethanol may be linked to the functions of ADH and ALDH [56].
3.3. Immunohistochemistry
Immunocytochemistry is a highly effective and sensitive method for detecting insulin and assessing its expression levels in pancreatic islets [57]. In this study, immunohistochemical labeling proved to be a valuable tool for identifying and distinguishing insulin-expressing cells in the pancreatic islets of C57BL/6 mice. The results showed that the islets predominantly contain insulin-producing cells with a lobular shape, consistent with the findings from previous studies [58,59].
This study employed ethanol exposure to induce diabetes in C57BL/6 mice. In the male mice of this strain, ethanol administration leads to elevated blood glucose levels, indicating that ethanol exerts a cytotoxic effect on pancreatic β-cells, likely through the generation of free radicals. The resulting destruction of β-cells reduces or entirely depletes insulin, thereby causing hyperglycemia [60]. However, in the mice that received β-carotene supplementation alongside ethanol, there was a notable increase in both the number and size of pancreatic islets (Figure 1D–F). The MA+B group exhibited the highest immunostaining intensity among the groups, as shown in Figure 1D. These findings suggest that β-carotene, as an antioxidant, may reduce free radical presence and mitigate ethanol’s cytotoxic effects on pancreatic cells.
3.4. Integrated Optical Density Analysis
Analyzing the mean gray value of each object within a digital image allows for the calculation of optical density (OD), a measurable parameter. Integrated optical density (IOD) represents the relationship between OD and a specific area of an image [61]. Pixel density quantifies the number of pixels per unit area, enabling an assessment of the object of interest relative to the image background. IOD facilitates the evaluation of immunohistochemical labeling by measuring color variations at the pixel level, which are then converted into numerical values to establish a quantifiable parameter.
In C57BL/6 mice, Table 2 presents the measured area and IOD characteristics of pancreatic islets. IOD properties varied across the experimental groups, with each group demonstrating statistically significant differences from the C group (p < 0.05). Notably, the β-cell mass area was larger in the β-carotene-supplemented groups (B, LA+B, and MA+B) compared to the unsupplemented groups (C, LA, and MA) (Table 2). Additionally, the β-carotene-supplemented groups showed elevated insulin concentration and potency in the islets, as displayed in Table 1.
Insulin resistance is defined as an inadequate response of tissues to insulin’s action in the bloodstream and is widely recognized as a key indicator for the development of metabolic disorders such as T2DM and metabolic syndrome [62]. Previous studies have explored the relationship between carotenoid consumption and impaired glucose tolerance, finding an inverse linear correlation between the concentrations of β-carotene and lycopene and the level of glucose tolerance, as shown by glucose tolerance test results [59]. These findings suggest that carotenoids may improve insulin sensitivity. Studies by Facchini et al. and Sugiura et al. have shown that lower plasma carotenoid levels are associated with higher insulin resistance in healthy individuals [63,64].
In this study, insulin expression in the pancreatic islets varied among the groups. The LA group demonstrated increased β-cell area and IOD (270.44 ± 107.99 μm2 and 35661.82 ± 13921.15 lum/μm2, respectively) compared to the C group (194.78 ± 38.30 μm2 and 26006.67 ± 5063.97 lum/μm2, respectively), potentially indicating heightened insulin sensitivity (p < 0.001). Although no significant differences in the serum glucose levels were found between the LA and C groups using the same experimental models, the LA group exhibited lower serum insulin content relative to the C group [60].
When β-carotene was administered concurrently with ethanol (in the LA+B and MA+B groups), there was a significant increase in the β-cell area and IOD compared to the groups without the β-carotene supplementation (LA and MA), although no differences were observed in the serum insulin or glucose levels. This effect could be attributed to several potential mechanisms: (1) β-carotene may reduce ethanol-induced β-cell damage while increasing insulin sensitivity [11,12,16]; (2) β-carotene alone may enhance insulin sensitivity [52,65,66]; or (3) β-carotene could mitigate ethanol damage in β-cells while jointly enhancing insulin sensitivity with ethanol [11,12,16,52,65,66].
3.5. Limitations
Our data offer new insights into the relationship between alcohol-induced diabetes and antioxidant therapies, specifically β-carotene. However, this study has a limitation in that it does not examine the interactions between alcohol metabolism byproducts and other pancreatic hormones, such as glucagon, somatostatin, amylin, or pancreatic polypeptide, in the context of antioxidant treatments. Future research should, therefore, focus on investigating the effects of β-carotene in cultured pancreatic cells and identifying the specific molecules and signaling pathways involved. Nevertheless, our findings suggest that β-carotene exposure may mitigate ethanol-induced damage to β-cells and enhance insulin sensitivity, even during ethanol consumption.
4. Materials and Methods
4.1. Sample Size
The study was a comparative analysis of independent groups, with parameters set at an alpha of 0.10, beta of 0.05, standard deviation of 0.05, a minimum detectable difference between groups of 0.1, and an anticipated follow-up loss proportion of 0.2 [67,68,69]. The sample size calculation was conducted using the G*Power 3.1.9.7 Software (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany).
4.2. Animals
Thirty-six male C57BL/6 mice (Mus musculus), aged fifty days, were obtained from the Public Health Institute of Chile. They were housed in the Animal Facility at the Center of Excellence in Morphological and Surgical Studies (CEMyQ), Universidad de La Frontera, for 30 days to acclimate to their environment. During this period, the mice were provided with a standard laboratory diet (AIN-93M) and water ad libitum. Lighting conditions were maintained on a 12 h light/dark cycle from 08:00 to 20:00 and 20:00 to 08:00. Animal care followed the guidelines from the Institute for Laboratory Animal Research’s Committee for the Update of the Guide for the Care and Use of Laboratory Animals [70].
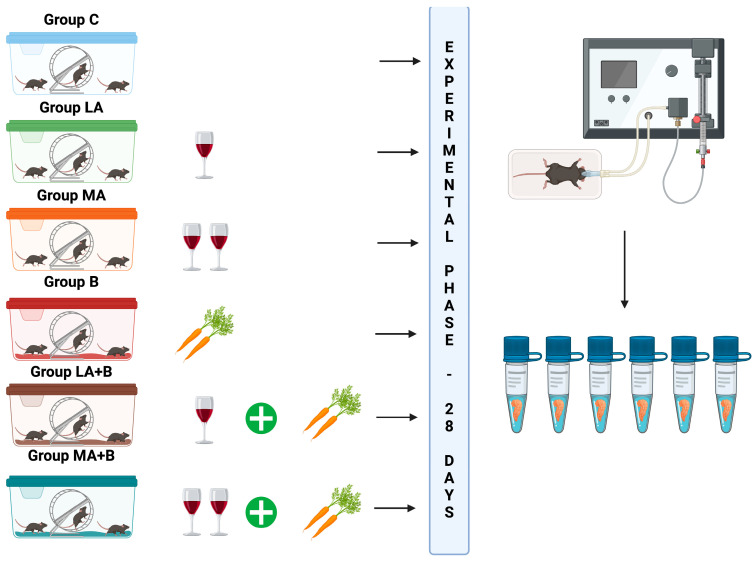
On the first day of the experiment, the mice were divided into six groups (n = 6 per group):
Control group (Group C): No alcohol or β-carotene administration.
Low-dose alcohol group (Group LA): Administered 3% v/v alcohol ad libitum for 28 days based on studies suggesting low alcohol intake enhances insulin sensitivity [49,71,72,73].
Moderate-dose alcohol group (Group MA): Administered 7% v/v alcohol ad libitum for 28 days, as moderate intake is also linked to increased insulin sensitivity [49,71,72,73].
β-carotene group (Group B): Administered 0.52 mg/kg body weight/day β-carotene for 28 days, as this dose has been shown to mitigate alcohol-induced liver damage by reducing oxidative stress and inhibiting apoptosis [30].
Low-dose alcohol + β-carotene group (Group LA+B): Administered low-dose alcohol plus 0.52 mg/kg body weight/day β-carotene for 28 days.
Moderate-dose alcohol + β-carotene group (Group MA+B): Administered moderate-dose alcohol plus 0.52 mg/kg body weight/day β-carotene for 28 days. Figure 2 illustrates the experimental design.
Figure 2.
Summary of the experimental design of the study. In this schematic representation, one glass of wine corresponds to an administration of a 28-day 3% alcohol dose, and two glasses of wine correspond to an administration of a 28-day 7% alcohol dose. Also, the carrots correspond to an administration of 28-day 0.52 mg/kg body weight/day of β-carotene. After the experimental phase, the animals were euthanized and samples (the blood and tissues) were obtained.
Chronic ethanol was administered using a modified Lieber–DeCarli liquid diet [71,73], while β-carotene was provided orally at a dosage of 0.52 mg/kg body weight per day [15]. This experimental model has been previously applied in animal studies [14,15,18,60].
4.3. Alcoholism and Treatments
A chronic plus single binge ethanol consumption (referred to as Lieber–DeCarli alcoholic diet) was used [71,74,75].
The ethanol groups were provided a liquid diet containing either 3% (LA or LA+B) or 7% (MA or MA+B) alcohol over a period of 28 days, while the control groups (C or B) were pair-fed an equivalent control diet for the same duration. On day 29, the mice in the ethanol groups were administered a single oral dose of ethanol (5 g/kg body weight, 20% ethanol), while the control groups received isocaloric dextrin maltose. Ethanol was sourced from Merck KGaA (107017, Darmstadt, Germany). The liquid diets were freshly prepared and administered daily [76].
β-carotene was administered in a dose of 0.52 mg/kg body weight/day (C9750, Sigma-Aldrich Co., St. Louis, MO, USA). β-carotene was diluted in water for the groups C, LA, MA, and B, whereas alcohol was used for the dissolution in the groups LA+B and MA+B. β-carotene was administered once a day by oral gavage (C9750, Sigma-Aldrich Co., St. Louis, MO, USA).
4.4. Euthanasia
On day 28, the animals were deprived of food for 6 h and then euthanized with sodium pentobarbital.
4.5. Biochemical Analyses
Centrifugation at 3500 rpm for 15 min separated the serum, which we then stored at −80 °C until analysis. In the biochemical analysis, we used a colorimetric kit (Sigma-Aldrich Co., St. Louis, MO, USA) to quantify the physiological concentration of glucose and a mouse-specific ELISA kit (Sigma-Aldrich Co., St. Louis, MO, USA) to measure insulin levels. The enzymatic activity of ADH and ALDH was assessed using the respective kit provided by Sigma-Aldrich Co. (St. Louis, MO, USA).
4.6. Homeostasis Model Assessment of β-Cell Function (HOMA-SS)
The HOMA-ß index was determined using Equation (1) [77]:
| (1) |
4.7. Homeostasis Model Assessment of Insulin Resistance (HOMA-IR)
The HOMA-IR index was determined using Equation (2) [78]:
| (2) |
4.8. Processing and Staining of Pancreas
To ensure random sampling, multiple sections were obtained from each pancreas, capitalizing on the tissue’s isotropic characteristics. After a 48 h fixation in 4% buffered formalin (1.27 mol/L formaldehyde in 0.1 M phosphate buffer, pH 7.2; Sigma-Aldrich, St. Louis, MO, USA), the samples were dehydrated and embedded in Paraplast Plus (Sigma-Aldrich, St. Louis, MO, USA). Each block was sectioned into four groups, with 5 μm cuts spaced 120 μm apart using a microtome (Leica® RM2255, Leica Biosystems, Nussloch, Germany).
4.9. Immunohistochemistry
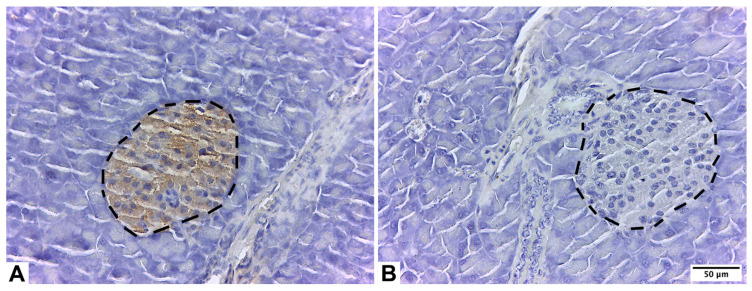
In summary, the paraffin sections were hydrated using a series of alcohols in decreasing concentrations following the standard procedure for traditional histological staining. Subsequently, they were immersed in distilled water for a duration of five minutes to restore their moisture content. Each histological section was washed in 1× PBS (Sigma-Aldrich Co., St. Louis, MO, USA) twice. They were treated with H2O2 (v/v) (ab64264, Abcam, Cambridge, UK) for 15 min to block the activity of endogenous peroxidase. Then, antigenic recovery was performed with HistoReveal (ab103720, Abcam, Cambridge, UK) for 10 min at room temperature. Next, each histological section was washed in 1× PBS three times. Then, the unspecific background was blocked using Protein Block (ab64207, Abcam, Cambridge, UK) for 15 min at room temperature. Each washing was performed with 1× PBS three times. First, the sections were incubated with anti-insulin IgG Guinea pig primary antibody (ab7842, Abcam, Cambridge, UK), dilution 1:50, in PBS overnight at 4 °C under a wet chamber. Second, each washing was performed with 1× PBS, four times. After washing with PBS, the sections were incubated with a biotinylated goat anti-polyvalent antibody (ab64207, Abcam, Cambridge, UK) for 10 min at room temperature in a wet chamber. Next, each wash was performed with 1× PBS four times. Then, the sections were incubated with streptavidin peroxidase (ab64207, Abcam, Cambridge, UK) for 10 min at room temperature. Afterward, each washing was performed with 1× PBS, four times. Finally, they were incubated with diaminobenzidine-peroxidase (ab64207, Abcam, Cambridge, UK) for visualization for ten minutes and washed with 1× PBS four times. The nuclear counterstain was performed with Harris hematoxylin (Sigma-Aldrich Co., St. Louis, MO, USA) for 50 s. The slides were dehydrated using a series of alcohols (Sigma-Aldrich Co., St. Louis, MO, USA) in increasing concentrations, following the standard procedure for traditional histological staining. A total of four pancreatic islets by slide were observed under a light microscope (Leica® LED750, Leica Biosystems, Nussloch, Germany) and photographed (Leica® ICC50W, Leica Biosystems, Nussloch, Germany). A total of 144 pancreatic islets were evaluated. For each immunohistochemical reaction, negative controls were used, which were incubated in PBS, omitting the primary antibody (ab7842, Abcam, Cambridge, UK). Table 3 shows the labeled process used for positive and negative control. The immunolabeling was quantified by the area (mm2) occupied in each field, and the IOD analysis was expressed as lum/μm2 [79]. Both measurements were made using the Image-ProPremier 9.1 software (Media Cybernetics, Warrendale, PA, USA). Figure 3 shows the positive and negative controls.
Table 3.
Labeled process used for positive and negative controls to determine insulin in pancreatic islets of C57BL/6 mice.
| Block Peroxidase |
Antigenic Recovery | Unspecific Background | Primary Antibody |
Secondary Antibody |
Labeled | Detection | |
|---|---|---|---|---|---|---|---|
| Positive control (protein of interest) | H2O2 | HistoReveal | Protein Block | Anti-insulin IgG guinea pig primary antibody | Biotinylated goat anti-polyvalent antibody | Streptavidin peroxidase | Diaminobenzidine-peroxidase |
| Negative control (without protein of interest) | H2O2 | HistoReveal | Protein Block | Saline solution | Biotinylated goat anti-polyvalent antibody | Streptavidin peroxidase | Diaminobenzidine-peroxidase |
| Samples (pancreas cuts) |
H2O2 | HistoReveal | Protein Block | Anti-insulin IgG guinea pig primary antibody | Biotinylated goat anti-polyvalent antibody | Streptavidin peroxidase | Diaminobenzidine-peroxidase |
Figure 3.
Exocrine pancreas of male C57BL/6 mice. Positive control for pancreatic islets stained with anti-insulin IgG Guinea pig primary antibody (A); negative control for pancreatic islets stained with primary antibody (anti-insulin IgG Guinea pig primary antibody was omitted) (B). Pancreatic islets are in segmented lines.
4.10. Statistical Analysis
Levene’s test was applied to assess the homoscedasticity of variances, while the Kolmogorov–Smirnov test was used to evaluate data normality. These tests were conducted to assess disparities in the quantitative data. Group differences were analyzed using one-way ANOVA, followed by either Dunnett’s T3 test or Tukey’s post hoc HSD test, as appropriate. Statistical significance was determined at a p-value of less than 0.05 using IBM SPSS Statistics, Version 21 (IBM Corp., Armonk, NY, USA).
5. Conclusions
Insulin expression in pancreatic islets varied among the groups. In mice, β-cell loss led to increased glucose release due to decreased insulin levels. β-carotene appeared to mitigate ethanol’s impact on these cells, resulting in reduced insulin degradation. In the pancreatic islets of the C57BL/6 mice, β-carotene exposure improved insulin sensitivity and lessened ethanol-induced β-cell damage. These findings suggest that antioxidant supplementation may be beneficial in treating ethanol-induced T2DM in animal models. Further research is necessary to better understand the interaction between alcohol consumption and antioxidant therapy, including studies using specific cell lines and clinical trials.
Acknowledgments
The authors thank SmartC-BIOREN (Service Management Analytical Research and Training Center), CCSS210005 Project, and Agencia Nacional de Investigación y Desarrollo de Chile (ANID). In addition, we would like to thank Isabela Pérez Núñez for her technical support to the Universidad de La Frontera, GI23-0021 Project.
Author Contributions
C.S., B.V., K.G., J.C. and J.F. carried out the conception and design of the research. C.S., B.V., K.G. and J.F. have participated in the experimental phase. L.C., Á.O., C.R., F.V. and Á.V. carried out the immunohistochemistry and integrated optical density analysis. C.S., F.T. and K.G. performed the statistical analysis. C.S., L.C., Á.O., C.R., F.V., Á.V., F.T., B.V., K.G., M.Z., J.C. and J.F. wrote the original draft preparation. C.S., L.C., Á.O., C.R., F.V., Á.V., F.T., B.V., K.G., M.Z., J.C. and J.F. wrote the review and editing. C.S. and J.F. carried out the supervision and project administration. C.S., K.G., M.Z. and J.F. participated in obtaining funding. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by the Scientific Ethics Committee of the Universidad de La Frontera (Nº034/22).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data presented in the study are openly available in FigShare at https://doi.org/10.6084/m9.figshare.27130587.v1 (accessed on 2 November 2024).
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was partially financed by Universidad de La Frontera, GI23-0021 Project; Universidad de La Frontera, DI22–0007 Project; and Universidad de La Frontera, PDT22-0001 Project and ANID, FONDECYT INI 11240623 Project.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Anderson P., Dalziel K., Davies E., Fitzsimmons D., Hale J., Hughes A., Isaac J., Onishchenko K., Phillips C., Pockett R. Survey of digestive health across Europe: Final report. Part 2: The economic impact and burden of digestive disorders. United Eur. Gastroenterol. J. 2014;2:544–546. doi: 10.1177/2050640614554155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Żorniak M., Beyer G., Mayerle J. Risk Stratification and Early Conservative Treatment of Acute Pancreatitis. Visc. Med. 2019;35:82–89. doi: 10.1159/000497290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conwell D.L., Banks P.A., Sandhu B.S., Sherman S., Al-Kaade S., Gardner T.B., Anderson M.A., Wilcox C.M., Lewis M.D., Muniraj T., et al. Validation of Demographics, Etiology, and Risk Factors for Chronic Pancreatitis in the USA: A Report of the North American Pancreas Study (NAPS) Group. Dig. Dis. Sci. 2017;62:2133–2140. doi: 10.1007/s10620-017-4621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lankisch P.G., Apte M., Banks P.A. Acute pancreatitis. Lancet. 2015;386:85–96. doi: 10.1016/S0140-6736(14)60649-8. [DOI] [PubMed] [Google Scholar]
- 5.Whitcomb D.C., LaRusch J., Krasinskas A.M., Klei L., Smith J.P., Brand R.E., Neoptolemos J.P., Lerch M.M., Tector M., Sandhu B.S., et al. Alzheimer’s Disease Genetics Consortium; Alkaade, S.; Amann, S.T.; Anderson, M.A.; Baillie, J.; et al. Alzheimer’s Disease Genetics Consortium. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat. Genet. 2012;44:1349–1354. doi: 10.1038/ng.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandol S.J., Lugea A., Mareninova O.A., Smoot D., Gorelick F.S., Gukovskaya A.S., Gukovsky I. Investigating the pathobiology of alcoholic pancreatitis. Alcohol Clin. Exp. Res. 2011;35:830–837. doi: 10.1111/j.1530-0277.2010.01408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maléth J., Balázs A., Pallagi P., Balla Z., Kui B., Katona M., Judák L., Németh I., Kemény L.V., Rakonczay Z., Jr., et al. Alcohol disrupts levels and function of the cystic fibrosis transmembrane conductance regulator to promote development of pancreatitis. Gastroenterology. 2015;148:427–439.e16. doi: 10.1053/j.gastro.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apte M.V., Wilson J.S., Lugea A., Pandol S.J. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210–1219. doi: 10.1053/j.gastro.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lugea A., Tischler D., Nguyen J., Gong J., Gukovsky I., French S.W., Gorelick F.S., Pandol S.J. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology. 2011;140:987–997. doi: 10.1053/j.gastro.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lugea A., Waldron R.T., Pandol S.J. Pancreatic adaptive responses in alcohol abuse: Role of the unfolded protein response. Pancreatology. 2015;15((Suppl 4)):S1–S5. doi: 10.1016/j.pan.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandoval C., Vásquez B., Mandarim-de-Lacerda C., del Sol M. Ethanol intake and toxicity: In search of new treatments. Int. J. Morphol. 2017;35:942–949. doi: 10.4067/S0717-95022017000300024. [DOI] [Google Scholar]
- 12.Sandoval C., Vásquez B., Souza-Mello V., Mandarim-de-Lacerda C., del Sol M. Role of Alcohol Consumption and Antioxidants on Global Methylation of DNA and Cancer. Int. J. Morphol. 2018;36:367–372. doi: 10.4067/S0717-95022018000100367. [DOI] [Google Scholar]
- 13.Wu H., Cai P., Clemens D.L., Jerrells T.R., Shakeel Ansari G.A., Kaphalia B.S. Metabolic basis of ethanol-induced cytotoxicity in recombinant HepG2 Cells: Role of nonoxidative metabolism. Toxicol. Appl. Pharmacol. 2006;216:238–247. doi: 10.1016/j.taap.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Sandoval C., Vásquez B., Souza-Mello V., Adeli K., Mandarim-de-Lacerda C., del Sol M. Morphoquantitative effects of oral β-carotene supplementation on liver of C57BL/6 mice exposed to ethanol consumption. Int. J. Clin. Exp. Pathol. 2019;12:1713–1722. [PMC free article] [PubMed] [Google Scholar]
- 15.Sandoval C., Vera A., Birditt K., Godoy K., Carmine F., Caamaño J., Farías J. β-Carotene Supplementation Improves Pancreas Function during Moderate Ethanol Consumption: Initial Characterization from a Morphological Overview. Int. J. Mol. Sci. 2024;25:1219. doi: 10.3390/ijms25021219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apte M.V., Wilson J.S., Korsten M.A., McCaughan G.W., Haber P.S., Pirola R.C. Effects of ethanol and protein deficiency on pancreatic digestive and lysosomal enzymes. Gut. 1995;36:287–293. doi: 10.1136/gut.36.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandoval C., Farías J., Zamorano M., Herrera C. Vitamin Supplements as a Nutritional Strategy against Chronic Alcohol Consumption? An Updated Review. Antioxidants. 2022;11:564. doi: 10.3390/antiox11030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandoval C., Mella L., Godoy K., Adeli K., Farías J. β-Carotene Increases Activity of Cytochrome P450 2E1 during Ethanol Consumption. Antioxidants. 2022;11:1033. doi: 10.3390/antiox11051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institute of Diabetes and Digestive and Kidney Diseases Symptoms and Causes of Pancreatitis. [(accessed on 30 June 2024)]; Available online: https://www.niddk.nih.gov/health-information/digestive-diseases/pancreatitis/symptoms-causes.
- 20.Kim J.Y., Lee D.Y., Lee Y.J., Park K.J., Kim K.H., Kim J.W., Kim W.H. Chronic alcohol consumption potentiates the development of diabetes through pancreatic β-cell dysfunction. World J. Biol. Chem. 2015;6:1–15. doi: 10.4331/wjbc.v6.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner K.H., Schwingshackl L., Draxler A., Franzke B. Impact of Dietary and Lifestyle Interventions in Elderly or People Diagnosed with Diabetes, Metabolic Disorders, Cardiovascular Disease, Cancer and Micronutrient Deficiency on Micronuclei Frequency—A Systematic Review and Meta-analysis. Mutat. Res. 2021;787:108367. doi: 10.1016/j.mrrev.2021.108367. [DOI] [PubMed] [Google Scholar]
- 22.Roshanravan N., Askari S.F., Fazelian S., Ayati M.H., Namazi N. The Roles of Quercetin in Diabetes Mellitus and Related Metabolic Disorders; Special Focus on the Modulation of Gut Microbiota: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2021;63:2990–3003. doi: 10.1080/10408398.2021.1983765. [DOI] [PubMed] [Google Scholar]
- 23.Wang X., Yang J., Qiu X., Wen Q., Liu M., Zhou D., Chen Q. Probiotics, Pre-biotics and Synbiotics in the Treatment of Pre-diabetes: A Systematic Review of Randomized Controlled Trials. Front. Public Health. 2021;9:645035. doi: 10.3389/fpubh.2021.645035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall D.C., Holloway M., Korer M., Woodman J., Brackenridge A., Hussain S. Do-It-Yourself Artificial Pancreas Systems in Type 1 Diabetes: Perspectives of Two Adult Users, A Caregiver and Three Physicians. Diabetes Ther. 2019;10:1553–1564. doi: 10.1007/s13300-019-00679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu X., Wen J., Yu W., Yang L., Pan W., Xu K., Chen X., Li Q., Chen G., Gu X. Associations of Early-life Exposure to Famine with Abdominal Fat Accumulation are Independent of Family History of Diabetes and Physical Activity. Br. J. Nutr. 2021;125:943–950. doi: 10.1017/S0007114520003414. [DOI] [PubMed] [Google Scholar]
- 26.Li A.L., Peng Q., Shao Y.Q., Fang X., Zhang Y.Y. The Interaction on Hypertension Between Family History and Diabetes and Other Risk Factors. Sci. Rep. 2021;11:4716. doi: 10.1038/s41598-021-83589-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J., Zhou Y., Hu H., Yang D., Yang F. Effects of β-carotene on glucose metabolism dysfunction in humans and type 2 diabetic rats. Acta Mater. Medica. 2022;1:138–153. doi: 10.15212/AMM-2021-0009. [DOI] [Google Scholar]
- 28.Lin W.T., Huang C.C., Lin T.J., Chen J.R., Shieh M.J., Peng H.C., Yang S.C., Huang C.Y. Effects of beta- carotene on antioxidant status in rats with chronic alcohol consumption. Cell Biochem. Funct. 2009;27:344–350. doi: 10.1002/cbf.1579. [DOI] [PubMed] [Google Scholar]
- 29.Takeda S., Bando N., Yamanishi R. Ingested β-carotene enhances glutathione level and up-regulates the activity of cysteine cathepsin in murine splenocytes. Biosci. Biotechnol. Biochem. 2008;72:1595–1600. doi: 10.1271/bbb.80102. [DOI] [PubMed] [Google Scholar]
- 30.Peng H.C., Chen Y.L., Yang S.Y., Ho P.Y., Yang S.S., Hu J.T., Yang S.C. The antiapoptotic effects of different doses of β-carotene in chronic ethanol-fed rats. Hepatobiliary Surg. Nutr. 2013;2:132–141. doi: 10.3978/j.issn.2304-3881.2013.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shete V., Quadro L. Mammalian metabolism of β-carotene: Gaps in knowledge. Nutrients. 2013;5:4849–4868. doi: 10.3390/nu5124849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mounien L., Tourniaire F., Landrier J.F. Anti-obesity effect of carotenoid: Direct impact on adipose tissue and adipose tissue-driven indirect effects. Nutrients. 2019;11:1562. doi: 10.3390/nu11071562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Concepcion M., Avalos J., Bonet M.L., Boronat A., Lourdes G.G., Hornero-Mendez D., Limon M.C., Meléndez-Martínez A.J., Olmedilla-Alonso B., Palou A., et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018;70:62–93. doi: 10.1016/j.plipres.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Perera C.R., Yen G.M. Functional properties of carotenoids in human health. Int. J. Food Prop. 2007;10:201–230. doi: 10.1080/10942910601045271. [DOI] [Google Scholar]
- 35.Rühl R., Landrier J.F. Dietary regulation of adiponectin by direct and indirect lipid activators of nuclear hormone receptors. Mol. Nutr. Food Res. 2016;60:175–184. doi: 10.1002/mnfr.201500619. [DOI] [PubMed] [Google Scholar]
- 36.Amengual J., Gouranton E., Helden Y.G.J., Hessel S., Ribot J., Kramer E., Kiec-Wilk B., Razny U., Lietz A., Wyss A., et al. Beta-carotene reduces body adiposity of mice via BCMO1. PLoS ONE. 2011;6:e20644. doi: 10.1371/journal.pone.0020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beydoun M.A., Chen X., Jha K., Beydoun H.A., Zonderman A.B., Canas J.A. Carotenoids, vitamin A, and their association with the metabolic syndrome: A systematic review and meta-analysis. Nutr. Rev. 2019;77:32–45. doi: 10.1093/nutrit/nuy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canas J.A., Lochrie A., McGowan A.G., Hossain J., Schettino C., Balagopal P.B. Effects of mixed carotenoids on adipokines and abdominal adiposity in children: A pilot study. J. Clin. Endocrinol. Metab. 2017;102:1983–1990. doi: 10.1210/jc.2017-00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asemi Z., Alizadeh S.A., Ahmad K., Goli M., Esmailzadeh A. Effects of beta-carotene fortified symbiotic food on metabolic control of patients with type 2 diabetes mellitus: A double-blind randomized cross-over controlled clinical trial. Clin. Nutr. 2016;35:819–825. doi: 10.1016/j.clnu.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Kim Y., Kim Y.K., Lim Y., Oh B., Kim J.Y., Bouwman J., Kwon O. Combination of diet quality score, plasma carotenoids, and lipid peroxidation to monitor oxidative stress. Oxid. Med. Cell Longev. 2018;2018:8601028. doi: 10.1155/2018/8601028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugiura M., Nakamura M., Ogawa K., Ikoma Y., Yano M. High serum carotenoids associated with lower risk for the metabolic syndrome and its components among Japanese subjects: Mikkabi cohort study. Br. J. Nutr. 2015;114:1674–1682. doi: 10.1017/S0007114515003268. [DOI] [PubMed] [Google Scholar]
- 42.Liu J., Shi W.Q., Cao Y., He L.P., Guan K., Ling W.H., Chen Y.M. Higher serum carotenoid concentrations associated with a lower prevalence of the metabolic syndrome in middle-aged and elderly Chinese adults. Br. J. Nutr. 2014;112:2041–2048. doi: 10.1017/S000711451400316X. [DOI] [PubMed] [Google Scholar]
- 43.Witt H., Apte M.V., Keim V., Wilson J.S. Chronic pancreatitis: Challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132:1557–1573. doi: 10.1053/j.gastro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 44.He Y., Shi B., Zhao X., Sui J. Sphingosine-1-phosphate induces islet β-cell proliferation and decreases cell apoptosis in high-fat diet/streptozotocin diabetic mice. Exp Ther Med. 2019;18:3415–3424. doi: 10.3892/etm.2019.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S., Wang Y., Cui L., Deng Y., Xu S., Yu J., Cichello S., Serrero G., Ying Y., Liu P. Morphologically and Functionally Distinct Lipid Droplet Subpopulations. Sci. Rep. 2016;6:29539. doi: 10.1038/srep29539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cullmann M., Hilding A., Ostenson C.G. Alcohol consumption and risk of pre-diabetes and type 2 diabetes development in a swedish population. Diabet. Med. 2012;29:441–452. doi: 10.1111/j.1464-5491.2011.03450.x. [DOI] [PubMed] [Google Scholar]
- 47.Carlsson S., Hammar N., Grill V. Alcohol consumption and type 2 diabetes Meta-analysis of epidemiological studies indicates a U-shaped relationship. Diabetologia. 2005;48:1051–1054. doi: 10.1007/s00125-005-1768-5. [DOI] [PubMed] [Google Scholar]
- 48.Sen D.B., Balaraman R., Sen A.K., Zanwar A.S., Greeshma K.P., Maheshwari R.A. Anti-diabetic activity of herbal remedies. J. Nat. Remedies. 2023;23:373–381. doi: 10.18311/jnr/2023/32182. [DOI] [Google Scholar]
- 49.Kiechl S., Willeit J., Poewe W., Egger G., Oberhollenzer F., Muggeo M., Bonora E. Insulin sensitivity and regular alcohol consumption: Large, prospective, cross sectional population study (Bruneck study) BMJ. 1996;313:1040–1044. doi: 10.1136/bmj.313.7064.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lazarus R., Sparrow D., Weiss S.T. Alcohol intake and insulin levels: The normative aging study. Am. J. Epidemiol. 1997;145:909–916. doi: 10.1093/oxfordjournals.aje.a009050. [DOI] [PubMed] [Google Scholar]
- 51.Villegas R., Salim A., O’Halloran D., Perry I.J. Alcohol intake and insulin resistance. A cross-sectional study. Nutr. Metab. Cardiovas. Dis. 2004;14:233–240. doi: 10.1016/S0939-4753(04)80049-8. [DOI] [PubMed] [Google Scholar]
- 52.Sandoval C., Herrera C., Schulz M., Vásquez B. Relationship Between Ethanol Intake and Insulin Sensitivity Metabolism in Men with no Comorbidities: A Systematic Review. Int. J. Morphol. 2021;39:829–838. doi: 10.4067/S0717-95022021000300829. [DOI] [Google Scholar]
- 53.Wallace T.M., Levy J.C., Matthews D.R. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 54.Lieber C., DeCarli L. Hepatic microsomal ethanol-oxidizing system. J. Biol. Chem. 1970;245:2505–2512. doi: 10.1016/S0021-9258(18)63099-6. [DOI] [PubMed] [Google Scholar]
- 55.Zakhari S., Li T.K. Determinants of alcohol use and abuse: Impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46:2032–2039. doi: 10.1002/hep.22010. [DOI] [PubMed] [Google Scholar]
- 56.Yoo Y., Jung E., Kang H., Choi I., Choi K., Jeung E. The sap of Acer okamotoanum decreases serum alcohol levels after acute ethanol ingestion in rats. Int. J. Mol. Med. 2011;28:489–495. doi: 10.3892/ijmm.2011.724. [DOI] [PubMed] [Google Scholar]
- 57.Edlund H. Developmental biology of the pancreas. Diabetes. 2001;50((Suppl. 1)):S5–S9. doi: 10.2337/diabetes.50.2007.S5. [DOI] [PubMed] [Google Scholar]
- 58.Steiner D.J., Kim A., Miller K., Hara M. Pancreatic islet plasticity: Interspecies comparison of islet architecture and composition. Islets. 2010;2:135–145. doi: 10.4161/isl.2.3.11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hawkins K.L., Summers B.A., Kuhajda F.P., Smith C.A. Immunocytochemistry of normal pancreatic islets and spontaneous islet cell tumors in dogs. Vet. Pathol. 1987;24:170–179. doi: 10.1177/030098588702400211. [DOI] [PubMed] [Google Scholar]
- 60.Sandoval C., Vásquez B., Vasconcellos A., Souza-Mello V., Adeli K., Mandarim-de-Lacerda C., del Sol M. Oral supplementation of b-carotene benefits the hepatic structure and metabolism in mice exposed to chronic ethanol consumption. Sains Malays. 2022;51:285–296. doi: 10.17576/jsm-2022-5101-23. [DOI] [Google Scholar]
- 61.Jafari S.M.S., Hunger R.E. IHC Optical Density Score: A New Practical Method for Quantitative Immunohistochemistry Image Analysis. Appl. Immunohistochem. Mol. Morphol. 2017;25:e12–e13. doi: 10.1097/PAI.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 62.Sluijs I., Cadier E., Beulens J.W., van der A D.L., Spijkerman A.M., van der Schouw Y.T. Dietary intake of carotenoids and risk of type 2 diabetes. Nutr Metab Cardiovasc. Dis. 2015;25:376–381. doi: 10.1016/j.numecd.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 63.Facchini F.S., Humphreys M.H., DoNascimento C.A., Abbasi F., Reaven G.M. Relation between insulin resistance and plasma concentrations of lipid hydroperoxides, carotenoids, and tocopherols. Am. J. Clin. Nutr. 2000;72:776–779. doi: 10.1093/ajcn/72.3.776. [DOI] [PubMed] [Google Scholar]
- 64.Sugiura M., Nakamura M., Ogawa K., Ikoma Y., Yano M. High-serum carotenoids associated with lower risk for developing type 2 diabetes among Japanese subjects: Mikkabi cohort study. BMJ Open Diabetes Res. Care. 2015;3:e000147. doi: 10.1136/bmjdrc-2015-000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roohbakhsh A., Karimi G., Iranshahi M. Carotenoids in the treatment of diabetes mellitus and its complications: A mechanistic review. Biomed. Pharmacother. 2017;91:31–42. doi: 10.1016/j.biopha.2017.04.057. [DOI] [PubMed] [Google Scholar]
- 66.Marcelino G., Machate D.J., Freitas K.C., Hiane P.A., Maldonade I.R., Pott A., Asato M.A., Candido C.J., Guimarães R.C.A. β-Carotene: Preventive Role for Type 2 Diabetes Mellitus and Obesity: A Review. Molecules. 2020;25:5803. doi: 10.3390/molecules25245803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duffau G. Tamaño muestral en estudios biomédicos. Rev. Chil. Ped. 1999;70:314–324. doi: 10.4067/S0370-41061999000400009. [DOI] [Google Scholar]
- 68.Rojo Amigo A. Cálculo del tamaño muestral en procedimientos de experimentación con animales. Valoración de las incidencias. Anim. Lab. 2014;62:31–33. [Google Scholar]
- 69.Bottaro F. Cómo lidiar con la pérdida en el seguimiento. Hematologia. 2016;20:133–137. [Google Scholar]
- 70.Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies & National Research Council . Guide for the Care and Use of Laboratory Animals. 8th ed. The National Academies Press; Washington, DC, USA: 2011. [Google Scholar]
- 71.Furuya D.T., Binsack R., Machado U.F. Low ethanol consumption increases insulin sensitivity in Wistar rats. Braz. J. Med. Biol. Res. 2003;36:125–130. doi: 10.1590/S0100-879X2003000100017. [DOI] [PubMed] [Google Scholar]
- 72.Klatsky A.L., Armstrong M.A., Friedman G.D. Alcohol and mortality. Ann. Intern. Med. 1992;117:646–654. doi: 10.7326/0003-4819-117-8-646. [DOI] [PubMed] [Google Scholar]
- 73.Doll R., Peto R., Hall E., Wheatley K., Gray R. Mortality in relation to consumption of alcohol: 13 years’ observations on male British doctors. BMJ. 1994;309:911–918. doi: 10.1136/bmj.309.6959.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diao Y., Nie J., Tan P., Zhao Y., Zhao T., Tu J., Ji H., Cao Y., Wu Z., Liang H., et al. Long-term low-dose ethanol intake improves healthspan and resists high-fat diet-induced obesity in mice. Aging. 2020;12:13128–13146. doi: 10.18632/aging.103401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ki S.H., Park O., Zheng M., Morales-Ibanez O., Kolls J.K., Bataller R., Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic- binge ethanol feeding: Role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi Y., Abdelmegeed M.A., Song B.J. Preventive effects of indole-3-carbinol against alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms: Role of gut-liver-adipose tissue axis. J. Nutr. Biochem. 2018;55:12–25. doi: 10.1016/j.jnutbio.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 78.Vogeser M., König D., Frey I., Predel H.G., Parhofer K.G., Berg A. Fasting serum insulin and the homeostasis model of insulin resistance (HOMA-IR) in the monitoring of lifestyle interventions in obese persons. Clin. Biochem. 2007;40:964–968. doi: 10.1016/j.clinbiochem.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 79.Boschi F., Rizzatti V., Zoico E., Montanari T., Zamboni M., Sbarbati A., Colitti M. Relationship between lipid droplets size and integrated optical density. Eur. J. Histochem. 2019;63:3017. doi: 10.4081/ejh.2019.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original data presented in the study are openly available in FigShare at https://doi.org/10.6084/m9.figshare.27130587.v1 (accessed on 2 November 2024).