Abstract
Streptococcus pneumoniae undergoes spontaneous phase variation between a transparent and an opaque colony phenotype, the latter being more virulent in a murine model of sepsis. Opaque pneumococci have previously been shown to express lower amounts of C polysaccharide (cell wall teichoic acid) and in this study were shown to have a higher content of capsular polysaccharide by immunoelectron microscopy. This report then examined the relationship between expression of these two cell surface carbohydrate structures and their relative contribution to the increased virulence of opaque variants. Comparison of genetically related strains showed that the differential content of capsular polysaccharide did not affect the amount of teichoic acid as measured by a capture enzyme-linked immunosorbent assay (ELISA). In contrast, when the teichoic acid structure was altered by replacing choline in the growth medium with structural analogs, the quantity of capsular polysaccharide as measured by a capture ELISA was decreased, demonstrating a linkage in the expression of the two surface carbohydrate structures. A standardized assay was used to assess the relative contribution of cell surface carbohydrates to opsonophagocytosis. The opaque variants required 1.2- to 30-fold more immune human serum to achieve 50% opsonophagocytic killing than did related transparent variants (types 6B and 9V). The opsonophagocytic titer was proportional to the quantity of capsular polysaccharide rather than teichoic acid. The major factor in binding of the opsonin, C-reactive protein (CRP), was also the amount of capsular polysaccharide rather than the teichoic acid ligand. Only for the transparent variant (type 6B), which bound more CRP, was there enhanced opsonophagocytic killing in the presence of this serum protein. Increased expression of capsular polysaccharide, therefore, appeared to be the major factor in the decreased opsonophagocytic killing of opaque pneumococci.
Streptococcus pneumoniae, the pneumococcus, colonizes the human nasopharynx and is a common etiologic agent of respiratory tract infection. In addition, infection with the pneumococcus frequently results in bacteremia and sepsis because of its capacity to invade the bloodstream. The ability to exist in these two host environments correlates with two distinct phenotypes observed in clinical isolates as compared in animal models of carriage and sepsis (7, 24). There is spontaneous back-and-forth switching or phase variation among opaque, transparent, and in some isolates intermediate colony morphologies. The more transparent forms are more efficient at adherence to human epithelial cells and colonization of the nasopharynx while only the opaque forms are able to cause sepsis in mice (3, 7, 24).
Comparison of cell surface factors that vary in association with opacity showed that transparent pneumococci have 2.1- to 3.8-fold more cell wall carbohydrate (C polysaccharide or teichoic acid) (7). The pneumococcal teichoic acid has an unusual structure including choline which is derived from the growth medium and is a nutritional requirement (5, 18). Choline in the form of phosphorylcholine (ChoP) on the teichoic acid has been implicated in direct adherence to host cells via the receptor for platelet-activating factor (2). In addition, a number of cell surface proteins, including several shown to contribute to the pathogenesis of pneumococcal infection, are anchored to the organism by noncovalent attachment to ChoP (13, 23, 26). The distribution of these choline-binding proteins differs in association with colony morphology and content of the ChoP anchor. ChoP is also a target for an acute-phase reactant in human serum, C-reactive protein (CRP), which has been shown to induce opsonophagocytic activity and to contribute to protection against invasive pneumococcal infection (6, 10, 11, 16, 22).
Opaque pneumococci, in contrast, have 1.2- to 5.6-fold-greater quantities of capsular polysaccharide, the major virulence determinant of the organism, than do related transparent organisms (7). The capsule acts to inhibit phagocytosis, the primary mechanism for clearance of the pneumococcus. Relatively small differences in the amount of capsular polysaccharide have been noted to be critical in the ability of the organism to cause experimental infection (9). The increased content of capsular polysaccharide in opaque pneumococci could account for the enhanced virulence associated with this phenotype in invasive infection in the mouse model.
It appears, therefore, that there is an inverse relationship in amounts of the two cell surface carbohydrates, with transparent variants expressing more teichoic acid and less capsular polysaccharide and opaque variants having less teichoic acid and more capsular polysaccharide. The purpose of this study was (i) to examine whether this inverse relationship results from an effect of one cell surface carbohydrate on the expression of the other and (ii) to determine the relative contribution of each of these factors in an opsonophagocytosis model of host clearance.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and growth medium.
Strains of pneumococcus used in this study are described in Table 1. Bacteria were grown in a semisynthetic medium (C + Y medium, pH 8.0) or in a chemically defined medium (Cden) at 37°C without shaking, unless otherwise specified (19). Broth cultures were plated onto tryptic soy plates with 1% agar, onto which 5,000 U of catalase (Worthington Biochemical, Freehold, N.J.) was spread, and incubated at 37°C in a candle extinction jar, as previously described (24). Colony morphology was determined under magnification and oblique transmitted illumination as previously described (24). Unless otherwise stated, chemicals and reagents were purchased from Sigma Chemical Co. (St. Louis, Mo.).
TABLE 1.
Pneumococcal strains
| Strain | Relevant characteristic(s) | Source or reference |
|---|---|---|
| D39 | Type 2, clinical isolate | 1 |
| R6x | Unencapsulated mutant derived from D39 | 17 |
| A66 | Type 3 | 1 |
| P156 | Type 3, R6x × A66 chromosomal DNA | This study |
| P125 | Unencapsulated, opaque variant | 23 |
| P126 | Unencapsulated, transparent variant | 23 |
| P11 | Type 9V, clinical isolate | This study |
| P13 | Spontaneous unencapsulated mutant of P11 | This study |
| P105 | Type 9V, spontaneous encapsulated revertant of P13 | This study |
| P62 | Type 9V, opaque variant of clinical isolate P10 | 24 |
| P63 | Type 9V, intermediate variant of clinical isolate P10 | 24 |
| P64 | Type 9V, transparent variant of clinical isolate P10 | 24 |
| P71 | Type 18C, opaque variant of clinical isolate P68 | 7 |
| P73 | Type 18C, transparent variant of clinical isolate P68 | 7 |
| P376 | Type 6A, opaque variant of clinical isolate P303 | 7 |
| P384 | Type 6A, transparent variant of clinical isolate P303 | 7 |
| P382 | Type 6B, opaque variant of clinical isolate P324 | 7 |
| P383 | Type 6B, transparent variant of clinical isolate P324 | 7 |
| P763 | Type 6B, opaque variant of clinical isolate P314 | This study |
| P764 | Type 6B, intermediate variant of clinical isolate P314 | This study |
| P765 | Type 6B, transparent variant of clinical isolate P314 | This study |
| P806 | Type 6B, spontaneous revertant of P765 to an opaque phenotype | This study |
Immunoelectron microscopy.
Techniques used in this study have previously been described in detail (14). Briefly, the pneumococci were cultured to mid-log phase (6 to 8 h) at 37°C and stabilized with formaldehyde. The bacterial cells were washed several times with phosphate-buffered saline (PBS) and then treated with an excess of type-specific pneumococcal antibody (Statens Seruminstitut, Copenhagen, Denmark). The same ratio of anticapsular antibody to cells was used in the different experiments for preparation of the specimens. The cells were washed again in order to remove unbound antibody. After the last centrifugation, the pellet of cells was mixed with a small amount of melted 1% agarose at 45°C. After cooling, the agarose block was cut into small cubes and handled according to a routine electron microscopy procedure (14).
DNA transformation.
Chromosomal DNA from type 3 strain A66 was used to transform competent R6x by the method of Lacks and Hotchkiss (8). Colonies were screened for acquisition of capsular polysaccharide by colony irridescence, and the presence of the type 3 capsule was confirmed by the Quellung reaction with type 3 antiserum purchased from Statens Seruminstitut.
Quantitation of total teichoic acid.
Phenotypic variants were grown to mid-log phase and sonicated as previously described. The quantity of teichoic acid was determined by a capture enzyme-linked immunosorbent assay (ELISA) method. A rabbit polyclonal antibody to C polysaccharide (Statens Seruminstitut) at a dilution of 1:5,000 in 0.05 M Na2CO3 (pH 9.6) was fixed onto 96-well microtiter plates (Greiner Labortechnik, Frickenhausen, Germany). Between each incubation step, the plate was washed five times with Tris buffer (10 mM Tris, 150 mM NaCl, 0.05% Brij, and 0.02% sodium azide). Samples of supernatant and sonicated cells were diluted across the plate and incubated at room temperature for 2 h with shaking. Standards consisted of purified lipoteichoic acid at a known concentration (5). After an additional five washes with Tris buffer, a mouse monoclonal immunoglobulin M (IgM) antibody (HAS) to ChoP (Statens Seruminstitut) was added at a concentration determined in pilot experiments, followed by incubation for 2 h at room temperature with shaking. After another five washes in Tris buffer, an alkaline phosphatase-conjugated anti-mouse IgM was added at a dilution of 1:10,000 and incubated at room temperature for 2 h with shaking, and the A415 was determined as previously described (7). Total cellular protein determination was carried out on sonicated cells with Micro-bicinchoninic acid according to the manufacturer’s directions (Pierce Chemical, Rockford, Ill.). Each experiment was performed three times in duplicate, and data were expressed as mean values.
Quantitation of capsular polysaccharide of bacteria grown in supplemented medium.
Type 6B opaque and transparent variants were grown in the semisynthetic medium, C + Y medium, as described above, to A620 = 0.3. A 1:50 dilution of PBS-washed bacteria was used to inoculate a chemically defined medium, Cden, and the bacteria were allowed to grow at 37°C without shaking, to A620 = 0.4. In parallel, Cden was altered by replacing choline (35.8 μM) with structural analogs 2-(methylamino)ethanol, 2-dimethylaminoethanol, or ethanolamine, each at a concentration of 35.8 μM (18, 19). Cells were washed in PBS and sonicated as described above. A capture ELISA technique was used to determine quantities of capsular polysaccharide present in variants grown in the different media compared to medium containing choline for each experiment. Type-specific rabbit antiserum (Statens Seruminstitut) at a dilution of 1:5,000 in 0.05 M Na2CO3 (pH 9.6) was fixed overnight at room temperature onto microtiter plates. Purified type 6B capsular polysaccharide at a known concentration purchased from the American Type Culture Collection (Manassas, Va.) was used as a standard. Capsular polysaccharide in cell sonicate fractions was detected with a mouse IgM monoclonal antibody (MAb), HASP 4, against type 6A and 6B capsular polysaccharides (obtained from Statens Seruminstitut) at a concentration determined in pilot experiments. These experiments were performed in duplicate at least three times, and data were expressed as mean values per total cellular protein concentration.
Western blotting.
Bacteria were grown to A620 = 0.4 in C + Y medium, washed in PBS, and resuspended in the same volume of 0.02 M Tris (pH 7.2)–0.15 M NaCl–10 mM CaCl2. Pooled human serum from 10 healthy adult donors at a volume of 1/10 the original culture volume was used as a source of human CRP as well as for buffering and blocking of nonspecific binding. CRP was removed from serum in controls by preincubation with ChoP agarose beads as previously described (25). The absence of CRP was confirmed by loss of reactivity with a MAb against this protein in Western blots. Following incubation for 30 min at 37°C with agitation, the cells were washed twice in an equal volume of PBS. The pelleted cells were resuspended in gel loading buffer and heated to 100°C for 5 min before separation by sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis and Western blot analysis as previously described (25). Equal loading of bacteria was confirmed by Ponceau S staining of membranes. Bound CRP was detected on immunoblots with a MAb directed against human CRP followed by alkaline phosphatase-conjugated anti-mouse Ig.
Opsonophagocytosis activity.
Opsonophagocytic differences among opaque, intermediate, and transparent variants of types 6B, 9V, and 18C were determined by using a panel of five quality control serum samples from adults vaccinated with the 23-valent pneumococcal polysaccharide vaccine and a purified IgG preparation, Sandoglobulin (Sandoz Pharmaceuticals Co., East Hanover, N.J.). Opsonophagocytic titers were measured as the reciprocal of the serum dilution giving ≥50% killing by differentiated HL-60 granulocytes as previously described (12). The source of complement was baby rabbit serum (Pel-Freez, Brown Bear, Wis.). All assays were performed in duplicate. Geometric mean titers (GMTs) were calculated after a log2 transformation of opsonophagocytic titers. To assess the role of CRP in the opsonophagocytosis of 6B strains P382 (opaque) and P383 (transparent), purified human CRP was added to a prevaccination serum previously shown to have no opsonophagocytic activity against type 6B pneumococci.
Statistical analysis.
All opsonophagocytic titers were log2 transformed before comparisons were made between groups. Since differences were not normally distributed, significant differences were determined by the Mann-Whitney rank sum test with a level of significance at P < 0.05 and by paired t test where appropriate.
RESULTS
Comparison of phenotypic variants by immunoelectron microscopy.
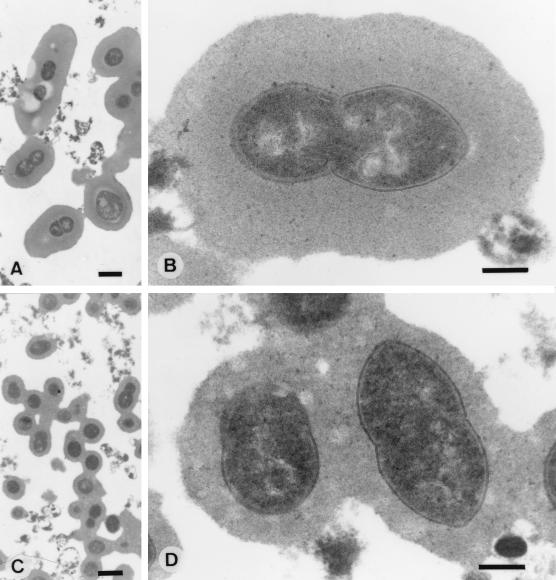
Differences between phenotypic variants of S. pneumoniae correlated with differences in the quantity of cell-associated capsular polysaccharide. The presence of higher amounts of capsular polysaccharide as previously determined by a capture ELISA was supported by examination of variants of the same isolate by immunoelectron microscopy with type-specific antisera for stabilization of the capsules (Fig. 1). Examination of a type 6B strain with the same ratio of anticapsular antibody to cells showed a larger zone of immunoreactive capsular polysaccharide surrounding the opaque variant (P382) than surrounding the related transparent variant (P383). C polysaccharide is not visualized by this procedure.
FIG. 1.
Immunoelectron microscopy of pneumococcal capsules showing an increased zone of capsular material in opaque (A and B) compared to transparent (C and D) variants of type 6B pneumococcal strain P324. Bar, 1 μm (A and C) and 0.3 μm (B and D).
Effect of capsular polysaccharide on the content of teichoic acid.
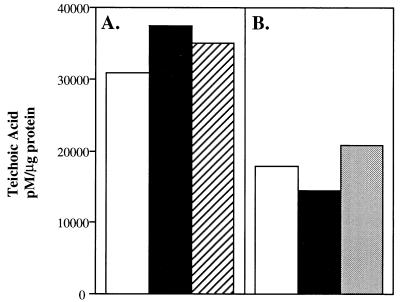
The previously documented inverse relationship in content of capsular polysaccharide and teichoic acid suggested that there could be a codependence in the expression of the two surface carbohydrate-containing structures. To define further this relationship, the amount of teichoic acid as measured by the content of ChoP was compared for mutants lacking capsule or expressing different capsular types. There were no significant differences in the quantity of cell-associated teichoic acid detected in a type 9V encapsulated parent strain, a spontaneous capsule-deficient mutant, and an encapsulated revertant of this strain (Fig. 2A). Similar results were shown with a type 2 encapsulated parent strain, an unencapsulated mutant, and a transformant expressing a type 3 capsule (Fig. 2B). These results demonstrated that the presence or type of capsular polysaccharide does not affect the amount of cell-associated teichoic acid.
FIG. 2.
The effect of encapsulation on the content of cellular teichoic acid. The capture ELISA technique using a MAb to ChoP was used to determine amounts of total cell-associated teichoic acid in mutants differing in encapsulation. (A) Amounts of teichoic acid in a type 9V strain, P11 (open bar); a spontaneously nonencapsulated mutant, P13 (solid bar); and a spontaneously encapsulated revertant, P105 (hatched bar), were compared. (B) Amounts of teichoic acid in a type 2 strain, D39 (open bar); a nonencapsulated mutant of D39, R6x (solid bar); and a transformant of R6x expressing a type 3 capsule, P156 (stippled bar), were compared. Values are the averages of two determinations and were calculated by comparison with standards consisting of purified lipoteichoic acid expressed as picomoles of teichoic acid per microgram of total cellular protein.
Effect of altered teichoic acid on the content of capsular polysaccharide.
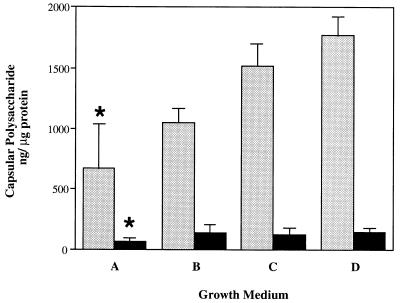
The possibility that differences in expression of the cell wall carbohydrate affect the amount of capsular polysaccharide was then addressed. No mutants lacking teichoic acid have been described. The composition of the cell wall carbohydrate, however, can be modified by replacing choline in the growth medium with structural analogs differing in the numbers of N-methyl groups (18). Opaque and transparent variants of a type 6B strain were grown in chemically defined media containing choline or equal concentrations of ethanolamine, 2-(methylamino)ethanol, or 2-dimethylaminoethanol in lieu of choline (19). The content of capsular polysaccharide in each growth condition was compared to that in choline-containing controls by the capture ELISA (Fig. 3). The absence of choline was confirmed by the loss of reactivity against ChoP in ELISAs with a MAb with specificity to this structure (data not shown). The more fully methylated the structural analog, the greater was the amount of cell-associated capsular polysaccharide expressed by the organism. These differences were statistically significant when growth in ethanolamine was compared to growth in choline (2.7-fold-more capsular polysaccharide for the opaque variant). This observation was seen for both the opaque and the transparent variants, though the amounts of capsular polysaccharide associated with the transparent organisms were about 12-fold less than those of the opaque organisms grown in the same medium. These findings suggested that structural differences in teichoic acid affect the content of capsular polysaccharide.
FIG. 3.
Relationship between teichoic acid structure and quantity of capsular polysaccharide. Opaque (stippled bars) and transparent (solid bars) variants of type 6B strain P324 were grown in chemically defined medium containing choline (D) or equal concentrations of structural analogs of choline including ethanolamine (A), 2-(methylamino)ethanol (B), and 2-dimethylaminoethanol (C). The capture ELISA technique using a type 6-specific MAb was used to determine amounts of cell-associated capsular polysaccharide. Values were calculated by comparison with standards consisting of purified type 6B capsular polysaccharide and are expressed as nanograms of capsular polysaccharide per microgram of total cellular protein ± standard deviation. The asterisks designate a significant difference (P < 0.05) from the control containing choline in the growth medium.
Relationship between phenotype and opsonophagocytic activity.
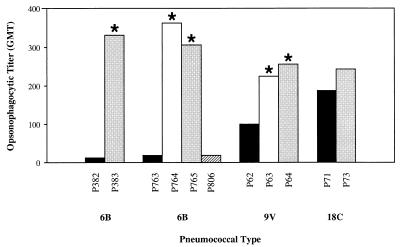
The effect of colony phenotype, content of capsular polysaccharide, and teichoic acid on opsonophagocytic killing was examined in a standardized assay which compared opsonophagocytic activity between opaque and transparent variants of strains for types 6B, 9V, and 18C (Table 2). There were statistically significant differences (P < 0.03) between the opaque and transparent variants for two isolates of type 6B and one isolate of type 9V. However, no significant differences in opsonophagocytic activity were observed between intermediate and transparent variants of the two types, 6B and 9V. A spontaneous transparent-to-opaque revertant (P806) was similar to the related opaque (P763) variant, confirming the relationship between opsonophagocytic activity and colony phenotype. Opaque variants which were associated with higher amounts of capsular polysaccharide and lower amounts of teichoic acid required higher opsonophagocytic titers of immune human serum than did transparent variants which were associated with less capsular polysaccharide and more teichoic acid. For types 6B and 9V, there was an association between the opsonophagocytic activity and the previously determined capsular polysaccharide content of each variant (Fig. 4) (7). There was, in contrast, no correlation between opsonophagocytic activity and previously determined teichoic acid content of each variant (Table 2). For example, no significant differences in opsonophagocytic GMTs were observed between type 18C variants which had similar quantities of capsular polysaccharide but different contents of teichoic acid. These findings emphasized the importance of the amount of capsular polysaccharide rather than teichoic acid or absolute antibody concentration in the capacity for opsonizing and phagocytizing pneumococci.
TABLE 2.
Opsonophagocytic activity, cell-associated capsular polysaccharide, and teichoic acid content of S. pneumoniae variants
| S. pneumoniae type | Strain | Colony phenotype | Opsonophagocytic activity (GMT) | Cell-associated capsular polysaccharide (ng/μg of protein) | Cell-associated teichoic acid (pmol/μg of protein) |
|---|---|---|---|---|---|
| 6B | P382 | Opaque | 11.3 | 233.4 | 19,500 |
| P383 | Transparent | 342.0 | 84.0 | 35,150 | |
| 6B | P763 | Opaque | 19.0 | 506.9 | NDa |
| P764 | Intermediate | 362.0 | 61.8 | ND | |
| P765 | Transparent | 304.0 | 23.2 | ND | |
| P806 | Opaque (revertant) | 18.0 | 361.0 | ND | |
| 9V | P62 | Opaque | 102.0 | 211.6 | 8,000 |
| P63 | Intermediate | 226.0 | 140.6 | 5,900 | |
| P64 | Transparent | 256.0 | 122.9 | 18,700 | |
| 18C | P71 | Opaque | 192.0 | 645.2 | 18,650 |
| P73 | Transparent | 242.0 | 576.5 | 38,680 |
ND, not determined.
FIG. 4.
Comparison of opsonophagocytic killing of S. pneumoniae phenotypic variants. Values are expressed as the GMT of quality control sera (n = 6) yielding 50% killing in an opsonophagocytic assay. Asterisks represent significant differences (P < 0.05) in opsonophagocytic titers between opaque and transparent or opaque and intermediate variants. Variants are opaque (solid bars), intermediate (open bars), transparent (stippled bars), and transparent-to-opaque revertant (hatched bar). Variants of the same isolate are grouped together, and the strain designation is indicated below. No intermediate phenotypes were isolated for type 6B strain P382 and for type 18C strain P71.
Relationship between colony phenotype and binding of CRP.
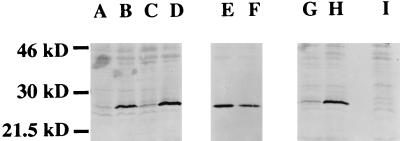
The relative binding of serum CRP to phenotypic variants was compared by incubating equivalent numbers of organisms in normal human serum. Opaque variant P376 showed minimal binding of CRP compared to a negative control with CRP-depleted serum (Fig. 5). Opaque variants of types 6A (P376) and 6B (P382) showed diminished binding of CRP in comparison to the transparent variants of the same isolates (P384 and P383, respectively). To distinguish whether these differences between phenotypes were due to differences in content of the teichoic acid ligand or capsular polysaccharide, opaque and transparent variants of an unencapsulated strain were compared. We have previously documented that unencapsulated strains can also display phenotypic variation (23). In the absence of capsular polysaccharide, the opaque variant (P125) bound as much or more CRP than its corresponding transparent variant (P126). Since the unencapsulated mutants differ in content of teichoic acid, this suggested that this cell surface component was not the determining factor in differential binding of CRP. The role of the capsular polysaccharide in binding of CRP was confirmed by showing that an encapsulated strain (D39) binds little CRP compared to the unencapsulated mutant (R6x) derived from the same strain. It was concluded that variation in amount of capsular polysaccharide, rather than amount of teichoic acid, is the major determinant of serum CRP binding to the pneumococcal cell surface. This is in agreement with the observation that C polysaccharide is not exposed on the surface of capsulated pneumococci (14).
FIG. 5.
Relationship between pneumococcal colony morphology and binding of purified human CRP. The amounts of bound CRP were compared in whole-cell lysates of equivalent numbers of bacteria by Western blot analysis with a MAb against human CRP. Opaque variants of type 6A (lane A) and 6B (lane C) isolates bound less CRP than did the transparent variants of the same isolate (lanes B and D, respectively). Opaque (P125) (lane E) and transparent (P126) (lane F) variants of an unencapsulated strain were compared to determine whether differences between opaque and transparent variants were present in the absence of the capsule. An encapsulated type 2 strain, D39 (lane G), was compared to a unencapsulated mutant of this strain, R6x (lane H), to confirm the role of capsular polysaccharide in CRP binding. Lane I shows control with a transparent type 6A variant, P384, with CRP-depleted serum.
The contribution of CRP to opsonophagocytic killing.
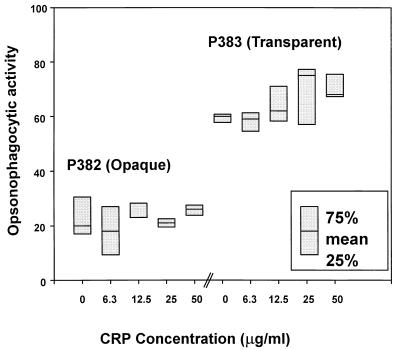
Based on these differences in binding of CRP to pneumococcal variants, the effect of CRP on opsonophagocytic activity was examined (Fig. 6). Phenotypic variants (type 6B) differed in their opsonophagocytic activity in the absence of exogenous CRP and a nonimmune serum (1:16 dilution). Addition of purified human CRP resulted in no enhancement of the opsonophagocytic activity in the opaque variant (P382). In the transparent variant (P383), however, there was a trend toward increased killing in the presence of CRP (>15.5 μg/ml).
FIG. 6.
Box plot showing the effect of human CRP on opsonophagocytic activity to type 6B variants P382 (opaque) and P383 (transparent). The percentage of killing with a 1:16 dilution of serum was determined in the absence of exogenous CRP and with purified human CRP added at the concentration indicated. The height of the box represents the range of values from the 25th to the 75th percentile in different samples. The serum opsonophagocytic titer with the opaque variant as target was <8 and with the transparent variant as target was 32. The serum anti-6B IgG antibody concentration was 0.2 μg/ml. The serum anti-C-polysaccharide antibody concentration was 1.5 μg/ml. No significant differences (P > 0.05) were obtained by the paired t test; however, there was a significant difference by the t test (P = 0.035) and a borderline significant difference by the paired t test (P = 0.078) in the transparent variant when 25 μg of CRP per ml was added to the nonimmune serum compared to the activity in nonimmune serum alone.
The possibility that the increased killing of the transparent variant may have been due to binding of anti-C-polysaccharide antibodies copurified with CRP with ChoP agarose was examined. However, the serum tested in these experiments contained a very low concentration of anti-C-polysaccharide antibodies (<1.5 μg/ml). In addition, preabsorption of type-specific antibodies with homologous 6B polysaccharide yielded a complete inhibition to an opsonophagocytic titer of 8 (the lowest level of detection in the assay). This indicated that anti-C-polysaccharide antibodies were not contributing to the enhanced opsonophagocytosis after addition of CRP. Similar experiments performed with sera containing 1.25 to 341 μg of anti-C-polysaccharide antibodies per ml also resulted in complete inhibition of the opsonophagocytic activity by preabsorption with homologous 6B polysaccharide. These results strengthen the role of type-specific antibodies in the opsonophagocytosis of the pneumococcus (12).
DISCUSSION
The focus of this study was to define the factors involved in the ability of opaque-phase variants to express increased quantities of capsular polysaccharide, decreased amounts of C polysaccharide, and enhanced virulence in invasive pneumococcal infection in comparison to the transparent phenotype (7). Different strains of S. pneumoniae have been reported to have considerable variation in the thickness of both C polysaccharide and capsular polysaccharide by immunoelectron microscopy (14). This technique was used in this study to confirm that variation in amount of capsular material occurs within an individual strain and is associated with colony opacity. A previous report comparing the electron microscopic appearances of opacity variants did not specifically visualize the capsule (24).
The possibility that variation in amounts of capsular polysaccharide was caused by differences in expression of the cell wall teichoic acid was addressed because of three lines of evidence indicating that expression of capsular polysaccharide and that of C polysaccharide may be linked. First, there is physical evidence that the capsular polysaccharide and cell wall both are covalently linked to the peptidoglycan and thereby indirectly to each other (15). Second, several of the 90 types of pneumococcal capsules contain unusual moieties such as ChoP also found in the teichoic acid, suggesting the possibility that the capsular material may have originated as a modified form of teichoic acid (20). Finally, there is the observation that for each of the isolates examined there is an inverse relationship between amounts of the two cell surface carbohydrates (12). In this study, the presence or type of capsular polysaccharide in the same genetic background had no effect on quantity of cell-associated teichoic acid as detected by the content of ChoP. It was not possible to experimentally manipulate the quantity of teichoic acid in a similar manner. However, altering the teichoic acid structure by replacement of choline was associated with as much as a 2.7-fold change in the amount of capsular polysaccharide in opaque pneumococci. This result provided evidence that expression of high levels of capsular polysaccharide is dependent on the native teichoic acid structure. Our data showing that qualitative differences in teichoic acid affect the content of capsular polysaccharide cannot be interpreted as evidence that quantitative differences in C polysaccharide have a similar effect. Our results do, however, suggest that the teichoic acid may be an important factor in the expression of capsular polysaccharide.
The primary mechanism of clearance of the pneumococcus is opsonophagocytosis. We took advantage of a recently described standardized opsonophagocytic assay to compare phenotypic variants and the relative contribution of cell surface carbohydrate structures (12). This assay utilizes HL-60 cells and provides more reproducible results necessary for the type of intrastrain comparisons carried out in this study than previously described methods. The effects of two serum factors, type-specific antibody in immune serum and purified human CRP, on opsonophagocytic killing were assessed. Our hypothesis was that the amount of capsular polysaccharide would affect opsonophagocytic activity mediated by type-specific antibody whereas the content of teichoic acid would determine sensitivity to CRP.
The opsonophagocytic activity of immune serum as measured by the average titer of serum necessary for 50% killing correlated with colony morphology and was 1.2- to 30-fold greater for opaque than for the related transparent isolate. This titer varied according to the quantity of capsular polysaccharide but not teichoic acid for variants of an individual strain. This result substantiated the role of encapsulation rather than teichoic acid in protection from phagocytosis and provided a plausible explanation for the greater virulence associated with the opaque phenotype (7). It is possible that the more virulent opaque phenotype requires higher concentrations of type-specific antibodies to be efficiently cleared from the host. This implies that the level of circulating antibody is not the only important factor in the clearance of pneumococcal infections. If the infecting strain is highly encapsulated, the minimum protective level of antibodies (to be established) may not be sufficient for clearance, leading to possible vaccine failures in an otherwise protected individual. Therefore, the level of expression of capsular polysaccharide could be considered a potential virulence marker. In vitro opsonophagocytic assays should use highly encapsulated strains, a factor that needs to be taken into consideration when selecting reference strains for a standardized opsonophagocytic assay.
The relative ability of pneumococci to bind to CRP was assessed by incubation of pneumococci in normal human serum. As expected, opaque variants bound less CRP than did the related transparent variants. The major determinant in binding of CRP in this assay, however, was not the amount of the ChoP ligand on the teichoic acid but the presence and amount of capsular polysaccharide. The larger capsule may inhibit the attachment of CRP to the ChoP anchor. CRP has been shown to enhance opsonophagocytic activity, although this effect could not be demonstrated for all pneumococcal types (4). In this study, the opsonophagocytic effect of CRP was shown only in the case of transparent pneumococci but required quantities of the protein found only during an inflammation response. CRP concentrations in infants likely to have a bacterial infection are ≥10 μg/ml; in adults, concentrations vary depending on the grade of the disease but generally are ≥50 μg/ml if there is a bacterial infection. Most normal individuals have circulating CRP concentrations under 3 μg/ml. This experiment required the use of baby rabbit serum as a source of complement since human complement alone was sufficient to kill the transparent but not the opaque variant of the type 6B isolate tested. Since bound CRP is reported to act through the activation of C1q, the source of complement may be important in determining the full contribution of CRP (21). Nonetheless, the data suggest that CRP may be a significant factor in opsonization of transparent variants and may explain, at least in part, the reduced virulence of this phenotype.
This study indicated that modifications in the teichoic acid structure altered the amount of cell-associated capsular polysaccharide, establishing a link in the expression of these two surface components. Transparent pneumococci bound higher amounts of CRP, and a trend toward increasing opsonophagocytosis was observed in this phenotype in the absence of type-specific antibodies. However, the ability of pneumococci to evade opsonophagocytosis was associated primarily with the amount of capsular polysaccharide present, rather than the amount of teichoic acid or the ability to bind CRP.
ACKNOWLEDGMENTS
J. O. Kim and S. Romero-Steiner contributed equally to this work.
Purified lipoteichoic acid was generously provided by Werner Fischer (University of Erlangen, Erlangen, Germany).
J.O.K. was supported by a training grant from the Public Health Service (AI07278-13). This work was supported by grants from the Lucille P. Markey Charitable Trust and the Public Health Service (AI38446) (J.N.W.).
REFERENCES
- 1.Avery O T, MacLeod C M, McCarty M. Studies on the nature of the chemical nature of the substance inducing transformation of pneumococcal types. J Exp Med. 1944;79:137–157. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cundell D R, Gerard N P, Gerard C, Idanpaan-Heikkila I, Tuomanen E I. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 3.Cundell D R, Weiser J N, Shen J, Young A, Tuomanen E I. Relationship between colonial morphology and adherence of Streptococcus pneumoniae. Infect Immun. 1995;63:757–761. doi: 10.1128/iai.63.3.757-761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Beaufort A J, Langermans J A M, Matze-Van der Lans A M, Hiemstra P S, Vossen J M, Van Furth R. Difference in binding of killed and live Streptococcus pneumoniae serotypes by C-reactive protein. Scand J Immunol. 1997;46:597–600. doi: 10.1046/j.1365-3083.1997.d01-171.x. [DOI] [PubMed] [Google Scholar]
- 5.Fischer W, Behr T, Hartmann R, Peter K C J, Egge H. Teichoic acid and lipoteichoic acid of Streptococcus pneumoniae possess identical chain structures. A reinvestigation of teichoid acid (C polysaccharide) Eur J Biochem. 1993;215:851–857. doi: 10.1111/j.1432-1033.1993.tb18102.x. [DOI] [PubMed] [Google Scholar]
- 6.Horowitz J, Volanakis J E, Briles D E. Blood clearance of Streptococcus pneumoniae by C-reactive protein. J Immunol. 1987;138:2598–2603. [PubMed] [Google Scholar]
- 7.Kim J, Weiser J. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J Infect Dis. 1998;177:368–377. doi: 10.1086/514205. [DOI] [PubMed] [Google Scholar]
- 8.Lacks S, Hotchkiss R D. A study of the genetic material determining an enzyme activity in pneumococcus. Biochim Biophys Acta. 1960;39:508–517. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- 9.MacLeod C M, Krauss M R. Relation of virulence of pneumococcal strains for mice to the quantity of capsular polysaccharide formed in vitro. J Exp Med. 1950;92:1–9. doi: 10.1084/jem.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mold C, Nakayama S, Holzer T, Gewurz H, Du Clos T. C-reactive protein is protective against Streptococcus pneumoniae infection in mice. J Exp Med. 1981;154:1703–1708. doi: 10.1084/jem.154.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama S, Mold C, Gewurz H, du Clos T. Opsonic properties of C-reactive protein in vivo. J Immunol. 1982;128:2435–2438. [PubMed] [Google Scholar]
- 12.Romero-Steiner S, Libutti D, Pais L B, Dykes J, Anderson P, Whitin J C, Keyserling H L, Carlone G M. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol. 1997;4:415–422. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenow C, Ryan P, Weiser J N, Johnson S, Fontan P, Ortqvist A, Masure H R. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol Microbiol. 1997;25:819–829. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 14.Sorensen U B S, Blom J, Birch-Andersen A, Henrichsen J. Ultrastructural localization of capsules, cell wall polysaccharide, cell wall proteins, and F antigen in pneumococci. Infect Immun. 1988;56:1890–1896. doi: 10.1128/iai.56.8.1890-1896.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorenson U B S, Henrichsen J, Chen H-C, Szu S C. Covalent linkage between the capsular polysaccharide and cell wall peptidoglycan of Streptococcus pneumoniae revealed by immunochemical methods. Microb Pathog. 1990;8:325–334. doi: 10.1016/0882-4010(90)90091-4. [DOI] [PubMed] [Google Scholar]
- 16.Szalai A J, Briles D E, Volanakis J E. Role of complement in C-reactive-protein-mediated protection of mice from Streptococcus pneumoniae. Infect Immun. 1996;64:4850–4853. doi: 10.1128/iai.64.11.4850-4853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiraby J G, Fox M S. Marker discrimination in transformation and mutation of pneumococcus. Proc Natl Acad Sci USA. 1973;70:3541–3545. doi: 10.1073/pnas.70.12.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci USA. 1968;59:86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomasz A. A chemically defined medium for Streptococcus pneumoniae. Bacteriol Proc. 1964;64:29. [Google Scholar]
- 20.van Dam J E G, Fleer A, Snippe H. Immunogenicity and immunochemistry of Streptococcus pneumoniae capsular polysaccharides. Antonie Leeuwenhoek. 1990;58:1–47. doi: 10.1007/BF02388078. [DOI] [PubMed] [Google Scholar]
- 21.Volanakis J E, Kaplan M H. Interaction of C-reactive protein complexes with the complement system. II. Consumption of guinea pig complement by CRP complexes. Requirement for human C1q. J Immunol. 1974;113:9–17. [PubMed] [Google Scholar]
- 22.Volanakis J E, Kaplan M H. Specificity of C-reactive protein for choline phosphate residues of pneumococcal C-polysaccharide. Proc Soc Exp Biol Med. 1971;136:612–614. doi: 10.3181/00379727-136-35323. [DOI] [PubMed] [Google Scholar]
- 23.Weiser J N, Markiewicz Z, Tuomanen E I, Wani J H. Relationship between phase variation in colony morphology, intrastrain variation in cell wall physiology, and nasopharyngeal colonization by Streptococcus pneumoniae. Infect Immun. 1996;64:2240–2245. doi: 10.1128/iai.64.6.2240-2245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiser J N, Austrian R, Sreenivasan P K, Masure H R. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect Immun. 1994;62:2582–2589. doi: 10.1128/iai.62.6.2582-2589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiser J N, Pan N, McGowan K L, Musher D, Martin A, Richards J C. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J Exp Med. 1998;187:631–640. doi: 10.1084/jem.187.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yother J, White J M. Novel surface attachment mechanism of the Streptococcus pneumoniae protein PspA. J Bacteriol. 1994;176:2976–2985. doi: 10.1128/jb.176.10.2976-2985.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]