Abstract
Phagocytosis of Plasmodium falciparum sexual stages in vitro and within the mosquito midgut was assayed in order to assess its role in transmission-blocking immunity to malaria. Both monocytes/macrophages (MM) and polymorphonuclear neutrophils (PMN) phagocytosed malarial gametes in vitro, but levels of phagocytosis were low. Intraerythrocytic gametocytes were not susceptible to phagocytosis. In vitro phagocytosis was positively correlated with levels of antibodies against the gamete surface proteins Pfs230 and Pfs48/45. Immunoglobulin G (IgG) subclass analysis revealed that phagocytosis was correlated with levels of antigamete IgG1. In vivo membrane-feeding experiments were performed in the presence of both pooled and individual malaria immune sera. The phagocytic process proceeded less efficiently in vivo than in vitro, which may be related to the lower ambient temperature (26°C, compared with 37°C). Finally, although we found a correlation between the ability of a serum to promote phagocytosis in vitro and the presence of antibodies against transmission-blocking target antigens, we were unable to demonstrate a role for MM- or PMN-mediated phagocytosis in reduction of infectivity of the malarial parasite to mosquitoes.
Transmission of malaria from one vertebrate host to another is absolutely dependent upon the Anopheles mosquito vector, in which the sexual phase of the life cycle occurs. Interruption of the mosquito phase of the life cycle—by vector control or vaccination—is thus a potentially powerful means of malaria control. Ingestion of intraerythrocytic gametocytes by the mosquito triggers gametogenesis, whereupon extracellular gametes are exposed to the other components of the blood meal, including leucocytes, antibodies, and complement. Antibodies directed to surface antigens of gametes have been shown to mediate agglutination (1) and complement-mediated lysis (10, 12) and to suppress the infectivity of gametocytes to mosquitoes (24). Thus, immunization with gamete- or gametocyte-specific antigens has the potential to induce transmission-blocking immunity, and such antigens may form a useful component of a malaria vaccine (25).
One potential mechanism of transmission-blocking immunity that has received relatively little attention is the role of phagocytosis of gametes within the mosquito midgut by leucocytes present in the blood meal. In vitro, intraerythrocytic schizonts and free merozoites of Plasmodium falciparum are phagocytosed by polymorphonuclear neutrophils (PMN) (30) and monocytes/macrophages (MM) (8). Schizont-infected erythrocytes are more efficiently phagocytosed than those infected with immature ring stages (30a), presumably due to differential expression of parasite-derived antigens on the erythrocyte surface. In contrast, little phagocytosis of gametocyte-infected erythrocytes occurs (28).
Recent attempts to correlate gamete phagocytosis with transmission-blocking activity (18, 19) have been somewhat inconclusive. In vitro studies suggest that (i) antigamete antibodies enhance activation of neutrophils by gametes and (ii) leucocytes enhance the transmission reduction potential of some immune sera (IS), but these two effects are not correlated in individual sera (19). In vivo studies suggest that infectivity of gametocytes from semi-immune carriers was independent of the presence of leucocytes (18).
In this study, we have attempted to quantify the extent of gametocyte and gamete phagocytosis in comparison to phagocytosis of asexual parasites and to relate this to the presence of antigamete antibodies. We also compared the phagocytic potential of PMN and MM and investigated the role of these two cell types in suppressing gamete infectivity to mosquitoes in membrane-feeding experiments.
MATERIALS AND METHODS
Parasites.
Gametocytes of P. falciparum clone 3D7 were grown in culture with fresh O+ erythrocytes, as described previously (4), in sterile medium composed of RPMI 1640 (Gibco, Paisley, Scotland), 10% heat-inactivated, non-malaria-exposed, O+ serum (Scottish Blood Transfusion Service), 25 mmol of HEPES buffer per liter, 0.4 mol of hypoxanthine per liter, and 5% NaHCO3 (all Sigma, Poole, United Kingdom).
After 14 to 17 days, gametocytes were harvested, and gametogenesis was stimulated by incubation for 1 h at room temperature in complete medium (pH 8.7), containing mosquito pupae extract (22).
Parasite separation was performed with a discontinuous Nycodenz (Nycomed AS, Oslo, Norway) gradient in medium 199 (Gibco). Gametes were harvested from the interface at between 6 and 11% Nycodenz, and gametocytes (stages II to IV) were harvested at between 11 and 16% Nycodenz (4). After being washed twice in RPMI, parasites were counted and used immediately. Schizonts were enriched from asexual synchronized cultures (17) on a 60% Percoll (Pharmacia, Uppsala, Sweden) gradient and treated as described above.
Sera.
Serum was collected, following the annual malaria transmission season, from 22 adults living in the village of Brefet, The Gambia (10). Malaria is seasonally endemic in this region (9). Aliquots of these sera were pooled, and a fraction was immunoglobulin G (IgG) depleted by affinity chromatography by using protein G-Sepharose Fast Flow (Sigma). Control sera were obtained from nonexposed European donors.
Preparation of monocytes and neutrophils.
Peripheral blood mononuclear cells (PBMC) were isolated from freshly obtained heparinized venous blood from healthy European donors and isolated by single-step Ficoll-Hypaque (Nycomed) density gradient centrifugation (1.077 ± 0.001-g/ml density [280 ± 15 mOsmol]). Cells were washed three times in RPMI and counted with a hemocytometer, and monocytes were left to adhere at 37°C to glass coverslips in 24-well plates. After 90 min, nonadherent cells were washed away with two washes of RPMI, and the plates were stored at 6°C until further use on the same day. About 30% of the cells added to the wells could be recovered through adherence to the coverslips; these were >90% pure for monocytes and contained less than 10% lymphocytes, erythrocytes, and platelets, as determined by microscopy after Giemsa staining.
Serum was added in a ratio of 1:1 (vol/vol) to the cell pellet obtained from the density centrifugation (above) and polymorphonuclear cells enriched by Ficoll-Hypaque centrifugation (Nycomed; 1.113 ± 0.001-g/ml density [460 ± 5 mOsmol]). Harvested cells were washed three times and counted as described above. The purity of the neutrophils was >98%, with less than 1% mononuclear cells.
Monocytes and neutrophils were derived from the same A+ donor in all in vitro experiments. For in vivo experiments, a healthy O+ European volunteered. Viability of the different cell populations was routinely determined by staining with trypan blue after blood cell separation and after incubation in in vitro experiments. Cells were 92 to 97% viable.
In vitro phagocytosis assays.
Optimal incubation time, concentration in serum, cell numbers, and parasite numbers were determined in preliminary experiments. A total of 4 × 105 purified schizonts, gametocytes, gametes, or uninfected erythrocytes were added either to the prepared 105 monocytes on coverslips or to 105 neutrophils in flat-bottom 96-well plates and incubated for 2 h at 37°C in a total volume of 250/μl (monocytes) or 50/μl (neutrophils), in the presence of 16% IS or nonimmune serum (NS). This assay was also carried out with IgG-depleted, pooled IS and RPMI as controls. Experiments were carried out in triplicate. Two hundred leucocytes were counted per sample (MM, Giemsa stained on coverslips; PMN, cytospin onto slides and Giemsa stained), and phagocytosis was expressed as the percentage of leucocytes containing phagocytosed target cells. Only phagocytes with recognizable parasites were considered positive to avoid counts of uptake of cell debris, pigment, or parasites with damaged cell membranes; the 2-h incubation time was chosen because this time gave maximal detection of phagocytosed whole parasites.
Phagocytosis under mosquito midgut conditions.
A total of 4 × 105 mature gametocytes were added to a mixture of 1 × 105 neutrophils and 1 × 105 adherent monocytes. These were incubated in 24-well plates at either 37 or 18°C for 2 h in the presence of 16% pooled IS or NS. Following incubation, coverslips (containing adherent monocytes) were removed for staining, and supernatants were taken for the cytospin onto slides for neutrophil counts. The experiments were carried out in duplicate.
Detection of gamete-specific antibodies.
Immunoprecipitation is the method of choice for detection of specific antibodies (4). Immunoprecipitation of surface-iodinated gamete proteins and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were performed as previously described (10). Approximately 107 125I-surface-labelled gametes were extracted in 50 μl of NETTI buffer (0.15 M NaCl, 5 mM EDTA, 50 mM Tris [pH 7.4], 0.5% Triton X-100, 0.05% NaNH3 and the following protease inhibitors [Sigma]: 1 mM phenylmethylsulfonyl fluoride, 0.1 μM pepstatin, 0.1 mM tolylsulfonyl phenylalanyl chloromethyl lectone, 1 mM EGTA, and 1 MM N-ethylmaleimide). The aqueous fraction was removed for immunoprecipitation with malaria IS or monoclonal antibodies (MAbs) (as positive control) and protein-G Sepharose (Pharmacia). The presence of gamete surface protein Pfs230 in these fractions was confirmed by immunoprecipitation with specific MAbs and SDS-PAGE before use. Quantitation of immunoprecipitated Pfs230 and Pfs48/45 was calculated by visual analysis of autoradiographs and scoring of the bands from 0 to 3 based on intensity.
ELISA for determination of IgG subclass of gamete-reactive antibodies in malaria IS.
Purified gametes were extracted in 500 μl of Triton X-114 (1% in 10 mM Tris-Cl [pH 7.4]). The aqueous fraction was diluted in phosphate-buffered saline (PBS) to give the equivalent of 106 gametes per ml, and 100 μl was adsorbed to each well of 96-well Immulon-4 microtiter plates (Dynex, Billinghurst, United Kingdom) overnight at 4°C. Between incubations, plates were washed three times in PBS containing 0.05% Tween (PBS-T). Plates were blocked for 4 h at room temperature with 2% skim milk in PBS. Sera were added to duplicate wells overnight at 4°C at a dilution of 1/200 in 1% skim milk—PBS-T, before washing and incubation of plates for 3 h at room temperature with horseradish peroxidase-conjugated rabbit anti-human IgG (Dako, Glostrup, Denmark) or sheep α-human IgG subclasses 1 to 4 (The Binding Site, Birmingham, United Kingdom). Plates were developed with H2O2 as a substrate and o-phenylenediamine (Sigma) as a chromogen. A492 was measured with a Titertek MultiScan (ICN Flow; Thame, Oxon, United Kingdom) and ElisaLite 30 software (Dynex, Billingshurst, United Kingdom). Specific absorbance values were calculated as [optical density (OD) for IS] − [mean OD + 2 standard deviations of the 12 control serum samples].
Infectivity experiments.
Five- to 6-day-old, non-blood-fed, adult female Anopheles stephensi mosquitoes were used for the infectivity experiments.
(i) Parasite phagocytosis within the mosquito.
Suspensions of gametocyte-infected erythrocytes were fed to mosquitoes via water-jacketed membrane feeders as described previously (23). Blood meals consisted of 16% packed gametocyte-infected erythrocytes, 20% packed O+ uninfected blood, 16% heat-inactivated pooled human serum (IS or NS), and 48% O+ heat-inactivated European NS. The final hematocrit of the feed was approximately 35%, and gametocytemia ranged from 1.5% to 2.0%. Blood meals were routinely examined for exflagellation under a light microscope, and the ratio of mature gametocytes to phagocytes and erythrocytes was determined. At the time of infection and at 0.5, 1, 2, 3, 4, and 5 h postinfection, mosquitoes were killed, and their midguts were smeared onto slides for Giemsa staining and microscopic examination. Staining with trypan blue showed viability of >82% for monocytes and >98% for neutrophils. A total of 200 phagocytes per gut in each group of 12 midguts were counted.
(ii) Infectivity assays in the presence of IS, leucocytes, and complement.
As a source of complement, serum was obtained from a healthy O+ European donor and tested for complement activity before use, by using freshly obtained sheep erythrocytes (Scottish Antibody Production Unit) and goat anti-sheep erythrocyte IgG (Sigma). An aliquot of the human serum was heat inactivated at 56°C for 30 min and tested for lack of complement activity. Freshly drawn O+ blood was also used as a source of leucocytes. A fraction of this blood was depleted of PMBC and neutrophils by centrifugation (as described above for the isolation of phagocytic cell populations).
Mosquitoes were allowed to feed on blood meals (in which complement and/or leucocytes had been depleted) containing either pooled or individual sera (in the same proportions described above), and nonengorged mosquitoes were removed. Mosquitoes were maintained at 26°C on a diet of glucose plus p-ominobenzoic acid for 9 to 10 days, after which they were dissected and the oocysts were counted. Twenty midguts were counted per serum tested.
The intensity of mosquito infection was determined for each serum by calculation of the geometric mean (GM) number of oocysts per gut.
Infectivity in the presence of each serum relative to its pair-matched control, was calculated by the following formula: Relative infectivity = GM for IS/GM for control serum.
Statistical analyses.
The t test was used to compare in vitro phagocytosis levels of different parasite stages, mediated by individual sera, by different cell populations and to compare the in vitro phagocytoses of gametes at different temperatures. Spearman’s rank test of correlation was used in the other analyses. Differences were considered significant where P < 0.05.
RESULTS
Phagocytosis of asexual and sexual stage parasites.
We compared the efficiency of phagocytosis of asexual parasites (schizont-infected erythrocytes) and sexual parasites (intraerythrocytic gametocytes and extracellular gametes) by either PMN or MM in the presence or absence of pooled human malaria IS.
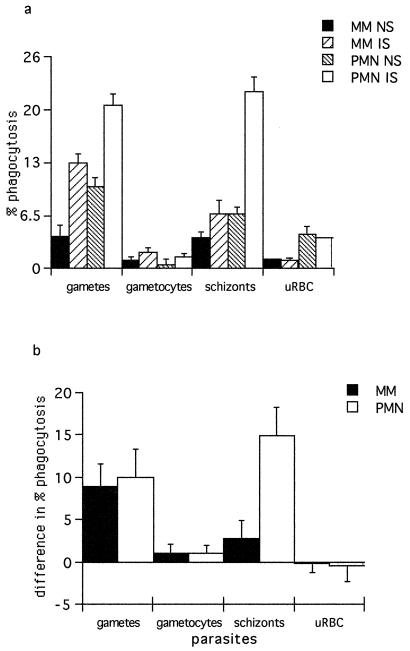
Background levels of phagocytosis of uninfected erythrocytes (uRBC) by MM were minimal and were not affected by the presence of IS. Phagocytosis of uRBC by PMN was marginally higher, but again there was no difference between IS and NS. Phagocytosis of intracellular gametocytes was also low and only slightly enhanced by IS. No differences were observed between rates of phagocytosis of gametocytes and uRBC or between PMN and MM (Fig. 1). In contrast, phagocytosis of schizont-infected erythrocytes and extracellular gametes was enhanced by IS (Fig. 1). Phagocytosis of schizonts by PMN was higher than that by MM, especially in the presence of IS. Levels of PMN phagocytosis of schizonts and gametes were not different, but in the presence of IS, MM phagocytosed more gametes than schizonts (Fig. 1b). Interestingly, intraerythrocytic schizonts were much more vulnerable to phagocytosis than were intraerythrocytic gametocytes. The phagocytosis-enhancing effect of IS was shown to be due to antibody, because IS which had been depleted of IgG by incubation with protein G-Sepharose did not enhance phagocytosis (data not shown).
FIG. 1.
(a) Percentage of phagocytosis of P. falciparum in vitro in the presence of pooled IS or NS by monocytes or neutrophils. The data represent the arithmetic mean of triplicates. Error bars show ±1 standard error. (b) Difference in percentage of phagocytosis (mean values for IS − mean values for NS). Error bars show 95% confidence intervals for the size of the difference.
Effect of individual human IS on parasite phagocytosis.
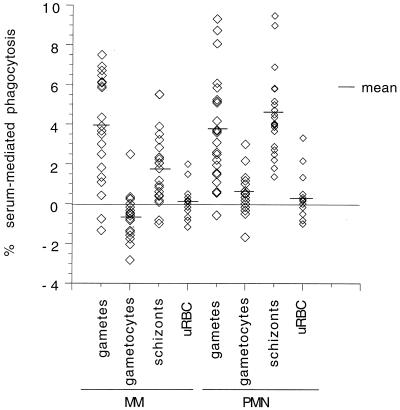
As shown above, serum pooled from a number of different immune donors enhanced phagocytosis of both schizonts and gametes. To determine whether all IS mediated phagocytosis, individual sera were tested in in vitro phagocytosis assays (Fig. 2). There was considerable variation from serum to serum in their ability to mediate phagocytosis, but in all cases, absolute levels of phagocytosis were rather low.
FIG. 2.
Percentage of phagocytosis of different P. falciparum stages by MM and PMN in the presence of individual malaria IS. The data represent the arithmetic mean of triplicates minus mean values for phagocytosis in the presence of nonexposed European sera.
Phagocytosis rates for gametocytes and schizonts were consistently higher in PMN than in MM (paired t test, t = 3.83, df = 21, P = 0.001 for gametocytes; t = 6.23, df = 21, P = <0.0001 for schizonts), but there was no significant difference between the two cell types for gamete phagocytosis (t = 0.28, df = 21, P = 0.78). However, phagocytosis rates for MM were significantly correlated with phagocytosis rates for PMN (correlation = 0.48, n = 22, P = 0.01).
As with the results obtained with pooled IS, mean phagocytosis values for gametes and schizonts were higher than for gametocytes in both cell types (Fig. 2). In MM, phagocytosis rates were significantly higher for gametes than for schizonts (paired t test, t = 2.67, df = 21, P = 0.02). In PMN, no differences were found in the rates of phagocytosis of gametes and schizonts (t = 0.73, df = 21, P = 0.48).
When we looked at the correlation between phagocytosis values for gametes and schizonts within individual sera, we found a marginally significant correlation for PMN (correlation = 0.37, n = 22, P = 0.09). No correlation was found for MM (correlation = 0.25, n = 22, P = 0.32).
Association between phagocytosis-enhancing ability of serum and the presence of antigamete antibodies.
Gamete-specific antibodies were measured by immunoprecipitation and ELISA and compared with in vitro phagocytosis (Table 1). All correlation analyses were performed by using Spearman’s rank test.
TABLE 1.
Characteristics of the malaria-IS used in this studya
| Serum | % Phagocytosis
|
Immunoprecipitation score
|
ELISA OD
|
Relative infectivity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MM | PMN | Pfs230 | Pfs48/45 | IgG | IgG1 | IgG2 | IgG3 | IgG4 | ||
| 1 | 7.5 | 6.08 | 3 | 3 | 1.525 | 0.691 | 0.467 | 0.298 | 0.032 | NDb |
| 2 | 3 | 8.75 | 3 | 3 | 1.485 | 0.818 | 0.253 | 1.400 | 0.952 | 0.31 |
| 3 | 3.67 | 1.95 | 2 | 3 | 1.102 | 0.203 | 0.084 | 0.777 | 0.240 | 0.21 |
| 4 | 3.5 | 8.08 | 3 | 2 | 1.498 | 0.317 | 0.169 | 0.924 | 0.693 | 2.5 |
| 5 | 6.46 | 3.58 | 2 | 2 | 1.235 | 0.214 | 0.114 | 0.343 | 0.001 | 1.62 |
| 6 | 4.34 | 9.33 | 2 | 2 | 1.563 | 0.177 | 0.033 | 1.010 | 0.419 | 0.81 |
| 7 | 6.71 | 5.25 | 2 | 1 | 0.780 | 0.076 | 0.077 | 0.578 | 0.015 | 2.5 |
| 8 | 0.42 | 1.5 | 1 | 1 | ND | ND | ND | ND | ND | ND |
| 9 | 6.09 | 4.17 | 1 | 1 | 1.459 | 0.447 | 0.109 | 0.197 | −0.010 | ND |
| 10 | 6.13 | 5.58 | 1 | 1 | 1.599 | 0.675 | 0.270 | 0.609 | 0.026 | 0.5 |
| 11 | 5.84 | 2.59 | 3 | 0 | 1.495 | 0.520 | 0.260 | 1.030 | 0.815 | 1.38 |
| 12 | 0.42 | 1.09 | 1 | 0 | 1.432 | 0.187 | 0.611 | 0.964 | 0.390 | 0.14 |
| 13 | 1.34 | 0.61 | 1 | 0 | 1.668 | 0.551 | 0.219 | 1.151 | 0.941 | 0.9 |
| 14 | 6.71 | 3.66 | 0 | 2 | 1.556 | 0.454 | 0.131 | 1.158 | 0.390 | 0.44 |
| 15 | 7.5 | 5.16 | 0 | 2 | 0.784 | 0.042 | 0.028 | 0.510 | −0.016 | 2 |
| 16 | −1.34 | 0.55 | 0 | 2 | 0.556 | −0.005 | −0.031 | 0.111 | −0.011 | 1.9 |
| 17 | 5.92 | 5.09 | 0 | 1 | 1.396 | 0.279 | 0.338 | 0.944 | 0.139 | 0.13 |
| 18 | 2.5 | 2.22 | 0 | 2 | 1.039 | 0.128 | 0.106 | 0.583 | 0.178 | 0.57 |
| 19 | −0.75 | 3.09 | 0 | 1 | 0.684 | 0.169 | −0.016 | 0.263 | 0.061 | 0.83 |
| 20 | 6.92 | 2.5 | 0 | 1 | 0.974 | 0.067 | 0.062 | 0.658 | 0.376 | 0.31 |
| 21 | 1.09 | 1.56 | 0 | 1 | 0.207 | 0.057 | 0.051 | 0.019 | −0.015 | 0.04 |
| 22 | 1.83 | −0.58 | 0 | 0 | 0.344 | 0.015 | −0.062 | 0.067 | −0.022 | ND |
Data are for individual malaria IS and represent the percentage of phagocytosis of P. falciparum gametes, immunoprecipitation scores against gametocyte and gamete surface proteins Pfs230 and Pfs48/45, absorbance values (A492) for total IgG and IgG subclasses against gamete surface proteins, and infectivity values for oocyst burdens in mosquitoes fed with serum plus leucocytes.
ND, not determined.
A positive correlation was found between gamete phagocytosis by neutrophils and the level of IgG antibodies to gamete surface proteins (rs = 0.48, n = 21, P = 0.03). This correlation was found for specific antibodies against Pfs230 (rs = 0.49, n = 22, P = 0.02) and Pfs48/45 (rs = 0.52, n = 22, P = 0.02), but not for antibodies to the Pfs230 precursor protein, Pfs260 (rs = 0.32, n = 22, P = 0.13). When IgG subclass responses to gamete proteins were compared with phagocytosis, it was found that the level of IgG1 antibodies against a gamete surface protein extract correlated with phagocytosis by PMN (r = 0.47, n = 21, P = 0.03), but not with phagocytosis by MM (r = 0.24, n = 21, P = 0.28). Levels of the other IgG subclasses, 2, 3, and 4, did not correlate significantly with phagocytosis in either MM or PMN (rs < 0.3, n = 21, P > 0.18 in all cases).
Parasite phagocytosis within the mosquito.
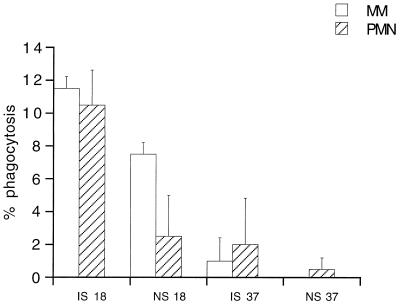
Phagocytes from vertebrates function optimally at body temperature (37°C in humans), but the temperature within the mosquito gut will reflect the ambient temperature of the environment, which will tend to be suboptimal for phagocytic cells. However, reducing the temperature of the blood meal from 37°C to 26°C or below also serves to induce gametogenesis and allows formation of extracellular gametes, which are susceptible to phagocytosis. To determine whether phagocytosis is affected by environmental temperature, phagocytosis rates of intracellular gametocytes were compared in in vitro assays conducted at 18 and 37°C. As expected, gametogenesis was enhanced at 18°C compared to 37°C (data not shown), and this was accompanied by enhanced rates of phagocytosis (Fig. 3).
FIG. 3.
Percentage of in vitro phagocytosis of P. falciparum gametes by monocytes (MM) and neutrophils (PMN) in the presence of pooled malaria IS or NS at different temperatures (°C).
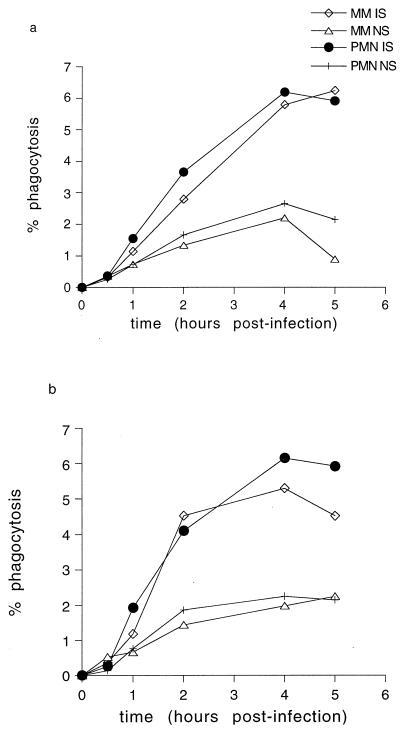
However, there are numerous other environmental differences between our in vitro system and the conditions inside the mosquito midgut (e.g., pH, cation concentrations, presence of digestive enzymes, etc). We therefore conducted in vivo phagocytosis assays in which mosquitoes were allowed to feed on infectious blood meals containing MM and PMN and maintained at 26°C. After time intervals ranging from 0 to 5 h, the mosquitoes were killed and dissected, and the contents of their midguts were smeared onto slides and examined. Phagocytosis rates in vivo were much lower than those seen in vitro; after 2 h of incubation, the phagocytosis rate was 4% for PMN in vivo, compared with 9.3% in vitro, and for MM, the rates were 2.7% in vivo and 7.5% in vitro. At the 4-h time point (peak phagocytosis rate), the phagocytosis rates were higher in the presence of IS than with NS (Fig. 4) (t = 8.096, n = 22, P = 0.015). Phagocytosis rates by MM and PMN were similar whether cells were present together in the same blood meal (Fig. 4a) or in discrete populations (Fig. 4b). No phagocytosis was seen until 1 to 2 h after feeding; this correlated with the time at which mature, extracellular gametes were seen in the blood meal (data not shown), indicating that the process of gametogenesis may be the rate-limiting step in the onset of phagocytosis.
FIG. 4.
Time course of in vivo phagocytosis of P. falciparum gametes in mosquito midguts (at 26°C). (a) Monocytes and neutrophils present in combination in mosquito blood meals. (b) Monocytes or neutrophils as discrete populations in mosquito blood meals.
Effects of leucocytes on transmission.
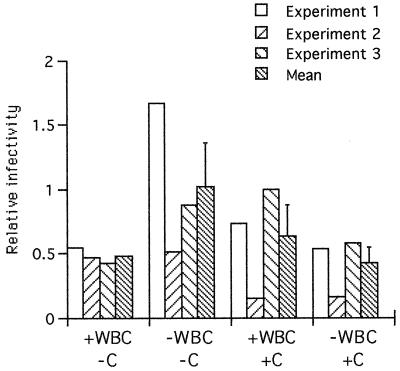
We tested whether the presence of phagocytic cells in the blood meal affected the infectivity of gametocytes to mosquitoes. Gametocytemic blood was fed to mosquitoes in the presence or absence of pooled IS, complement, MM, or PMN. Engorged mosquitoes were dissected after 10 days, the number of oocysts on the midgut wall was counted, and the relative infectivity was calculated (Fig. 5). When no leucocytes were present, the presence of complement tended to suppress infectivity, and where no complement was present in the blood meal, the presence of leucocytes tended to suppress infectivity, but no significant differences were detected in the numbers of oocysts developing in the different groups of mosquitoes. Neither was there any additive suppressive effect of complement plus leucocytes.
FIG. 5.
Relative infectivity of P. falciparum gametocytes to mosquitoes in the presence (+) or absence (−) of leucocytes (WBC) and complement (c). The data represent three independent experiments and the mean infectivity for each group. Infectivity values were derived from calculations of the GM numbers of oocysts in mosquitoes given pooled malaria IS divided by the GM for mosquitoes given nonexposed European serum.
Finally, gametocytes were fed to mosquitoes in the presence of individual malaria IS, leucocytes, and complement. Relative infectivity values are shown in Table 1. These ranged from 0.04 (transmission blocking) to 2.5 (transmission enhancement), but there was no correlation between levels of phagocytosis and infectivity for individual sera (rs = 0.17, n = 18, P = 0.48 for MM; rs = 0.23, n = 18, P = 0.35 for PMN).
DISCUSSION
Monocyte- and neutrophil-mediated killing of schizont-infected erythrocytes and merozoites has been proposed as a potentially important mechanism of protective immunity to malaria (5, 13, 14, 31), but the extent to which this is dependent upon phagocytosis per se, as opposed to activation of other antiparasitic mechanisms, is not clear (3, 7, 16). In contrast to the large body of work on cellular killing of asexual parasites, relatively little is known about cellular control of sexual stage parasites.
Cell-mediated inactivation of intracellular P. falciparum and Plasmodium vinckei petteri gametocytes, associated with markedly reduced infectivity to mosquitoes, has been attributed to cytokine-mediated activation of peripheral blood leucocytes and release of nitric oxide (20, 21); this effect is not dependent on the presence of IS or antigamete antibodies. In contrast, Lensen et al. (18, 19) have found that the ability of leucocytes to reduce the infectivity of P. falciparum gametocytes is dependent upon the presence of IS and that individual sera vary both in their requirement for leucocytes in order to reduce transmission and in their ability to mediate leucocyte-dependent transmission reduction. Although Lensen et al. suggest that the mechanism of cell-mediated transmission blocking is phagocytosis of opsonized gametes, phagocytosis of either gametocytes or gametes was not directly assessed in that study (19).
In this study, we have shown quite clearly that both MM and PMN can phagocytose schizont-infected erythrocytes and extracellular gametes and that phagocytosis is significantly enhanced in the presence of IS. Interestingly, mature intracellular gametocytes were only poorly phagocytosed, suggesting that few parasite-specific antigens are expressed on the erythrocyte surface. Erythrocytes infected with immature gametocytes are known to express parasite-derived cytoadherence ligands, allowing the developing gametocytes to shelter in the deep vasculature (26), but in a recent study, Day et al. (6) found that the gametocyte cytoadherence phenotype changes as the gametocyte matures. Whereas early gametocytes and asexual stages bind to CD36, later-stage gametocytes do not, suggesting loss of the original cytoadherence ligand from the surface of stage II gametocyte-infected erythrocytes. However, because stage II to IV gametocytes continue to cytoadhere in vivo, they presumably express other ligands on the surface of the infected erythrocyte membrane. Our data suggest that these ligands are not widely recognized by IS.
PMN were more efficient at phagocytosing schizonts and gametocytes than MM, but, overall, levels of phagocytosis were quite low, with less than 20% of phagocytic cells containing ingested parasites after the optimal time of incubation. This may reflect the true ability of these cells to phagocytose malaria parasites, or it may suggest that phagocytes need to be activated by extrinsic factors in order to become fully effective. All of our in vitro assays were conducted with leucocytes from nonimmune donors, and all lymphocytes had been removed from the system. Because immune lymphocytes are the major source of the inflammatory cytokines which are required to activate both MM and PMN (15, 27), it is possible that the phagocytic ability of these cells may be greatly enhanced in the blood of immune individuals. However, Lensen et al. found that purified neutrophils were efficiently activated (as assessed by a chemiluminescence assay) in the presence of P. falciparum gametes and IS (19), suggesting that extrinsic factors such as cytokines may not be required.
The marked variation between IS in their ability to promote phagocytosis of gametes is consistent with the data of Lensen et al. (19), who found similar variation in the ability of IS to activate neutrophils.
Importantly, we have shown that phagocytosis of extracellular gametes by PMN correlates with the presence of antibodies to the gamete surface proteins Pfs230 and Pfs48/45. The IgG1 subclass of antibody was positively correlated with phagocytosis, which is consistent with the cytophilic nature of this IgG subclass. Although very high levels of gamete-specific IgG3 were also present in the sera, these antibodies do not appear to mediate phagocytosis. IgG1 is known to be a more efficient opsonin than IgG3 and has higher affinity for FcγRI, FcγIIA, and FcγIIB than IgG3 (11).
Considerable caution must be exercised in extrapolating in vitro data to the mosquito, because the environment within the mosquito midgut is very different from that in the culture vessel. We have shown that phagocytosis of gametocytes is enhanced at 18°C compared with at 37°C, because, although phagocytes might be expected to function less efficiently at 18°C, the lower temperature stimulates gametogenesis and exposes surface antigens of the extracellular gamete to antibody and phagocytic cells. However, in common with a previous study, we found that phagocytosis rates were significantly lower in the mosquito than in vitro (28), indicating that the efficiency of phagocytosis in the mosquito midgut may be too low to be functionally significant. This was true even when phagocytes were present with complement, which is known to enhance Ig-mediated phagocytosis by MM and PMN (29). Accordingly, we were unable to show any significant effect of leucocytes on the transmission-reducing effect of either pooled or individual IS. Although some individual serum samples blocked transmission, this was not correlated with their ability to mediate phagocytosis in vitro.
In summary, our data suggest that although both MM and PMN can phagocytose malarial gametes in vitro, absolute levels of phagocytosis are low, and phagocytosis of intracellular gametocytes is extremely inefficient. In vivo, although conditions within the mosquito midgut facilitate the formation of extracellular gametes which are susceptible to phagocytosis, the phagocytic process itself proceeds less efficiently than in vitro. This may be due to unfavorable conditions inside the mosquito midgut, with respect to pH and the presence of digestive enzymes (2). Finally, although we found a correlation between the ability of a serum to promote phagocytosis in vitro and the presence of antibodies against transmission-blocking target antigens, we were unable to demonstrate a role for MM or PMN in transmission reduction in mosquitoes. However, it remains to be seen whether phagocytes from immune individuals, in the presence of immune T lymphocytes, may mediate effective transmission-blocking immunity.
ACKNOWLEDGMENTS
We thank Lisa Ranford-Cartwright for generously providing infectious gametocyte cultures, Margaret Mooney for maintenance of the insectaries, Brian Greenwood and the villagers of Brefet, The Gambia, for access to the endemic sera, and Alan Gemmill for help with statistical methods.
This work was funded by the Wellcome Trust.
REFERENCES
- 1.Aikawa M, Rener J, Carter R, Miller L H. An electron microscopical study of the interaction of monoclonal antibodies with gametes of the malarial parasite Plasmodium gallinaceum. J Protozool. 1981;28:383–388. doi: 10.1111/j.1550-7408.1981.tb02871.x. [DOI] [PubMed] [Google Scholar]
- 2.Billingsley P F, Sinden R E. Determinants of malaria-mosquito specificity. Parasitol Today. 1997;13:297. [Google Scholar]
- 3.Bouharoun-Tayoun H, Oeuvra C, Lunel F, Druilhe P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med. 1995;182:409–418. doi: 10.1084/jem.182.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter R, Ranford Cartwright L, Alano P. The culture and preparation of gametocytes of Plasmodium falciparum for immunochemical, molecular, and mosquito infectivity studies. Methods Mol Biol. 1993;21:67–88. doi: 10.1385/0-89603-239-6:67. [DOI] [PubMed] [Google Scholar]
- 5.Celada A, Cruchaud A, Perrin L H. Phagocytosis of Plasmodium falciparum-parasitized erythrocytes by human polymorphonuclear leukocytes. J Parasitol. 1983;69:49–53. [PubMed] [Google Scholar]
- 6.Day K P, Hayward R E, Smith D, Culvenor J G. CD-36-dependent adhesion and knob expression of the transmission stages of Plasmodium falciparum is stage specific. Mol Biochem Parasitol. 1998;93:166–177. doi: 10.1016/s0166-6851(98)00040-1. [DOI] [PubMed] [Google Scholar]
- 7.Druilhe P, Perignon J L. A hypothesis about the chronicity of malaria infection. Parasitol Today. 1997;13:353–357. doi: 10.1016/s0169-4758(97)01095-8. [DOI] [PubMed] [Google Scholar]
- 8.Ferrante A L, Kumartilake L, Rzepczyk C M, Dayer J M. Killing of Plasmodium falciparum by cytokine activated effector cells (neutrophils and macrophages) Immunol Lett. 1990;25:179–187. doi: 10.1016/0165-2478(90)90112-4. [DOI] [PubMed] [Google Scholar]
- 9.Greenwood B M, Bradley A K, Greenwood A M, Byass P, Jammeh K, Marsh K, Tulloch S, Oldfield F S, Hayes R. Mortality and morbidity from malaria among children in a rural area of The Gambia, West Africa. Trans R Soc Trop Med Hyg. 1987;81:478–486. doi: 10.1016/0035-9203(87)90170-2. [DOI] [PubMed] [Google Scholar]
- 10.Healer J, McGuinness D, Hopcroft P, Haley S, Carter R, Riley E. Complement-mediated lysis of Plasmodium falciparum gametes by malaria-immune human sera is associated with antibodies to the gamete surface antigen Pfs230. Infect Immun. 1997;65:3017–3023. doi: 10.1128/iai.65.8.3017-3023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janeway C A, Travers P. Immunobiology: the immune system in health and disease. Edinburgh, United Kingdom: Churchill Livingstone; 1997. pp. 8:18–8:27. [Google Scholar]
- 12.Kaushal D C, Carter R, Rener J, Grotendorst C A, Miller L H, Howard R J. Monoclonal antibodies against surface determinants on gametes of Plasmodium gallinaceum block transmission of malaria parasites to mosquitoes. J Immunol. 1983;131:2557–2562. [PubMed] [Google Scholar]
- 13.Kharazami A, Jepsen S. Enhanced inhibition of in vitro multiplication of Plasmodium falciparum by stimulated human polymorphonuclear leukocytes. Clin Exp Immunol. 1984;57:287–292. [PMC free article] [PubMed] [Google Scholar]
- 14.Khushmith S, Druilhe P. Antibody-dependent ingestion of P. falciparum merozoites by human blood monocytes. Parasite Immunol. 1983;5:357. doi: 10.1111/j.1365-3024.1983.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 15.Kumaratilake L M, Ferrante A, Rzepczyk C. The role of T lymphocytes in immunity to Plasmodium falciparum. Enhancement of neutrophil-mediated parasite killing by lymphotoxin and IFN-gamma: comparisons with tumor necrosis factor effects. J Immunol. 1991;146:762–767. [PubMed] [Google Scholar]
- 16.Kumaratilake L M, Ferrante A, Rzepczyk C M. Tumor necrosis factor enhances neutrophil-mediated killing of Plasmodium falciparum. Infect Immun. 1990;58:788–793. doi: 10.1128/iai.58.3.788-793.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambros C, Vanderberg J P. Synchronisation of Plasmodium falciparum erythrocytic cultures. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 18.Lensen A, Mulder L, Tchuinkam T, Willemsen L, Eling W, Sauerwein R. Mechanisms that reduce transmission of Plasmodium falciparum malaria in semiimmune and nonimmune persons. J Infect Dis. 1998;177:1358–1363. doi: 10.1086/515263. [DOI] [PubMed] [Google Scholar]
- 19.Lensen A H W, Bolmer-van de Vegte M, Van Gemert G J, Eling W M C, Sauerwein R W. Leukocytes in a Plasmodium falciparum-infected blood meal reduce transmission of malaria to Anopheles mosquitoes. Infect Immun. 1997;65:3834–3837. doi: 10.1128/iai.65.9.3834-3837.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motard A, Landau I, Nussler A, Grau G, Baccam D, Mazier D, Targett G A. The role of reactive nitrogen intermediates in modulation of gametocyte infectivity of rodent malaria parasites. Parasite Immunol. 1993;15:21–26. doi: 10.1111/j.1365-3024.1993.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 21.Naotunne T S, Karunaweera N D, Mendis K N, Carter R. Cytokine-mediated inactivation of malarial gametocytes is dependent on the presence of white blood cells and involves reactive nitrogen intermediates. Immunology. 1993;78:555–562. [PMC free article] [PubMed] [Google Scholar]
- 22.Nijhout M M. Plasmodium gallinaceum: exflagellation stimulated by a mosquito factor. Exp Parasitol. 1979;48:75–80. doi: 10.1016/0014-4894(79)90056-0. [DOI] [PubMed] [Google Scholar]
- 23.Read D, Lensen A H W, Bergarnie S, Haley S, Raza A, Carter R. Transmission-blocking antibodies against multiple, non-variant target epitopes of the Plasmodium falciparum gamete surface antigen Pfs 230 are all complement fixing. Parasite Immunol. 1994;16:511–519. doi: 10.1111/j.1365-3024.1994.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 24.Rener J, Carter R, Rosenberg Y, Miller L H. Anti-gamete monoclonal antibodies synergistically block transmission of malaria by preventing fertilization in the mosquito. Proc Natl Acad Sci USA. 1980;77:6797–6799. doi: 10.1073/pnas.77.11.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rener J, Graves P M, Carter R, Williams J L, Burkot T R. Target antigens of transmission-blocking immunity on gametes of Plasmodium falciparum. J Exp Med. 1983;158:976–781. doi: 10.1084/jem.158.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers N J, Daramola O, Targett G A T, Hall B S. CD36 and intercellular adhesion molecule 1 mediate adhesion of developing Plasmodium falciparum gametocytes. Infect Immun. 1996;64:1480–1483. doi: 10.1128/iai.64.4.1480-1483.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shalaby M R, Aggarwal B B, Rinderknecht E, Svedersky L P, Finkle B S, Palladino M A. Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumour necrosis factors. J Immunol. 1985;135:2069–2073. [PubMed] [Google Scholar]
- 28.Sinden R E, Smalley M E. Gametocytes of Plasmodium falciparum: phagocytosis by leucocytes in vivo and in vitro. Trans R Soc Trop Med Hyg. 1976;70:344–345. doi: 10.1016/0035-9203(76)90096-1. [DOI] [PubMed] [Google Scholar]
- 29.Snapper C M, Finkelman F D. Immunoglobulin class switching. In: Paul W E, editor. Fundamental immunology. New York, N.Y: Raven Press; 1993. p. 837. [Google Scholar]
- 30.Trubowitz S, Masek B. Plasmodium falciparum: phagocytosis by polymorphonuclear leukocytes. Science. 1968;162:273–274. doi: 10.1126/science.162.3850.273. [DOI] [PubMed] [Google Scholar]
- 30a.Turrini F H, Ginsburg H, Bussolino F, Pescasmona G P, Serra M V, Arese P. Phagocytosis of Plasmodium falciparum-infected human red blood cells by human monocytes—dependence on parasite developmental stages. Blood. 1992;80:801–808. [PubMed] [Google Scholar]
- 31.Wozencraft A O, Dockrell H M, Taverne J, Targett G A, Playfair J H L. Killing of human malaria parasites by macrophage secretory products. Infect Immun. 1984;43:664–669. doi: 10.1128/iai.43.2.664-669.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]