Abstract
Background: The reintroduction of hemp production has resulted in increased consumption of cannabidiol (CBD) products, particularly CBD oil, yet their effects on intestinal health are not fully understood. Proper mitochondrial function and antioxidant defenses are vital for maintaining the intestinal epithelial barrier. AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor gamma coactivator (PGC)1α are key mediators of mitochondrial metabolism. Methods & Results: Using Caco-2 cells, we found that CBD oil promoted AMPK phosphorylation, upregulated differentiation markers, and enhanced PGC1α/SIRT3 mitochondrial signaling. CBD oil reduced reactive oxygen species production and increased antioxidant enzymes. Moreover, CBD oil also increased levels of citrate, malate, and succinate—key metabolites of the tricarboxylic acid cycle—alongside upregulation of pyruvate dehydrogenase and isocitrate dehydrogenase 1. Similarly, pure CBD induced metabolic and antioxidant signaling. Conclusions: CBD enhances mitochondrial metabolic activity and antioxidant defense in Caco-2 cells, making it a promising candidate for treating intestinal dysfunction.
Keywords: AMPK, antioxidant activity, cannabidiol, intestinal epithelium, mitochondria
1. Introduction
The intestinal epithelium is a self-renewing, tightly regulated barrier essential for nutrient absorption, tissue protection, and the coordination of immune responses [1]. Dysregulation of the epithelium, such as impaired barrier function, is characteristic of gut diseases such as inflammatory bowel disease (IBD) [2]. The intestinal epithelium is constantly renewed, depending on the proliferation and differentiation of stem cells residing in crypts. Extensive oxidative metabolic transition and mitochondrial biogenesis occur during epithelial differentiation [3]. Proper cellular energetics, orchestrated by functional mitochondria, are vital for epithelial health. Mice with enhanced intestinal oxidative phosphorylation and ATP production develop less severe colitis than their peers [4]. In humans, patients with colitis exhibit decreased levels of mitochondrial respiratory chain complexes compared to healthy counterparts [5].
Mitochondria are major producers of free radicals, and their dysfunction results in increased oxidative damage that contributes to the progression of pathological intestinal conditions [6]. The inability of the body to balance the production of free radicals, such as reactive oxygen species (ROS), with appropriate defense mechanisms results in damaging oxidative stress, which plays a role in the pathology of numerous conditions including IBD [7]. Managing ROS is an intricate process vital for maintaining redox homeostasis. The body employs various defenses to control levels of oxidative stress, including antioxidative enzymes, whose levels can be influenced by lifestyle factors and aging [8]. Patients with gastrointestinal diseases exhibit both elevated levels of ROS and decreased activity of antioxidative enzymes [9]. Inhibition of these mediators increases ROS levels and promotes inflammasome activation [10]. Thus, it is important to identify strategies to maintain adequate antioxidative activity.
NF-E2-related factor 2 (NRF2) is an important transcription factor that regulates the expression of antioxidant genes and controls ROS levels in the intestines [11]. NRF2 is regulated by Kelch-like ECH-associated protein 1 (KEAP1), and it binds to the antioxidant response element (ARE) to promote the expression of enzymes such as NAD(P)H quinone dehydrogenase 1 (NQO1) and heme oxygenase 1 (HO-1) [12]. In the canonical pathway of NRF2 activation, oxidative stress decreases the ability of KEAP1 to sequester NRF2, allowing NRF2 to translocate to the nucleus and activate ARE-driven gene expression [13]. Alternatively, the scaffold protein p62 plays a role in the noncanonical activation of NRF2 by disrupting the interaction between KEAP1 and NRF2 [14,15]. Several upstream pathways regulate NRF2/HO-1, including phosphoinositide 3-kinase (PI3K)/AKT [16], p38 mitogen-activated protein kinase (MAPK) [17], and AMP-activated protein kinase (AMPK) [18]. AMPK also regulates intestinal differentiation and barrier function through the activation of caudal type homeobox 2 (CDX2) [19], illustrating the interconnection between metabolic and antioxidant pathways, which is essential for maintaining intestinal homeostasis.
Cannabidiol (CBD) is a non-psychoactive cannabinoid found in cannabis that can reach the gut depending on the method of delivery [20,21]. In the human colorectal carcinoma cell line Caco-2, CBD reduces ROS production and improves transepithelial resistance upon exposure to H2O2 or H2O2/Fe2+ [22,23]. We previously demonstrated that mice with dextran sodium sulfate (DSS)-induced colitis that received 200 mg kg−1 CBD in their diet for 5 weeks showed reduced inflammation, associated with increased phosphorylation and activation of AMPK [24].
Several studies showed the effects of CBD in improving mitochondrial energetics. Hippocampal neurons treated with 5 µM CBD exhibit greater mitochondrial respiration compared to untreated neurons [25]. In mice, intraperitoneal injections of CBD ameliorate the negative effects of doxorubicin on mitochondrial biogenesis in the myocardium [26]. The effect of CBD on intestinal mitochondrial energetics and signaling remains to be elucidated. This study used human epithelial Caco-2 cells to investigate the effects of CBD on the antioxidant and metabolic activities of colon cells. We hypothesized that CBD treatment upregulates the phosphorylation of AMPK in Caco-2 cells, which is accompanied by enhanced mitochondrial energetics and the upregulation of antioxidant enzymes.
2. Materials and Methods
2.1. Cannabidiol
A commercial full-spectrum CBD hemp oil was purchased directly from the manufacturer (Nutra Pure LLC, Vancouver, WA, USA). As per the test results provided by the producer, analysis by a published method [27] determined the CBD content in the oil to be 18.3 mg/mL, while other cannabinoids were below the limit of quantification. A CBD stock solution (10 mM) was prepared by diluting the oil in dimethylsulfoxide (DMSO) (VWR, Radnor, PA, USA). Additionally, cannabidiol (≥98%) was purchased from Cayman Chemical (Ann Arbor, MI, USA) and dissolved in DMSO to prepare a stock solution at 10 mM. All CBD stock solutions were stored at −20 °C.
2.2. Cell Line
Caco-2 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were routinely cultured in Dulbecco’s modified Eagle medium (DMEM) (Sigma; St. Louis, MO, USA) supplemented with 10% fetal bovine serum (Sigma), 100 units/mL of penicillin G, and 100 µg/mL of streptomycin (Sigma) at 37 °C with 5% CO2. Cells were seeded into 12-well plates for analyses unless stated otherwise. Following overnight incubation, cells were treated with or without 10µM CBD for 1, 2, or 4 days.
2.3. Intracellular Reactive Oxygen Species (ROS) Measurement
Levels of intracellular ROS were evaluated as previously described [28]. In brief, Caco-2 cells were seeded into 96-well plates and cultured in complete DMEM overnight, followed by incubation with media containing the cell-permeable fluorescent probe, 2,7-dichlorofluorescein diacetate (H2DCFDA) (MilliporeSigma, Burlington, MA, USA), for 45 min. After replacing the media, the cells were treated with or without CBD and with or without H2O2 for 24 h. Fluorescence was measured using a BioTek Synergy H1 microplate reader (Agilent Technologies, Palo Alto, CA, USA) with an excitation wavelength of 485 nm and an emission wavelength of 530 nm. The fluorescence of H2DCFDAobtained from the plate reader was reported in arbitrary units and normalized to the values of the untreated controls.
2.4. Immunoblotting
Proteins were extracted from Caco-2 cells, separated by SDS-PAGE gels, and transferred onto nitrocellulose membrane as described previously [29,30]. Antibodies against acetyl-CoA carboxylase (ACC), AMPK, CDX2, catalase, HO-1, isocitrate dehydrogenase 1 (IDH1), p62, p-ACC, p-AMPK, pyruvate dehydrogenase (PDH), sirtuin3 (SIRT3), and superoxide dismutase (SOD)2 were purchased from Cell Signaling Technology (Danvers, MA, USA). The antibody for claudin-2 was purchased from Thermo Fisher Scientific (Waltham, MA, USA), while the antibody for proliferator-activated receptor gamma coactivator 1 alpha (PGC1α) was obtained from ProteinTech (Rosemont, IL, USA). The antibody for SOD1 was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Horseradish peroxidase-coupled anti-rabbit or anti-mouse IgGs were used for visualization by chemiluminescence. Band density quantification was normalized to the signal of β-tubulin. Data are presented as relative to the control group.
2.5. Quantitative Reverse Transcription PCR (qRT-PCR) Analysis
Total mRNA was extracted from Caco-2 cells or animal tissue using Trizol Reagent (Thermo Fisher) per the manufacturer’s instructions. Reverse transcription was completed using an iScript™ kit (Bio-Rad, Hercules, CA, USA). The produced cDNA served as templates for qRT-PCR analysis, using SYBR Green Master Mix (Bio-Rad) and a CFX96™ Real-Time PCR Detection System (Bio-Rad). The primers used for qRT-PCR are listed in Supplementary Materials Table S1, with 18S serving as the housekeeping gene.
2.6. Tricarboxylic Acid Cycle Metabolite Analysis by GC-MS
Caco-2 cells were treated with or without CBD for 2 days. Following the treatment period, the cells were collected and processed for analysis using an Agilent 7890B gas chromatography system equipped with a 5977A single-quadrupole mass spectrometer and a 7693 autosampler system (Agilent Technologies), following previously established procedures [31]. The column utilized was an HP-5 ms column (30 m × 250 µM i.d., 0.25 µM film thickness; Agilent Technologies). Ribitol, purchased from Sigma, served as the internal standard. Citrate and malate standards were purchased from Sigma. The succinate standard was purchased from ThermoFisher Scientific. The relative abundances of metabolites were determined by calculating the area ratios of the target peaks to the ribitol (internal standard) peaks.
2.7. Statistical Analysis
Statistical analysis was performed as previously described using GraphPad Prism 7 [31]. The data are presented relative to the control group as mean ± SEM (standard error of the mean). Treatments were compared using either a two-tailed Student’s t-test or one-way ANOVA. Significance was determined using a p-value ≤ 0.05.
3. Results
3.1. CBD Oil Induces Phosphorylation of AMPK and Upregulates Downstream Targets
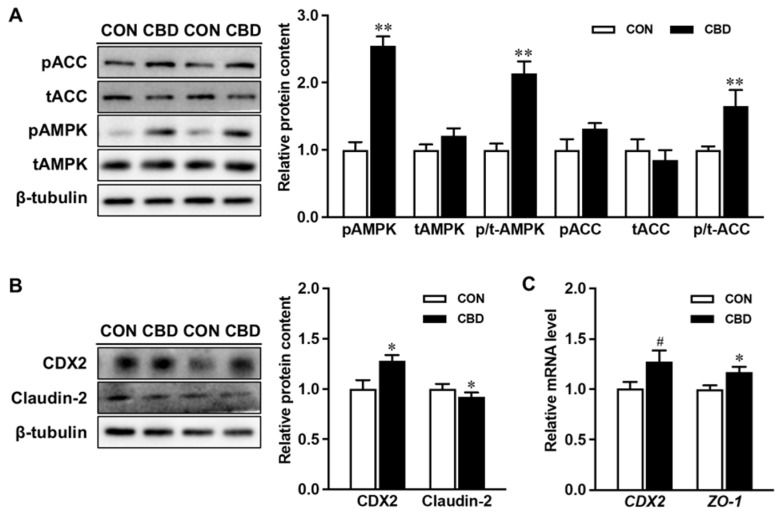
Incubation with CBD oil increased the phosphorylation of AMPK compared to controls (Figure 1A). CBD oil treatment also enhanced the phosphorylation of ACC, a downstream target of AMPK (Figure 1A). Moreover, at both the protein and mRNA levels, CBD oil exhibited a promotive effect on CDX2, another downstream target of AMPK and the key transcription factor governing epithelial differentiation (Figure 1B,C). Additionally, exposure to CBD oil decreased the protein level of claudin-2 and increased the mRNA level of zonula occludens-1 (ZO-1), indicating positive effects on epithelial differentiation and barrier function (Figure 1B,C).
Figure 1.
Cannabidiol oil induces AMP-activated protein kinase (AMPK) phosphorylation and upregulates downstream targets overseeing epithelial differentiation and barrier function. (A) Protein contents of p-AMPK, t-AMPK, p- acetyl-CoA carboxylase (ACC), and t-ACC. (B) The protein content of caudal type homeobox 2 (CDX2) and claudin-2. (C) mRNA expression of CDX2 and zonula occludens-1 (ZO-1). CON: untreated Caco-2 cells; CBD: Caco-2 cells treated with 10 µM cannabidiol (CBD) oil. Mean ± SEM, n = 4, #: p ≤ 0.10; *: p ≤ 0.05; **: p ≤ 0.01.
3.2. CBD Oil Treatment Promotes Mitochondrial Energetics
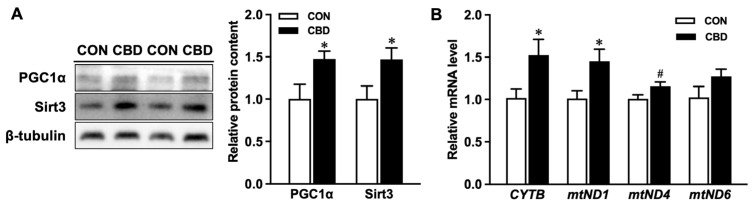
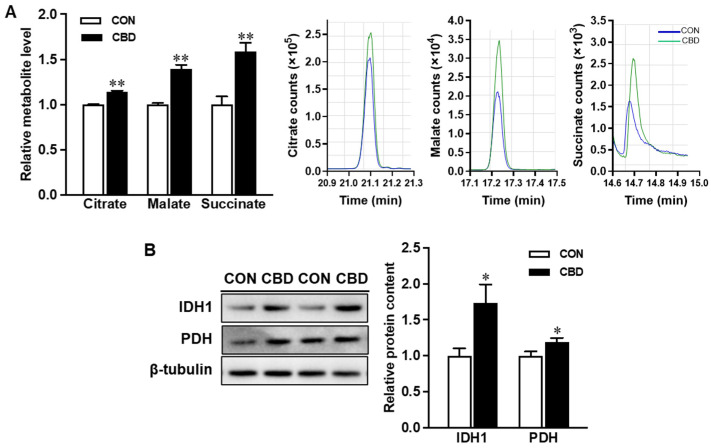
CBD oil treatment increased protein contents of the transcription factor Peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1α) and the deacetylase enzyme, Sirtuin 3 (SIRT3) (Figure 2A). Treatment with CBD oil also upregulated the mRNA expression of the mitochondrially encoded NADH-ubiquinone oxidoreductase core subunit 1 (mtND1), mtND4, and cytochrome b (CYTB) (Figure 2B). Additionally, CBD oil promoted oxidative phosphorylation in Caco-2 cells. GC-MS analysis of key tricarboxylic acid (TCA) cycle metabolites revealed elevated contents of citrate, malate, and succinate in CBD-treated cells (Figure 3A). This effect was accompanied by increased protein contents of isocitrate dehydrogenase 1 (IDH1), an enzyme in the TCA cycle responsible for the conversion of isocitrate to alpha-ketoglutarate (αKG), and pyruvate dehydrogenase (PDH), the enzyme responsible for directing pyruvate to oxidative phosphorylation (Figure 3B).
Figure 2.
Cannabidiol oil enhances mitochondrial signaling and activity. (A) Protein contents of Peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1α) and sirtuin 3 (SIRT3). (B) mRNA expression of cytochrome b (CYTB), mitochondrially encoded NADH-ubiquinone oxidoreductase core subunit 1 (mtND1), mtND4, and mtND6. CON: untreated Caco-2 cells; CBD: Caco-2 cells treated with 10 µM cannabidiol (CBD) oil. Mean ± SEM, n = 4, #: p ≤ 0.10; *: p ≤ 0.05.
Figure 3.
Cannabidiol oil alters tricarboxylic acid (TCA) cycle activity and metabolite levels. (A) Levels of TCA cycle metabolites obtained from gas chromatography/mass spectrometry and representative peaks for citrate, malate, and succinate. (B) Protein contents of isocitrate dehydrogenase 1 (IDH1) and pyruvate dehydrogenase (PDH). CON: untreated Caco-2 cells; CBD: Caco-2 cells treated with 10 µM cannabidiol (CBD) oil. Mean ± SEM, n = 3–4, *: p ≤ 0.05; **: p ≤ 0.01.
3.3. CBD Oil Suppresses ROS Formation and Upregulates Antioxidants
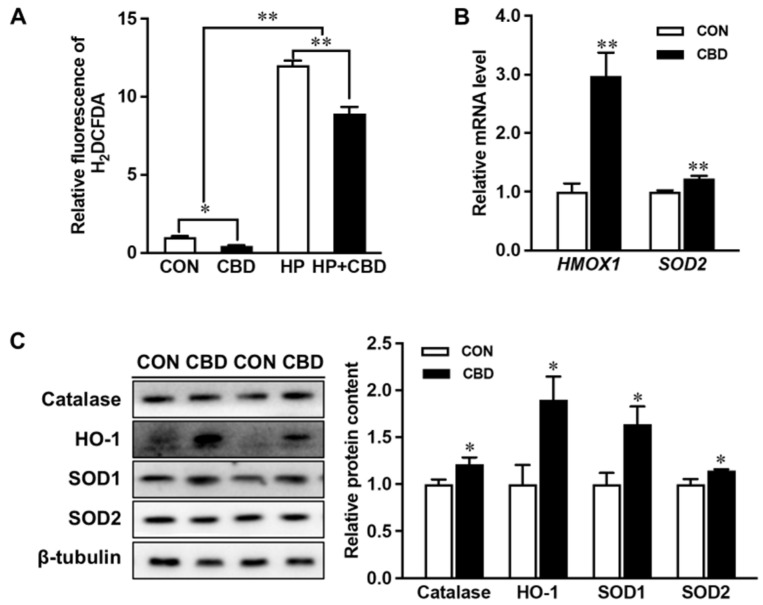
Treating Caco-2 cells with 10 µM CBD oil lowered levels of ROS with or without the H2O2 challenge (Figure 4A). CBD oil also induced the expression of the antioxidant enzyme, HO-1 (Figure 4B). Moreover, the protein contents of catalase SOD1, and to a lesser extent SOD2, were increased (Figure 4B). At the mRNA level, CBD oil upregulated heme oxygenase 1 gene (HMOX1) and SOD2 expression (Figure 4C).
Figure 4.
Cannabidiol oil mitigates reactive oxygen species (ROS) production and enhances antioxidant expression. (A) ROS production measured with H2DCFDA. (B) mRNA expression of heme oxygenase 1 gene (HMOX1) and superoxide dismutase 2 (SOD2). (C) Protein contents of catalase, heme oxygenase 1 (HO-1), SOD1, and SOD2. CON: untreated Caco-2 cells; CBD: Caco-2 cells treated with 10 µM cannabidiol (CBD) oil. Mean ± SEM, n = 4, *: p ≤ 0.05; **: p ≤ 0.01.
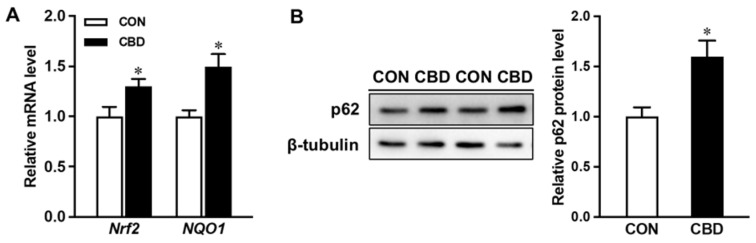
3.4. Exposure to CBD Oil Upregulates NRF2 Signaling Pathway
Consistent with the upregulation of HO-1 protein and mRNA (HMOX1) levels in CBD oil-treated cells, the mRNA expression of NRF2 was also elevated (Figure 5A). Accordingly, the mRNA level of NQO1, a target gene of NRF2 encoding the detoxification enzyme, was increased in CBD oil-treated cells. The content of p62 was also upregulated in CBD oil-treated cells (Figure 5B).
Figure 5.
Cannabidiol oil targets NF-E2-related factor 2 (NRF2) signaling. (A) mRNA expression of Nrf2 and NAD(P)H quinone dehydrogenase 1 (NQO1). (B) Protein content of p62. CON: untreated Caco-2 cells; CBD: Caco-2 cells treated with 10 µM cannabidiol (CBD) oil. Mean ± SEM, n = 4, *: p ≤ 0.05.
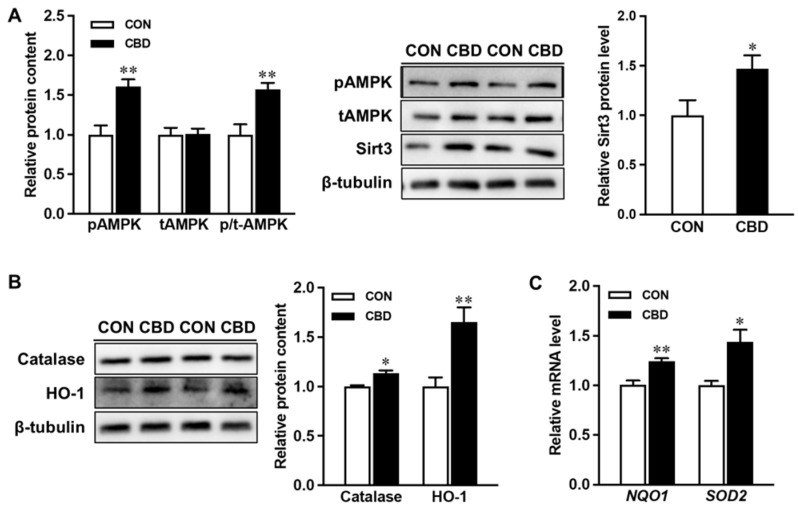
3.5. Pure CBD Promotes Signaling Pathways Similarly to CBD Oil
While CBD is the major constituent in commercial CBD oils, it is common for them to contain other cannabinoids at much smaller levels. To confirm that CBD exerts positive effects on AMPK and related signaling, Caco-2 cells were further treated with 10 µM pure CBD compound. Consistently, pure CBD increased phosphorylation of AMPK and upregulated SIRT3 (Figure 6A). Pure CBD exerted beneficial effects on antioxidant signaling, elevating protein levels of HO-1 and catalase (Figure 6B), as well as the mRNA expression of NQO1 and SOD2 (Figure 6C). Together these findings show that CBD targets mitochondrial and antioxidant signaling to improve intestinal epithelial health.
Figure 6.
Pure cannabidiol induces phosphorylation of AMPK and upregulates both mitochondrial and antioxidant signaling. (A) Protein contents of p- AMP-activated protein kinase (AMPK), t-AMPK, and sirtuin3 (SIRT3). (B) Protein contents of catalase and heme oxygenase 1 (HO-1). (C) mRNA expression of NAD(P)H quinone dehydrogenase 1 (NQO1) and superoxide dismutase 2 (SOD2). CON: untreated Caco-2 cells; CBD: Caco-2 cells treated with 10 µM cannabidiol (CBD). Mean ± SEM, n = 4, *: p ≤ 0.05; **: p ≤ 0.01.
4. Discussion
The average daily intake of CBD among adults who regularly consume CBD-containing products is 50.3 ± 40.7 mg [32]. CBD exhibits poor bioavailability, hampered by its low water solubility [33], and approximately one-third of orally ingested CBD is excreted in the feces [34]. Pharmacokinetic studies with humans have largely focused on serum levels following oral intake. After ingesting an oral capsule containing 10 mg CBD, the maximum concentration measured in serum was 2.47 ng/mL (7.85 nM) [35]. In a different study, a higher oral dose of 200 mg CBD resulted in a maximum serum concentration of 148 ng/mL (470.63 nM) [36]. Multiple factors affect the oral absorption of CBD, including the presence of lipids, which enhances CBD absorption [35,37]. Following oral delivery in rats, CBD levels detected in intestinal lymph far exceeded serum levels, being 250-fold greater [37], suggesting the importance of understanding its effects on gut epithelial health. If the same holds following oral intake in humans, micromolar levels of CBD may be obtainable in the intestines.
Among CBD product consumers, drops are the most commonly used type of product [38]. In this experiment, we employed commercially available CBD oil to best reflect exposure to the average consumers. This CBD oil utilized hempseed oil as a carrier, which contains essential fatty acids and bioactive tocopherols [39]. To account for this, we also utilized pure CBD in the present study. Given that concentration and dosage instructions differ between CBD products, the use of a single treatment condition is a limitation of the present study.
The present study employed the Caco-2 cell line to model the intestinal epithelium of the colon. The cultured cells mimic epithelial differentiation upon achieving confluence which dictated our choice of treatment times [19]. In Caco-2 cell monolayers, 10 µM CBD treatment potentiated the recovery of transepithelial resistance following a challenge with ethylenediaminetetraacetic acid [40]. CBD treatment also resulted in elevated mRNA expression of ZO-1 [40]. In the present study, CBD oil treatment similarly increased the mRNA expression of ZO-1. In agreement with our previous observations in mice with DSS-induced colitis, CBD oil and pure CBD increased the phosphorylation of AMPK in Caco-2 cells. AMPK, a regulator of energy homeostasis, plays a key role in the homeostasis of the intestinal epithelium [19,41]. Considering that AMPK activation upregulates differentiation transcription factor CDX2 at both the mRNA and protein levels [42], the ability of both CBD to activate AMPK and upregulate its downstream targets holds promise as a means to strengthen intestinal epithelial barrier function.
Proper differentiation relies on mitochondrial function, characterized by increased oxidative phosphorylation and mitochondrial biogenesis as epithelial cells differentiate and migrate away from the crypts [43,44]. In intestinal epithelial cells, the proinflammatory signal, tumor necrosis factor-alpha (TNF-α) induces mitochondrial dysfunction, reducing oxygen consumption and mitochondrial membrane potential [45]. It also decreases the expression of alkaline phosphatase, a marker for intestinal epithelial differentiation [46]. CBD oil treatment upregulated the protein contents of PGC1α and SIRT3, a deacetylase controlling mitochondrial quantity, metabolism, and antioxidant activity [47,48].
Furthermore, CBD oil upregulated the TCA cycle enzyme IDH1, responsible for synthesizing α-ketoglutarate, a vital cofactor for epigenetic modifications that is required for the proper differentiation of epithelial cells [49,50]. PDH was also increased in CBD-treated cells. Impairment of the PDH in cancer cells favors greater glycolysis at the expense of mitochondrial oxidation [51]. Changes to TCA cycle enzyme protein levels were accompanied by changes in metabolite levels. CBD treatment increased the intracellular content of citrate, malate, and succinate in Caco-2 cells. In pigs, succinate increased epithelial tight junction protein content [52]. Together, our findings suggest that CBD enhances mitochondrial metabolic function in colon epithelial cells, which may contribute to its beneficial effects.
CBD oil treatment ameliorated ROS production in Caco-2 cells both in the presence or absence of H2O2. This effect was accompanied by the upregulation of antioxidant enzymes including HO-1. Similar observations of NRF2 and HO-1 induction have been reported in keratinocytes [53] and endothelial cells [54] treated with 1 and 6 µM CBD, respectively. The scaffold protein p62 can facilitate the degradation of KEAP1, impeding its interactions with NRF2 [55], and also participates in a positive feedback loop with NRF2 [56]. We observed the upregulation of p62 in CBD oil-treated Caco-2 cells. Given its role in regulating the expression of important antioxidants and detoxification enzymes, proper NRF2 expression is crucial for intestinal health. Mice deficient in NRF2 showed increased susceptibility to colitis induced by DSS [57]. Conversely, the activation of NRF2 and the upregulation of its antioxidant signaling pathways in mice mitigated DSS-induced disease severity [58]. In DSS-induced colitis, HO-1 is negatively regulated by transcription factor BTB domain and CNC homology 1 (BACH1), and mice deficient in BACH1 display elevated levels of colonic HO-1 and decreased disease activity [59]. Co-treatment with ZnPP, an inhibitor of HO-1, negated the beneficial effects of BACH1 deficiency on disease activity [59]. Promoting proper expression of HO-1 and other antioxidant defenses through phytochemicals such as CBD may serve as an approach for combatting intestinal dysfunction.
5. Conclusions
As the burden generated by IBD persists, multiple prevention and treatment strategies are needed. The beneficial effects of plant-derived bioactive compounds, such as resveratrol, which ameliorate disease activity of IBD in experimental animal models of colitis, are partially through the promotion of antioxidant activity [60]. Our findings showcase the ability of CBD to combat oxidative stress in colonic epithelial cells, shedding light on potential mechanisms behind these effects such as induction of NRF2/HO-1 signaling. Additionally, our study reveals that CBD treatment impacts mitochondrial energetics and related signaling, inducing the phosphorylation of AMPK and the upregulation of PGC1α and SIRT3. Given that the maintenance of the intestinal epithelium is an energy-demanding process reliant on intricate regulation of mitochondrial activity, our findings help understand the potential of CBD to safeguard against intestinal epithelial dysregulation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16223843/s1, Table S1: Lists the primer sets used for quantitative RT-qPCR. References [61,62,63] are cited in Supplementary Materials.
Author Contributions
Conceptualization, A.B.I. and M.-J.Z.; methodology, A.B.I., Q.S., Q.C. and M.-J.Z.; validation, M.-J.Z.; formal analysis, A.B.I.; writing—original draft preparation, A.B.I.; writing—review and editing, M.D. and M.-J.Z.; visualization, A.B.I. and M.-J.Z.; supervision, M.-J.Z.; project administration, M.-J.Z.; funding acquisition, M.-J.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the United States Department of Agriculture-National Institute of Food and Agriculture (USDA-NIFA) (2018-67017-27517), the Washington State University Agricultural Research Center Emerging Research Issues Competitive Grant, and the Washington State University Dedicated Marijuana Account (DMAc) Grant.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Martini E., Krug S.M., Siegmund B., Neurath M.F., Becker C. Mend your fences: The epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol. Gastroenterol. Hepatol. 2017;4:33–46. doi: 10.1016/j.jcmgh.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeissig S., Burgel N., Gunzel D., Richter J., Mankertz J., Wahnschaffe U., Kroesen A.J., Zeitz M., Fromm M., Schulzke J.D. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodríguez-Colman M.J., Schewe M., Meerlo M., Stigter E., Gerrits J., Pras-Raves M., Sacchetti A., Hornsveld M., Oost K.C., Snippert H.J., et al. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature. 2017;543:424–427. doi: 10.1038/nature21673. [DOI] [PubMed] [Google Scholar]
- 4.Bär F., Bochmann W., Widok A., von Medem K., Pagel R., Hirose M., Yu X., Kalies K., König P., Böhm R., et al. Mitochondrial gene polymorphisms that protect mice from colitis. Gastroenterology. 2013;145:1055–1063. doi: 10.1053/j.gastro.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Sifroni K.G., Damiani C.R., Stoffel C., Cardoso M.R., Ferreira G.K., Jeremias I.C., Rezin G.T., Scaini G., Schuck P.F., Dal-Pizzol F., et al. Mitochondrial respiratory chain in the colonic mucosal of patients with ulcerative colitis. Mol. Cell Biochem. 2010;342:111–115. doi: 10.1007/s11010-010-0474-x. [DOI] [PubMed] [Google Scholar]
- 6.Beltrán B., Nos P., Dasí F., Iborra M., Bastida G., Martínez M., O’Connor J.E., Sáez G., Moret I., Ponce J. Mitochondrial dysfunction, persistent oxidative damage, and catalase inhibition in immune cells of naïve and treated Crohn’s disease. Inflamm. Bowel Dis. 2010;16:76–86. doi: 10.1002/ibd.21027. [DOI] [PubMed] [Google Scholar]
- 7.Vona R., Pallotta L., Cappelletti M., Severi C., Matarrese P. The impact of oxidative stress in human pathology: Focus on gastrointestinal disorders. Antioxidants. 2021;10:201. doi: 10.3390/antiox10020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouzid M.A., Filaire E., Matran R., Robin S., Fabre C. Lifelong voluntary exercise modulates age-related changes in oxidative stress. Int. J. Sports Med. 2018;39:21–28. doi: 10.1055/s-0043-119882. [DOI] [PubMed] [Google Scholar]
- 9.Jiménez P., Piazuelo E., Sánchez M.T., Ortego J., Soteras F., Lanas A. Free radicals and antioxidant systems in reflux esophagitis and Barrett’s esophagus. World J. Gastroenterol. 2005;11:2697–2703. doi: 10.3748/wjg.v11.i18.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z., Zhong H., Wei J., Lin S., Zong Z., Gong F., Huang X., Sun J., Li P., Lin H., et al. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res. Ther. 2019;21:300. doi: 10.1186/s13075-019-2085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hochmuth C.E., Biteau B., Bohmann D., Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell. 2011;8:188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Z., Zhang S., Chan J.Y., Zhang D.D. Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol. Cell Biol. 2007;27:6334–6349. doi: 10.1128/MCB.00630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva-Islas C.A., Maldonado P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018;134:92–99. doi: 10.1016/j.phrs.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Komatsu M., Kurokawa H., Waguri S., Taguchi K., Kobayashi A., Ichimura Y., Sou Y.S., Ueno I., Sakamoto A., Tong K.I., et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 15.Lau A., Wang X.J., Zhao F., Villeneuve N.F., Wu T., Jiang T., Sun Z., White E., Zhang D.D. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: Direct interaction between Keap1 and p62. Mol. Cell Biol. 2010;30:3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu L., He S., Yin P., Li D., Mei C., Yu X., Shi Y., Jiang L., Liu F. Punicalagin induces Nrf2 translocation and HO-1 expression via PI3K/Akt, protecting rat intestinal epithelial cells from oxidative stress. Int. J. Hyperth. 2016;32:465–473. doi: 10.3109/02656736.2016.1155762. [DOI] [PubMed] [Google Scholar]
- 17.Hsu J.T., Kan W.H., Hsieh C.H., Choudhry M.A., Schwacha M.G., Bland K.I., Chaudry I.H. Mechanism of estrogen-mediated intestinal protection following trauma-hemorrhage: p38 MAPK-dependent upregulation of HO-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R1825–R1831. doi: 10.1152/ajpregu.00112.2008. [DOI] [PubMed] [Google Scholar]
- 18.Liu X.M., Peyton K.J., Shebib A.R., Wang H., Korthuis R.J., Durante W. Activation of AMPK stimulates heme oxygenase-1 gene expression and human endothelial cell survival. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H84–H93. doi: 10.1152/ajpheart.00749.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X., Yang Q., Rogers C.J., Du M., Zhu M.J. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017;24:819–831. doi: 10.1038/cdd.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birnbaum A.K., Karanam A., Marino S.E., Barkley C.M., Remmel R.P., Roslawski M., Gramling-Aden M., Leppik I.E. Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy. Epilepsia. 2019;60:1586–1592. doi: 10.1111/epi.16093. [DOI] [PubMed] [Google Scholar]
- 21.Lucas C.J., Galettis P., Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018;84:2477–2482. doi: 10.1111/bcp.13710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cocetta V., Governa P., Borgonetti V., Tinazzi M., Peron G., Catanzaro D., Berretta M., Biagi M., Manetti F., Dall’Acqua S., et al. Cannabidiol isolated from Cannabis sativa L. protects intestinal barrier from in vitro inflammation and oxidative stress. Front. Pharmacol. 2021;12:641210. doi: 10.3389/fphar.2021.641210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borrelli F., Aviello G., Romano B., Orlando P., Capasso R., Maiello F., Guadagno F., Petrosino S., Capasso F., Di Marzo V., et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J. Mol. Med. 2009;87:1111–1121. doi: 10.1007/s00109-009-0512-x. [DOI] [PubMed] [Google Scholar]
- 24.Sun Q., Bravo Iniguez A., Tian Q., Du M., Zhu M.J. Dietary cannabidiol activates PKA/AMPK signaling and attenuates chronic inflammation and leaky gut in DSS-induced colitis mice. Mol. Nutr. Food Res. 2024;68:e2300446. doi: 10.1002/mnfr.202300446. [DOI] [PubMed] [Google Scholar]
- 25.Sun S., Hu F., Wu J., Zhang S. Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biol. 2017;11:577–585. doi: 10.1016/j.redox.2016.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hao E., Mukhopadhyay P., Cao Z., Erdélyi K., Holovac E., Liaudet L., Lee W.S., Haskó G., Mechoulam R., Pacher P. Cannabidiol protects against doxorubicin-induced cardiomyopathy by modulating mitochondrial function and biogenesis. Mol. Med. 2015;21:38–45. doi: 10.2119/molmed.2014.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giese M.W., Lewis M.A., Giese L., Smith K.M. Method for the analysis of cannabinoids and terpenes in cannabis. J. AOAC Int. 2015;98:1503–1522. doi: 10.5740/jaoacint.15-116. [DOI] [PubMed] [Google Scholar]
- 28.Xue Y., Zhang S., Du M., Zhu M.-J. Dandelion extract suppresses reactive oxidative species and inflammasome in intestinal epithelial cells. J. Funct. Foods. 2017;29:10–18. doi: 10.1016/j.jff.2016.11.032. [DOI] [Google Scholar]
- 29.Xue Y., Zhang H., Wang H., Hu J., Du M., Zhu M.-J. Host inflammatory response inhibits Escherichia coli O157: H7 adhesion to gut epithelium through augmentation of mucin expression. Infect. Immun. 2014;82:1921–1930. doi: 10.1128/IAI.01589-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu M.J., Du M., Hess B.W., Means W.J., Nathanielsz P.W., Ford S.P. Maternal nutrient restriction upregulates growth signaling pathways in the cotyledonary artery of cow placentomes. Placenta. 2007;28:361–368. doi: 10.1016/j.placenta.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Sun Q., Bravo Iniguez A., Tian Q., Du M., Zhu M.-J. PGC-1α in mediating mitochondrial biogenesis and intestinal epithelial differentiation promoted by purple potato extract. J. Funct. Foods. 2022;98:105291. doi: 10.1016/j.jff.2022.105291. [DOI] [Google Scholar]
- 32.Kaufmann R., Aqua K., Lombardo J., Lee M. Observed impact of long-term consumption of oral cannabidiol on liver function in healthy adults. Cannabis Cannabinoid Res. 2023;8:148–154. doi: 10.1089/can.2021.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perucca E., Bialer M. Critical aspects affecting cannabidiol oral bioavailability and metabolic elimination, and related clinical implications. CNS Drugs. 2020;34:795–800. doi: 10.1007/s40263-020-00741-5. [DOI] [PubMed] [Google Scholar]
- 34.Wall M.E., Perez-Reyes M. The metabolism of delta 9-tetrahydrocannabinol and related cannabinoids in man. J. Clin. Pharmacol. 1981;21:178S–189S. doi: 10.1002/j.1552-4604.1981.tb02594.x. [DOI] [PubMed] [Google Scholar]
- 35.Millar S.A., Stone N.L., Yates A.S., O’Sullivan S.E. A systematic review on the pharmacokinetics of cannabidiol in humans. Front. Pharmacol. 2018;9:1365. doi: 10.3389/fphar.2018.01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor L., Crockett J., Tayo B., Morrison G. A phase 1, open-label, parallel-group, single-dose trial of the pharmacokinetics and safety of cannabidiol (CBD) in subjects with mild to severe hepatic impairment. J. Clin. Pharmacol. 2019;59:1110–1119. doi: 10.1002/jcph.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zgair A., Lee J.B., Wong J.C.M., Taha D.A., Aram J., Di Virgilio D., McArthur J.W., Cheng Y.K., Hennig I.M., Barrett D.A., et al. Oral administration of cannabis with lipids leads to high levels of cannabinoids in the intestinal lymphatic system and prominent immunomodulation. Sci. Rep. 2017;7:14542. doi: 10.1038/s41598-017-15026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodman S., Wadsworth E., Schauer G., Hammond D. Use and perceptions of cannabidiol products in Canada and in the United States. Cannabis Cannabinoid Res. 2022;7:355–364. doi: 10.1089/can.2020.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alonso-Esteban J.I., González Fernández M., Fabrikov D., Sánchez-Mata M., Torija-Isasa E., Guil-Guerrero J. Fatty acids and minor functional compounds of hemp (Cannabis sativa L.) seeds and other Cannabaceae species. J. Food Compos. Anal. 2022;115:104962. doi: 10.1016/j.jfca.2022.104962. [DOI] [Google Scholar]
- 40.Alhamoruni A., Lee A.C., Wright K.L., Larvin M., O’Sullivan S.E. Pharmacological effects of cannabinoids on the Caco-2 cell culture model of intestinal permeability. J. Pharmacol. Exp. Ther. 2010;335:92–102. doi: 10.1124/jpet.110.168237. [DOI] [PubMed] [Google Scholar]
- 41.Olivier S., Diounou H., Pochard C., Frechin L., Durieu E., Foretz M., Neunlist M., Rolli-Derkinderen M., Viollet B. Intestinal epithelial AMPK deficiency causes delayed colonic epithelial repair in DSS-induced colitis. Cells. 2022;11:590. doi: 10.3390/cells11040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun X., Du M., Navarre D.A., Zhu M.-J. Purple potato extract promotes intestinal epithelial differentiation and barrier function by activating AMP-activated protein kinase. Mol. Nutr. Food Res. 2018;62:1700536. doi: 10.1002/mnfr.201700536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stringari C., Edwards R.A., Pate K.T., Waterman M.L., Donovan P.J., Gratton E. Metabolic trajectory of cellular differentiation in small intestine by Phasor Fluorescence Lifetime Microscopy of NADH. Sci. Rep. 2012;2:568. doi: 10.1038/srep00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Errico I., Salvatore L., Murzilli S., Lo Sasso G., Latorre D., Martelli N., Egorova A.V., Polishuck R., Madeyski-Bengtson K., Lelliott C., et al. Peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC1alpha) is a metabolic regulator of intestinal epithelial cell fate. Proc. Natl. Acad. Sci. USA. 2011;108:6603–6608. doi: 10.1073/pnas.1016354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baregamian N., Song J., Bailey C.E., Papaconstantinou J., Evers B.M., Chung D.H. Tumor necrosis factor-alpha and apoptosis signal-regulating kinase 1 control reactive oxygen species release, mitochondrial autophagy, and c-Jun N-terminal kinase/p38 phosphorylation during necrotizing enterocolitis. Oxid. Med. Cell Longev. 2009;2:297–306. doi: 10.4161/oxim.2.5.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malo M.S., Biswas S., Abedrapo M.A., Yeh L., Chen A., Hodin R.A. The pro-inflammatory cytokines, IL-1beta and TNF-alpha, inhibit intestinal alkaline phosphatase gene expression. DNA Cell Biol. 2006;25:684–695. doi: 10.1089/dna.2006.25.684. [DOI] [PubMed] [Google Scholar]
- 47.Torrens-Mas M., Hernández-López R., Pons D.G., Roca P., Oliver J., Sastre-Serra J. Sirtuin 3 silencing impairs mitochondrial biogenesis and metabolism in colon cancer cells. Am. J. Physiol. Cell Physiol. 2019;317:C398–C404. doi: 10.1152/ajpcell.00112.2019. [DOI] [PubMed] [Google Scholar]
- 48.Zhao C., Sakaguchi T., Fujita K., Ito H., Nishida N., Nagatomo A., Tanaka-Azuma Y., Katakura Y. Pomegranate-derived polyphenols reduce reactive oxygen species production via SIRT3-mediated SOD2 activation. Oxid. Med. Cell Longev. 2016;2016:2927131. doi: 10.1155/2016/2927131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tran T.Q., Hanse E.A., Habowski A.N., Li H., Gabra M.B.I., Yang Y., Lowman X.H., Ooi A.M., Liao S.Y., Edwards R.A., et al. α-Ketoglutarate attenuates Wnt signaling and drives differentiation in colorectal cancer. Nat. Cancer. 2020;1:345–358. doi: 10.1038/s43018-020-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian Q., Iniguez A.B., Sun Q., Wang H., Du M., Zhu M.J. Dietary alpha-ketoglutarate promotes epithelial metabolic transition and protects against DSS-induced colitis. Mol. Nutr. Food Res. 2021;65:e2000936. doi: 10.1002/mnfr.202000936. [DOI] [PubMed] [Google Scholar]
- 51.McFate T., Mohyeldin A., Lu H., Thakar J., Henriques J., Halim N.D., Wu H., Schell M.J., Tsang T.M., Teahan O., et al. Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J. Biol. Chem. 2008;283:22700–22708. doi: 10.1074/jbc.M801765200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X., Mao M., Zhang Y., Yu K., Zhu W. Succinate modulates intestinal barrier function and inflammation response in pigs. Biomolecules. 2019;9:486. doi: 10.3390/biom9090486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jastrząb A., Gęgotek A., Skrzydlewska E. Cannabidiol regulates the expression of keratinocyte proteins involved in the inflammation process through transcriptional regulation. Cells. 2019;8:827. doi: 10.3390/cells8080827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Böckmann S., Hinz B. Cannabidiol promotes endothelial cell survival by Heme Oxygenase-1-mediated autophagy. Cells. 2020;9:1703. doi: 10.3390/cells9071703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Copple I.M., Lister A., Obeng A.D., Kitteringham N.R., Jenkins R.E., Layfield R., Foster B.J., Goldring C.E., Park B.K. Physical and functional interaction of sequestosome 1 with Keap1 regulates the Keap1-Nrf2 cell defense pathway. J. Biol. Chem. 2010;285:16782–16788. doi: 10.1074/jbc.M109.096545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jain A., Lamark T., Sjøttem E., Larsen K.B., Awuh J.A., Øvervatn A., McMahon M., Hayes J.D., Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khor T.O., Huang M.T., Kwon K.H., Chan J.Y., Reddy B.S., Kong A.N. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66:11580–11584. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y., Yan T., Sun D., Xie C., Wang T., Liu X., Wang J., Wang Q., Luo Y., Wang P., et al. Rutaecarpine inhibits KEAP1-NRF2 interaction to activate NRF2 and ameliorate dextran sulfate sodium-induced colitis. Free Radic. Biol. Med. 2020;148:33–41. doi: 10.1016/j.freeradbiomed.2019.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takagi T., Naito Y., Mizushima K., Hirai Y., Harusato A., Okayama T., Katada K., Kamada K., Uchiyama K., Handa O., et al. Heme oxygenase-1 prevents murine intestinal inflammation. J. Clin. Biochem. Nutr. 2018;63:169–174. doi: 10.3164/jcbn.17-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao J., Wang J.Y., Liu L., Li Y.X., Xun A.Y., Zeng W.S., Jia C.H., Wei X.X., Feng J.L., Zhao L., et al. Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch. Med. Res. 2010;41:288–294. doi: 10.1016/j.arcmed.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 61.Spandidos A., Wang X., Wang H., Seed B. PrimerBank: A resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010;38:D792–D799. doi: 10.1093/nar/gkp1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xue Y., Du M., Zhu M.-J. Quercetin suppresses NLRP3 inflammasome activation in epithelial cells triggered by Escherichia coli O157:H7. Free Radic. Biol. Med. 2017;108:760–769. doi: 10.1016/j.freeradbiomed.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 63.Xue Y., Du M., Zhu M.-J. Raspberry extract prevents NLRP3 inflammasome activation in gut epithelial cells induced by pathogenic Escherichia coli. J. Funct. Foods. 2019;56:224–231. doi: 10.1016/j.jff.2019.03.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available upon request.