Abstract
We have examined the roles of gamma interferon (IFN-γ), nitric oxide (NO), and natural killer (NK) cells in the host resistance to infection with the blood-stage malarial parasite Plasmodium berghei XAT, an irradiation-induced attenuated variant of the lethal strain P. berghei NK65. Although the infection with P. berghei XAT enhanced NK cell lytic activity of splenocytes, depletion of NK1.1+ cells caused by the treatment of mice with anti-NK1.1 antibody affected neither parasitemia nor IFN-γ production by their splenocytes. The P. berghei XAT infection induced a large amount of NO production by splenocytes during the first peak of parasitemia, while P. berghei NK65 infection induced a small amount. Unexpectedly, however, mice deficient in inducible nitric oxide synthase (iNOS−/−) cleared P. berghei XAT after two peaks of parasitemia were observed, as occurred for wild-type control mice. Although the infected iNOS−/− mouse splenocytes did not produce a detectable level of NO, they produced an amount of IFN-γ comparable to that produced by wild-type control mouse splenocytes, and treatment of these mice with neutralizing anti-IFN-γ antibody led to the progression of parasitemia and fatal outcome. CD4−/− mice infected with P. berghei XAT could not clear the parasite, and all these mice died with apparently reduced IFN-γ production. Furthermore, treatment with carrageenan increased the susceptibility of mice to P. berghei XAT infection. These results suggest that neither NO production nor NK cell activation is critical for the resistance to P. berghei XAT infection and that IFN-γ plays an important role in the elimination of malarial parasites, possibly by the enhancement of phagocytic activity of macrophages.
Both cell-mediated immunity and humoral immunity play important roles in the mechanisms of defense against intracellular pathogens. Although these defense mechanisms largely depend on antigen-specific T helper (Th) cell activation, early innate mechanisms associated with natural killer (NK) cells, cytokines, and nitric oxide (NO) produced by phagocytic cells are also important.
Cytolytic activity and gamma interferon (IFN-γ) production of NK1.1+ cells were shown to be important for innate resistance to a variety of pathogens (1). Strains of mice resistant to Plasmodium chabaudi infection were reported to exhibit high NK cell activity (1), and NK cells were suggested to play a role in protection from malarial parasites (14, 23, 34, 39). However, resistance to P. chabaudi and P. vinckei petteri infection was shown not to be impaired in beige mutant C57BL/6 mice with reduced NK cell activity (19, 33, 45). Thus, the role of NK cells in the protection against malarial infection has not been elucidated.
NO produced by the activation of inducible nitric oxide synthase (iNOS) has been indicated to be an important effector molecule to kill a variety of pathogens, since iNOS antagonists inhibited macrophage killing of pathogens in vitro and in vivo (6, 16, 20). NO production pathways have been shown to be activated by IFN-γ or lipopolysaccharide in various types of cells, including macrophages (37), endothelial cells (24), and hepatocytes (21). Resistance to blood-stage P. chabaudi AS infection was reported to correlate with the amount of NO produced by splenocytes at an early stage of the infection (12). Moreover, adoptive transfer of a Th1 clone was shown to protect the host from P. chabaudi AS infection, and an NO-dependent mechanism was asserted to play a critical role in the protection, because treatment with an iNOS inhibitor, aminoguanidine, made mice susceptible to the infection and the increase in susceptibility was correlated with reduction in serum NO2− level (12). Furthermore, administration of recombinant interleukin-12 (IL-12) was reported to promote resistance to P. chabaudi AS infection via an NO-dependent mechanism (36). We have recently shown that IL-12 produced by splenocytes plays an important role in protection against infection with P. berghei XAT, an irradiation-induced attenuated variant of the lethal strain P. berghei NK65, through stimulation of IFN-γ production (46). In liver-stage infection, IFN-γ produced by CD8+ T cells was shown to stimulate NO production of liver cells (31). The authors of that study proposed that the NO production is critical for the destruction of infected hepatocytes. The administration of recombinant IL-12 was indicated to cure P. yoelii sporozoite infection of mice by stimulating the production of IFN-γ and NO (30).
In the present study, we have examined the roles of IFN-γ, NO, and NK1.1+ cells in the host defense against P. berghei XAT infection by using iNOS-deficient (iNOS−/−) mice and mice depleted of NK1.1+ cells by treatment with anti-NK1.1. Our results indicate that neither NO nor NK1.1+ cells play a crucial role in the resistance against P. berghei XAT infection, although the P. berghei XAT infection induced NO production and NK cell activation more efficiently than the infection with P. berghei NK65. IFN-γ was indicated to play a critical role in the resistance against P. berghei XAT infection. Although the cells that produced IFN-γ in mice with P. berghei XAT infection were not formally identified, CD4+ cells were suggested to play a role.
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice were purchased from Japan SLC (Hamamatsu, Japan). iNOS−/− mice backcrossed onto C57BL/6 mice were kindly provided by J. D. MacMicking and C. Nathan (Cornell University Medical College, New York, N.Y.) and J. S. Mudgett (Merck Research Laboratories, Rahway, N.J.) (17); in some experiments, we also used iNOS−/− mice purchased from Jackson Laboratory, Bar Harbor, Maine (15). CD4−/− mice on C57BL/6 background were a generous gift from T. W. Mak (26) (University of Toronto, Toronto, Ontario, Canada). C57BL/6 mice were used as controls in all experiments. Mice were used for experiments at 6 to 10 weeks of age.
Culture media.
RPMI 1640 (JRH Biosciences, Lenexa, Kans.) supplemented with 10% fetal calf serum (Summit Biotechnology, Fort Collins, Colo.), 5 × 10−5 M 2-mercaptoethanol (Wako Pure Chemical Industries, Osaka, Japan), and kanamycin (100 μg/ml) (Meiji Seika, Tokyo, Japan) was used. Eagle’s minimal essential medium (JRH Biosciences) was used for cell washing.
Parasite infection.
For malarial infection, mice were injected intravenously (i.v.) with erythrocyte (RBC) suspension containing 104 RBC parasitized (PRBC) with a lethal strain, P. berghei NK65, or its irradiation-induced attenuated variant P. berghei XAT (41). Parasitemia was assessed by the microscopic examination of Giemsa-stained smears of tail blood. The percent parasitemia was calculated as follows: parasitemia (%) = [(number of infected RBC)/(total number of RBC counted)] × 100.
NK cell activity.
YAC-1 lymphoma cells were labeled with 51Cr by incubation at 37°C (5% CO2) for 1 h with Na251CrO4 (Amersham, Arlington Heights, Ill.) and washed extensively. The 51Cr-labeled YAC-1 cells (104 cells) were incubated at 37°C (5% CO2) for 4 h with effector splenocytes in 0.2 ml of RPMI 1640 medium in a round-bottom 96-well plate (Corning, New York, N.Y.). 51Cr released into the supernatant was estimated in a gamma counter (1470 WIZARD; Wallac, Turku, Finland). The activity was assayed at effector-to-target-cell ratios of 100:1, 50:1, 25:1, and 12.5:1. The percent specific 51Cr release was calculated as follows: [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. The maximum release was obtained by target cell lysis with 1% Triton X-100 (Wako). In all experiments, spontaneous release did not exceed 11% of the maximum release.
Depletion of NK cells in vivo with monoclonal antibody (MAb).
To deplete NK cells in vivo, each mouse was injected intraperitoneally (i.p.) with rat anti-mouse NK1.1 (PK136, rat immunoglobulin G1 [IgG1]) at 0.3 mg/injection once daily for 3 consecutive days before the day of the parasite inoculation and then once daily every other day for 20 days. Anti-NK1.1 was purified from ascites on a protein G column (Pharmacia, Uppsala, Sweden). Normal rat IgG (Sigma, St. Louis, Mo.) was used as a control.
FACScan analysis.
One million splenocytes were stained with phycoerythrin (PE)-labeled anti-NK1.1 (PK136, rat IgG1; PharMingen, San Diego, Calif.) or PE-labeled anti-IL-2Rβ (TM-β1, rat IgG2b; PharMingen) and analyzed for NK (NK1.1+ or IL-2Rβ+) cells on a FACScan by using Lysis II software (Becton Dickinson, Mountain View, Calif.) for data analysis.
Detection of NO production.
Splenocytes were incubated for 72 h at 6 × 106 cells/ml without addition of parasite antigen in RPMI 1640 medium. Culture supernatants were assayed for NO2− by the Griess reaction (12). Briefly, 100 μl of the supernatant was incubated with 100 μl of Griess reagent for 5 min at room temperature, and NO2− concentration was determined by measuring the optical density at 550 nm (OD550) in reference to the OD550 of standard NaNO2 solution.
Assay for IFN-γ production by splenocytes.
Splenocytes were incubated for 48 h at 6 × 106 cells/ml without addition of parasite antigen in RPMI 1640 medium, and culture supernatants were assayed for IFN-γ in a sandwich enzyme-linked immunosorbent assay (ELISA) by using two different clones of rat MAbs against mouse IFN-γ (R4-6A2, rat IgG1, and XMG1.2, rat IgG1; PharMingen) according to the manufacturer’s instructions.
Neutralization of IFN-γ in vivo with MAb.
To neutralize IFN-γ in vivo, each mouse was injected i.p. with rat anti-mouse IFN-γ (XMG1.2, rat IgG1) (4) at 0.2 mg/injection once daily for 4 consecutive days starting on the day of the parasite inoculation and then twice a week for 3 weeks. Anti-IFN-γ was purified from ascites on a protein G column. Normal rat IgG was used as a control.
Treatment of mice with CGN.
Carrageenan (CGN) type II (Sigma) in sterile phosphate-buffered saline (PBS) was injected i.p. into each mouse at 1 mg/injection on days −9, −7, −5, −3, −1, +1, +3, +5, +7, and +9 in relation to the day of PRBC inoculation.
Assay for phagocytosis.
Ten million splenocytes obtained from P. berghei XAT-infected mice and uninfected mice treated with CGN or PBS were incubated at 37°C (10% CO2) for 2 h on a culture dish, and then nonadherent cells were removed by washing three times with warmed Eagle’s minimal essential medium. Fluorescein isothiocyanate (FITC)-conjugated beads (Fluoresbrite Plain YG 0.75-μm Microspheres; Polysciences, Inc., Warrington, Pa.) were added to the adherent cells and incubated at 37°C (5% CO2) for 2 h. These adherent cells were then collected with cold 5 mM EDTA–PBS and stained with PE-labeled anti-Mac-1 (M1/70, rat IgG2b; Serotec, Oxford, United Kingdom), followed by analysis for the cells containing FITC-conjugated beads.
Statistical analysis.
Statistical analysis was performed by using Student’s t test.
RESULTS
No effect of NK cell lytic activity on the resistance to P. berghei XAT infection.
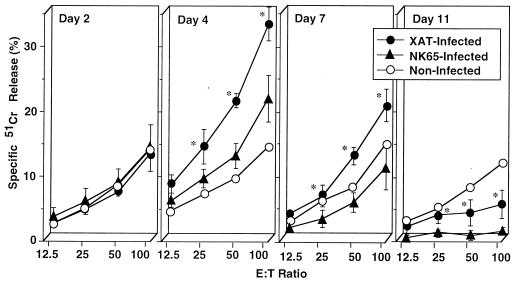
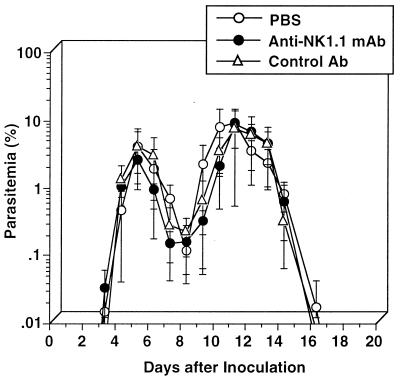
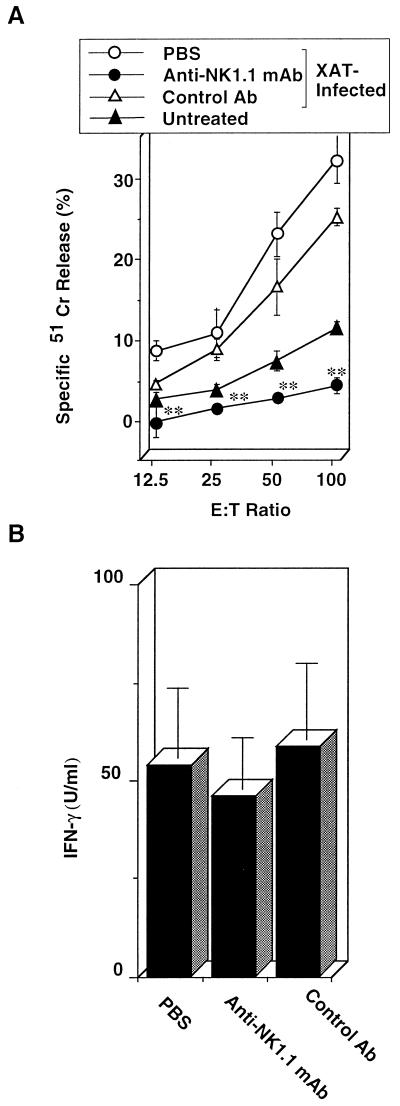
We first compared splenocytes from C57BL/6 mice infected with P. berghei XAT to those from P. berghei NK65-infected mice for NK cell lytic activity using YAC-1 cells as a target. The NK cell lytic activity was significantly increased by P. berghei XAT infection; the peak activity was observed on day 4 after the inoculation of the parasites, and the activity gradually decreased thereafter (Fig. 1). In contrast, P. berghei NK65 infection barely increased the activity on day 4, and the activity was rapidly decreased thereafter (Fig. 1). We repeated the experiments and obtained essentially the same results. These results suggest the correlation of NK cell lytic activities with the host resistance against P. berghei XAT and P. berghei NK65 infections. To examine further the role of NK1.1+ cells, mice were treated with anti-NK1.1 antibody to deplete NK1.1+ cells, infected with P. berghei XAT, and assayed for parasitemia. Unexpectedly, the parasites were cleared in mice treated with anti-NK1.1 antibody after these mice showed temporal kinetics of parasitemia similar to that for control mice (Fig. 2). To confirm the depletion of NK1.1+ cells in these mice, NK cell lytic activities for splenocytes from these mice were assayed 4 days after the infection. The activity was confirmed to be abrogated by the anti-NK1.1 treatment (Fig. 3A). Neither NK1.1+ cells nor IL-2Rβ+ cells were detected in these splenocytes (data not shown). NK+ cells are known to produce IFN-γ. Therefore, we also assayed IFN-γ production by splenocytes from anti-NK1.1-treated mice 4 days after the P. berghei XAT infection. In our preliminary experiments, IFN-γ production of splenocytes reached the maximum 4 days after the infection and decreased rapidly thereafter. We observed no significant difference in IFN-γ production between anti-NK1.1-treated mice and mice treated with control antibody or PBS (Fig. 3B). These results were all confirmed to be reproducible in experiments performed twice. These results suggest that NK cell activity is not critical for the host resistance against infection with P. berghei XAT.
FIG. 1.
Increased NK cell lytic activity in splenocytes caused by the infection with blood-stage P. berghei XAT but not with P. berghei NK65. After i.v. inoculation of normal mice with 104 PRBC, splenocytes were obtained at various time intervals and assayed for NK cell lytic activity by using YAC-1 cells as a target. The percentages of spontaneous 51Cr release in assays of splenocytes obtained on days 2, 4, 7, and 11 were 5.7, 6.1, 10.7, and 5.7% of the maximum release, respectively. Data are means ± SD for three mice. ∗, P < 0.05, compared with data for NK65-infected mice. These results were confirmed to be reproducible by performing a repeat experiment. E:T, effector-to-target-cell.
FIG. 2.
No effect of NK cell depletion on the resistance to infection with blood-stage P. berghei XAT. NK1.1+ cells were depleted by treatment with anti-NK1.1 MAb at 0.3 mg/injection/mouse once daily for 3 consecutive days before the day of inoculation i.v. of 104 PRBC and then every other day for 20 days. Normal rat IgG was used as a control antibody. Parasitemia was assessed by the microscopic examination of Giemsa-stained smears of tail blood. Data are means ± SD for five mice. These results were confirmed to be reproducible by performing a repeat experiment.
FIG. 3.
NK cell lytic activity was reduced in mice treated with anti-NK1.1 without impairment of IFN-γ production. Mice were injected i.p. at 0.3 mg/injection/mouse with anti-NK1.1 once daily for 3 consecutive days before the day of the 1 × 104 PRBC i.v. inoculation and then every other day for 20 days. (A) Spleens were obtained 4 days after the inoculation of parasites and assayed for NK cell lytic activity by using YAC-1 cells as a target. E:T, effector-to-target-cell. The percent spontaneous 51Cr release in the assay was 8.7% of the maximum release. Data are means ± SD for three mice. (B) Splenocytes obtained 4 days after the parasite inoculation were cultured without addition of parasite antigen for 48 h, and the culture supernatants were assayed for IFN-γ by using an ELISA. Data are means ± SD for three mice. ∗∗, P < 0.01, compared with the data in PBS- or control antibody-treated mice. These results were confirmed to be reproducible by performing a repeat experiment.
Splenocytes from mice infected with P. berghei XAT produced more NO than those from P. berghei NK65-infected mice.
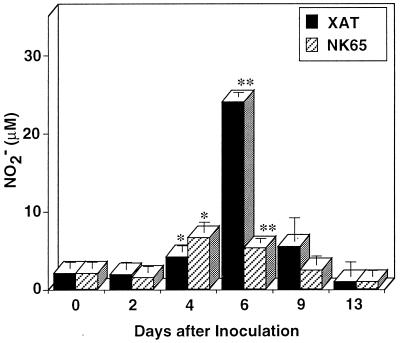
We next analyzed in vitro NO production of splenocytes from mice infected with P. berghei XAT or P. berghei NK65. For splenocytes obtained 4 days after P. berghei XAT infection, significant NO production was observed, and the peak production was observed for splenocytes obtained 6 days after the parasite inoculation (Fig. 4), coincident with the first peak of parasitemia. In contrast, only a weak, though significant, enhancement of NO production was observed for splenocytes obtained from mice 4 and 6 days after the inoculation with P. berghei NK65 (Fig. 4). The experiment was performed twice, and essentially the same results were obtained both times. The difference in NO production on days 4 and 6 postinfection was further confirmed in additional experiments performed four times. These results indicate that P. berghei XAT infection induced NO production in an early phase of infection more efficiently than P. berghei NK65 infection. Thus, NO production by splenocytes seems to correlate with the different degrees of resistance to P. berghei XAT and P. berghei NK65 infection.
FIG. 4.
Splenocytes obtained from mice infected with blood-stage P. berghei XAT produced more NO than those obtained from mice infected with P. berghei NK65. After i.v. inoculation of 104 PRBC, splenocytes were obtained at various intervals and cultured in vitro without addition of parasite antigen for 72 h. The culture supernatants were assayed for NO2−. Data are means ± SD for three mice. ∗, P < 0.05, and ∗∗, P < 0.01, compared with the data for splenocytes obtained on day 0. Similar experiments were performed three times, and essentially the same results were obtained.
No difference in parasitemia between iNOS−/− mice and wild-type control mice inoculated with P. berghei XAT.
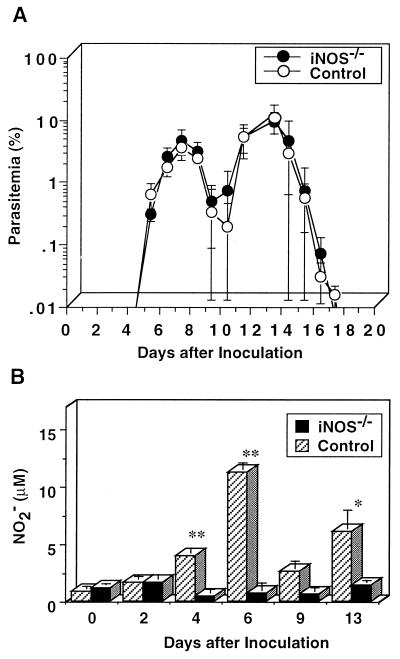
To examine the role of NO in the host defense against P. berghei XAT infection, we inoculated P. berghei XAT into iNOS−/− mice and wild-type control mice and assayed for parasitemia. Surprisingly, iNOS−/− mice showed temporal kinetics of parasitemia similar to that in wild-type control mice and cleared parasites (Fig. 5A). The results were confirmed in experiments carried out twice by using the same protocol. We also obtained similar results in experiments in which 105 PRBC were inoculated (data not shown). The results were essentially the same for two different strains of iNOS−/− mice obtained from different sources (data not shown). We inoculated PRBC with P. berghei XAT into more than 30 iNOS−/− mice in total; however, all these mice cleared the parasites. NO production by splenocytes from iNOS−/− mice was confirmed not to be induced by the P. berghei XAT infection, although infected wild-type control mice produced a large amount of NO (Fig. 5B). The results were confirmed in three repeated experiments. Inability of iNOS−/− mice infected with P. berghei XAT to produce NO was also confirmed by stimulation of their splenocytes with lipopolysaccharide (data not shown). These results suggest that NO production is not critical for the defense against P. berghei XAT infection.
FIG. 5.
Comparable susceptibilities of iNOS−/− mice and wild-type control mice to the infection with blood-stage P. berghei XAT. (A) After iNOS−/− mice or wild-type control mice were inoculated i.v. with 104 PRBC, parasitemia was assessed by the microscopic examination of Giemsa-stained smears of tail blood. Data are means ± SD for five mice. (B) After iNOS−/− mice or wild-type control mice were inoculated i.v. with 104 PRBC, splenocytes were obtained at various intervals and cultured in vitro without addition of parasite antigen for 72 h. The culture supernatants were assayed for NO2−. Data are means ± SD for three mice. ∗, P < 0.05, and ∗∗, P < 0.01, compared with the data for splenocytes obtained on day 0. These experiments were performed three times, and similar results were obtained.
Important role of IFN-γ in the resistance of iNOS−/− mice to infection with blood-stage P. berghei XAT.
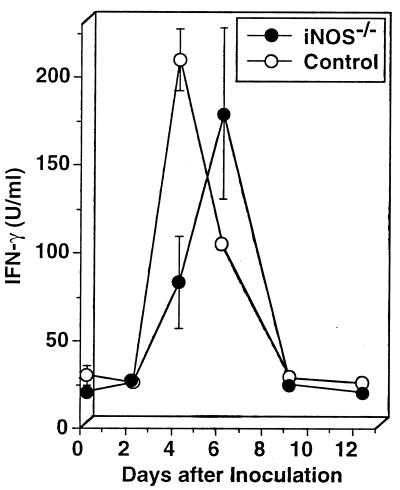
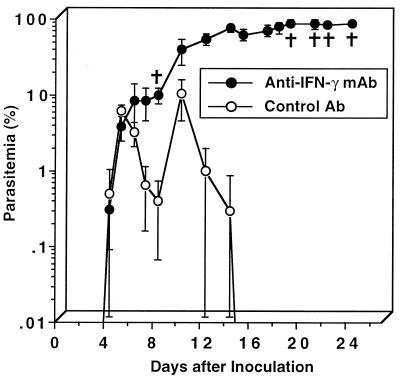
To examine the mechanism of clearing of the parasite in iNOS−/− mice, we analyzed IFN-γ production by their splenocytes. IFN-γ production by wild-type control mouse splenocytes was increased after the inoculation of P. berghei XAT and peaked on day 4 (Fig. 6). iNOS−/− mouse splenocytes also produced a level of IFN-γ comparable to that produced by wild-type control mouse splenocytes, although the peak response was delayed by 2 days (Fig. 6). Since IFN-γ was demonstrated to play a critical role in protective immunity against P. berghei infection in wild-type control mice (42, 43), we next examined whether neutralization of IFN-γ by anti-IFN-γ antibody made iNOS−/− mice susceptible to P. berghei XAT infection. Parasitemia in iNOS−/− mice treated with anti-IFN-γ antibody progressively increased, and all mice eventually died (Fig. 7). On the other hand, the treatment with control antibody did not affect the parasitemia. We obtained essentially the same results in three repeated experiments. These results indicate that IFN-γ plays a critical role in the host defense against P. berghei XAT infection in iNOS−/− mice.
FIG. 6.
Comparable production of IFN-γ in splenocytes from iNOS−/− mice and in those from wild-type control mice caused by the infection with blood-stage P. berghei XAT. After iNOS−/− mice or wild-type control mice were inoculated i.v. with 104 PRBC, splenocytes were obtained at various intervals and cultured in vitro without addition of parasite antigen for 48 h. The culture supernatants were assayed for IFN-γ by using an ELISA. Data are means ± SD for three mice. Similar results were obtained in two successive experiments.
FIG. 7.
Important role for IFN-γ in the host resistance of iNOS−/− mice against the infection with blood-stage P. berghei XAT. After iNOS−/− mice were inoculated i.v. with 104 PRBC, endogenously produced IFN-γ was neutralized by treatment at 0.2 mg/mouse with anti-IFN-γ once daily for 4 consecutive days starting from the day of the inoculation and then twice a week for 3 weeks. Parasitemia was assessed by the microscopic examination of Giemsa-stained smears of tail blood. Normal rat IgG was used as a control antibody. Data are means ± SD for five mice. †, days on which individual mice died. We obtained essentially the same results in three successive experiments.
Role of CD4+ T cells in IFN-γ production in P. berghei XAT infection.
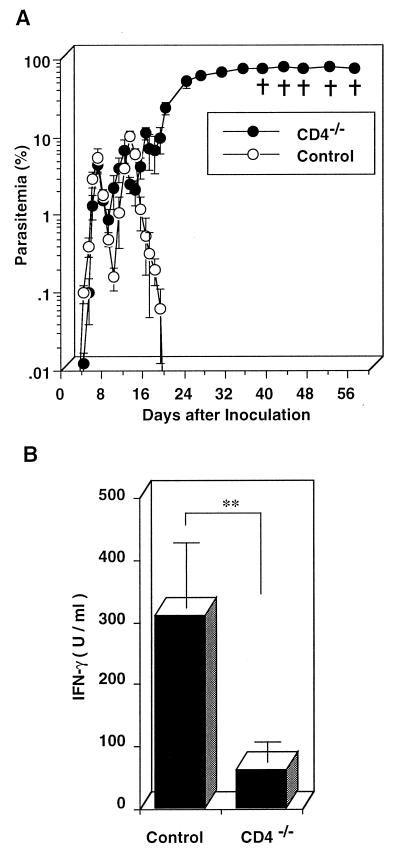
We examined the role of CD4+ T cells in IFN-γ production in P. berghei XAT infection using CD4−/− mice. When CD4−/− mice were injected with 104 PRBC, the PRBC progressively increased in number after small peaks in parasitemia were observed; all these mice eventually died (Fig. 8A). When splenocytes from CD4−/− mice inoculated with PRBC were assayed for IFN-γ production in vitro, spleen cells obtained from both CD4−/− mice and wild-type mice on day 4 after the parasite inoculation were found to show peak responses in IFN-γ production. The results for their peak responses are shown in Fig. 8B. These results indicate that CD4+ T cells play a critical role in the production of IFN-γ, which plays an important role in the clearance of P. berghei XAT.
FIG. 8.
Increased susceptibility to blood-stage P. berghei XAT infection of CD4−/− mice with reduced IFN-γ production. (A) After CD4−/− mice or wild-type control mice were inoculated i.v. with 104 PRBC, parasitemia was assessed by the microscopic examination of Giemsa-stained smears of tail blood. Data are means ± SD for five mice. †, days on which individual mice died. (B) Splenocytes were obtained on day 4 after the parasite inoculation and cultured in vitro without addition of parasite antigen for 48 h. The culture supernatants were assayed for IFN-γ by using an ELISA. Data are means ± SD for three mice. ∗∗, P < 0.01. The results presented in both panels A and B were confirmed to be reproducible in two successive experiments.
Impairment of P. berghei XAT clearance by treatment of mice with CGN.
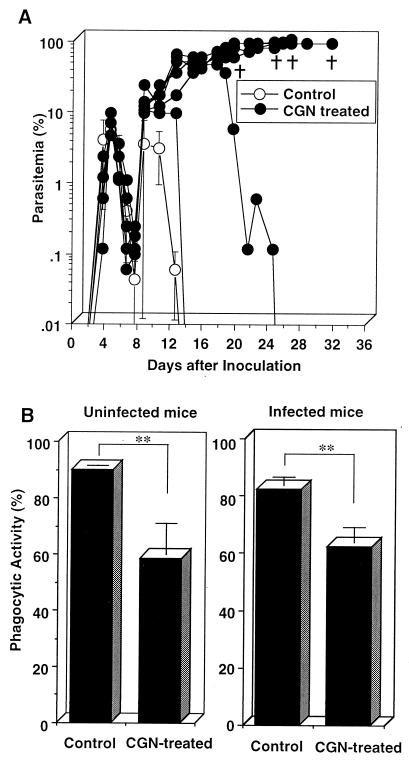
Macrophage function has been suggested to play an important role in the defense mechanisms against malarial infection (2, 32, 35). To investigate the macrophage involvement in the mechanism of defense against P. berghei XAT infection, we examined the effect of CGN treatment on parasitemia of mice infected with P. berghei XAT, since CGN was reported to block macrophage function (11, 27). Parasitemia was progressively increased in four of six mice treated with CGN (Fig. 9A). We obtained similar results in the repeat experiments performed according to the same protocol. Splenocytes obtained from P. berghei XAT-infected mice treated with CGN produced amounts of IFN-γ and NO comparable to those produced by splenocytes from infected mice which had not received the CGN injection. Splenocytes from infected mice treated and not treated with CGN produced 206.3 ± 17.7 and 202.2 ± 67.0 U of IFN-γ/ml and 12.1 ± 4.3 and 13.9 ± 2.2 μM NO2−, respectively, 6 days after the parasite inoculation (values are means ± standard deviations [SD] for three mice). To examine the effect of the CGN treatment on macrophage phagocytic function, adherent spleen cells from infected and uninfected mice treated with CGN were obtained 14 days after the parasite inoculation, incubated with FITC-conjugated beads, and analyzed for their phagocytosis on a FACScan. The percents Mac-1high macrophages containing FITC-conjugated beads for both infected and uninfected mice treated with CGN were significantly reduced compared with those for control mice (Fig. 9B). These results suggest that phagocytic function of macrophages is important for the host defense against the P. berghei XAT infection.
FIG. 9.
Increased susceptibility of mice treated with CGN to blood-stage P. berghei XAT infection with reduced phagocytic activity of spleen macrophages. (A) Mice treated with CGN as described in Materials and Methods were inoculated i.v. with 104 PRBC, and parasitemia was assessed by the microscopic examination of Giemsa-stained smears of tail blood. †, days on which individual mice died. (B) Adherent spleen cells obtained from CGN-treated uninfected mice and also from mice infected for 14 days were incubated with FITC-conjugated beads for 2 h. Phagocytic activity was assayed by FACScan analysis with gating on a Mac-1high population. Data are means ± SD for eight mice. ∗∗, P < 0.01. These results were confirmed to be reproducible in two successive experiments.
DISCUSSION
NK cells play an important role in the innate resistance to a variety of pathogens through their target cell lysis and IFN-γ production (1) and were suggested to be involved in the host resistance to P. falciparum in humans (23, 39), to P. berghei in rats (34), and to P. chabaudi in mice (9). However, beige mutant mice with reduced NK activity were shown to resolve P. chabaudi and P. vinckei petteri infection like the control mice did (19, 33, 45), suggesting that NK cell activity is not crucial for the resistance. The treatment with anti-NK1.1 has been shown to increase the mortality of mice infected with blood-stage P. chabaudi (14). Treatment with anti-asialo GM1 was also reported to increase the parasitemia of mice infected with blood-stage P. chabaudi AS (19) and P. yoelii coincident with impairment in IFN-γ production, especially in the early phase of infection (8). Our present results suggest that NK1.1+ cells do not play a critical role in the resistance to P. berghei XAT infection. In addition, in our present experiments, CD4−/− mice showed increased susceptibility to the infection and their IFN-γ production was shown to be impaired during P. berghei XAT infection. The parasitemia was increased after initial small fluctuations in CD4−/− mice, although in wild-type control mice treated with anti-IFN-γ it increased consistently without regression. The findings may indicate that IFN-γ produced by cells other than CD4+ cells, such as NK cells, plays a role in the defense mechanisms during an early phase of infection with P. berghei XAT. The above results also indicate that CD4+ cells play a more important role in the resistance against P. berghei XAT infection than NK1.1+ cells, although NK cells may play a role in an early phase of the infection and the involvement of NK cells in the defense mechanism could vary depending on the kind of malarial parasite.
The present results show that P. berghei XAT infection induced a larger amount of NO production by splenocytes as compared with P. berghei NK65 infection. NO production by splenocytes was apparently increased in mice infected with P. berghei XAT, and peaked 6 days after the inoculation, coincident with the first peak of parasitemia. In contrast, NO production of splenocytes was only weakly increased by the infection with P. berghei NK65. The mechanism of the difference in NO production between splenocytes infected with lethal and nonlethal strains remains unknown. However, it is possible that the activation of macrophages downstream to IFN-γ stimulation may be impaired in the P. berghei NK65 infection. The correlation of the NO production with host resistance observed in the present experiments seems to suggest an important role of NO in mechanisms of defense against P. berghei XAT infection, as reported for P. chabaudi AS infection (12). Surprisingly, however, iNOS−/− mice infected with P. berghei XAT showed a profile of parasitemia, in terms of both temporal kinetics and percent PRBC, similar to that of wild-type control mice, and all the mice used in our experiments recovered from infection. In the present experiments, we used iNOS−/− mice obtained from two different sources to confirm the results, and essentially the same results were obtained. Splenocytes from iNOS−/− mice were confirmed not to produce NO as a result of the infection with P. berghei XAT (Fig. 2). These results indicate that NO is not critical for the protective immunity to P. berghei XAT infection. iNOS−/− mice were shown to display increased susceptibility to infection with Listeria monocytogenes (17), Leishmania major (44), or Mycobacterium tuberculosis (18). These results indicate that NO plays at least some role in defense mechanisms against these pathogens. Although macrophages from these mice were defective in killing of Toxoplasma gondii in vitro, iNOS−/− mice were shown to survive the acute infection and the protective role of NO in the late stage of the infection was indicated to be tissue specific (29). Taken together, these results suggest a possibility that iNOS−/− mice develop an alternative pathway(s) of pathogen clearance. In our previous experiments, IL-12 was shown to play an important role in the clearance of P. berghei XAT (46). It is possible that IL-12-dependent mechanisms play a compensatory role in the clearance of P. berghei XAT in iNOS−/− mice. In T. gondii infection, IL-12 was shown to be able to enhance protection in the absence of an NO pathway by engaging both IFN-γ-dependent and -independent pathways (13). In our present study, IFN-γ was shown to play a critical role in the parasite clearance in iNOS−/− mice. These results indicate that there is some important mechanism(s) downstream to IFN-γ other than the activation of iNOS for the clearance of P. berghei XAT. Induction of respiratory burst and upregulation of natural resistance-associated macrophage protein 1 (NRAMP1) could be possible mechanisms. NRAMP1−/− mice showed impaired resistance to infection with intracellular parasites (40).
The in vivo role of NO in host resistance to blood-stage P. chabaudi AS was previously investigated by treating resistant C57BL/6 mice with iNOS inhibitors (12, 38). The treatment with aminoguanidine was shown to reduce serum NO3− levels in P. chabaudi AS-infected mice to a level similar to that observed in uninfected control mice, and mortality was increased to 80% without affecting parasitemia (12). Parasitemia with P. chabaudi AS in mice depleted of CD4+ T cells that received transferred Th1 cells was also demonstrated to be increased by treatment with the iNOS inhibitor l-Nγ monomethyl arginine (l-NMMA), although these mice cleared the parasites without the l-NMMA treatment (38). These results might be caused by adverse side effects of these iNOS inhibitors. Aminoguanidine was reported to bind to reactive aldehydes formed by oxidative stress during malarial infection (3, 5, 10, 25).
NO has also been suggested to play a crucial role in protection from blood-stage malarial parasites in humans. Plasma NO levels were reported to increase in patients infected with P. falciparum and P. vivax (7, 22), and the duration of coma due to cerebral malaria was reported to be short in children with high plasma NO levels (7). Moreover, NO-generating compounds were shown to be able to kill blood-stage P. falciparum in vitro (28). It is possible that involvement of iNOS in the clearance of malarial parasites varies depending upon the kind of parasite.
To investigate the involvement of macrophages in the host resistance to P. berghei XAT infection, we examined the effect of the macrophage-toxic substance CGN (11, 27) on parasitemia. Splenocytes obtained from P. berghei XAT-infected mice treated with CGN produced amounts of IFN-γ and NO comparable to those produced by splenocytes from mice which had not been treated with CGN. However, treatment with CGN was shown to increase the parasitemia, resulting in high mortality of mice infected with P. berghei XAT, as previously reported to occur in infection with other parasites (27). The CGN treatment did not affect the first peak of parasitemia (Fig. 9A). The finding suggests that the mechanism(s) of resistance to P. berghei XAT during the early phase of the infection is different from that during the second peak of parasitemia. The CGN treatment may affect only the mechanism involved in the late phase. Thus, the function, possibly phagocytic activity, of macrophages activated by IFN-γ may play an important role in the resistance to P. berghei XAT infection.
Taken together, the present results suggest that neither NO production nor NK cell activation plays a critical role in the resistance to blood-stage P. berghei XAT infection, although both NO production and NK cell activity correlate with the resistance of mice to infections with P. berghei XAT and P. berghei NK65. IFN-γ was indicated to play an important role in the protective immunity through macrophage activation. Although the cells that produce IFN-γ in P. berghei XAT infection were not formally identified, CD4+ cells were indicated to play an important role in the IFN-γ production.
ACKNOWLEDGMENTS
We thank J. D. MacMicking and C. Nathan (Cornell University Medical College, New York, N.Y.) for providing iNOS−/− mice. We also thank J. S. Mudgett (Merck Research Laboratories, Rahway, N.J.) and T. W. Mak (University of Toronto, Toronto, Ontario, Canada) for kindly providing iNOS−/− mice and CD4−/− mice, respectively.
This study was supported by a Grant-in-Aid for Scientific Research on Priority Areas, by a Grant-in-Aid for International Scientific Research (Joint Research), and by a Grant-in-Aid for Encouragement of Young Scientists from the Ministry of Education, Science, Sports and Culture, Japan, and from the Japanese Ministry of Public Health and Welfare.
REFERENCES
- 1.Bancroft G J. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993;5:503–510. doi: 10.1016/0952-7915(93)90030-v. [DOI] [PubMed] [Google Scholar]
- 2.Brown K M, Kreier J P. Effect of macrophage activation on phagocyte-plasmodium interaction. Infect Immun. 1986;51:744–749. doi: 10.1128/iai.51.3.744-749.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buffinton G D, Hunt N H, Cowden W B, Clark I A. Detection of short-chain carbonyl products of lipid peroxidation from malaria-parasite (Plasmodium vinckei)-infected red blood cells exposed to oxidative stress. Biochem J. 1988;249:63–68. doi: 10.1042/bj2490063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherwinski H M, Schumacher J H, Brown K D, Mosmann T R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166:1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark I A, Butcher G A, Buffinton G D, Hunt N H, Cowden W B. Toxicity of certain products of lipid peroxidation to the human malaria parasite Plasmodium falciparum. Biochem Pharmacol. 1987;36:543–546. doi: 10.1016/0006-2952(87)90364-9. [DOI] [PubMed] [Google Scholar]
- 6.Clark I A, Rockett K A. Nitric oxide and parasitic disease. Adv Parasitol. 1996;37:1–56. doi: 10.1016/s0065-308x(08)60218-3. [DOI] [PubMed] [Google Scholar]
- 7.Cot S, Ringwald P, Mulder B, Miailhes P, Yap Yap J, Nussler A K, Eling W M. Nitric oxide in cerebral malaria. J Infect Dis. 1994;169:1417–1418. doi: 10.1093/infdis/169.6.1417. [DOI] [PubMed] [Google Scholar]
- 8.De Souza J B, Williamson K H, Otani T, Playfair J H. Early gamma interferon responses in lethal and nonlethal murine blood-stage malaria. Infect Immun. 1997;65:1593–1598. doi: 10.1128/iai.65.5.1593-1598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eugui E M, Allison A C. Differences in susceptibility of various mouse strains to haemoprotozoan infections: possible correlation with natural killer activity. Parasite Immunol. 1980;2:277–292. doi: 10.1111/j.1365-3024.1980.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 10.Goode H F, Webster N R. Free radicals and antioxidants in sepsis. Crit Care Med. 1993;21:1770–1776. doi: 10.1097/00003246-199311000-00029. [DOI] [PubMed] [Google Scholar]
- 11.Ishizaka S, Otani S, Morisawa S. Effect of carrageenan on immune responses. II. A possible regulatory role of macrophages in the immune responses of low-responder mice. J Immunol. 1978;120:61–65. [PubMed] [Google Scholar]
- 12.Jacobs P, Radzioch D, Stevenson M M. Nitric oxide expression in the spleen, but not in the liver, correlates with resistance to blood-stage malaria in mice. J Immunol. 1995;155:5306–5313. [PubMed] [Google Scholar]
- 13.Khan I, Matsuura A T, Fonseka S, Kasper L H. Production of nitric oxide (NO) is not essential for protection against acute Toxoplasma gondii infection in IRF-1−/− mice. J Immunol. 1996;156:636–643. [PubMed] [Google Scholar]
- 14.Kitaguchi T, Nagoya M, Amano T, Suzuki M, Minami M. Analysis of roles of natural killer cells in defense against Plasmodium chabaudi in mice. Parasitol Res. 1996;82:352–357. doi: 10.1007/s004360050125. [DOI] [PubMed] [Google Scholar]
- 15.Laubach V E, Shesely E G, Smithies O, Sherman P A. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci USA. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacMicking J, Xie Q W, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 17.MacMicking J D, Nathan C, Hom G, Chartrain N, Fletcher D S, Trumbauer M, Stevens K, Xie Q W, Sokol K, Hutchinson N. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 18.MacMicking J D, North R J, LaCourse R, Mudgett J S, Shah S K, Nathan C F. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohan K, Moulin P, Stevenson M M. Natural killer cell cytokine production, not cytotoxicity, contributes to resistance against blood-stage Plasmodium chabaudi AS infection. J Immunol. 1997;159:4990–4998. [PubMed] [Google Scholar]
- 20.Nathan C. Natural resistance and nitric oxide. Cell. 1995;82:873–876. doi: 10.1016/0092-8674(95)90019-5. [DOI] [PubMed] [Google Scholar]
- 21.Nussler A, Drapier J C, Renia L, Pied S, Miltgen F, Gentilini M, Mazier D. l-Arginine-dependent destruction of intrahepatic malaria parasites in response to tumor necrosis factor and/or interleukin 6 stimulation. Eur J Immunol. 1991;21:227–230. doi: 10.1002/eji.1830210134. [DOI] [PubMed] [Google Scholar]
- 22.Nussler A K, Eling W, Kremsher P G. Patients with Plasmodium falciparum malaria and Plasmodium vivax malaria show increased nitrite and nitrate plasma levels. J Infect Dis. 1994;169:1418–1419. doi: 10.1093/infdis/169.6.1418. [DOI] [PubMed] [Google Scholar]
- 23.Orago A S, Facer C A. Cytotoxicity of human natural killer (NK) cell subsets for Plasmodium falciparum erythrocytic schizonts: stimulation by cytokines and inhibition by neomycin. Clin Exp Immunol. 1991;86:22–29. doi: 10.1111/j.1365-2249.1991.tb05768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oswald I P, Eltoum I, Wynn T A, Schwartz B, Caspar P, Paulin D, Sher A, James S L. Endothelial cells are activated by cytokine treatment to kill an intravascular parasite, Schistosoma mansoni, through the production of nitric oxide. Proc Natl Acad Sci USA. 1994;91:999–1003. doi: 10.1073/pnas.91.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picard S, Parthasarathy S, Fruebis J, Witztum J L. Aminoguanidine inhibits oxidative modification of low density lipoprotein protein and the subsequent increase in uptake by macrophage scavenger receptors. Proc Natl Acad Sci USA. 1992;89:6876–6880. doi: 10.1073/pnas.89.15.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahemtulla A, Fung Leung W P, Schilham M W, Kundig T M, Sambhara S R, Narendran A, Arabian A, Wakeham A, Paige C J, Zinkernagel R M. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 27.Rao U R, Vickery A C, Kwa B H, Nayar J K, Subrahmanyam D. Effect of carrageenan on the resistance of congenitally athymic nude and normal BALB/c mice to infective larvae of Brugia malayi. Parasitol Res. 1992;78:235–240. doi: 10.1007/BF00931732. [DOI] [PubMed] [Google Scholar]
- 28.Rockett K A, Awburn M M, Cowden W B, Clark I A. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun. 1991;59:3280–3283. doi: 10.1128/iai.59.9.3280-3283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scharton Kersten T M, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sedegah M, Finkelman F, Hoffman S L. Interleukin 12 induction of interferon-γ-dependent protection against malaria. Proc Natl Acad Sci USA. 1994;91:10700–10702. doi: 10.1073/pnas.91.22.10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seguin M C, Klotz F W, Schneider I, Weir J P, Goodbary M, Slayter M, Raney J J, Aniagolu J U, Green S J. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei infected mosquitoes: involvement of interferon-γ and CD8+ T cells. J Exp Med. 1994;180:353–358. doi: 10.1084/jem.180.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shear H L, Nussenzweig R S, Bianco C. Immune phagocytosis in murine malaria. J Exp Med. 1979;149:1288–1298. doi: 10.1084/jem.149.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skamene E, Stevenson M M, Lemieux S. Murine malaria: dissociation of natural killer (NK) cell activity and resistance to Plasmodium chabaudi. Parasite Immunol. 1983;5:557–565. doi: 10.1111/j.1365-3024.1983.tb00772.x. [DOI] [PubMed] [Google Scholar]
- 34.Solomon J B. Natural cytotoxicity for Plasmodium berghei in vitro by spleen cells from susceptible and resistant rats. Immunology. 1986;59:277–281. [PMC free article] [PubMed] [Google Scholar]
- 35.Stevenson M M, Ghadirian E, Phillips N C, Rae D, Podoba J E. Role of mononuclear phagocytes in elimination of Plasmodium chabaudi AS infection. Parasite Immunol. 1989;11:529–544. doi: 10.1111/j.1365-3024.1989.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 36.Stevenson M M, Tam M F, Wolf S F, Sher A. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-γ and TNF-α and occurs via a nitric oxide-dependent mechanism. J Immunol. 1995;155:2545–2556. [PubMed] [Google Scholar]
- 37.Stuehr D J, Marletta M A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci USA. 1985;82:7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor Robinson A W, Phillips R S, Severn A, Moncada S, Liew F Y. The role of TH1 and TH2 cells in a rodent malaria infection. Science. 1993;260:1931–1934. doi: 10.1126/science.8100366. [DOI] [PubMed] [Google Scholar]
- 39.Theander T G, Pedersen B K, Bygbjerg I C, Jepsen S, Larsen P B, Kharazmi A. Enhancement of human natural cytotoxicity by Plasmodium falciparum antigen activated lymphocytes. Acta Trop. 1987;44:415–422. [PubMed] [Google Scholar]
- 40.Vidal S, Tremblay M L, Govoni G, Gaouthin S, Sebastioni G, Malo D, Skamene E, Olivier M, Jothy S, Gras P. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J Exp Med. 1995;182:655–666. doi: 10.1084/jem.182.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waki S, Tamura J, Imanaka M, Ishikawa S, Suzuki M. Plasmodium berghei: isolation and maintenance of an irradiation attenuated strain in the nude mouse. Exp Parasitol. 1982;53:335–340. doi: 10.1016/0014-4894(82)90076-5. [DOI] [PubMed] [Google Scholar]
- 42.Waki S, Uehara S, Kanbe K, Nariuchi H, Suzuki M. Interferon-γ and the induction of protective IgG2a antibodies in non-lethal Plasmodium berghei infections of mice. Parasite Immunol. 1995;17:503–508. doi: 10.1111/j.1365-3024.1995.tb00880.x. [DOI] [PubMed] [Google Scholar]
- 43.Waki S, Uehara S, Kanbe K, Ono K, Suzuki M, Nariuchi H. The role of T cells in pathogenesis and protective immunity to murine malaria. Immunology. 1992;75:646–651. [PMC free article] [PubMed] [Google Scholar]
- 44.Wei X Q, Charles I G, Smith A, Ure J, Feng G J, Huang F P, Xu D, Muller W, Moncada S, Liew F Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 45.Wood P R, Clark I A. Apparent irrelevance of NK cells to resolution of infections with Babesia microti and Plasmodium vinckei petteri in mice. Parasite Immunol. 1982;4:319–327. doi: 10.1111/j.1365-3024.1982.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 46.Yoshimoto T, Yoneto T, Waki S, Nariuchi H. Interleukin-12-dependent mechanisms in the clearance of blood-stage murine malaria parasite Plasmodium berghei XAT, an attenuated variant of P. berghei NK65. J Infect Dis. 1998;177:1674–1681. doi: 10.1086/515301. [DOI] [PubMed] [Google Scholar]