Abstract
Cysticercosis is a parasitic infection and neglected tropical disease caused by Taenia solium, or the pork tapeworm. Cysticercosis with central nervous system involvement, or neurocysticercosis, is a leading cause of chronic headaches and epilepsy in endemic regions, including Latin America and Asia. In the United States, the epidemiology of cysticercosis has not been well described. We conducted a cross-section serosurvey of Mexican-American adults residing along the Texas–Mexico border (Starr County, Texas) and identified an overall seroprevalence of 7.4% (45/605) for cysticercosis. Brain imaging studies conducted on seropositive study participants identified lesions consistent with calcified neurocysticercosis in 2 of the 45 seropositive individuals. Female sex (p = 0.021), employment in healthcare, caregiving, or social service (p = 0.002), and indoor occupation (p < 0.001) were found to be significantly associated with seropositivity. Further study is needed to evaluate the burden of neurocysticercosis and local transmission risk in this community.
Keywords: taeniasis, tropical medicine, cysticercosis, neurocysticercosis, cestode infections
1. Introduction
Cysticercosis is a neglected tropical disease (NTD) caused by pork tapeworms (Taenia solium). Humans become infected by the ingestion of eggs shed in the stool of a person harboring an adult pork tapeworm infection in their small intestine. Following exposure, metacestodes (colloquially known as cysts) can develop in tissues and organs throughout the body. When cysts develop in the central nervous system, the infection is called neurocysticercosis (NCC). Clinical presentation of NCC varies largely based on the number and location of cysts and can range from asymptomatic to significant neurologic sequelae. Symptoms can include headaches, increased intracranial pressure, and/or seizures/epilepsy [1]. Parenchymal NCC typically presents as seizures, while extraparenchymal NCC can present as headache, dizziness, or increased intracranial pressure from hydrocephalus [1]. The identification of extraparenchymal infection and appropriate treatment is critical, as mortality in these cases is 16% [2]. Chronic symptoms caused by this infection have been associated with significant socioeconomic loss in heavily affected areas [3]. Cysticercosis involving the subcutaneous tissue and muscle is often asymptomatic.
Cysticercosis and NCC are endemic in low- and middle-income countries in Latin America, sub-Saharan Africa, and South, East, and Southeast Asia [1]. In the United States, the majority of recognized NCC cases have been described in immigrants and travelers who acquired the infection in endemic countries [4]. While sporadic reports of autochthonous infection have been documented in the USA [5], the epidemiology of cysticercosis and autochthonous transmission dynamics remains ill defined.
The Texas–Mexico border has been identified as a high-risk area for autochthonous NTD transmission, including cysticercosis and NCC [6]. Starr County, Texas, located on the Texas–Mexico border, consists almost entirely of Mexican Americans, has a high poverty rate (34.9%), and is designated as a Health Professional Shortage Area (HPSA) [7,8]. Other NTDs, such as Chagas disease, have been described in this population [9]. These factors, combined with a high rate of immigration from endemic regions, may put this population disproportionately at risk for cysticercosis and NCC. In the following study, we investigated the prevalence of cysticercosis and NCC in a population living along the Texas–Mexico border. Our overarching study goal was to better define the epidemiology of this disease in an at-risk population living in the USA.
2. Materials and Methods
2.1. Serological Testing and Neuroimaging
We conducted a cross-sectional serologic survey of Mexican-American adults residing in Starr County, Texas, who were enrolled between 2018 and 2020. This convenience sampling utilized samples from an existing cohort that was originally established to investigate the relationship between the gut microbiome and diabetes status [10]. From this cohort (n = 616), plasma from 605 participants (98.2%) was available for cysticercosis serology testing. Samples were tested for reactivity against cysticercosis-specific antigens T24H, GP50, and Ts18var3 via a triplex enzyme-linked immunoassay (ELISA). This triplex ELISA was previously validated and found to have 98% positive and 100% negative concordance when compared to an electroimmunotransfer blot (EITB) as the reference standard [11]. Brain magnetic resonance imaging (MRI) with and without intravenous contrast was performed in qualifying seropositive participants. The brain MRI protocol included axial T1- and T2-weighted images, axial FLAIR (fluid attenuation inversion recovery), and SWI (susceptibility weighted imaging) to evaluate for calcifications and T2-FIESTA (fast imaging employing steady-state acquisition) to assess for subarachnoid NCC. The brain MRI was independently read by two neuroradiologists, one with more than 20 years of experience (F.M.). This study was reviewed and approved by the institutional review board (IRB) at the University of Texas Health Science Center (HSC-SPH-06-0225).
2.2. Risk Factor Analysis
To better understand community- and individual-level drivers for seropositivity, we conducted a risk factor analysis using self-reported survey data and neighborhood-level variables. The survey data included information on participant demographic, socioeconomic, and lifestyle variables. Each participant’s address at enrollment was geocoded to assess for community-level variables, including the area deprivation index (ADI) at a census tract level and if their residence was in a known colonia, a community with substandard housing and a lack of basic municipal services commonly found on the Texas–Mexico border [12]. ADI is a composite score (ranging from 1 to 10) used to assess neighborhood socioeconomic disadvantage [13]. We evaluated the association between these variables and cysticercosis seropositivity using Chi-square and Kruskal–Wallis tests for categorical values and the Mann–Whitney test for continuous variables. All statistical analyses were conducted in Stata v16 [Stata Corp, College Station, TX, USA].
3. Results
We identified an overall seropositivity of 7.4% (45/605) for cysticercosis in the cohort. Of the 45 participants testing positive, 40 were positive for Ts18var3 only, 1 was positive for T24H only, 1 was positive for Ts18var3 and T24H, and 3 were positive for all three antigens. Female sex and type of occupation were found to be significantly associated with positive cysticercosis serology (Table 1). Of the 45 seropositive participants, 39 (86.7%) were female (p = 0.021), though it should be noted that the overall cohort is 71.7% female. Additionally, 26 (58%) were employed in healthcare, caregiving, or social service (p = 0.002), and 42 were employed in predominately indoor occupations (p < 0.001).
Table 1.
Demographic and risk factor analysis of cysticercosis seropositivity. Values are no. (%) except as indicated.
| Demographic | Cysticercosis Seronegative | Cysticercosis Seropositive | p-Value | |
|---|---|---|---|---|
| n = 560 | n = 45 | |||
| Sex | 0.021 ⍭ | |||
| Male | 165 (29.5%) | 6 (13.3%) | ||
| Female | 395 (70.5%) | 39 (86.7%) | ||
| Age (years) | 0.424 * | |||
| 30–39 | 68 (12.1%) | 3 (6.7%) | ||
| 40–49 | 201 (35.9%) | 16 (35.5%) | ||
| 50–59 | 207 (37.0%) | 19 (42.2%) | ||
| >60 | 84 (15.0%) | 7 (15.6%) | ||
| Years of education | 0.954 ⍭ | |||
| ≤12 | 446 (79.6%) | 36 (80.0%) | ||
| >12 | 114 (20.4%) | 9 (20.0%) | ||
| Income (household) | 0.284 * | |||
| <USD 20,000 | 223 (39.8%) | 21 (46.7%) | ||
| USD 20,001–USD 30,000 | 129 (23.0%) | 10 (22.2%) | ||
| USD 30,001–USD 40,000 | 50 (8.9%) | 6 (13.3%) | ||
| USD 40,001–USD 50,000 | 54 (9.6%) | 4 (8.9%) | ||
| >USD 50,001 | 95 (17.0%) | 4 (8.9%) | ||
| Health insurance status | 0.217 ⍭ | |||
| Uninsured | 297 (53.0%) | 20 (44.4%) | ||
| Insured | 253 (45.2%) | 25 (55.6%) | ||
| Employment status | 0.352 * | |||
| Working full-time | 246 (44.9%) | 22 (48.9%) | ||
| Working part-time | 108 (19.3%) | 13 (28.9%) | ||
| Unemployed | 140 (25.0%) | 7 (15.6%) | ||
| Retired | 20 (3.6%) | 1 (2.2%) | ||
| Extended sick leave | 1 (0.2%) | 0 (0%) | ||
| Disabled | 36 (6.4%) | 2 (4.4%) | ||
| Current or previous occupation | 0.002 * | |||
| Management, business, creators, teaching, administration | 71 (12.7%) | 6 (13.3%) | ||
| Healthcare, caregiving, social service | 189 (33.8%) | 26 (57.8%) | ||
| Sales, customer service | 62 (11.1%) | 8 (17.8%) | ||
| Maintenance, construction, farming, transportation | 165 (29.5%) | 3 (6.7%) | ||
| Other (self-employed, housewife, or missing data) | 73 (13.0%) | 2 (4.4%) | ||
| Outdoor occupation | <0.001 ς | |||
| No | 394 (70.4%) | 42 (93.3%) | ||
| Yes | 166 (29.6%) | 3 (6.7%) | ||
| Residency in colonia | 0.904 ⍭ | |||
| No | 281 (50.2%) | 23 (51.1%) | ||
| Yes | 279 (49.8%) | 22 (48.9%) | ||
| ADI (area deprivation index) Mean (95%CI) |
9.26 (9.20–9.33) | 9.33 (9.12–9.54) | 0.734 # | |
| Years lived in Starr County | 0.957 * | |||
| 3–24 | 150 (26.8%) | 7 (15.6%) | ||
| 25–32 | 134 (23.9%) | 21 (46.7%) | ||
| 33–41 | 145 (25.9%) | 6 (13.3%) | ||
| 42–65 | 131 (23.4%) | 11 (24.4%) | ||
| Marriage status | 0.585 ⍭ | |||
| Not married | 165 (29.5%) | 15 (33.3%) | ||
| Married | 395 (70.5%) | 30 (66.7%) | ||
| Birthplace | 0.376 ⍭ | |||
| Mexico | 396 (70.7%) | 29 (64.4%) | ||
| USA | 164 (29.3%) | 16 (35.6%) | ||
⍭ indicates χ2 test; ς indicates Fisher’s exact test; * indicates Kruskal–Wallis test; and # indicates Mann–Whitney test.
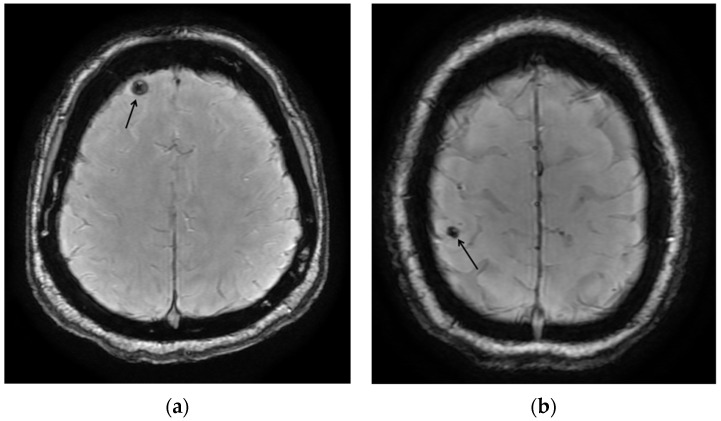
A total of 37 participants underwent MRI (82% of seropositive participants), 4 participants declined the MRI, and 4 were lost to follow-up. We identified two participants with calcifications consistent with inactive NCC (5.4%). One participant exhibited focal calcification in the right superior parietal lobe, while the other participant had a calcification in the right frontal lobe (Figure 1). Both participants were born in Mexico, were employed in healthcare, caregiving, or social service, and have lived in Starr County for more than 30 years. In addition, both participants were positive for Ts18var3 but not T24H or GP50 (Table 2).
Figure 1.
MRI images: (a) the susceptibility-weighted image shows a round hypointense calcification (arrow) in the right anterior frontal lobe, with no surrounding edema or mass effect, consistent with the calcified nodular stage of NCC; (b) the susceptibility-weighted image shows a round hypointense calcification (arrow) in the right superior parietal lobule, with no surrounding edema or mass effect, consistent with the calcified nodular stage of NCC.
Table 2.
Demographics, risk factors, serological testing results, and neuroimaging results.
| Study ID | Sex | Income >USD 30,000 | Birthplace | Age | Current or Previous Occupation Category | Outdoor Occupation |
Ts18var3 | T24H | GP50 | MRI Results |
|---|---|---|---|---|---|---|---|---|---|---|
| NC0120 | Female | No | Mexico | 40–59 | Sales, customer service, and community-oriented | No | Yes | No | No | Negative |
| NC0067 | Female | No | Mexico | 40–59 | Healthcare, caregiving, and social service | No | Yes | Yes | No | Negative |
| NC0260 | Male | Yes | United States | 40–59 | Healthcare, caregiving, and social service | No | Yes | No | No | Negative |
| NC0061 | Female | No | Mexico | 40–59 | Healthcare, caregiving, and social service | No | Yes | No | No | Negative |
| NC0354 | Female | No | United States | 40–59 | Healthcare, caregiving, and social service | No | Yes | No | No | Negative |
| NC0127 | Female | No | United States | 40–59 | Supervisors, creators, teaching, professionals, and office |
No | Yes | No | No | Negative * |
| NC0108 | Female | No | Mexico | 40–59 | Healthcare, caregiving, and social service | No | Yes | No | No | Negative * |
| NC0075 | Female | No | United States | 40–59 | Healthcare, caregiving, and social service | No | Yes | No | No | Negative |
| NC0172 | Male | Yes | United States | 40–59 | Supervisors, creators, teaching, professionals, and office |
No | Yes | No | No | Negative |
| NC0095 | Female | No | Mexico | 40–59 | Healthcare, caregiving, and social service | No | Yes | No | No | Negative |
| NC0074 | Female | Yes | Mexico | 40–49 | Healthcare, caregiving, and social service | No | Yes | No | No | Positive |
| NC0098 | Female | Yes | Mexico | 40–59 | Sales, customer service, and community-oriented | No | Yes | Yes | No | Negative |
| NC0126 | Female | No | Mexico | 40–59 | Healthcare, caregiving, and social service | No | Yes | No | No | Positive |
| NC0363 | Female | No | United States | >60 | Healthcare, caregiving, and social service | No | Yes | Yes | Yes | Negative |
| NC0081 | Female | No | Mexico | 40–59 | Healthcare, caregiving, and social service | No | Yes | Yes | Yes | Negative |
| NC0179 | Female | No | Mexico | >60 | Outdoor and manual labor, and workforce | Yes | Yes | No | No | Negative |
| NC0096 | Female | Yes | United States | 40–59 | Healthcare, caregiving, and social service | No | Yes | No | No | Negative |
| NC0340 | Female | Yes | Mexico | 40–59 | Healthcare, caregiving, and social service | No | Yes | No | No | Negative |
| NC0090 | Female | No | Mexico | 40–49 | Sales, customer service, and community-oriented | No | Yes | No | No | Negative |
| NC0289 | Male | No | Mexico | 40–59 | Sales, customer service, and community-oriented | No | Yes | No | No | Negative * |
| NC0089 | Female | Yes | United States | <40 | Supervisors, creators, teaching, professionals, office |
No | Yes | No | No | Negative |
| NC0259 | Male | No | United States | 60–69 | Outdoor and manual labor, and workforce | Yes | Yes | No | No | Negative |
| NC0228 | Male | Yes | Mexico | 40–59 | Outdoor and manual labor, and workforce | Yes | Yes | No | No | Negative |
| NC0119 | Female | No | Mexico | 40–59 | Sales, customer service, and community-oriented | No | Yes | No | No | Negative |
| NC0129 | Female | No | United States | 40–59 | Healthcare, caregiving, and social service | No | Yes | No | No | Negative |
| NC0160 | Female | Yes | Mexico | 40–59 | Healthcare, caregiving, and social service | No | Yes | No | No | Negative |
| NC0123 | Female | Yes | Mexico | 40–59 | Sales, customer service, and community-oriented | No | Yes | No | No | Negative |
| NC0485 | Female | Yes | United States | 40–59 | Healthcare, caregiving, and social service | No | Yes | No | No | Negative |
| NC0161 | Female | No | Mexico | 40–59 | Healthcare, caregiving, and social service | No | Yes | No | No | Negative |
| NC0454 | Female | No | Mexico | >60 | Healthcare, caregiving, and social service | No | Yes | Yes | Yes | Negative |
| NC0262 | Female | No | Mexico | 40–59 | Supervisors, creators, teaching, professionals, and office |
No | Yes | No | No | Negative |
| NC0117 | Female | No | Mexico | 40–59 | Healthcare, caregiving, and social service | No | Yes | No | No | Negative |
| NC0464 | Female | No | Mexico | 40–59 | Healthcare, caregiving, and social service | No | Yes | No | No | Negative * |
| NC0300 | Male | No | Mexico | 40–59 | Sales, customer service, and community-oriented | No | Yes | No | No | Negative |
| NC0072 | Female | No | Mexico | <40 | Healthcare, caregiving, and social service | No | Yes | No | No | Negative |
| NC0097 | Female | No | Mexico | 40–59 | Healthcare, caregiving, and social service | No | Yes | No | No | Negative |
| NC0164 | Female | Yes | United States | <40 | Supervisors, creators, teaching, professionals, and office |
No | Yes | No | No | Negative |
Note: Age is at time of enrollment in parent study; * MRI completed without contrast.
4. Discussion
We found an overall cysticercosis seroprevalence of 7.4%, with two cases of confirmed calcified NCC based on neuroimaging. The seropositivity of a population likely reflects a combination of parasite exposure, muscle or subcutaneous disease, old calcified NCC, and least common viable or degenerating NCC. Additionally, false-positive serologic results are possible. The seroprevalence identified in this cohort is similar to rates described in endemic countries in Central and South America. Previous studies have found a cysticercosis seroprevalence of 4.9–12.2% in Mexico and 5–35% in Peru [14,15]. Studies of cysticercosis prevalence in the United States are limited to specific populations and are largely not generalizable. Previous work has identified a seroprevalence of 1.8% in migrant farmers and local residents in rural southern California [16], 10% in Hispanic and Haitian migrant farmworkers in North Carolina [17], and 1.3% in an Orthodox Jewish community [18].
In our study, two seropositive participants had MRI findings consistent with calcified NCC disease; both were reactive to only the Ts18var3 antigen. Previous research has identified an association with Ts18var3 reactivity and parenchymal disease, consistent with our findings [11]. Notably, neuroimaging cannot detect already-healed lesions or lesions that may be present elsewhere in the body, such as in subcutaneous or muscle cysticercosis. Previous studies have demonstrated that a relatively small portion of seropositive individuals have evidence of infection detected with neuroimaging [19,20,21], and our findings are consistent with these.
Seroprevalence studies, such as this one, can provide useful information on the burden and distribution of T. solium exposure in a community, especially when distinct risk factors are identified [15]. In our cohort, we found that female sex, indoor occupations, and occupations specifically in the healthcare, caregiving, and social service domains were significantly associated with cysticercosis seropositivity. Previous research into the association between cysticercosis and sex is mixed, with studies identifying no association or positive association in either men or women [19,21,22,23,24]. The associations found with occupations in healthcare, caregiving, and social service domains could possibly represent a risk for transmission through common tasks such as aiding in toileting and bathing, as cysticercosis is typically acquired through fecal–oral contamination by someone with intestinal T. solium tapeworm infection.
Interestingly, we did not identify living in high-poverty communities (ADI or colonias) as a significant risk factor for seropositivity, perhaps due to the general high poverty rate in the area impacting our ability to detect significant differences between groups (mean ADI for seropositive, 9.33, and seronegative, 9.26). Household income was also found to not be significant between the seropositive and seronegative participants. However, specific risk factors such as travel to endemic regions and/or activities with non-commercial pork have not yet been assessed in this population.
Our study has some noteworthy limitations. The convenience sampling study design may have resulted in a cohort not representative of the population of Starr County or the surrounding communities. Further research should be conducted to determine if our findings are consistent with the larger Texas–Mexico border community. Additionally, due to the nature of serologic testing, we cannot determine if seropositive participants with no neuroimaging findings had this result due to a false positive, muscle or subcutaneous disease, or a previous lesion that has since healed.
Given our preliminary findings and the limitations of this testing, there is a critical need to further investigate the burden of disease in this community and the risk of local transmission along the Texas–Mexico border. Determining whether there is autochthonous transmission in these communities is an important next step in defining the local risk of disease. The answers to these questions can be used to inform public health interventions to mitigating disease transmission and increase targeted screening in high-risk populations to prevent the morbidity and mortality of this often-hidden disease.
Acknowledgments
We would like to thank the study participants for their participation in our study, as well as the Starr County study team, without whom this study would not have been possible.
Author Contributions
Conceptualization of study: S.M.G., E.L.B. and M.M.D.; Funding acquisition: S.M.G. and E.L.B.; Investigation: N.L.T., E.M.O., M.M.D. and L.M.L.; Data analysis: M.M.D., S.M.G. and F.E.M.; Visualization: M.M.D. and M.J.; Writing—original draft preparation: M.M.D.; Writing—review and editing: M.J. and C.L.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board at the University of Texas Health Science Center (HSC-SPH-06-0225).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data that support the findings are available on request from the corresponding author, M.M.D. The data are not publicly available due to them containing protected health information (PHI).
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This study was funded by the Texas Department of State Health Services (HHS000427700001). Author M.M.D. was funded as part of an NIAID T32 (T32 AI 055413-19).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Garcia H.H., Gonzalez A.E., Gilman R.H. Taenia solium Cysticercosis and Its Impact in Neurological Disease. Clin. Microbiol. Rev. 2020;33:e00085-19. doi: 10.1128/CMR.00085-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abanto J., Blanco D., Saavedra H., Gonzales I., Siu D., Pretell E.J., Bustos J.A., Garcia H.H., Cysticercosis Working Group in Peru Mortality in Parenchymal and Subarachnoid Neurocysticercosis. Am. J. Trop. Med. Hyg. 2021;105:176–180. doi: 10.4269/ajtmh.20-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattarai R., Budke C.M., Carabin H., Proaño J.V., Flores-Rivera J., Corona T., Ivanek R., Snowden K.F., Flisser A. Estimating the non-monetary burden of neurocysticercosis in Mexico. PLoS Negl. Trop. Dis. 2012;6:e1521. doi: 10.1371/journal.pntd.0001521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serpa J.A., Graviss E.A., Kass J.S., White A.C., Jr. Neurocysticercosis in Houston, Texas: An update. Medicine. 2011;90:81–86. doi: 10.1097/MD.0b013e318206d13e. [DOI] [PubMed] [Google Scholar]
- 5.Sorvillo F., Wilkins P., Shafir S., Eberhard M. Public health implications of cysticercosis acquired in the United States. Emerg. Infect. Dis. 2011;17:e101210. doi: 10.3201/eid1701.101210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotez P.J., Bottazzi M.E., Dumonteil E., Valenzuela J.G., Kamhawi S., Ortega J., Rosales S.P., Cravioto M.B., Tapia-Conyer R. Texas and Mexico: Sharing a legacy of poverty and neglected tropical diseases. PLoS Negl. Trop. Dis. 2012;6:e1497. doi: 10.1371/journal.pntd.0001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Census Bureau Poverty Status in the Past 12 Months, American Community Survey, ACS 1-Year Estimates Subject Tables, Table S1701. [(accessed on 30 July 2024)]; Available online: https://data.census.gov/table/ACSST1Y2022.S1701?g=050XX00US48427.
- 8.U.S. Department of Health and Human Services HPSA Find: Health Resources & Services Administration. [(accessed on 23 November 2022)]; Available online: https://data.hrsa.gov/tools/shortage-area/hpsa-find.
- 9.Nolan M.S., Aguilar D., Brown E.L., Gunter S.M., Ronca S.E., Hanis C.L., Murray K.O. Continuing evidence of Chagas disease along the Texas-Mexico border. PLoS Negl. Trop. Dis. 2018;12:e0006899. doi: 10.1371/journal.pntd.0006899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Essigmann H.T., Aguilar D.A., Perkison W.B., Bay K.G., Deaton M.R., Brown S.A., Hanis C.L., Brown E.L. Epidemiology of Antibiotic Use and Drivers of Cross-Border Procurement in a Mexican American Border Community. Front. Public Health. 2022;10:832266. doi: 10.3389/fpubh.2022.832266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang N.L., Nash T.E., Corda M., Nutman T.B., O’Connell E.M. Triplex ELISA for Assessing Durability of Taenia solium Seropositivity After Neurocysticercosis Cure. Emerg. Infect. Dis. 2023;29:1340–1348. doi: 10.3201/eid2907.230364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colonias. [(accessed on 7 February 2023)]; Available online: https://www.texasattorneygeneral.gov/divisions/colonias.
- 13.Singh G.K. Area deprivation and widening inequalities in US mortality, 1969–1998. Am. J. Public Health. 2003;93:1137–1143. doi: 10.2105/AJPH.93.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flisser A., Sarti E., Lightowlers M., Schantz P. Neurocysticercosis: Regional status, epidemiology, impact and control measures in the Americas. Acta Trop. 2003;87:43–51. doi: 10.1016/S0001-706X(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 15.Coral-Almeida M., Gabriël S., Abatih E.N., Praet N., Benitez W., Dorny P. Taenia solium Human Cysticercosis: A Systematic Review of Sero-epidemiological Data from Endemic Zones around the World. PLoS Negl. Trop. Dis. 2015;9:e0003919. doi: 10.1371/journal.pntd.0003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeGiorgio C., Pietsch-Escueta S., Tsang V., Corral-Leyva G., Ng L., Medina M.T., Astudillo S., Padilla N., Leyva P., Martinez L., et al. Sero-prevalence of Taenia solium cysticercosis and Taenia solium taeniasis in California, USA. Acta Neurol. Scand. 2005;111:84–88. doi: 10.1111/j.1600-0404.2005.00373.x. [DOI] [PubMed] [Google Scholar]
- 17.Ciesielski S., Seed J.R., Estrada J., Wrenn E. The seroprevalence of cysticercosis, malaria, and Trypanosoma cruzi among North Carolina migrant farmworkers. Public Health Rep. 1993;108:736–741. [PMC free article] [PubMed] [Google Scholar]
- 18.Moore A.C., Lutwick L.I., Schantz P.M., Pilcher J.B., Wilson M., Hightower A.W., Chapnick E.K., Abter E.I., Grossman J.R., Fried J.A., et al. Seroprevalence of cysticercosis in an Orthodox Jewish community. Am. J. Trop. Med. Hyg. 1995;53:439–442. doi: 10.4269/ajtmh.1995.53.439. [DOI] [PubMed] [Google Scholar]
- 19.Moyano L.M., O’Neal S.E., Ayvar V., Gonzalvez G., Gamboa R., Vilchez P., Rodriguez S., Reistetter J., Tsang V.C., Gilman R.H., et al. High Prevalence of Asymptomatic Neurocysticercosis in an Endemic Rural Community in Peru. PLoS Negl. Trop. Dis. 2016;10:e0005130. doi: 10.1371/journal.pntd.0005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez A.L., Ljungstrom I., Medina M.T. Diagnosis of human neurocysticerocosis in endemic countries: A clinical study in Honduras. Parasitol. Int. 1999;48:81–89. doi: 10.1016/S1383-5769(99)00007-0. [DOI] [PubMed] [Google Scholar]
- 21.Goodman K.A., Ballagh S.A., Carpio A. Case-control study of seropositivity for cysticercosis in Cuenca, Ecuador. Am. J. Trop. Med. Hyg. 1999;60:70–74. doi: 10.4269/ajtmh.1999.60.70. [DOI] [PubMed] [Google Scholar]
- 22.Galipó E., Dixon M.A., Fronterrè C., Cucunubá Z.M., Basáñez M.G., Stevens K., Flórez Sánchez A.C., Walker M. Spatial distribution and risk factors for human cysticercosis in Colombia. Parasit. Vectors. 2021;14:590. doi: 10.1186/s13071-021-05092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia H.H., Araoz R., Gilman R.H., Valdez J., Gonzalez A.E., Gavidia C., Bravo M.L., Tsang V.C. Increased prevalence of cysticercosis and taeniasis among professional fried pork vendors and the general population of a village in the Peruvian highlands. Cysticercosis Working Group in Peru. Am. J. Trop. Med. Hyg. 1998;59:902–905. doi: 10.4269/ajtmh.1998.59.902. [DOI] [PubMed] [Google Scholar]
- 24.Kanobana K., Praet N., Kabwe C., Dorny P., Lukanu P., Madinga J., Mitashi P., Verwijs M., Lutumba P., Polman K. High prevalence of Taenia solium cysticerosis in a village community of Bas-Congo, Democratic Republic of Congo. Int. J. Parasitol. 2011;41:1015–1018. doi: 10.1016/j.ijpara.2011.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings are available on request from the corresponding author, M.M.D. The data are not publicly available due to them containing protected health information (PHI).