Abstract
Antibodies reactive with capsular polysaccharides are considered the principal mediators of immunity against invasive diseases caused by Streptococcus pneumoniae. In this study, we tested the hypothesis that anti-pneumococcal capsular polysaccharide (PPS) antibody avidity can influence protective efficacy. We measured the avidities of individual adult postvaccination immunoglobulin G2 (IgG2) antibodies to PPS serotypes 6B and 23F and examined the relationship between avidity and opsonophagocytic and mouse-protective activities. The avidities of PPS 6B- and PPS 23F-specific IgG2 antibodies ranged from 6 to 31 nM−1 and from 3 to 20 nM−1, respectively. We observed an inverse correlation between the magnitude of avidity and the amount of antibody required to protect mice against lethal bacteremia caused by serotype 6B pneumococci. Similarly, higher-avidity antibodies were more effective than lower-avidity antibodies in vitro in mediating complement-dependent opsonophagocytosis of both 6B and 23F pneumococci. These data suggest that in adults, PPS antibodies are sufficiently polymorphic to possess biologically significant variations in avidity. We conclude that avidity functions as an important determinant of anticapsular antibody protective efficacy against pneumococci.
Streptococcus pneumoniae causes meningitis, bacteremia, pneumonia, and acute otitis media and is responsible for approximately 40,000 deaths per year in the United States (1, 5, 29). The capsular polysaccharide (PS) functions as a virulence determinant, and although 90 or more different pneumococcal capsular PS (PPS) serotypes have been identified worldwide, only a subset of these are responsible for the majority of disease (1, 5, 29). Complement-dependent phagocytosis mediated by anti-PPS antibodies provides protection against pneumococcal disease; accordingly, pneumococcal vaccine development has focused upon the induction of these antibodies (9, 17, 24, 32). The presently licensed pneumococcal vaccine, which consists of a mixture of 23 different PPS serotypes, appears to be efficacious in healthy adults; however, this vaccine is poorly immunogenic in children under 2 to 3 years of age, a population at increased risk for developing invasive pneumococcal disease. New pneumococcal vaccines are being developed with protein-conjugated forms of PPS (18, 20, 32), a strategy that has proven to be successful in generating immunogenic and efficacious pediatric vaccines for Haemophilus influenzae type b (Hib) (11).
The evaluation of pneumococcal vaccines involves a serological assessment of anti-PPS antibody responses, with the aim of developing reliable surrogates of vaccine protective efficacy. While the serum anti-PPS antibody concentration is typically considered the primary index of response and protection (32, 33), properties such as avidity (14) and opsonophagocytic activity (9, 30) may be important surrogates as well. Recently, Anttila and colleagues observed avidity differences among PPS-specific antibodies elicited in infants following vaccination with different PPS-protein conjugate vaccines and showed that avidity may be influenced by the type of PPS conjugate used for vaccination (4).
The relationship between anti-PPS antibody avidity and protective efficacy has not been investigated to date. Although studies of the human antibody response to Hib PS have directly implicated avidity as a determinant of antibody bactericidal and rat-protective activities (2, 14, 22, 31), it is not known whether these conclusions are generally applicable to antibody responses to the various capsular serotypes of pneumococci. This issue is particularly important since, unlike the situation with immunity to Hib, which is mediated principally by bactericidal antibody, opsonophagocytosis functions as the primary effector mechanism in immunity to pneumococci.
In the present report, we determined the avidities of immunoglobulin G2 (IgG2) antibodies specific for PPS 6B and 23F elicited in adults following PPS vaccination and examined the relationship between avidity and protective activities. We studied antibodies to PPS capsular serotypes 6B and 23F because these two serotypes of pneumococci are a frequent cause of disease, are components of experimental conjugate vaccines presently under evaluation in clinical trials (18, 20, 32), and are structurally disparate. PPS 6B is a straight-chain negatively charged polymer consisting of repeating units of galactose-glucose-rhamnose-ribitol phosphate, whereas PPS 23F is a branched-chain negatively charged polymer of glucose-galactose-rhamnose with glycerol phosphate and rhamnose attached to the galactose unit via esterification.
MATERIALS AND METHODS
Human subjects and vaccinations.
The sera available for analysis were either from a previous study (23) or from a group of 20 healthy adults who were vaccinated with 23-valent PPS vaccine essentially as described before (23). Informed consent was obtained from all volunteers, and protocols were reviewed by the Institutional Review Board of Children’s Hospital Oakland. Peripheral blood samples were taken prior to vaccination and approximately 30 days after vaccination. Serum was heat inactivated by heating at 56°C for 30 min and was stored frozen until used. The antibodies used for avidity and functional analyses were derived from a subset of the vaccinated subjects. The principal criteria for selection of subjects were availability of sufficient serum and the exclusive presence of IgG2 anti-PPS 6B and 14 antibodies following protein G purification (see below).
Preparation of IgG fractions.
Gamma globulins were prepared by precipitation of serum (30 day postvaccination) with 50% saturated ammonium sulfate. IgG fractions were isolated from gamma globulins with protein G (PerSeptive Biosystems, Framingham, Mass.) by high-pressure liquid chromatography. The IgG fractions were adsorbed with S. pneumoniae common cell wall PS (Statens Seruminstitut, Copenhagen, Denmark) coupled to agarose, spun at 100,000 × g for 1 h, sterilized by filtration, and stored at 4°C. Anti-PPS antibodies in the protein G fractions were evaluated for the expression of IgM, IgA, and IgG subclasses by a previously described enzyme-linked immunosorbent assay (18). The IgG samples chosen for avidity and functional analyses contained anti-PPS 6B and 14 antibodies restricted to the IgG2 subclass. IgM and IgA antibodies were undetectable, and comparison to the respective assigned values of reference serum 89SF (28) indicated that IgA and IgM, if present, comprised less than 0.5% of the anti-PPS antibody activity.
Measurement of anti-PPS antibody concentrations and avidity.
The preparation of radiolabeled PPS and the radioantigen binding assay for determination of concentrations of antibodies to PPS 6B and 23F have been described elsewhere (23). A modification of this assay, similar to that previously described for anti-Hib PS antibodies (16), was used to measure anti-PPS antibody avidity. Briefly, PPS binding was assessed at two radioantigen concentrations, ∼0.1 and ∼0.007 nM (assuming an average molecular weight of 100,000 for each PPS). Binding at the higher radioantigen concentration was used to calculate anti-PPS antibody concentrations as calibrated with reference serum 89SF (23, 28). Avidity was determined by evaluating binding at the lower radioantigen concentration. Avidity was calculated as Fb/{(1 − Fb) × [IgG]}, where Fb is the fraction of radioantigen bound and [IgG] is the molar concentration of PPS-specific IgG. Fb values were determined at several IgG concentrations, and the mean avidity was calculated from values falling on the linear portion of the binding curve. Avidity indices reported for individual IgG2 anti-PPS 6B and 23F antibodies represent the arithmetic mean of at least three independent determinations.
Opsonophagocytosis assay.
Opsonophagocytosis was performed essentially as described elsewhere (30). Briefly, HL-60 myelomonocytic cells were cultured for 5 days in the presence of 100 mM dimethylformamide. Opsonophagocytosis reaction mixtures consisted of 4 × 105 human HL-60 myelomonocytic cells per 40-μl volume, 10 μl of antibody, ∼1,000 mid-log-phase pneumococci per 20-μl volume, and 10 μl of newborn rabbit serum as a source of complement. After 60 min at 37°C, aliquots of the mixtures were diluted and plated on blood agar. The number of pneumococcal colonies was counted after overnight incubation. Percent killing (opsonophagocytic activity) was calculated as 100 × [(CFUT0 − CFUT60)/CFUT0], where CFUT0 is the number of viable pneumococci added per well at the start of the assay and CFUT60 is the number of viable pneumococci after 60 min of incubation. Antibodies were tested in duplicate at several concentrations. The 6B strain BG7322 was provided by David Briles, University of Alabama, Birmingham, and the 23F strain 92A-3248 was obtained from the State of California Department of Health Services, Berkeley.
Mouse protection assay.
The mouse protection assay was based upon the procedure of Briles and colleagues (7, 8). Briefly, CBA/N xid mice received a subcutaneous injection of sterile phosphate-buffered saline or antibody. After 24 h, mice received an intraperitoneal injection of 10 to 15 6B pneumococci (strain BG7322) freshly grown to the mid-log phase. The number of pneumococci in the inoculum was determined by plating on blood agar. Peripheral blood samples were taken 24 h later, and the number of pneumococci in the blood was determined by plating on blood agar. Each antibody preparation was tested at multiple doses with at least five mice per dose. For purposes of summarizing the results, a protective dose was defined as the amount of antibody required to prevent lethal bacteremia, i.e., 104 organisms per ml of blood.
RESULTS AND DISCUSSION
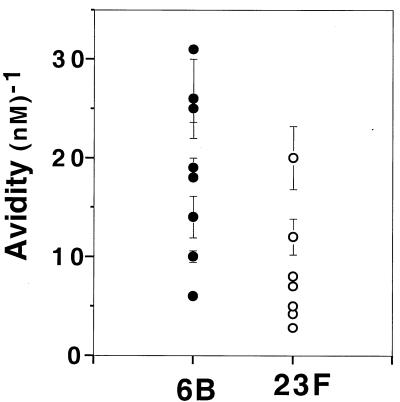
We determined the avidities of IgG antibodies specific for PPS 6B and 23F isolated from sera of adults who had been vaccinated with a polyvalent pneumococcal vaccine. We chose to evaluate antibodies of the IgG class, since it is the predominant isotype of serum PPS-specific antibodies. The PPS 6B- and 23F-specific antibodies in these fractions were restricted to the IgG2 subclass, as determined by an enzyme-linked immunosorbent assay. By controlling for heavy-chain isotype, we avoided the possible confounding properties of complement fixation and multivalence that can differ between the different immunoglobulin classes and subclasses. The pneumococcal vaccine used for immunization contains, in addition to PPS, the common cell wall PS of S. pneumoniae. To restrict our analysis to capsular serotype-specific antibodies, antibodies reactive to the common cell wall PS were removed by adsorption. As shown in Fig. 1, avidity heterogeneity was present among these adult vaccine-induced pneumococcal antibodies; the avidities of PPS 6B- and PPS 23F-specific IgG2 antibodies ranged from 6 to 31 nM−1 and from 3 to 20 nM−1, respectively.
FIG. 1.
Avidities of anti-PPS 6B and anti-PPS 23F IgG2 antibodies. Each circle represents the mean avidity of an individual adult IgG fraction prepared from serum obtained 30 days after PPS vaccination. Avidities were determined with a radioantigen binding assay, and the values shown represent the means ± standard errors of the means for at least three independent assays.
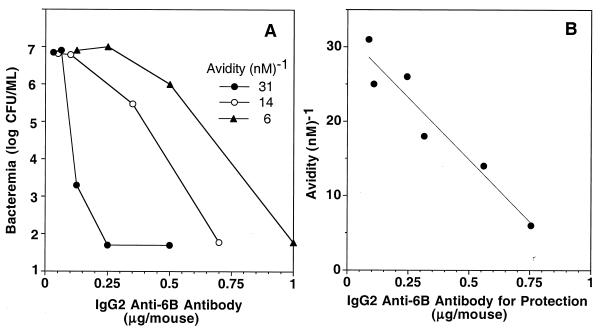
We evaluated PPS 6B-specific antibodies for their ability to protect CBA/N xid mice from lethal 6B pneumococcal bacteremia. These experiments were limited to serotype 6B pneumococci since serotype 23F pneumococci were essentially avirulent. In this model of infection, intraperitoneal injection of as few as 10 6B pneumococci elicited bacteremia within 24 h. Mice having ≥104 organisms per ml of blood 24 h after inoculation died within 48 to 72 h (data not shown). The administration of graded doses of PPS 6B-specific antibody 24 h prior to infection resulted in dose-dependent protection against lethal bacteremia, and the amount of antibody required for prevention of lethal bacteremia depended upon avidity (Fig. 2A). A summary of these experiments showed a strong inverse correlation between the magnitude of avidity and the amount of antibody required for the prevention of lethal bacteremia (Fig. 2B).
FIG. 2.
Protection of mice from lethal pneumococcal serotype 6B bacteremia correlates with anti-PPS 6B antibody avidity. (A) Prevention of pneumococcal 6B bacteremia in CBA/N xid mice by three IgG anti-PPS 6B antibodies with different avidities. (B) Correlation between avidity and amount of anti-PPS 6B antibody required to prevent lethal bacteremia. Each circle represents the amount of PPS 6B-specific IgG2 antibody required to prevent lethal pneumococcal 6B bacteremia (104 organisms per ml of blood). Values shown were derived by testing each IgG antibody in at least three independent experiments. Within an experiment, each antibody was tested at several concentrations with five mice per antibody dose. Linear regression gave a correlation coefficient of 0.92.
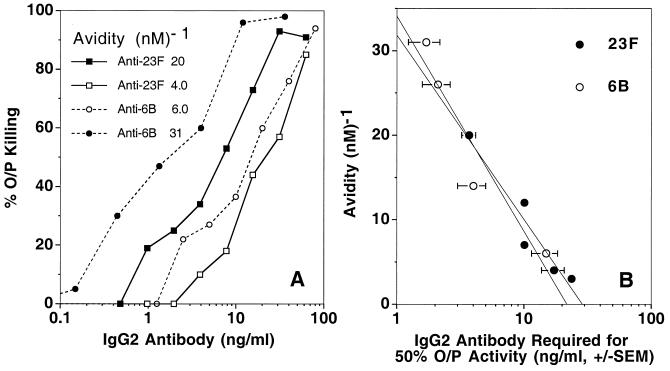
To determine whether the relationship between avidity and protective function was demonstrable in vitro and applicable to another pneumococcal serotype, we examined the IgG2 antibodies for their ability to mediate opsonophagocytosis of 6B and 23F organisms. The degree of opsonophagocytosis of 6B and 23F pneumococci depended upon both antibody concentration and avidity (Fig. 3A). As was observed in vivo with serotype 6B pneumococcal infection, avidity and opsonophagocytic activity were inversely related (Fig. 3B). Higher-avidity antibodies to PPS 6B and 23F were more effective on a weight basis than lower-avidity antibodies in mediating opsonophagocytosis.
FIG. 3.
Opsonophagocytosis of pneumococci correlates with anti-PPS antibody avidity. (A) Opsonophagocytosis (O/P Killing) of serotype 6B and 23F pneumococci by two anti-PPS 6B and two anti-PPS 23F IgG2 antibodies. (B) Correlation between avidity and the amount of antibody required for opsonophagocytosis of pneumococci. Each circle represents the amount of PPS 6B- or PPS 23F-specific IgG2 antibody required to achieve 50% opsonophagocytosis activity (O/P Activity). Values shown were derived by testing each IgG antibody at several doses in at least three independent experiments. Linear regression gave a correlation coefficient of 0.96 for both PPS 6B- and PPS 23F-specific antibodies. SEM, standard error of the mean.
The findings presented here demonstrate that avidities vary among individual antibodies to PPS 6B and 23F, and this variation in avidities is sufficient to confer differences in antibody protective efficacy, as assessed in vivo and in vitro. Since the heavy-chain isotype of the antibodies studied here was uniformly of the IgG2 subclass, the variation in protective efficacy cannot be attributed to differences in effector functions, such as complement fixation, but rather is due to the differential ability of anti-PPS variable (V) domains to bind PPS. The IgG preparations studied here should be considered polyclonal in the strict sense; therefore, the avidity of an individual sample represents a net value that could result from PPS binding by different V domains. However, several studies indicate that within individuals, serum PPS-specific antibody populations are markedly oligoclonal and in some cases may approach monoclonality (21, 23, 26). Thus, V-region diversity within individuals is likely to be limited; accordingly, avidity would reflect PPS binding by the predominant clone(s). Avidity variation among anti-PPS antibodies could arise from intrinsic differences in their ability to recognize a single immunodominant PPS epitope or from differences in their antigenic fine specificity (25).
Our data showing that avidity is a determinant of pneumococcal antibody protective efficacy parallel previous studies of human antibodies to Hib PS (2, 14, 22, 31) and murine IgM antibodies to group B streptococci (27). Avidity differences among anti-Hib PS antibodies elicited in infants by immunization with Hib PS conjugate vaccines are sufficient to confer marked differences in bactericidal and rat-protective activities (2, 22, 31) and, interestingly, the distribution of serum antibody avidities correlates with the type of Hib PS conjugate used for vaccination (2, 15, 22, 31). A similar phenomenon may occur in the responses of infants to experimental PPS conjugate vaccines (4) which, like Hib PS conjugate vaccines, differ in the sizes of the PS moieties, the carrier proteins, and their conjugation chemistry (18, 20, 32).
Disparities in anti-PPS antibody avidity would assume greater importance in vivo when antibody concentrations are limiting, for example, in the naive repertoires of infants following their primary vaccination or at later times following vaccination when antibody levels have declined. Furthermore, avidity differences among PPS-specific precursor B-lymphocyte populations could influence not only which clones initially are activated but also which clones enter the memory pool or assume prominence during the course of the response as they compete for diminishing levels of available antigen. In keeping with this suggestion is the finding that the avidity of antibodies to Hib PS serves as a surrogate for the successful induction of immunological memory in infants (13).
Avidity is germane to the concept that a single concentration of circulating antibody can be assigned as a surrogate for protective efficacy; this concept has been invoked in a theoretical context (10) and in the practical need to establish reliable indicators of protective immunity for both immunotherapy and vaccine evaluation (3, 14, 19, 33). Our data show that on a weight basis, higher-avidity anti-PPS antibodies are more effective than lower-avidity antibodies in mediating protective functions, a result suggesting that immunity against invasive pneumococcal disease depends on both antibody concentration and avidity. Thus, assignment of a single concentration of anti-PPS antibody may not suffice as an adequate surrogate for protection. The relevance of this issue for vaccine evaluation is underscored by the recent demonstration of avidity heterogeneity of anti-PPS antibodies elicited in infants vaccinated with new experimental PPS-protein conjugates (4).
Finally, it is important to note that avidity may not serve as a universal indicator of antibody protective efficacy. A recent study of murine monoclonal antibodies to vesicular stomatitis virus showed a lack of correlation between antivirus avidity and protective efficacy in a mouse model of infection (6). The majority of the antiviral antibodies apparently had exceeded a minimum avidity threshold for protection such that further increases in avidity did not lead to discernible improvements in protective function (6, 12). Vaccine-induced human PPS-specific antibodies differ from murine monoclonal antibodies to vesicular stomatitis virus in that they have not exceeded this putative threshold; therefore, their avidity variation is still meaningful with respect to protective function. Indeed, unlike antibodies to viral surface proteins, antibodies reacting with carbohydrate determinants of bacterial capsules may never achieve avidities sufficiently high to cross a threshold such that their protective function becomes independent of concentration. The results presented here lead us to conclude that avidity plays a fundamental role in human immunity to pathogenic encapsulated pneumococci.
ACKNOWLEDGMENTS
We thank C. Connolly for technical assistance, N. Kennedy and J. Simon for performing vaccination and phlebotomy, D. Reason and P. Klebba for reviewing the manuscript, D. Briles and J. King for sharing their pneumococcal isolates with us and for advice in performing the mouse protection assay, and S. Romero-Steiner and G. Carlone for sharing their opsonophagocytosis protocol prior to publication.
This work was supported by National Institutes of Health grants AI-25008, RR-01271, and AI-45250.
REFERENCES
- 1.Alonso deVelasco E, Verheul A F M, Verhoef J, Snippe H. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol Rev. 1995;59:591–603. doi: 10.1128/mr.59.4.591-603.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amir J, Liang X, Granoff D M. Variability in the functional activity of vaccine-induced antibody to Haemophilus influenzae type b. Pediatr Res. 1990;27:358–364. doi: 10.1203/00006450-199004000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Anderson P. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1984;149:1034. doi: 10.1093/infdis/149.6.1034. [DOI] [PubMed] [Google Scholar]
- 4.Anttila M, Eskola J, Ähman H, Käyhty H. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J Infect Dis. 1998;177:1614–1621. doi: 10.1086/515298. [DOI] [PubMed] [Google Scholar]
- 5.Austrian R. Pneumococcal infections. In: Germanier R, editor. Bacterial vaccines. Orlando, Fla: Academic Press, Inc.; 1984. pp. 257–288. [Google Scholar]
- 6.Bachmann M F, Kalinke U, Althage A, Freer G, Burkhart C, Roost H-P, Auget M, Hengartner H, Zinkernagel R M. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–2027. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- 7.Benton K A, Paton J C, Briles D E. Differences in virulence for mice among Streptococcus pneumoniae strains of capsular types 2, 3, 4, 5, and 6 are not attributable to differences in pneumolysin production. Infect Immun. 1997;65:1237–1244. doi: 10.1128/iai.65.4.1237-1244.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briles D E, Crain B, Gray B M, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruyn G A W, Zegers B J M, van Furth R. Mechanisms of host defense against infection with Streptococcus pneumoniae. Clin Infect Dis. 1992;14:251–262. doi: 10.1093/clinids/14.1.251. [DOI] [PubMed] [Google Scholar]
- 10.Cohn M, Langman R E. The protecton: the unit of humoral immunity selected by evolution. Immunol Rev. 1990;115:11–147. doi: 10.1111/j.1600-065x.1990.tb00783.x. [DOI] [PubMed] [Google Scholar]
- 11.Ellis R W, Granoff D M. Development and clinical uses of Haemophilus b conjugate vaccines. New York, N.Y: Marcel Dekker, Inc.; 1994. [Google Scholar]
- 12.Foote J, Eisen H N. Kinetic and affinity limits on antibodies produced during immune responses. Proc Natl Acad Sci USA. 1995;92:1254–1256. doi: 10.1073/pnas.92.5.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldblatt D, Pinto Vaz A R, Miller E. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J Infect Dis. 1998;177:1112–1115. doi: 10.1086/517407. [DOI] [PubMed] [Google Scholar]
- 14.Granoff D M, Lucas A H. Laboratory correlates of protection against Haemophilus influenzae type B disease: importance of assessment of antibody avidity and immunologic memory. Ann N Y Acad Sci. 1995;754:278–288. doi: 10.1111/j.1749-6632.1995.tb44461.x. [DOI] [PubMed] [Google Scholar]
- 15.Granoff D M, Shackelford P G, Holmes S J, Lucas A H The Collaborative Vaccine Study Group. Variable region expression in the antibody responses of infants vaccinated with Haemophilus influenzae type b polysaccharide-protein conjugates: description of a new λ light chain-associated idiotype and the relation between idiotype expression, avidity, and vaccine formulation. J Clin Investig. 1993;91:788–796. doi: 10.1172/JCI116298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griswold W R, Lucas A H, Bastian J F, Garcia G. Functional affinity of antibody to the Haemophilus influenzae b polysaccharide. J Infect Dis. 1989;159:1084–1087. doi: 10.1093/infdis/159.6.1083. [DOI] [PubMed] [Google Scholar]
- 17.Heidelberger M, MacLeod C M, diLappi M M. The human antibody response to simultaneous injection of six specific polysaccharides of pneumococcus. J Exp Med. 1948;88:369–372. doi: 10.1084/jem.88.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Käyhty H, Eskola J. New vaccines for the prevention of pneumococcal infections. Emerg Infect Dis. 1996;2:289–298. doi: 10.3201/eid0204.960404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Käyhty H, Peltola H, Karanko V, Mäkelä P H. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1983;147:1100. doi: 10.1093/infdis/147.6.1100. [DOI] [PubMed] [Google Scholar]
- 20.Klein D L, Ellis R W. Conjugate vaccines against Streptococcus pneumoniae. In: Levine M M, Woodrow G C, Kaper J C, Cobon G S, editors. New generation vaccines. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1997. p. 503. [Google Scholar]
- 21.Konradsen H B, Hahn-Zoric M, Hanson L Å. Differences within mono- and dizygotic twin-pairs in spectrotypes and clones of IgG2 antibodies to pneumococcal polysaccharide type-1 and C-polysaccharide after vaccination. Scand J Immunol. 1994;40:423–428. doi: 10.1111/j.1365-3083.1994.tb03484.x. [DOI] [PubMed] [Google Scholar]
- 22.Lucas A H, Granoff D M. Functional differences in idiotypically defined IgG1 anti-polysaccharide antibodies elicited by vaccination with Haemophilus influenzae type b polysaccharide-protein conjugates. J Immunol. 1995;154:4195–4202. [PubMed] [Google Scholar]
- 23.Lucas A H, Granoff D M, Mandrell R E, Connolly C C, Shan A S, Powers D C. Oligoclonality of serum immunoglobulin G antibody responses to Streptococcus pneumoniae capsular polysaccharide serotypes 6B, 14, and 23F. Infect Immun. 1997;65:5103–5109. doi: 10.1128/iai.65.12.5103-5109.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musher D M, Johnson B, Jr, Watson D A. Quantitative relationship between anticapsular antibody measured by enzyme-linked immunosorbent assay or radioimmunoassay and protection of mice against challenge with Streptococcus pneumoniae serotype 4. Infect Immun. 1990;58:3871–3876. doi: 10.1128/iai.58.12.3871-3876.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nahm M H, Olander J V, Magyarlaki M. Identification of cross-reactive antibodies with low opsonophagocytic activity for Streptococcus pneumoniae. J Infect Dis. 1997;176:698–703. doi: 10.1086/514093. [DOI] [PubMed] [Google Scholar]
- 26.Park M K, Sun Y, Olander J V, Hoffmann J W, Nahm M H. The repertoire of human antibodies to the carbohydrate capsule of Streptococcus pneumoniae 6B. J Infect Dis. 1996;174:75–82. doi: 10.1093/infdis/174.1.75. [DOI] [PubMed] [Google Scholar]
- 27.Pincus S H, Shigeoka A O, Moe A A, Ewing L P, Hill H R. Protective efficacy of IgM monoclonal antibodies in experimental group B streptococcal infection is a function of antibody avidity. J Immunol. 1988;140:2779–2785. [PubMed] [Google Scholar]
- 28.Quataert S A, Kirch C S, Wiedl L J Q, Phipps D C, Strohmeyer S, Cimino C O, Skuse J, Madore D V. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin Diagn Lab Immunol. 1995;2:590–597. doi: 10.1128/cdli.2.5.590-597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robbins J B, Austrian R, Lee C-J, Rastogi S C, Schiffman G, Henrichsen J, Mäkela P H, Broome C V, Facklam R R, Tiesjema R H, Parke J C. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis. 1983;148:1136–1159. doi: 10.1093/infdis/148.6.1136. [DOI] [PubMed] [Google Scholar]
- 30.Romero-Steiner S, Libutti D, Pais L B, Dykes J, Anderson P, Whitin J C, Keyserling H L, Carlone G M. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol. 1997;4:415–422. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlesinger Y, Granoff D M The Collaborative Vaccine Study Group. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. JAMA. 1992;267:1489–1494. [PubMed] [Google Scholar]
- 32.Siber G R. Pneumococcal disease: prospects for a new generation of vaccines. Science. 1994;265:1385–1387. doi: 10.1126/science.8073278. [DOI] [PubMed] [Google Scholar]
- 33.Stack A M, Malley R, Thompson C M, Kobzik L, Siber G R, Saldino A S. Minimum protective serum concentration of pneumococcal anti-capsular antibodies in infant rats. J Infect Dis. 1998;177:986–990. doi: 10.1086/515259. [DOI] [PubMed] [Google Scholar]