Abstract
Conventional adhesives used in wood-based panels typically contain volatile organic compounds, including formaldehyde, which can potentially lower indoor air quality and damage human health. Lignin, a natural adhesive present in wood, offers significant advantages over other materials due to its ready availability, renewable nature, rich aromatic rings, and aliphatic and aromatic hydroxyl groups, as well as quinone groups. However, when modified as an adhesive for wood-based panels, lignin suffers from poor water resistance and formaldehyde release. Dehydrogenation polymer (DHP), as a lignin model compound, possesses a structure similar to lignin and excellent water resistance, making it a potential substitute for lignin as a formaldehyde-free adhesive. A DHP-xylose complex was obtained from a condensation reaction between DHP and xylose in hemicellulose in a simulated hot-pressing environment. The feasibility of DHP bonding with hemicellulose components was verified using FT-IR and NMR spectroscopic methods. In addition, the structure of the adduct and condensation process were also studied. DHP and xylose underwent condensation under simulated hot-pressing conditions. Xylose and DHP may be linked by C-C bonds. The thermal condensation of DHP with xylose was investigated. This may contribute to a better understanding of the adhesive bonding process for xylose during hot-pressing and offer support for practical applications.
Keywords: dehydrogenation polymer, xylose, thermal condensation mechanism, adhesives
1. Introduction
In the contemporary world, there is a continuously increasing demand in the construction and furniture industries for high-performance, environmentally friendly materials. As an indispensable part of this field, wood-based panels occupy an important position in several areas. Traditional wood-based panels are fabricated from wood or other non-wood plant materials, which are subjected to mechanical processing to segregate them into distinct unit materials, which are subsequently bonded with adhesives to produce boards or molded products. The core of wood-based panel production lies in using adhesives to firmly bond wood particles or fibers together, forming a sturdy panel structure. Adhesives play a crucial role in the production of wood-based panels and directly influence the performance, quality, and lifespan of the resulting boards. Traditional adhesives used in the production of wood-based panels often include urea-formaldehyde, phenol-formaldehyde, melamine, and their modified derivatives [1]. Although these adhesives have strong bonding strength and water resistance, they also release harmful volatile organic compounds (VOCs) such as formaldehyde and phenol, posing potential risks to indoor air quality and human health [2].
To mitigate or eliminate formaldehyde pollution in wood-based panels, more environmentally friendly biomass adhesives have become a focus. Among numerous biomass adhesives, lignin-based adhesives have been extensively studied. They are typically derived from industrial lignin, a by-product of the paper industry, which has the characteristics of being easy to obtain and low-cost compared with traditional adhesives. Lignin-based adhesives are usually prepared by blending modified industrial lignin with “three-aldehyde” resins, which can reduce the release of toxic substances [3,4,5,6,7,8,9,10,11,12]. However, the issue of formaldehyde emissions has not been completely solved. After enzymatic modification, industrial lignin promotes the formation of a large number of radicals during the production of wood-based panels [13,14]. These radicals promote the cross-linking of industrial lignin through mutual coupling, thereby improving its bonding performance while completely eliminating the problem of formaldehyde emission. Wood-based panels with high strength have been obtained by treating industrial lignin with laccase [13,14,15]. However, the enzyme modification of lignin is only effective in an aqueous solution. Although the modified wood boards have good dry strength, their water resistance is relatively poor [13,14]. Therefore, the potential for using enzymatically modified industrial lignin to prepare formaldehyde-free adhesives is subject to significant limitations. The enzyme-catalyzed transformation of phenolic substances or lignin precursors can generate a dehydrogenation polymer (DHP) with a structure resembling that of lignin. This material may serve as an adhesive [16,17,18]. The polymer can couple with free phenolic groups at the surface of thermomechanical pulp (TMP) fibers under catalytic oxidation promoted by enzymes to significantly improve the wet strength of paper. Laccase and peroxidase have been utilized to prepare DHP on the fiber surface. A significant enhancement in water resistance for paper and paperboard was observed [17,18,19,20,21,22,23,24]. In summary, the use of DHP to function as an adhesive in wood panels has been investigated.
In natural wood, lignin and cellulose fibers are primarily cross-linked through high-molecular-weight lignin and hemicellulose. The lignin and hemicellulose can form lignin–carbohydrate complexes (LCCs) through covalent bonding. These two interactions account for the excellent physical and mechanical properties of wood [25]. Therefore, two conditions need to be met for DHP to function as an effective adhesive. Firstly, it must efficiently self-condense during hot-pressing to form high-molecular-weight polymers that cross-link wood fibers. Secondly, high-molecular-weight DHP must condense with hemicellulose to form covalently bonded structures similar to LCCs. This allows DHP to tightly bind plant cell walls together through both physical adhesion and chemical bonding. This ensures good adhesive performance in wood-based panels. The fact that DHP can efficiently undergo self-condensation under hot-pressing conditions to form high polymers [26] has previously been demonstrated. This suggests that DHP has potential to be used as an adhesive.
Consequently, whether or not high-molecular-weight DHP can undergo condensation with hemicellulose under hot-pressing conditions to form covalently bonded structures similar to those of LCCs has been investigated. Specifically, the reaction of DHP with xylose in hemicellulose under simulated hot-pressing conditions has been examined. Moreover, the structure and thermal condensation process for DHPs with hemicellulose have been investigated using FT-IR and NMR spectroscopic methods. Groundwork for the development of high-performance wood-based panel adhesives that are environmentally friendly and non-polluting has been established.
2. Materials and Methods
2.1. Materials
Acetic Acid, Toluene, and Dimethyl Sulfoxide (DMSO), all from Analytical Reagent, were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and xylose was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). The synthesis of DHP is referenced in [27,28].
2.2. Preparation of DHP-Xylose Complex
Xylose (200 mg), DHP (120 mg), and 200 μL of acetic acid solution (10% v/v) were added to a 2 mL stainless steel sealed vessel and heated in an oil bath at 140 °C for 25 min. After the reaction, we performed repeated washes and centrifugation with distilled water and removed the supernatant to ensure that the xylose was completely removed until the wash solution was neutral. The resulting insoluble substance was the final product, which was then dried to obtain the DHP-xylose complex. These experimental conditions were based on previous research conducted by our group [26].
2.3. Structural Characterization of DHP-Xylose Complex
The DHP-xylose complex was ball-milled for about 22 h. The ball-milled product was washed with toluene, centrifuged at a low temperature, and dried to obtain the powdered product. A 2 mg sample was mixed with 250 mg KBr (ground in an agate mortar) and pressed into a pellet, and the infrared spectra of the DHP self-condensation product (DHP SC) and the DHP-xylose complex (DHP-Xylose) were recorded using a Fourier transform infrared spectrometer (model: NICOLET 6700, Waltham, MA, USA). An amount of 100 mg of DHP-xylose was dissolved in 1.2 mL of DMSO-d6 in a 5 mm NMR tube and detected using a 500 MHz AVANCE III Nuclear Magnetic Resonance Spectrometer (Bruker, Berlin, Germany) with 9000 scans. Then, 80 mg of DHP-xylose was dissolved in 1 mL of DMSO-d6 in a 5 mm NMR tube and analyzed using a 500 MHz AVANCE III Nuclear Magnetic Resonance Spectrometer (Bruker, Berlin, Germany) with 32 scans. Resolution: 1H: 0.45 Hz, 13C: 0.2 Hz; sensitivity: 1H ≥ 730:1 (0.1% EB), 13C ≥ 250:1 (ASTM).
3. Results and Discussions
3.1. FT-IR Analysis of DHP-Xylose Complex
The infrared spectra of DHP self-condensation and the DHP-xylose complex are shown in Figure 1. The absorption peaks at 1078 cm−1, 1652 cm−1, and 1726 cm−1 in the spectrum correspond to the vibration of C-O in the ether bonds, the conjugated carbonyl group, and the non-conjugated carbonyl group, respectively. The signals of benzene rings were observed at 1600 cm−1, 1510 cm−1, and 1424 cm−1 in the spectrum of the DHP-xylose complex, indicating the presence of the benzene ring framework. Compared with self-condensed DHP, the absorption peaks at 1078 cm−1 and 1030 cm−1 in the spectrum of the DHP-xylose complex were intensified. The peak at 1078 cm−1 corresponded to the C-O vibration in the ether bond, while the peak at 1030 cm−1 was associated with the C=O stretching vibration of xylose [29], indicating the possible condensation of DHP with xylose. Further characterization required analysis via 13C-NMR.
Figure 1.
FT-IR spectra of DHP self-condensation and DHP-xylose complex.
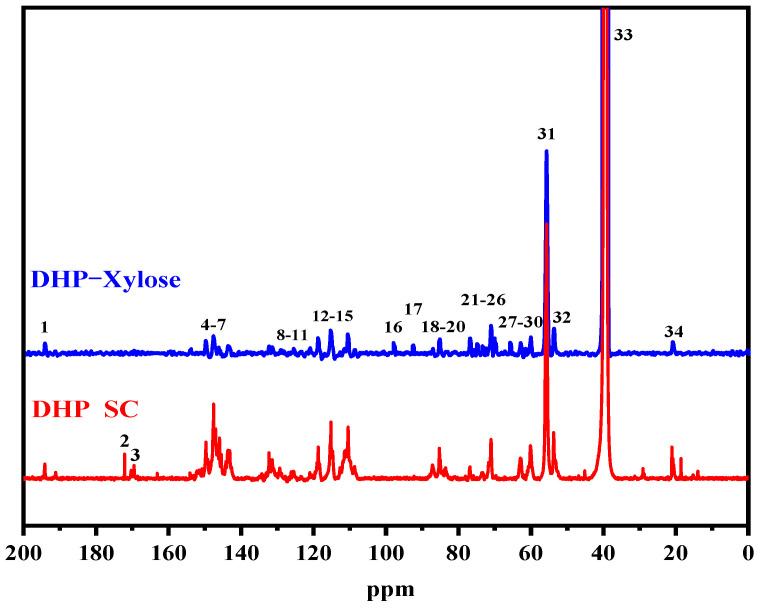
3.2. 13C-NMR Analysis of DHP-Xylose Complex
The ball-milled DHP-xylose complex was completely dissolved in DMSO. The 13C NMR spectra of self-condensed DHP and the DHP-xylose complex are shown in Figure 2, and the functional group assignments for both are provided in Table 1 [30]. As shown in Figure 2, the DHP-xylose complex exhibited several new absorption peaks compared to the self-condensed DHP. These include the C1 absorption peak of β-D-xylose at 98.3 ppm, the C1 absorption peak of α-D-xylose at 93 ppm, the C3 absorption peak of xylose at 75.3 ppm, the C2 absorption peak of β-D-xylose at 73.7 ppm, the C2 absorption peak of α-D-xylose at 72.9 ppm, the C4 absorption peak of xylose at 70.1 ppm, and the C5 absorption peak of xylose at 66.1 ppm [29]. Additionally, within the range of 60–64 ppm, a new absorption peak at 62.1 ppm (No. 29) appeared for the DHP-xylose complex, while the peaks at 63.2 ppm and 60.4 ppm remained largely unchanged. The peak at 62.1 ppm corresponded to the γ-C absorption peak of lignin, indicating a substantial increase in the lignin structure within the condensed DHP-xylose complex [31]. Moreover, a signal peak appeared around 85 ppm, corresponding to Cα and Cβ [29]. These findings thoroughly demonstrate the thermal condensation of DHP with xylose under hot-pressing conditions.
Figure 2.
13C-NMR spectra of DHP self-condensation and DHP-xylose complex.
Table 1.
Functional group assignments of DHP self-condensation and DHP-xylose complex.
| Signal | Chemical Shifts (δ, ppm) | Assignments | |
|---|---|---|---|
| DHP SC | DHP-Xylose | ||
| 1 | 194.1 | 194.5 | γ-CHO in cinnamaldehyde |
| 2 | 172.1 | — | Cγ in Ferulic acid |
| 3 | 169.5 | — | -COO- in Ferulic acid ester |
| 4 | 150.6 | 150.1 | C3/C4 in guaiacyl |
| 5 | 147.5 | 148.1 | C3/C5 in guaiacyl, etherified |
| 6 | 145.9 | 146.6 | C4/C4′ in 5-5′, etherified |
| 7 | 143.6 | 144 | C4 in 5-5 |
| 8 | 132.2 | 132.7 | C1 in β-O-4 guaiacyl, non-etherified |
| 9 | 131.3 | 131.9 | C1/C1′ in β-5 |
| 10 | 129.3 | 129.4 | Cα in cinnamaldehyde |
| 11 | 128.7 | 128.6 | Cβ in cinnamaldehyde |
| 12 | 119.5 | 119.3 | C6 in guaiacyl |
| 13 | 118.7 | 118.6 | C5 in guaiacyl |
| 14 | 115.3 | 115.8 | C5 in guaiacyl |
| 15 | 110.4 | 110.9 | C2 in guaiacyl |
| 16 | — | 98.3 | C1 in β-D-Xylose |
| 17 | — | 93 | C1 in α-D-Xylose |
| 18 | 87.1 | 87.5 | Cα in β-5 |
| 19 | 85 | 85.5 | Cα(β-β), Cβ(β-O-4) |
| 20 | 83.8 | 83.5 | Cβ in β-O-4 |
| 21 | 76.9 | 77.3 | Cα in β-O-4 |
| 22 | — | 75.3 | C3 in Xylose |
| 23 | — | 73.7 | C2 in β-D-Xylose |
| 24 | — | 72.9 | C2 in α-D-Xylose |
| 25 | 71 | 71.5 | Cγ in β-β |
| 26 | — | 70.1 | C4 in Xylose |
| 27 | — | 66.1 | C5 in Xylose |
| 28 | 62.9 | 63.2 | Cγ in β-5 |
| 29 | — | 62.1 | Cγ in cinnamylalcohol |
| 30 | 60.1 | 60.4 | Cγ in β-O-4 |
| 31 | 55.6 | 56.2 | -OCH3 |
| 32 | 53.7 | 54 | Cβ in β-5 |
| 33 | 39.4 | 40 | DMSO |
| 34 | 21 | 21.3 | -CH3 in acetyl group |
3.3. Two-Dimensional-HSQC NMR Analysis of DHP-Xylose Complex
To further support the evidence of the condensation of DHP with xylose, 2D-HSQC NMR analysis was performed on the DHP-xylose complex. The side-chain region (δC/δH 50~100/2.2~6.2) and the aromatic ring region (δC/δH 90~135/5.9~7.8) of the 2D-HSQC NMR spectrum of the DHP-xylose complex are shown in Figure 3, and the functional group assignments of the main signals in the 2D-HSQC NMR spectrum are presented in Table 2 [30], while the primary linkage structures within the DHP-xylose complex are illustrated in Figure 4.
Figure 3.
Two-dimensional HSQC NMR spectra of the DHP-xylose complex: (a) side-chain region and (b) aromatic ring region.
Table 2.
Two-dimensional HSQC NMR functional group assignments of the DHP-xylose complex.
| Label | Chemical Shift | Assignments |
|---|---|---|
| δC/δH (ppm) | ||
| Cβ | 53.29/3.46 | Cβ-Hβ in phenylcoumaran (C) |
| Bβ | 53.82/3.04 | Cβ-Hβ in β-β (resinol) (B) |
| OCH3 | 55.82/3.76 | C-H in methoxyls |
| Aγ | 60.19/3.59 | Cγ-Hγ in β-O-4 substructures (A) |
| Fγ | 61.73/4.08 | Cγ-Hγ in cinnamyl alcohol end-groups (F) |
| Cγ | 62.96/3.71 | Cγ-Hγ in phenylcoumaran (C) |
| Bγ | 71.10/3.73 | Cγ-Hγ in β-β resinol (B) |
| 71.13/4.13 | ||
| Aα | 71.41/4.73 | Cα-Hα in β-O-4 unit (A) |
| A′α | 74.26/5.91 | β-O-4, Cα-Hα in guaiacyl units (G) |
| Aβ | 83.99/4.29 | Cβ-Hβ in β-O-4 substructures (A) |
| 81.18/4.56 | ||
| 83.50/4.48 | ||
| Bα | 85.28/4.62 | Cα-Hα in β-β resinol (B) |
| B′α | 83.40/4.83 | Cα-Hα in β-β (B′, tetrahydrofuran) |
| Cα | 87.22/5.46 | Cα-Hα in phenylcoumaran (C) |
| X1 | 92.62/4.86 | C1-H1 in α-D-Xylose |
| 97.83/4.22 | C1-H1 in β-D-Xylose | |
| G2 | 108.55/6.92 | C2-H2 in guaiacyl units (G) |
| 110.38/6.90 | ||
| G′2 | 110.71/7.39 | α C2-H2 in G-type structural units with oxidized sites |
| 112.58/7.31 | ||
| G5 | 115.13/6.74 | C5-H5 in guaiacyl units (G) |
| 115.37/6.98 | ||
| G6 | 118.61/6.77 | C6-H6 in guaiacyl units (G) |
| 120.84/6.74 | ||
| G′6 | 118.81/7.32 | α C6-H6 in G-type structural units with oxidized sites |
| FA6 | 123.30/7.21 | C6-H6 in ferulate (p-FA) |
| Eβ | 126.17/6.78 | Cβ-Hβ in cinnamyl aldehyde end-groups (E) |
Figure 4.
The main basic junction structures in the 2D-HSQC NMR of the DHP-xylose complex.
In the NMR spectra (Figure 3a,b, side-chain region and aromatic ring region), the side-chain structures (β-O-4, β-5, and β-β) and benzene ring structures (G2, G5, and G6) of DHP were observed. The signals at 92.62/4.86 ppm and 97.83/4.22 ppm (X1) [30,32,33] were attributed to the C1 signals of α-D-xylose and β-D-xylose, respectively. The signal at 74.26/5.91 ppm was identified as the β-O-4 and Cα-Hα signal bond. DHP underwent thermal condensation with xylose under high-temperature, high-pressure, and acidic conditions, which was consistent with the previous analysis of FT-IR and 13C-NMR spectra.
In conclusion, the 13C NMR spectrum revealed the absorption signals of xylose C1, C2, C3, C4, and C5, confirming the presence of xylose. In the side-chain region of the 2D-HSQC NMR spectrum, along with the functional group assignment table, a chemical linkage between DHP and xylose was also found. It is speculated that the C1 of xylose and the C6 of DHP may be linked by C-C bonds [34,35]. The related formation process is illustrated in Figure 5.
Figure 5.
Thermal condensation of DHP with xylose.
4. Conclusions
DHP and xylose were used as raw materials to prepare DHP-xylose composites, catalyzed by a 10% volume fraction of acetic acid at 140 °C. The appearance of signals corresponding to xylose in the infrared spectrum of the DHP-xylose complex confirmed that DHP had undergone condensation with xylose under simulated hot-pressing conditions. Additionally, xylose-related signals were also observed in the NMR spectrum, indicating that DHP and xylose may be linked by C-C bonds, forming covalently bonded structures similar to LCCs. This represents a new opportunity to replace traditional formaldehyde adhesives, thereby promoting development in the formaldehyde-free adhesive field.
Author Contributions
Conceptualization, P.W.; Methodology, P.W.; Validation, J.X. (Jiaju Xie) and J.X. (Junxian Xie); Formal analysis, W.P., G.Z. and J.C.; Investigation, J.X. (Jiaju Xie) and J.A.; Data curation, J.X. (Jiaju Xie); Writing—original draft, P.W. and J.X. (Jiaju Xie); Writing—review & editing, W.P., J.X. (Junxian Xie), J.A., G.Z. and J.C.; Visualization, J.C.; Supervision, W.P.; Project administration, J.C.; Funding acquisition, P.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
There are no conflicts to declare.
Funding Statement
The authors are grateful for the support from the National Natural Science Foundation of China (No. 32071722).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Shen R., Hu R., Chen Z. Discussions on the Developments of Aldehyde-Free Adhesive Technology and Aldehyde-Free Wood-based Panels in China. Mod. Chem. Res. 2022;17:6–9. doi: 10.3969/j.issn.1672-8114.2022.17.003. [DOI] [Google Scholar]
- 2.Shi Y., Cui H. Patent Analysis of Formaldehyde-free Adhesive for Wood-based Panels. Stand. Sci. 2022;S1:21–26. doi: 10.3969/j.issn.1674-5698.2022.z1.003. [DOI] [Google Scholar]
- 3.Vázquez G., González J., Freire S., Antorrena G. Effect of chemical modification of lignin on the gluebond performance of lignin-phenolic resins. Bioresour. Technol. 1997;60:191–198. doi: 10.1016/S0960-8524(97)00030-8. [DOI] [Google Scholar]
- 4.Khan M.A., Ashraf S.M., Malhotra V.P. Development and characterization of a wood adhesive using bagasse lignin. Int. J. Adhes. Adhes. 2004;24:485–493. doi: 10.1016/j.ijadhadh.2004.01.003. [DOI] [Google Scholar]
- 5.Alonso M., Oliet M., Rodríguez F., Astarloa G., Echeverría J. Use of a methylolated softwood ammonium lignosulfonate as partial substitute of phenol in resol resins manufacture. J. Appl. Polym. Sci. 2004;94:643–650. doi: 10.1002/app.20887. [DOI] [Google Scholar]
- 6.Mu Y., Wang C., Zhao L., Chu F. Study on Composite Adhesive of Hydroxymethylated Lignosulfonate/Phenol-formaldehyde Resin with Low Free Formaldehyde. Chem. Ind. For. Prod. 2009;29:38–42. doi: 10.3321/j.issn:0253-2417.2009.03.007. [DOI] [Google Scholar]
- 7.Mu Y., Wang C., Zhao L., Chun F. Study on composite adhesive of alkali lignin/phenol formaldehyde resin for E0 grade plywood; Proceedings of the Biorefining Technology Exchange and Industrialization Seminar and the 3rd National Chemical Application Technology Development Hot Spot Seminar; Xiamen, China. 12 November 2008; pp. 221–224. [Google Scholar]
- 8.Alonso M.V., Oliet M., Rodrıguez F., Garcıa J., Gilarranz M., Rodrıguez J. Modification of ammonium lignosulfonate by phenolation for use in phenolic resins. Bioresour. Technol. 2005;96:1013–1018. doi: 10.1016/j.biortech.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Liu G., Qiu X., Xing D. Phenolation Modification of Wheat Straw Soda Lignin and its Utilization in Preparation of Lignin-Based Phenolic Formaldehyde Resins Adhesive. J. Chem. Eng. Chin. Univ. 2007;21:678–684. doi: 10.3321/j.issn:1003-9015.2007.04.024. [DOI] [Google Scholar]
- 10.Khan M., Ashraf S. Studies on thermal characterization of lignin: Substituted phenol formaldehyde resin as wood adhesives. J. Therm. Anal. Calorim. 2007;89:993–1000. doi: 10.1007/s10973-004-6844-4. [DOI] [Google Scholar]
- 11.An X. Application of Demethylated Lignosulfonate as a Substitute for Phenol in Wood Adhesives. Chem. Ind. For. Prod. 1995;3:36–42. [Google Scholar]
- 12.Guo T., Chen K., Yang S., Li S. Isolation of Lignin from Masson pine Kraft pulping black liqour and Preparation of Lignin-based Adhesives. China For. Prod. Ind. 1999;26:25–28. doi: 10.19531/j.issn1001-5299.1999.01.007. [DOI] [Google Scholar]
- 13.Hüttermann A., Milstein O., Nicklas B., Trojanowski J., Haars A., Kharazipour A. Enzymatic Modification of Lignin for Technical Use: Strategies and Results. ACS Symp. Ser. 1989;27:361–370. [Google Scholar]
- 14.Haars A., Kharazipour A., Zanker H., Huttermann A. Room-Temperature Curing Adhesives Based on Lignin and Phenoloxidases. ACS Symp. Ser. 1989;385:126–134. [Google Scholar]
- 15.Cao Y., Duan X., Cao Y., Lu J. Effect of Laccase Treatment Conditions of Lignosulphonate on Shearing Strength of Plywood. China For. Prod. Ind. 2007;34:13–17. doi: 10.3969/j.issn.1001-5299.2007.02.003. [DOI] [Google Scholar]
- 16.Yamaguchi H., Maeda Y., Sakata I. Bonding among woody fibers by use of enzymatic phenol dehydrogenative polymerization. Mechanism of generation of bonding strength. Mokuzai Gakkaishi. 1994;40:185–190. [Google Scholar]
- 17.Yamaguchi H., Maeda Y., Sakata I. Applications of phenol dehydrogenative polymerization by laccase to bonding among woody-fibers. Mokuzai Gakkaishi. 1992;38:931–937. [Google Scholar]
- 18.Yamaguchi H., Nagamori N., Sakata I. Application of phenol dehydrogenative polymerization of vanillic acid to bonding of woody fibers. Mokuzai Gakkaishi. 1993;37:220–226. [Google Scholar]
- 19.Lund M., Felby C. Wet strength improvement of unbleached kraft pulp through laccase catalyzed oxidation. Enzym. Microb. Technol. 2001;28:760–765. doi: 10.1016/S0141-0229(01)00339-8. [DOI] [PubMed] [Google Scholar]
- 20.Chandra R.P., Ragauskas A.J. Evaluating laccase-facilitated coupling of phenolic acids to high-yield kraft pulps. Enzym. Microb. Technol. 2002;30:855–861. doi: 10.1016/S0141-0229(02)00020-0. [DOI] [Google Scholar]
- 21.Chandra R.P., Lehtonen L.K., Ragauskas A.J. Modification of high lignin content kraft pulps with laccase to improve paper strength properties. 1. Laccase treatment in the presence of gallic acid. Biotechnol. Prog. 2004;20:255–261. doi: 10.1021/bp0300366. [DOI] [PubMed] [Google Scholar]
- 22.Chandra R.P., Felby C., Ragauskas A.J. Improving laccase-facilitated grafting of 4-hydroxybenzoic acid to high-kappa kraft pulps. J. Wood Chem. Technol. 2005;24:69–81. doi: 10.1081/WCT-120035945. [DOI] [Google Scholar]
- 23.Aracri E., Roncero M.B., Vidal T. Studying the effects of laccase-catalysed grafting of ferulic acid on sisal pulp fibers. Bioresour. Technol. 2011;102:7555–7560. doi: 10.1016/j.biortech.2011.05.046. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X., Pei J., Zhang F., Yu X., Hu H. Modification of OCC Pulp by Laccase with the Phenols Contained in APMP Waste Liquor. Trans. China Pulp Pap. 2011;26:16–20. [Google Scholar]
- 25.Bolker H.I. A lignin carbohydrate bond as revealed by infra-red spectroscopy. Nature. 1963;197:489–490. doi: 10.1038/197489a0. [DOI] [Google Scholar]
- 26.Wang X. Master’s Thesis. Hubei University of Technology; Wuhan, China: 2022. Study on Thermal Condensation Reaction Mechanism of Dehydrogenation Polymer Catalyzed with Acid. [Google Scholar]
- 27.Terashima N., Ralph S.A., Landucci L.L. New facile syntheses of monolignol glucosides; p-glucocoumaryl alcohol, coniferin and syringin. Holzforschung. 1996;50:151–155. [Google Scholar]
- 28.Yao L., Yang H., Yoo C.G., Chen C., Meng X., Dai J., Yang C., Yu J., Ragauskas A.J., Chen X. A mechanistic study of cellulase adsorption onto lignin. Green Chem. 2021;23:333–339. doi: 10.1039/D0GC02463E. [DOI] [Google Scholar]
- 29.Zhang M., Yang H., Yao L., Xie Y. Study on the Chemical Bond Connection between Lignin Dehydrogenation Polymer and Xylose. HuBei ZaoZhi. 2012;3:41–45. [Google Scholar]
- 30.Wen J.L., Sun S.L., Xue B.L., Sun R.C. Recent Advances in Characterization of Lignin Polymer by Solution-State Nuclear Magnetic Resonance (NMR) Methodology. Materials. 2013;6:359–391. doi: 10.3390/ma6010359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmgren A., Zhang L., Henriksson G. Monolignol dehydrogenative polymerization in vitro in the presence of dioxane and a methylated β-β′ dimer model compound. Holzforschung. 2008;62:508–513. doi: 10.1515/HF.2008.099. [DOI] [Google Scholar]
- 32.Richel A., Nicks F., Laurent P., Wathelet B., Wathelet J.-P., Paquot M. Efficient microwave-promoted synthesis of glucuronic and galacturonic acid derivatives using sulfuric acid impregnated on silica. Green Chem. Lett. Rev. 2012;5:179–186. doi: 10.1080/17518253.2011.607852. [DOI] [Google Scholar]
- 33.Nicola G., Martin L. Structural Insights on Recalcitrance during Hydrothermal Hemicellulose Extraction from Wood. ACS Sustain. Chem. Eng. 2017;5:5156–5165. [Google Scholar]
- 34.Matsushita Y., Kakehi A., Miyawaki S., Yasuda S. Formation and chemical structures of acid-soluble lignin II: Reaction of aromatic nuclei model compounds with xylan in the presence of a counterpart for condensation, and behavior of lignin model compounds with guaiacyl and syringyl nuclei in 72% sulfuric acid. J. Wood Sci. 2004;50:136–141. doi: 10.1007/s10086-003-0543-9. [DOI] [Google Scholar]
- 35.Yasuda S., Murase N. Chemical Structures of Sulfuric Acid Lignin. Part XII. Reaction of Lignin Models with Carbohydrates in 72% H2SO4. Holzforschung. 1995;49:418–422. doi: 10.1515/hfsg.1995.49.5.418. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.