Abstract
Pseudomonas aeruginosa is a persistent pathogen in the airways of patients with cystic fibrosis or bronchiectasis from other causes and appears to have evolved strategies to survive the inflammatory response of the host. We hypothesized that the secreted hemolytic phospholipase C (PLC) of P. aeruginosa (PlcHR) would decrease neutrophil respiratory burst activity. We found that while intact wild-type P. aeruginosa cells stimulated moderate respiratory burst activity from human neutrophils, an isogenic mutant pseudomonas (ΔHR strain) containing a targeted deletion of the plcHR operon induced a much more robust oxidative burst from neutrophils. In contrast, a second pseudomonas mutant (ΔN) containing a disruption in the gene encoding the nonhemolytic PLC (PlcN) was not different from the wild type in stimulating neutrophil O2·− production. Readdition of purified PlcHR to the ΔHR strain suppressed neutrophil O2·− production to levels stimulated by wild-type bacteria. Interestingly, purified PlcHR decreased phorbol myristate acetate (PMA)- but not formyl methionyl-leucyl-proline (fMLP)-induced respiratory burst activity, suggesting interference by PlcHR with a protein kinase C (PKC)-specific signaling pathway. Accordingly, the PKC inhibitor bisindolylmaleimide inhibited the oxidative burst induced by either PMA or intact pseudomonas, but not by fMLP, whereas the p38 kinase inhibitor SB-203580 fully inhibited the respiratory burst induced by fMLP or the PlcHR-replete wild-type bacteria, but not PMA or the PlcHR-deficient ΔHR bacterial mutant. We conclude that expression of PlcHR by P. aeruginosa suppresses bacterium-induced neutrophil respiratory burst by interfering with a PKC-dependent, non-p38 kinase-dependent pathway.
Chronic lung infection appears to play a central role in perpetuating bronchial inflammation in cystic fibrosis, and Pseudomonas aeruginosa has emerged as a key bacterial pathogen in this condition. Although the high salt content of bronchial secretions of cystic fibrosis patients has been shown to suppress bactericidal activity of bronchial mucosa (23), P. aeruginosa is also a predominant pathogen in bronchiectasis associated with a variety of other conditions, suggesting that the bacterium itself can withstand the immune response of the host. While the neutrophil is the principal effector cell responsible for clearance of P. aeruginosa, it is notable that the bacterium persists in lungs of affected individuals despite the heavy accumulation of granulocytes in the airway walls and lumen. This suggests that P. aeruginosa may elaborate substances which suppress neutrophil activation, thus enabling it to survive despite inflammatory cell recruitment.
P. aeruginosa elaborates two known phospholipases C (PLCs), PlcHR (hemolytic) and PlcN (nonhemolytic) (17). While PlcN has no demonstrated pathogenic activity, PlcHR may be an important virulence factor. Indeed, purified PlcHR causes vascular permeability, end organ damage, and death when injected into mice in high doses (1, 14). The plcHR operon has been cloned and consists of the structural gene plcH and the two downstream in-phase, overlapping genes plcR1 and plcR2, whose products are necessary for secretion and solubility of PlcHR (6). Because of the induction of PlcHR by phosphate starvation, it is thought to function in phosphate-scavenging pathways. This induction may be of pathogenic significance, since humans infected with gram-negative pathogens have circulating Pi levels reduced to a level suboptimal for bacterial growth (27).
Interestingly, however, both PLCs recognize phospholipids found predominantly on eukaryotic (e.g., phosphatidylcholine and sphingomyelin) rather than prokaryotic membranes. Since metazoan PLCs play a central role in host inflammatory cell signaling, P. aeruginosa PLCs may represent the evolution not only of nutritional enzymes, but also of secreted products which specifically alter the host’s immune response to the bacterium. In the present study, we demonstrate, using deletion mutants of P. aeruginosa, that bacterial expression of PlcHR suppresses neutrophil respiratory burst activity and thus may facilitate chronic persistent infection by P. aeruginosa.
MATERIALS AND METHODS
Reagents.
Catalase (81,536 U/mg; bovine liver) was purchased from Worthington Biochemical (Freehold, N.J.), the p38 inhibitor SB-203580 was a gift of SmithKline Beecham (King of Prussia, Pa.), and the protein kinase C (PKC) inhibitor bisindolylmaleimide I was from Calbiochem (San Diego, Calif.). Rabbit polyclonal antiserum against human neutrophil p47phox was a kind gift of B. Babior and R. Faust. [3H]dipalmitoylphosphatidylcholine (50 Ci/mmol) was from NEN (Boston, Mass.). Cytochrome c (horse heart, type VI), superoxide dismutase (SOD; bovine erythrocyte; 3,000 U/ml), formyl methionyl-leucyl-proline (fMLP), phorbol myristate acetate (PMA), and all other reagents were from Sigma (St. Louis, Mo.).
Bacterial strains and growth conditions.
The P. aeruginosa strains used in this study included the wild-type isolate, PAO1 (13), and its isogenic derivatives, ΔHR and ΔN. ΔHR was derived by deletion of the plcHR operon (18), consisting of the structural gene plcH and the two downstream in-phase, overlapping genes plcR1 and plcR2, whose products are necessary for secretion of PlcHR (6). PlcR remains tightly associated with PlcH after secretion into the culture media and through the purification process and is itself devoid of enzymatic activity. The stoichiometry of PlcH to PlcR is 1:1, and PlcH is insoluble without PlcR (26a). The ΔN derivative contains a deletion in the structural gene for the nonhemolytic PLC, plcN (22). Bacteria were grown at 37°C for approximately 16 h after inoculation from isolated colonies maintained on Luria-Bertani agar plates, selected with tetracycline (50 μg/ml) and/or gentamicin (50 μg/ml). Bacteria were grown in a mixture of 0.1 M HEPES (pH 7.0), 0.5 mM MgSO4, 7 mM (NH4)2SO4, 20 mM lactate, 1.78 μM FeCl3, 1.62 μM MnCl2, 2.45 μM CaCl2, 13.914 μM ZnCl2, and 4.69 μM H3BO4, without selection, under phosphate-deficient conditions (0.2 mM K2HPO4), to induce expression of the PLC enzymes. Bacteria were harvested by centrifugation at 10,000 × g for 10 min, washed once in phosphate-buffered saline (PBS), resuspended at 6.5 × 108 cells/ml in Hank’s buffered salt solution (HBSS), and used within 30 min.
Purification of PlcHR.
The plcHR operon was cloned into a T7 expression system (3), and was overexpressed in P. aeruginosa PAO1. PlcHR, the predominant protein complex secreted into culture media, was passed over a DEAE anion-exchange column and eluted with a 50 to 500 mM NaCl gradient. Following precipitation with 70% ammonium sulfate, PlcHR was further purified by preparative native gel electrophoresis. Only PlcH and PlcR proteins were detected on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels stained with Coomassie ammonical silver or SYPRO-orange (Molecular Probes, Oreg.), which can detect as little as 1 ng of material and is not protein selective (24). These preparations have undetectable quantities of lipopolysaccharide by the Limulus amoebocyte lysate assay.
The purified PlcHR was found to be active on the synthetic substrate ρ-nitrophenylphosphorylcholine and on the natural substrates phosphatidylcholine and sphingomyelin. A vesicle assay using [3H]dipalmitoylphosphatidylcholine (2) yielded a specific activity of the purified PlcHR preparation of 20 nmol of [3H]phosphorylcholine released/min/mg of protein. In this assay, water-soluble [3H]phosphorylcholine is released by PLC, whereas [3H]choline is released by phospholipase D. Release of [3H]phosphorylcholine but not [3H]choline by the PlcHR preparation was verified by thin-layer chromatography (data not shown).
Neutrophil respiratory burst activity.
Neutrophils were isolated from normal human subjects by Percoll and hydroxyethyl starch sedimentation (25), which resulted in >99% granulocytes. Neutrophils (106/ml), normal pooled human serum (5%), cytochrome c (0.75 mg/ml), catalase (5 μg/ml), and bacteria (2.5 × 108/ml) were combined in 96-well plates in a final volume of 200 μl in HBSS. A bacterium/neutrophil ratio of 1:250 was used in all experiments. Baseline wells contained 100 μg of SOD per ml, and the SOD-inhibitable change in A550 was monitored with an enzyme-linked immunosorbent assay plate reader (Biotek EL-340). In some experiments, PMA (100 nM) or fMLP (100 nM) was added instead of bacteria. In selected experiments, neutrophils were pretreated at 37°C with either bisindolylmaleimide I (1 μM) or SB-203580 (4 μM) for 30 min or with PlcHR (0.1 pg/ml to 0.1 μg/ml) for 15 min prior to addition of bacteria or agonist. Working stocks of PlcHR were made in 0.1% bovine serum albumin as a carrier protein. Each experiment was repeated with at least three separate neutrophil donors.
p47phox translocation.
Human neutrophils were incubated in Hank’s buffer containing 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 μg of aprotinin per ml, and 10 μg of leupeptin per ml for 15 min on ice and then resuspended in PBS with 7.5 mM glucose at 5 × 107 cells/ml. Cells were preincubated with PlcHR (10 pg/ml) at 37°C for 15 min in some experiments and then stimulated with either PMA (100 nM) or fMLP (100 nM) for 12 min. Cells were quickly pelleted, resuspended in relaxation buffer {3 mM NaCl, 100 mM KCl, 1.5 mM EGTA, 3.5 mM MgCl2, 10 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], 1 mM PMSF, 50 μg of leupeptin per ml, 25 μg of pepstatin per ml, 25 μg of aprotinin per ml, 1 mM ATP}, and sonicated for two 5-s pulses. The postnuclear fraction was spun through a discontinuous 15%/50% sucrose gradient at 100,000 × g at 4°C for 30 min. The supernatant fraction, largely cytosolic factors, was acetone precipitated to recover the majority of p47phox, which was used as a marker. The membrane fraction was recovered from the sucrose interface, washed twice with 0.25 M sucrose, and loaded on 10% PAGE gels. Western blots were performed with the p47phox antisera by using the ECL (enhanced chemiluminescence) detection system (Amersham, Arlington Heights, Ill.).
Statistical analysis.
Multiple groups were compared by using one-way analysis of variance with Student-Newman-Keuls multiple-comparison tests.
RESULTS
Effect of PlcHR on bacterium-induced respiratory burst activity.
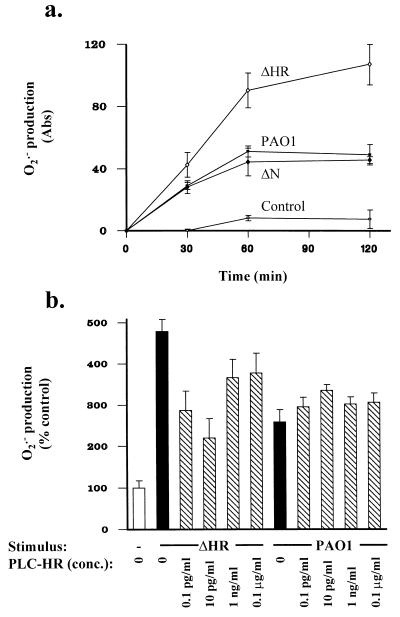
Addition of wild-type P. aeruginosa (PAO1) stimulated human neutrophils to produce O2·− for about 60 min, after which time, respiratory burst activity plateaued (Fig. 1a). Stimulation of neutrophils with the ΔHR bacterial mutant, deficient in PlcHR, led to an increase in both the rate of O2·− production and the total amount of O2·− produced, compared to stimulation with wild-type bacteria (P < 0.05). In contrast, stimulation of neutrophils with the ΔN mutant, deficient in PlcN, resulted in O2·− production that was not different from that elicited by wild-type bacteria (P > 0.05 at all time points). Further, treatment of neutrophils with purified PlcHR decreased the respiratory burst response to the ΔHR bacteria, to levels not different from that stimulated by the wild-type PAO1 bacteria (Fig. 1b). Addition of exogenous PlcHR to neutrophils did not alter respiratory burst activity in response to wild-type bacteria.
FIG. 1.
(a) Respiratory burst by neutrophils exposed to intact P. aeruginosa, as measured by cytochrome c reduction. Neutrophils had increased O2·− production when exposed to the ΔHR strain at 30 min (P < 0.05), 60 min (P < 0.001), and 120 min, compared to wild-type bacteria (PAO1). O2·− production induced by the ΔN strain was not different (P > 0.05) from that following exposure to wild-type bacteria. Neutrophils exposed to all bacterial strains produced greater O2·− levels than unstimulated neutrophils (P < 0.001). Abs, absorbance. (b) Neutrophils were pretreated with PlcHR (0.1 pg/ml to 0.1 μg/ml) for 15 min prior to addition of bacteria. Addition of ΔHR or PAO1 increased O2·− production by neutrophils (P < 0.01). PlcHR decreased (P < 0.05) O2·− production stimulated by ΔHR but not that by wild-type bacteria.
Effect of PlcHR on agonist-stimulated neutrophil respiratory burst activity.
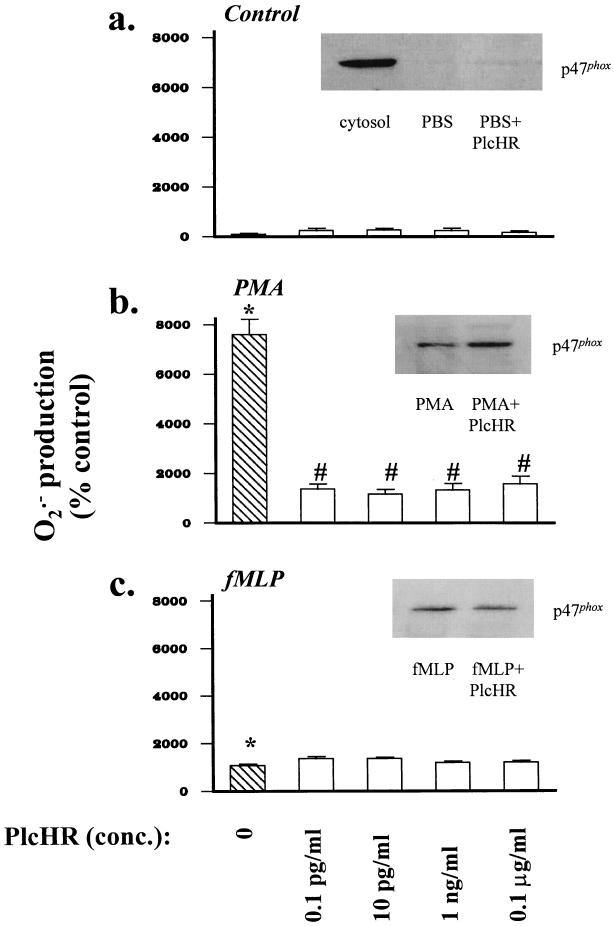
Purified PlcHR did not significantly alter O2·− production and only weakly increased translocation of cytosolic p47phox by unstimulated neutrophils (P > 0.05 [Fig. 2a]). However, PlcHR profoundly suppressed PMA-stimulated neutrophil respiratory burst activity (P < 0.001), at concentrations as low as 0.1 pg/ml (Fig. 2b). Despite this effect, PlcHR did not diminish and in fact somewhat enhanced p47phox translocation. In contrast, PlcHR did not affect neutrophil respiratory burst activity or p47phox translocation in response to a second soluble agonist, fMLP (Fig. 2c).
FIG. 2.
(a) PlcHR (0.1 pg/ml to 0.1 μg/ml) did not affect baseline O2·− production by unstimulated neutrophils (P > 0.05). Neutrophils were pretreated with PlcHR for 15 min prior to measurement of O2·− production. O2·− production was measured over 60 min. (Inset) PlcHR alone (10 pg/ml) weakly stimulated translocation of p47phox to the neutrophil membrane fraction. The cytosolic fraction of unstimulated neutrophils, containing the majority of p47phox, is shown in the first lane as a control. All other lanes shown are membrane fractions. (b) PMA increased O2·− production by neutrophils compared to that in the control (∗, P < 0.001). PlcHR (15-min pretreatment prior to agonist stimulation) decreased (#, P < 0.001) O2·− production by PMA-stimulated neutrophils. (Inset) PlcHR increased PMA-stimulated membrane translocation of p47phox. (c) fMLP increased O2·− production by neutrophils (∗, P < 0.001), and PlcHR did not decrease O2·− production by fMLP-stimulated neutrophils (P > 0.05). (Inset) PlcHR did not alter fMLP-stimulated membrane translocation of p47phox.
Effect of PKC and p38 kinase inhibitors on neutrophil respiratory burst activity.
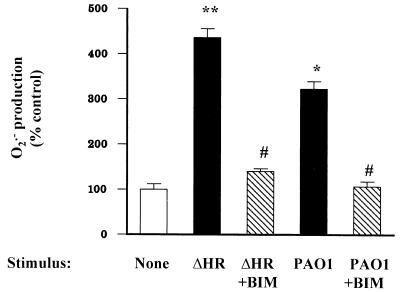
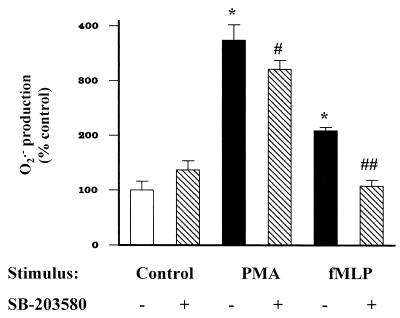
The specific PKC inhibitor bisindolylmaleimide decreased neutrophil O2·− production stimulated by either wild-type or ΔHR bacteria, to baseline levels (Fig. 3), suggesting that bacteria stimulate the respiratory burst through PKC-dependent mechanisms. As expected, bisindolylmaleimide decreased neutrophil O2·− production stimulated by the direct PKC agonist PMA (Fig. 4a); however, bisindolylmaleimide failed to inhibit fMLP-stimulated O2·− production (Fig. 4b). In contrast, the p38 kinase inhibitor SB-203580 fully suppressed fMLP-stimulated neutrophil O2·− production (P < 0.001), but only partially suppressed PMA-stimulated O2·− production (P < 0.05 [Fig. 5]). SB-203580 also partially suppressed neutrophil O2·− production stimulated by the ΔHR mutant (P < 0.001), but fully suppressed O2·− production when neutrophils were stimulated with the wild-type PAO1 strain (P < 0.001 [Fig. 6]).
FIG. 3.
Neutrophils exposed to wild-type pseudomonas (PAO1) had increased O2·− production over 60 min compared to unstimulated neutrophils (∗, P < 0.001). The ΔHR mutant stimulated more O2·− production than the wild type (∗∗, P < 0.001). Bisindolylmaleimide (BIM; 1 μM, 30-min pretreatment prior to addition of bacteria) decreased (#, P < 0.001) O2·− production by neutrophils exposed to either wild-type (PAO1) or ΔHR pseudomonas strains to levels not different from that of the control (P > 0.05).
FIG. 4.
Bisindolylmaleimide (BIM; 1 μM, 30-min pretreatment prior to addition of agonist) decreased (∗, P < 0.001) O2·− production by neutrophils stimulated with PMA (100 nM, 60 min) (a), but did not affect (P > 0.05) O2·− production by neutrophils stimulated with fMLP (100 nM, 60 min) (b).
FIG. 5.
PMA (100 nM) and fMLP (100 nM) increased neutrophil O2·− production over 60 min (∗, P < 0.001). Pretreatment of neutrophils with SB-203580 (4 μM) 30 min prior to addition of agonist partially decreased PMA-stimulated neutrophil O2·− production (#, P < 0.05) and fully suppressed fMLP-stimulated O2·− production (#, P < 0.001) to levels not different from that of the control.
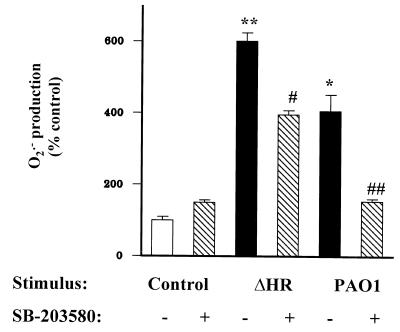
FIG. 6.
Neutrophils exposed to wild-type pseudomonas (PAO1) for 60 min had increased O2·− production compared to unstimulated neutrophils (∗, P < 0.001). The ΔHR mutant stimulated more O2·− production than the wild type (∗∗, P < 0.001). SB-203580 (4 μM, added 30 min prior to exposure to bacteria) partially decreased neutrophil O2·− production stimulated by the ΔHR bacterial strain (#, P < 0.001) and fully suppressed O2·− production in response to the wild-type pseudomonas (##, P < 0.001) to levels not different from that of the control. SB-203580 alone had no effect on O2·− production (P > 0.05).
DISCUSSION
Bacterial phospholipases are thought to constitute important virulence factors for a variety of organisms, although the mechanism for their pathogenic effects is quite varied. Divergent examples, for instance, include the clostridial alpha-toxin, a PLC which appears to act as a direct hemolysin in its ability to cause intravascular hemolysis, capillary injury, and myonecrosis, versus the phosphatidylinositol-specific listerial PLC, which appears to specifically allow bacteria to escape from intracellular phagolysosomes (4). P. aeruginosa PLC has been considered to be pathogenic, since purified pseudomonal PLC injected into mice causes hepatonecrosis and renal tubular necrosis (14), and intradermal injection of sheep with PlcHR causes superficial inflammatory lesions characteristic of a pseudomonal skin infection of sheep called fleecerot (5). Indeed, pseudomonas mutants with either disruptions or deletions in plcHR are markedly less virulent in different mouse burn models of sepsis (18, 19), providing more direct evidence that PlcHR affects bacterial virulence in vivo. Chronic infection of rabbit lungs with a plcHR deletion mutant also resulted in lower levels of the inflammatory mediators 6-keto-PGF1α and thromboxane B2 (10). In addition, a mutant deficient in both PlcHR and PlcN produces less lung injury and disseminates less than the wild-type strain in a rabbit model of pneumonia (28). Our findings suggest that this virulence associated with pseudomonal PLC may in part be due to suppression of neutrophil respiratory burst activity.
We found that P. aeruginosa mutants which do not elaborate PlcHR (ΔHR) elicit an exaggerated respiratory burst from normal human neutrophils, compared with their isogenic wild-type parental strain. This appeared to be explained by the specific loss of PlcHR and not some unanticipated or secondary change in bacterial properties, since readdition of purified PlcHR to the ΔHR strain caused suppression of the respiratory burst to levels induced by wild-type bacteria. Further evidence for the suppressive effects of PlcHR was seen in its inhibition of PMA-stimulated respiratory burst activity. The latter observation also indicated that PlcHR affects neutrophils directly and not through alterations in other bacterial characteristics.
Surprisingly, PlcHR did not diminish neutrophil respiratory burst activity induced by the bacterial peptide fMLP. This selectivity suggests that PlcHR does not act as a nonspecific NADPH oxidase inhibitor or interfere with substrate availability but rather that it may inhibit part of the activation pathway upstream of the oxidase complex. Interestingly, PlcHR did not suppress and in fact enhanced translocation of the cytosolic NADPH oxidase subunit p47phox from neutrophil cytosol to membrane. This observation suggests that PlcHR likely targets some other aspect of the multistep oxidase assembly and activation process. Other events which appear to occur independently of p47phox translocation during assembly of a functional oxidase are translocation of cytosolic Rac 2 (12), phosphorylation of p67phox (9), and posttranslocation phosphorylation of p47phox (8). In addition, since PMA stimulates neutrophils largely through direct activation of PKC, inhibition of the neutrophil respiratory burst by PlcHR likely occurs at or distal to PKC. Consistent with this inference is the observation that P. aeruginosa appears to stimulate neutrophil respiratory burst through a PKC-dependent pathway, since bisindolylmaleimide completely suppressed O2·− production of bacterium-stimulated neutrophils.
In contrast, fMLP-stimulated respiratory burst was inhibited by neither PlcHR nor bisindolylmaleimide, indicating a divergence in the proximal signaling pathways initiated by fMLP on the one hand and PMA or intact bacteria on the other. Further evidence for the existence of dual activation pathways was found in the differential effect of SB-203580, a specific inhibitor of the p38 MAP kinases. While the p38 kinase inhibitor completely suppressed fMLP-induced O2·− production, it had a much lesser, though significant, effect on PMA-induced O2·− production. This is consistent with prior studies linking neutrophil p38 kinase-dependent respiratory burst activity more closely with fMLP than PMA stimulation. For instance, a p38 kinase inhibitor decreased fMLP- but not PMA-stimulated neutrophil O2·− production in one study (16), implying a non-p38 activation of neutrophils by PMA. Supporting this inference, treatment of neutrophils with a peptide inhibitory for MAPKAP kinase 2, an important p38 substrate, only marginally suppressed PMA-stimulated O2·− production, whereas it greatly suppressed fMLP-stimulated O2·− production (29).
Importantly, the p38 kinase inhibitor only partially suppressed respiratory burst activity induced by the PlcHR-deficient ΔHR bacterial strain, suggesting that these bacteria activate neutrophils through redundant pathways which involve and bypass p38 MAP kinase, respectively. In contrast, the p38 kinase inhibitor fully suppressed the respiratory burst stimulated by the PlcHR-replete wild-type pseudomonas, indicating that these bacteria initiate signaling entirely through the p38 kinase-dependent pathway. PlcHR, therefore, appears to inhibit neutrophil signaling at a PKC-dependent, non-p38-dependent step.
The suppressive effects of pseudomonas PlcHR are somewhat surprising in light of the participation of endogenous PLCs in neutrophil activation. However, PLCs known to be involved in neutrophil activation are phosphatidylinositol specific (15), whereas PlcHR hydrolyzes exclusively phosphatidylcholine and sphingomyelin (17). Phosphatidylcholine-specific PLCs have recently been shown to participate in MAP kinase and NF-κB signaling pathways in other cell types (7, 21, 26), raising the possibility that phosphatidylcholine-PLC activity may be important in neutrophil signaling as well. Interestingly, the ΔN pseudomonas mutant, which lacks the phosphatidylcholine and phosphatidylserine-specific PlcN, did not affect bacterium-induced respiratory burst activity in this study. This may be a function of the significantly different physical properties of PlcN and PlcHR (17), or it may reflect the difference in substrate specificities between the two enzymes. Additionally, an unrecognized property of PlcHR may be responsible for respiratory burst suppression. Indeed, a partially homologous (15% identity, 51% similarity) acid phosphatase from Francisella tularensis also inhibits neutrophil respiratory burst, although the mechanism for this effect is also not known (20).
In summary, we have demonstrated that PlcHR, a major secreted product of P. aeruginosa, potently suppresses the neutrophil respiratory burst response to whole bacteria. The biological relevance of this effect is heightened by the enhancement of burst activity in response to a bacterial PlcHR knockout mutant. In cystic fibrosis patients, titers of antibodies specific for pseudomonas PLC increase as chronic colonization with this bacterium becomes established, documenting the presence of significant quantities of the protein in the inflammatory environment in vivo (11). We suggest that PlcHR is an important bacterial product which facilitates pseudomonas survival in tissues despite the abundance of neutrophils, an inevitable situation in the bronchiectasis of cystic fibrosis.
ACKNOWLEDGMENTS
This work was supported by the American Heart Association, the Cystic Fibrosis Foundation, and the National Institutes of Health (R29-HL52591 and R01-HL61897 to L.S.T. and RO1-HL62608 to M.L.V.). L. S. Terada is an Established Investigator of the American Heart Association, and K. A. Johansen is a recipient of a Postdoctoral Research Fellowship from the Cystic Fibrosis Foundation.
REFERENCES
- 1.Berk R S, Brown D, Coutinho I, Meyers D. In vivo studies with two phospholipase C fractions from Pseudomonas aeruginosa. Infect Immun. 1987;55:1728–1730. doi: 10.1128/iai.55.7.1728-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown H A, Gutowski S, Moomaw C R, Slaughter C, Sternweis P C. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75:1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- 3.Brunschwig E, Darzins A. A two-component T7 system for the overexpression of genes in Pseudomonas aeruginosa. Gene. 1992;111:35–41. doi: 10.1016/0378-1119(92)90600-t. [DOI] [PubMed] [Google Scholar]
- 4.Camilli A, Tilney L G, Portnoy D A. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chin J C, Watts J E. Biological properties of phospholipase C purified from a fleecerot isolate of Pseudomonas aeruginosa. J Gen Microbiol. 1988;134:2567–2575. doi: 10.1099/00221287-134-9-2567. [DOI] [PubMed] [Google Scholar]
- 6.Cota-Gomez A, Vasil A I, Kadurugamuwa J, Beveridge T J, Schweizer H P, Vasil M L. PlcR1 and PlcR2 are putative calcium-binding proteins required for secretion of the hemolytic phospholipase C of Pseudomonas aeruginosa. Infect Immun. 1997;65:2904–2913. doi: 10.1128/iai.65.7.2904-2913.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowen D S, Sowers R S, Manning D R. Activation of a mitogen-activated protein kinase (ERK2) by the 5-hydroxytryptamine 1A receptor is sensitive not only to inhibitors of phosphatidylinositol 3-kinase, but to an inhibitor of phosphatidylcholine hydrolysis. J Biol Chem. 1996;271:22297–22300. doi: 10.1074/jbc.271.37.22297. [DOI] [PubMed] [Google Scholar]
- 8.DeLeo F R, Quinn M T. Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase proteins. J Leukoc Biol. 1996;60:677–691. doi: 10.1002/jlb.60.6.677. [DOI] [PubMed] [Google Scholar]
- 9.El Benna J, Dang P M, Gaudry M, Fay M, Morel F, Hakim J, Gougerot-Pocidalo M A. Phosphorylation of the respiratory burst oxidase subunit p67(phox) during human neutrophil activation. Regulation by protein kinase C-dependent and independent pathways. J Biol Chem. 1997;272:17204–17208. doi: 10.1074/jbc.272.27.17204. [DOI] [PubMed] [Google Scholar]
- 10.Graham L M, Vasil A, Vasil M L, Voelkel N F, Stenmark K R. Decreased pulmonary vasoreactivity in an animal model of chronic Pseudomonas pneumonia. Am Rev Respir Dis. 1990;142:221–229. doi: 10.1164/ajrccm/142.1.221. [DOI] [PubMed] [Google Scholar]
- 11.Granstrom M, Ericsson A, Strandvik B, Wretlind B, Pavlovskis O R, Berka R, Vasil M L. Relation between antibody response to Pseudomonas aeruginosa exoproteins and colonization/infection in patients with cystic fibrosis. Acta Paediatr Scand. 1984;73:772–777. doi: 10.1111/j.1651-2227.1984.tb17774.x. [DOI] [PubMed] [Google Scholar]
- 12.Heyworth P G, Knaus U G, Xu X, Uhlinger D J, Conroy L, Bokoch G M, Curnutte J T. Requirement for posttranslational processing of Rac GTP-binding proteins for activation of human neutrophil NADPH oxidase. Mol Biol Cell. 1993;4:261–269. doi: 10.1091/mbc.4.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holloway B W, Krishnapillai V, Morgan A F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979;43:73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyers D J, Palmer K C, Bale L A, Kernacki K, Preston M, Brown T, Berk R S. In vivo and in vitro toxicity of phospholipase C from Pseudomonas aeruginosa. Toxicon. 1992;30:161–169. doi: 10.1016/0041-0101(92)90469-l. [DOI] [PubMed] [Google Scholar]
- 15.Mullmann T J, Cheewatrakoolpong B, Anthes J C, Siegel M I, Egan R W, Billah M M. Phospholipase C and phospholipase D are activated independently of each other in chemotactic peptide-stimulated human neutrophils. J Leukoc Biol. 1993;53:630–635. doi: 10.1002/jlb.53.6.630. [DOI] [PubMed] [Google Scholar]
- 16.Nick J A, Avdi N J, Young S K, Knall C, Gerwins P, Johnson G L, Worthen G S. Common and distinct intracellular signaling pathways in human neutrophils utilized by platelet activating factor and FMLP. J Clin Investig. 1997;99:975–986. doi: 10.1172/JCI119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostroff R M, Vasil A I, Vasil M L. Molecular comparison of a nonhemolytic and a hemolytic phospholipase C from Pseudomonas aeruginosa. J Bacteriol. 1990;172:5915–5923. doi: 10.1128/jb.172.10.5915-5923.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostroff R M, Wretlind B, Vasil M L. Mutations in the hemolytic-phospholipase C operon result in decreased virulence of Pseudomonas aeruginosa PAO1 grown under phosphate-limiting conditions. Infect Immun. 1989;57:1369–1373. doi: 10.1128/iai.57.5.1369-1373.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahme L G, Stevens E J, Wolfort S F, Shao J, Tompkins R G, Ausubel F M. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 20.Reilly T J, Baron G S, Nano F E, Kuhlenschmidt M S. Characterization and sequencing of a respiratory burst-inhibiting acid phosphatase from Francisella tularensis. J Biol Chem. 1996;271:10973–10983. doi: 10.1074/jbc.271.18.10973. [DOI] [PubMed] [Google Scholar]
- 21.Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell. 1992;71:765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 22.Shortridge V D, Lazdunski A, Vasil M L. Osmoprotectants and phosphate regulate expression of phospholipase C in Pseudomonas aeruginosa. Mol Microbiol. 1992;6:863–871. doi: 10.1111/j.1365-2958.1992.tb01537.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg T H, Jones L J, Haugland R P, Singer V L. SYPRO orange and SYPRO red protein gel stains: one-step fluorescent staining of denaturing gels for detection of nanogram levels of protein. Anal Biochem. 1996;239:223–228. doi: 10.1006/abio.1996.0319. [DOI] [PubMed] [Google Scholar]
- 25.Terada L S, Hybertson B M, Connelly K G, Weill D, Piermattei D, Repine J E. Xanthine oxidase increases neutrophil adherence to endothelial cells by a dual ICAM-1 and P-selectin mechanism. J Appl Physiol. 1997;82:866–873. doi: 10.1152/jappl.1997.82.3.866. [DOI] [PubMed] [Google Scholar]
- 26.van Dijk M C, Muriana F J, de Widt J, Hilkmann H, van Blitterswijk W J. Involvement of phosphatidylcholine-specific phospholipase C in platelet-derived growth factor-induced activation of the mitogen-activated protein kinase pathway in Rat-1 fibroblasts. J Biol Chem. 1997;272:11011–11016. doi: 10.1074/jbc.272.17.11011. [DOI] [PubMed] [Google Scholar]
- 26a.Vasil, M. L., and A. I. Vasil. Unpublished observations.
- 27.Weinberg E D. Iron and susceptibility to infectious disease. Science. 1974;134:952–955. doi: 10.1126/science.184.4140.952. [DOI] [PubMed] [Google Scholar]
- 28.Wiener-Kronish J, Sakuma T, Kudoh I, Pittet J, Frank D, Dobbs L, Vasil M, Matthay M. Alveolar epithelial injury and pleural empyema in acute P. aeruginosa pneumonia in anesthetized rabbits. J Appl Physiol. 1993;75:1661–1669. doi: 10.1152/jappl.1993.75.4.1661. [DOI] [PubMed] [Google Scholar]
- 29.Zu Y L, Ai Y, Gilchrist A, Labadia M E, Sha’afi R I, Huang C K. Activation of MAP kinase-activated protein kinase 2 in human neutrophils after phorbol ester or fMLP peptide stimulation. Blood. 1996;87:5287–5296. [PubMed] [Google Scholar]