Abstract
ASPND1 and ASPF2 are immunodominant antigens from Aspergillus nidulans and A. fumigatus, respectively, that are readily synthesized in infections in the human host, as demonstrated by their reactivity with more than 80% of sera from patients with aspergilloma or allergic bronchopulmonary aspergillosis. We demonstrate here that both antigens are exclusively produced under situations of low bioavailability of free Zn2+. Addition of micromolar concentrations of Zn2+ to the culture medium strongly stimulated Aspergillus growth but totally inhibited ASPND1 or ASPF2 production. This effect was specific, since other divalent metals had no effect. Removal of endogenous Zn2+ by a chelator also stimulated ASPND1 production, and the effect was specifically reversed by Zn2+. These results suggest a possible role of these antigens in the survival of the fungus in the lungs.
Several Aspergillus species are opportunistic pathogens causing respiratory diseases (aspergilloma and allergic bronchopulmonary aspergillosis [ABPA]) in normal hosts and invasive or disseminated infections in immunosuppressed patients (16). Immunocompetent affected individuals often have high levels of antibodies directed at fungal components. Although these antibodies may not always provide an effective defense against the fungus, they can be useful for diagnostic purposes.
ASPND1 is an Aspergillus nidulans immunodominant antigen that is highly reactive to sera from aspergilloma-affected individuals. Recent molecular cloning and characterization of the aspnd1 gene (5) has revealed a high degree of homology to a well-characterized A. fumigatus allergen (ASPF2) which is consistently reactive to sera from individuals suffering from ABPA (1). Both cross-reactive antigens (of unknown function) have been overproduced in bacteria as recombinant proteins which retain the ability to react with both immunoglobulin G- and immunoglobulin E-specific antibodies. Characteristics common to the antigens are that they are detected only when the fungi are grown in certain conditions (especially in Czapek-Dox medium) and that they elicit a strong immune response (4, 15). These observations may reflect how the expression of these proteins is regulated in vivo and could provide some clues about their function, if any, as determinants of virulence.
In bacterial systems, virulence genes seem to be integrated into complex regulatory networks which determine the expression of virulence factors only when needed (19). In most cases, these regulatory circuits switch on in response to a few signals, such as a temperature of 37°C, iron deprivation, or contact with eukaryotic cells, which are all environmental conditions expected to be found in the bodies of mammals (6). In the case of Aspergillus, only iron deprivation has been shown to stimulate the synthesis of products involved in the survival of the invading fungus (3).
We report here that the factor responsible for the production of ASPND1 and ASPF2 is the lack of Zn2+ in the culture medium. We started our study with A. nidulans, observing that identical regulatory mechanisms are present in A. fumigatus and other Aspergillus species. Our results are consistent with the idea that zinc deprivation could induce the in vivo synthesis of specific zinc-regulated fungal proteins in the human body. Zinc starvation could therefore be considered a new signal for fungal pathogens from the host environment.
MATERIALS AND METHODS
Organisms and growth conditions.
A. nidulans G1059wt (adF17 pabaA1 yA2) was obtained from A. J. Clutterbuck, Glasgow, Scotland. Two different A. fumigatus isolates were used: ATCC 9197 (here after called A. fumigatus C), from the American Type Culture Collection and A. fumigatus R, a clinical isolate from the sputum of a patient with pulmonary aspergilloma. A. flavus, isolated from a lymphadenopathy in a patient with breast cancer and cutaneous invasive aspergillosis, was a gift from A. del Palacio (12 de Octubre Hospital, Madrid, Spain). A. terreus is a strain from the Spanish Type Culture Collection (CECT 2748).
The organisms were maintained on solid YED medium (1% [wt/vol] d-glucose, 1% [wt/vol] Difco yeast extract, 2% [wt/vol] agar). To obtain high yields of conidia, the fungi were grown on solid Aspergillus complete minimal medium (Amm) or AMM containing 1% glucose, 0.6% NaNO3, 0.052% MgSO4, 0.052% KCl, 0.15% KH2PO4, traces of FeSO4 and ZnSO4, and 1.5% (wt/vol) agar (pH 6.5). Plates were incubated at 28°C for at least 4 days.
For liquid growth cultures, four different media were used: YED, AMM, Bacto Sabouraud dextrose broth (SAB), and Bacto Czapek-Dox broth alone (CD) or mixed 1:1 with Bacto synthetic broth AOAC (CDA). SAB, CD, and CDA media were obtained from Difco Laboratories (Detroit, Mich.). For A. nidulans, AMM, SAB, CD, and CDA media, both solid and liquid, were supplemented with 10 mg of p-aminobenzoic acid and 200 mg of adenine per liter.
All Aspergillus species were grown by inoculation of 105 conidia per ml in 1-liter Erlenmeyer flasks containing 300 ml of the corresponding liquid medium followed by incubation at 28 or 37°C in an Adolph Kühner orbital shaker at 250 rpm. Mycelia were harvested from liquid medium cultures by filtering through Whatman GF/C paper and washed thoroughly with double-distilled H2O. The wet cake was immediately frozen and kept at −70°C until used.
Preparation of cell extracts.
Frozen mycelia were thawed and mixed with lysing buffer (100 mM Tris-HCl [pH 7.5] containing 1 mM EDTA, 5 mM dithiothreitol, 1 mM freshly added phenylmethylsulfonyl fluoride [Sigma Chemical Co.], 5 μg of aprotinin per ml, and 5 μg of pepstatin A per ml [both obtained from Boehringer Mannheim]) to give a dense suspension. Samples were then disrupted in the 20,000-lb/in2 cell of an SLM Aminco French press, previously refrigerated at −20°C, at a pressure of 16,000 lb/in2. Complete breakage was monitored by microscopic observation. Sodium dodecyl sulfate (2%, final concentration) was added to the lysed mycelia, and the lysate was incubated for 10 min at 100°C. Clarified extracts (12,000 × g, 15 min) were aliquoted and stored at −70°C.
Protein was quantitated by a modification of the Lowry method (24). Extracts containing less than 1 mg of protein per ml were concentrated by precipitation with 7 volumes of cold acetone at −70°C for at least 3 h. Precipitated protein was pelleted by spinning for 20 min at 12,000 × g at 4°C, dried in a vacuum evaporator (Savant Instruments), carefully resuspended in 2% SDS to the desired concentration, and clarified by centrifugation at 3,000 × g 15 min.
SDS-polyacrylamide gel electrophoresis (PAGE).
Electrophoreses were carried out on a Protean II or Mini-Protean apparatus (Bio-Rad Laboratories) on isotropic 14% (wt/vol) acrylamide slab gels (16 by 18 by 0.1 cm or 8 by 6 by 0.1 cm), using the discontinuous buffer system of Laemmli (17). Molecular weight protein standards were Bio-Rad Low or GIBCO-BRL molecular weight standards.
Proteins in gels were detected by a sensitive silver stain (21) or by staining for 30 min with 0.5% Coomassie brilliant blue R-250 in acetic acid-isopropanol-water (1:3:6) and destaining in acetic acid-methanol-water (10:5:85).
Electrophoretic blotting procedures and immunological detection of proteins.
Proteins from extracts were first subjected to SDS-PAGE as described above and then transferred to nitrocellulose sheets (0.45 μm; Schleicher & Schuell) in a Trans-Blot cell (Bio-Rad) as previously described (4). Blots were developed with a monospecific anti-ASPND1 (5) or an antimembrane (25) rabbit serum (for detection of the loading control AgMb [antigen from membranes]) at 1/500 dilution as the first antibody. Whenever two proteins were detected in the same sample, the blotted membrane was treated with antibodies to one protein, washed with Tris-buffered saline, blocked for 1 h, and incubated with antibodies against the second protein.
Densitometric quantification of blots.
The amount of immunoreactive protein in the bands was quantitated by volume integration, with subtraction of the local background, by using the Bioimage Whole Band Analyzer software (Genomic Solutions, Ann Arbor, Mich.). The values obtained as integrated optical density (IOD) units were used directly for comparison. When needed, the values for ASPND1 bands were corrected for the corresponding value of the loading control (AgMb).
RESULTS
The production of ASPND1 antigen is dependent on the culture medium.
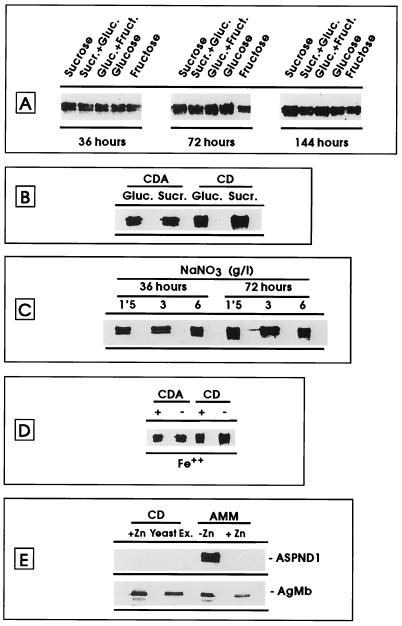
Total protein extracts from A. nidulans grown in four different culture media (YED, SAB, AMM, and CDA) were analyzed by immunoblotting (Fig. 1) for the presence of the immunodominant antigen ASPND1. An anti-ASPND1 rabbit serum, previously shown to be monospecific (5), confirmed that the characteristic ASPND1 double antigenic band (running around the 50-kDa region) was present at high levels only when the fungus was grown in CDA medium, and this was independent of the temperature of incubation (28 or 37°C). The antigen was detected in all growth phases analyzed (exponential, deceleration, or stationary).
FIG. 1.
Immunoblot of total mycelial extracts from A. nidulans cultured in different media (20 μg of protein/lane), separated on an SDS–14% polyacrylamide gel, and developed by using as the first antibody a monospecific anti-ASPND1 (ASPND1) or antimembrane (AgMb) rabbit serum at 1/500 dilution. Lanes: E, exponential phase; D, deceleration phase; S, stationary phase.
To identify proteins synthesized in all media that could be used as positive controls for constitutive expression, the same blots were reprobed with a rabbit antiserum raised by injecting A. nidulans total membranes (25). Under these circumstances, several reactive bands were consistently present in all extracts analyzed. One of these bands, labeled in Fig. 1 as AgMb (running at about 33 kDa), was selected as the internal control for protein loading in further experiments.
Zn2+ starvation induces ASPND1 production.
To determine which components of CDA medium were responsible for the production of ASPND1, we made a detailed comparison of the two defined media, CDA and AMM, and studied the effects of all of their different components (both quantitatively and qualitatively) on the presence or absence of the antigen. The type of sugar used as the carbon source in CDA medium did not influence ASPND1 production since the amount of antigen was roughly the same regardless of the sugar (Fig. 2A). ASPND1 was undetectable when grown in AMM, also regardless of the carbon source (data not shown). When the AOAC amino acid mixture was omitted from CDA medium to give the CD medium, ASPND1 production was unaffected, also independently of the carbon source (Fig. 2B). Differences in the levels of nitrate (analyzed in the absence of AOAC amino acid mixture with either glucose or sucrose as the carbon source) did not have any effect on ASPND1 production (Fig. 2C). The absence of Fe2+ in both CDA and CD media did not influence the production of ASPND1 (Fig. 2D). However, addition of Zn2+ to CD medium abolished ASPND1 production; conversely, when Zn2+ was omitted in the preparation of AMM medium, high levels of ASPND1 were detected on the immunoblots (Fig. 2E, line ASPND1). Again, AgMb was used as a constitutive control (Fig. 2E) and did not significantly change. Yeast extract was also inhibitory when added to CD (Fig. 2E) or CDA (not shown) medium, probably because of its undetermined Zn2+ content.
FIG. 2.
Immunoblots of total mycelial extracts (20 μg of protein/lane) from A. nidulans cultured in CD medium (with the variations and during the times indicated), separated on an SDS–14% polyacrylamide gel, and developed by using as the first antibody a monospecific anti-ASPND1 (panels A to D and ASPND1 in panel E) or an antimembrane (AgMb in panel E) rabbit serum at 1/500 dilution. Experimental details are described in the text.
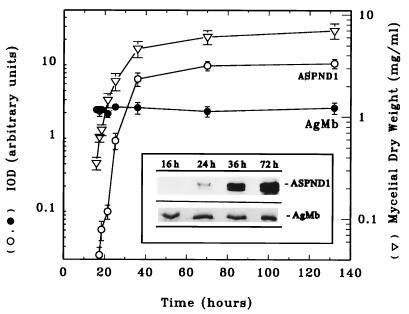
Kinetics of ASPND1 production.
ASPND1 seemed to be produced continuously during growth in CDA (Fig. 1). However, in these early experiments the first samples had been withdrawn after 24 h at 37°C or 39 h at 28°C. To examine earlier time points, cultures were inoculated with 105 conidia/ml and grown in CD medium (the simplest producing medium) at 28°C. ASPND1 was not evident on immunoblots until 24 h of growth at 28°C, and its concentration increased exponentially with the increase in mycelial dry weight (Fig. 3), indicating that the antigen was not constitutively produced at early time points. When CDA medium was used, the results were analogous, but ASPND1 was not detectable until around 36 h at 28°C. AgMb used as a loading control was detected at the same level throughout the growth period in all of the media and can therefore be used as a true constitutively expressed protein.
FIG. 3.
Kinetics of ASPND1 and AgMb production with time. A. nidulans (105 conidia/ml) was cultured at 28°C in CD medium. At the indicated times, samples were withdrawn to determine mycelial dry weight (triangles) and to obtain total mycelial extracts, which were separated on an SDS–14% polyacrylamide gel (20 μg of protein per lane) and immunoblotted by using as the first antibody anti-ASPND1 (insert, ASPND1) or antimembrane (insert, AgMb) rabbit serum at 1/500 dilution. ASPND1 and AgMb bands were quantified by densitometry, and the IOD values obtained were plotted (on a semilogarithmic scale) against time. AgMb was constitutively produced (black circles), whereas the synthesis of ASPND1 (white circles) increased with time in parallel with the increase in mycelial dry weight (white triangles). Results are means (± standard deviations) of three separate determinations.
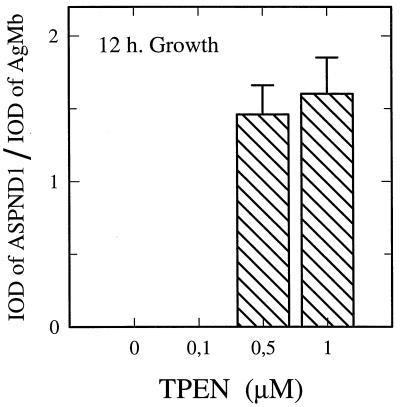
The lack of ASPND1 in the early hours of growth may be due to an inhibitor, such as Zn2+, present in CD medium. To test this hypothesis, we used the specific chelator tetrakis-(2-pyrydylmethyl)ethylenediamine (TPEN) (8). If Zn2+ is the inhibitor, then lowering the free Zn2+ should enhance ASPND1 production at early time points. Figure 4 shows that this is indeed the case. Concentrations of 0.5 to 1 μM chelator were enough to allow high levels of ASPND1 production in the previously nonproducing 12-h cultures (106 conidia/ml), whereas the biosynthesis of the loading control AgMb was not affected by TPEN (not shown). These results are consistent with the idea that trace amounts of Zn2+ in the culture medium were responsible for the delay in the onset of ASPND1 production. Concentrations of TPEN between 5 to 100 μM prevented conidium germination and were not tested. The effects of TPEN were specifically reversed by the addition of Zn2+ (data not shown), and it therefore follows that the onset of ASPND1 production is dependent on the concentration of available Zn2+.
FIG. 4.
Effect of zinc chelation on ASPND1 production. A. nidulans was cultured for 12 h at 28°C by inoculation of 106 conidia/ml in CD medium supplemented with 0.1, 0.5, and 1.0 μm TPEN (a zinc-specific chelator). At the end of growth, total mycelial extracts were separated on an SDS–14% polyacrylamide gel (20 μg of protein per lane) and immunoblotted with anti-ASPND1 and anti-membrane rabbit serum as the first antibody. Results are means (± standard deviations) of three separate determinations and are expressed as the IOD ratio between ASPND1 and AgMb.
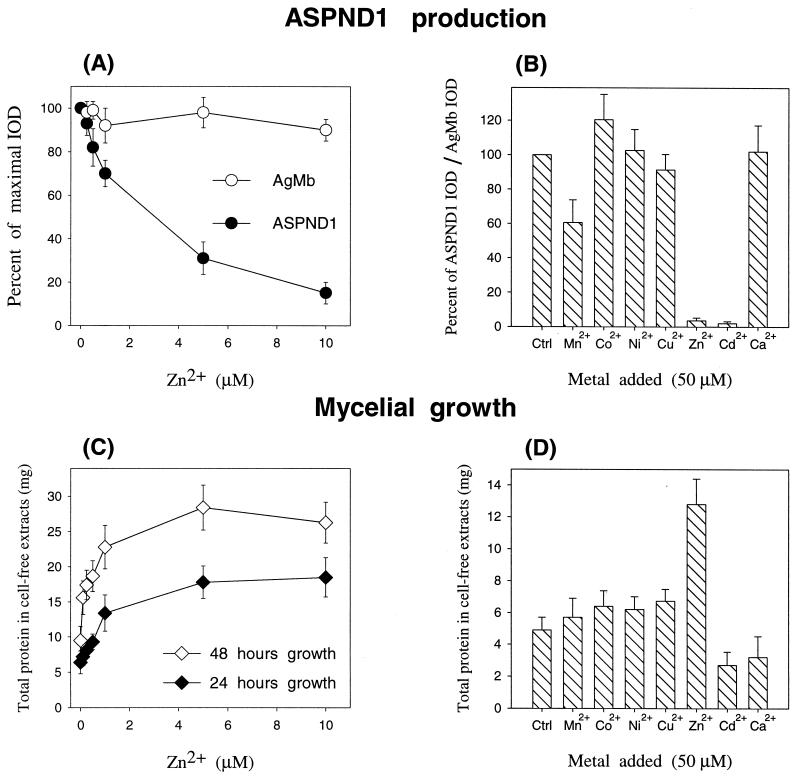
Addition of Zn2+ specifically inhibits ASPND1 production but stimulates mycelial growth.
The extent of inhibition of ASPND1 biosynthesis was dependent on the amount of Zn2+ added to the culture medium (Fig. 5). To prevent any interference in conidium germination the cultures (106 conidia/ml) were allowed to grow for 12 h at 28°C. At this point they were supplemented with increasing concentrations of Zn2+ (0 to 10 μM); after a further 12 or 36 h of incubation, the amounts of ASPND1 and AgMb were determined by immunoblotting and densitometry (the results were identical at 12 and 36 h, and only the first are shown in Fig. 5A). Concentrations as low as 5 μM Zn2+ reduced ASPND1 levels by more than 50%, and a total absence of the antigen was observed with concentrations above 20 μM (data not shown). When the cation was added from the start of culture, 5 μM Zn2+ was sufficient to fully prevent ASPND1 production (data not shown). Mycelial growth (measured as total protein in cell extracts) increased with the addition of Zn2+ to the medium. Concentrations of 1 μM and higher almost doubled the amount of mycelia with respect to the control without added Zn2+, demonstrating that Zn2+ is limiting for growth in CD medium (Fig. 5C).
FIG. 5.
Effect of the addition of increasing concentrations of Zn2+ or of 50 μM divalent ions on ASPND1 production. Several parallel cultures of A. nidulans (106 conidia/ml) were grown at 28°C for 12 h. Each of the cultures was then supplemented with ZnSO4 to obtain final concentrations of 0, 0.1, 0.5, 1.0, 5.0, and 10.0 μM Zn2+ (A and C) or with the indicated divalent metal salts (sulfate form) to achieve a final concentration of 50 μM (B and D). The amount of immunoreactive ASPND1 or AgMb present in total mycelial extracts obtained from all cultures after a further 12 h of growth was determined as described in the text by densitometry of the corresponding immunoblots (A and B). Results in panel A are expressed as the percentage of the maximal IOD measured for ASPND1 or AgMb; results in panel B are expressed as the percentage of the maximal IOD ratio between ASPND1 and AgMb. The amount of total protein in cell extracts (C and D) was determined as indicated in Materials and Methods. Results are means (± standard deviations) of three separate determinations. Ctrl, control.
To determine whether other divalent metal ions had similar effects on the production of ASPND1 protein, we treated cultures with 50 μM Co2+, Ni2+, Cu2+, or Ca2+. These metal ions did not inhibit ASPND1 production (Fig. 5B). Zn2+ and Cd2+ totally prevented ASPND1 biosynthesis, and Mn2+ afforded a 40% inhibitory effect. Mycelial growth was maximal in the presence of Zn2+ (Fig. 5D). At these levels, Mn2+, Co2+, Ni2+, and Cu2+ did not affect significantly growth. Cd2+ and Ca2+ inhibited growth by about 30%. The biosynthesis of the loading control AgMb was not affected by any of the metals (not shown).
The ASPF2 allergen from A. fumigatus is another zinc-responsive protein.
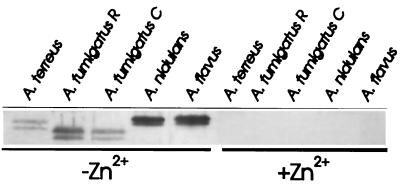
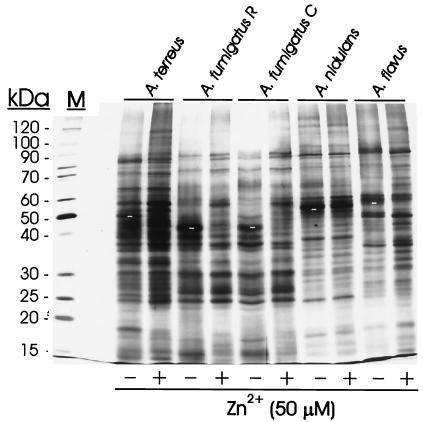
Due to the close homology of ASPND1 with the immunodominant allergen ASPF2 from A. fumigatus, and since this species is the main etiological agent of the different forms of aspergillosis, we were interested in knowing whether the zinc-dependent response observed in A. nidulans also occurred in A. fumigatus or other Aspergillus species. When two different A. fumigatus strains, A. fumigatus C and A. fumigatus R, were grown 48 h in CD medium with or without 50 μM Zn2+, a double band (of faster electrophoretic mobility than ASPND1 and corresponding to the 40- and 37-kDa components of ASPF2) was detected in extracts from both strains only when Zn2+ was absent from the growth medium (Fig. 6). A relative of the ASPND1 family was also expressed in A. terreus and A. flavus only in low-zinc culture medium. Immunoblots developed with anti-ASPND1 (Fig. 6) and anti-ASPF2 rabbit monospecific antibodies (data not shown) yielded identical results. The absence of zinc did not dramatically modify the protein patterns, except for ASPND1 and its related antigens (Fig. 7). The patterns of A. terreus, A. nidulans, and A. flavus were very similar, and only minor differences were observed for both strains of A. fumigatus.
FIG. 6.
Presence of zinc-regulated proteins in other Aspergillus species. Immunoblots of total mycelial extracts (20 μg of protein/lane) from the indicated Aspergillus spp. (105 conidia/ml) cultured for 48 h at 28°C in CD medium with or without 50 μM Zn2+, separated on an SDS–14% polyacrylamide gel, and developed by using as the first antibody a monospecific anti-ASPND1 rabbit serum at 1/500 dilution. Experimental details are described in the text.
FIG. 7.
Protein patterns of different Aspergillus species in presence or absence of zinc. Silver-stained SDS–14% polyacrylamide gel profiles of total mycelial extracts (5 μg of protein/lane) from the indicated Aspergillus spp. (105 conidia/ml inoculum) cultured for 48 h at 28°C in CD medium with or without 50 μM Zn2+. ASPND1 and related antigens are indicated in − Zn2+ lanes by white dashes. M, molecular mass markers.
DISCUSSION
The influence of the culture medium on the biosynthesis of Aspergillus antigens was first described many years ago (14, 32) and has been confirmed on different occasions (4, 13, 15). For instance, antigens harboring proteolytic activities are mainly synthesized in response to low nitrogen or carbon contents in the culture medium (29) or can be induced by the presence of specific substrates such as elastin (26–28) or collagen (30), both of which are susceptible to proteolytic degradation. Apart from these cases, no other defined inducing signals have been reported. However, the production of some immunodominant antigens is dependent on a particular culture medium; e.g., the closely related A. fumigatus ASPF2 and A. nidulans ASPND1 antigens were almost exclusively produced in CDA medium (4, 15). Information about the particular components of the media responsible for induction is lacking. To obtain insight into the in vivo signals that direct the fungus to produce a particular protein and into the possible function of that protein as a virulence determinant, we decided to carefully analyze which factors determined the onset of the synthesis of one of these antigens, ASPND1. Here we have shown that ASPND1 synthesis does not depend on the type or amount of the carbon or nitrogen source but instead is strongly inhibited by micromolar concentrations of Zn2+ or Cd2+ in the culture medium. This regulatory system is not exclusive to the A. nidulans strain used for the study since the same response was found in other strains tested (data not shown). Furthermore, the response seems to be characteristic of this group of fungi since related species such as A. fumigatus (the main pathogenic agent), A. flavus, and A. terreus also synthesize ASPND1-related antigens in response to zinc starvation.
In trace quantities, zinc is known to be a micronutrient essential to fungal growth, and several Aspergillus species have been reported to grow poorly in zinc-deficient cultures (12). Zinc is a functional component of a variety of transcription factors and fungal metalloenzymes ranging from those involved in intermediary metabolism to those involved in the synthesis of nucleic acids. Omission of this cation from the culture medium may have widespread physiological consequences such as a decrease in DNA, RNA, and protein synthesis or a decrease in the number of mitochondria (12). Despite this, very little is known about the molecular mechanisms that cells use to obtain zinc. So far, only two fungal transporter genes, ZRT1 and ZRT2 from Saccharomyces cerevisiae (34, 35), have been isolated. These two genes define two separate systems: one system (corresponding to ZRT1) shows a strong affinity for zinc, and its activity markedly increases in zinc-deprived cells (34). The second system (corresponding to ZRT2) has a lower affinity for zinc and is not regulated by zinc availability (35).
The human body has developed a metal-withholding defense system in which metal-binding proteins restrict access of microbial invaders to the host’s metals (2). Iron deprivation induces the synthesis of siderophores and several bacterial outer membrane proteins involved in iron acquisition (18). These iron-repressible outer membrane proteins are found in many bacterial pathogens both in vitro and in vivo (22, 33), and there is increasing evidence that these proteins are important virulence determinants (10, 23, 31). Siderophore production induced by low iron concentrations has also been found in Aspergillus, although the direct involvement of iron in pathogenesis has not yet been demonstrated (3).
The metal content of lung tissues has never been correlated with the expression of Aspergillus virulence factors. It would be interesting to determine the environmental signals that are responsible for enhancing virulence in the lung. Zn2+ levels in human plasma and tissues may vary with aging and in several pathological states, but in any case, physiological levels of free zinc are below 20 nM (9, 20). Zinc limitation (independently or together with iron limitation) could indicate to the fungus that it is within a living mammal and function as a switch to induce the synthesis of proteins required to acquire and transport zinc, ensuring that these proteins are synthesized only when needed. ASPND1 and ASPF2 may be the first reported members of a family of zinc-responsive proteins, apparently widely distributed among the aspergilli. Whereas these proteins are only expressed in vitro under conditions of Zn2+ starvation, they are readily synthesized in vivo, as demonstrated by the existence of high levels of circulating antibodies specifically directed against them in several forms of aspergillosis (ABPA and aspergilloma) (1, 4, 7).
To our knowledge, this is the first description of zinc-regulated proteins in Aspergillus. Negative regulation by zinc in eukaryotes has been reported for at least two other cases. The most similar is represented by the above-mentioned S. cerevisiae ZRT1 gene (the first gene of this class described in any organism), whose expression was induced at the transcriptional level by zinc limitation (34). In a human cell culture system, addition of Zn2+ to the medium inhibited the induction of ornithine decarboxylase (ODC) activity in ODC-overproducing L1210-DFMO cells. The decrease in activity was accompanied by a proportional decrease in the content of immunoreactive ODC protein, whereas the level of ODC mRNA was not affected significantly (11). Preliminary experiments suggest that in the case of ASPND1, major regulation takes place at the transcriptional level. Confirmation of these data and identification and characterization of putative zinc-responsive elements probably present in the 5′ regulatory region of the A. nidulans ASPND1 gene are currently been addressed at our laboratory.
ACKNOWLEDGMENTS
We are grateful to John Doonan for critical reading of the manuscript, Carlos Belinchón for photographic services, and Nicholas Skinner for revising the English version.
This work was supported by grant PM97-0157 from the DGES of the Ministerio de Educación y Ciencia (Spain) and by the Acción Integrada HB1997-0199 between the British Council (U.K.) and the Ministerio de Educación y Ciencia (Spain).
REFERENCES
- 1.Banerjee B, Kurup V P, Phadnis S, Greenberger P A, Fink J N. Molecular cloning and expression of a recombinant Aspergillus fumigatus protein AspfII with significant immunoglobulin E reactivity in allergic bronchopulmonary aspergillosis. J Lab Clin Med. 1996;127:253–262. doi: 10.1016/s0022-2143(96)90093-1. [DOI] [PubMed] [Google Scholar]
- 2.Barclay R. The role of iron in infection. Med Lab Sci. 1985;42:166–177. [PubMed] [Google Scholar]
- 3.Bouchara J P, Tronchin G, Larcher G, Chabasse D. The search for virulence determinants in Aspergillus fumigatus. Trends Microbiol. 1995;3:327–330. doi: 10.1016/s0966-842x(00)88965-9. [DOI] [PubMed] [Google Scholar]
- 4.Calera J A, López-Medrano R, Ovejero M C, Puente P, Leal F. Variability of Aspergillus nidulans antigens with media and time and temperature of growth. Infect Immun. 1994;62:2322–2333. doi: 10.1128/iai.62.6.2322-2333.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calera J A, Ovejero M C, López-Medrano R, Segurado M, Puente P, Leal F. Characterization of the Aspergillus nidulans aspnd1 gene demonstrates that the ASPND1 antigen, which it encodes, and several A. fumigatus immunodominant antigens belong to the same family. Infect Immun. 1997;65:1335–1344. doi: 10.1128/iai.65.4.1335-1344.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornelis G R. Contact with eukaryotic cells: a new signal triggering bacterial gene expression. Trends Microbiol. 1997;5:43–45. doi: 10.1016/S0966-842X(96)30040-1. [DOI] [PubMed] [Google Scholar]
- 7.Coulot P, Bouchara J P, Renier G, Annaix V, Planchenault C, Tronchin G, Chabasse D. Specific interaction of Aspergillus fumigatus with fibrinogen and its role in cell adhesion. Infect Immun. 1994;62:2169–2177. doi: 10.1128/iai.62.6.2169-2177.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuajungco M P, Lees G J. Prevention of zinc neurotoxicity in vivo by N,N,N′,N′-tetrakis (2-pyridylmethyl) ethylene-diamine (TPEN) Neuroreport. 1996;7:1301–1304. doi: 10.1097/00001756-199605170-00017. [DOI] [PubMed] [Google Scholar]
- 9.Fabris N, Mocchegiani E. Zinc, human diseases and aging. Aging (Milano) 1995;7:77–93. doi: 10.1007/BF03324297. [DOI] [PubMed] [Google Scholar]
- 10.Fernández-Beros M E, González C, McIntosh A, Cabello F C. Immune response to the iron deprivation-induced proteins of Salmonella typhi in typhoid fever. Infect Immun. 1989;57:1271–1274. doi: 10.1128/iai.57.4.1271-1275.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flamigni F, Campana G, Carboni L, Rossoni C, Spampinato S. Post-transcriptional inhibition of ornithine decarboxylase induction by zinc in a difluoromethylornithine resistant cell line. Biochim Biophys Acta. 1994;1201:101–105. doi: 10.1016/0304-4165(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 12.Garraway M O, Evans R C. Fungal nutrition and physiology. New York, N.Y: John Wiley & Sons; 1984. pp. 154–157. [Google Scholar]
- 13.Hearn V M. Antigenicity of Aspergillus species. J Med Vet Mycol. 1992;30:11–25. [PubMed] [Google Scholar]
- 14.Kim S J, Chaparas S D. Characterization of antigens from Aspergillus fumigatus. I. Preparation of antigens from organisms grown in completely synthetic medium. Am Rev Respir Dis. 1978;118:547–552. doi: 10.1164/arrd.1978.118.3.547. [DOI] [PubMed] [Google Scholar]
- 15.Kurup V P, Kumar A. Immunodiagnosis of aspergillosis. Clin Microbiol Rev. 1991;4:439–456. doi: 10.1128/cmr.4.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon-Chung K J, Bennett J E. Medical mycology. Philadelphia, Pa: Lea & Febiger; 1992. pp. 201–247. [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Litwin C M, Calderwood B. Role of iron in regulation of virulence genes. Clin Microbiol Rev. 1993;6:137–149. doi: 10.1128/cmr.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mekalanos J J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milne P D. Trace elements. In: Burtis C A, Ashwood E R, editors. Tietz textbook of clinical chemistry. W. B. Philadelphia, Pa: Saunders; 1998. pp. 1029–1055. [Google Scholar]
- 21.Morrisey J H. Silver stain for proteins in polyacrylamide gel: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981;117:307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- 22.Otto B R, Verweij W R, Sparrius M, Verweij-van Vught A M J J, Nord C E, MacLaren D M. Human immune response to an iron-repressible outer membrane protein of Bacteroides fragilis. Infect Immun. 1991;59:2999–3003. doi: 10.1128/iai.59.9.2999-3003.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Payne S M. Iron acquisition in microbial pathogenesis. Trends Microbiol. 1993;1:66–69. doi: 10.1016/0966-842x(93)90036-q. [DOI] [PubMed] [Google Scholar]
- 24.Peterson G L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 25.Puente P, Fernández N, Ovejero M C, Leal F. Immunogenic potential of Aspergillus nidulans subcellular fractions and their polypeptide components. Mycoses. 1992;35:235–241. doi: 10.1111/j.1439-0507.1992.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 26.Ramesh M V, Sirakova T, Kolattukudy P E. Isolation, characterization, and cloning of cDNA and the gene for an elastinolytic serine proteinase from Aspergillus flavus. Infect Immun. 1994;62:79–85. doi: 10.1128/iai.62.1.79-85.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhodes J C, Amlung T W, Miller M S. Isolation and characterization of an elastinolytic proteinase from Aspergillus flavus. Infect Immun. 1990;58:2529–2534. doi: 10.1128/iai.58.8.2529-2534.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sirakova T D, Markaryan A, Kolattukudy P E. Molecular cloning and sequencing of the cDNA and gene for a novel elastinolytic metalloproteinase from Aspergillus fumigatus and its expression in Escherichia coli. Infect Immun. 1994;62:4208–4218. doi: 10.1128/iai.62.10.4208-4218.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang C M, Cohen J, Holden D W. An Aspergillus fumigatus alkaline protease mutant constructed by gene disruption is deficient in extracellular elastase activity. Mol Microbiol. 1992;6:1663–1671. doi: 10.1111/j.1365-2958.1992.tb00891.x. [DOI] [PubMed] [Google Scholar]
- 30.Tomee J F C, Kauffman H F, Klimp A H, de Monchy J G R, Köeter G H, Dubois A E J. Immunologic significance of a collagen-derived culture filtrate containing proteolytic activity in Aspergillus-related diseases. J Allergy Clin Immunol. 1994;93:768–778. doi: 10.1016/0091-6749(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 31.Trivier D, Courcol R J. Iron depletion and virulence in Staphylococcus aureus. FEMS Microbiol Lett. 1996;141:117–127. doi: 10.1111/j.1574-6968.1996.tb08373.x. [DOI] [PubMed] [Google Scholar]
- 32.van der Heide S, Kauffman H F, de Vries K. Cultivation of fungi in synthetic and semi-synthetic liquid medium. II. Immunochemical properties of the antigenic and allergenic extracts. Allergy. 1985;40:592–598. doi: 10.1111/j.1398-9995.1985.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 33.Worst D J, Sparrius M, Kuipers E J, Kusters J G, de Graaf J. Human serum antibody response against iron-repressible outer membrane proteins of Helicobacter pylori. FEMS Microbiol Lett. 1996;144:29–32. doi: 10.1111/j.1574-6968.1996.tb08504.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhao H, Eide D. The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc Natl Acad Sci USA. 1996;93:2454–2458. doi: 10.1073/pnas.93.6.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao H, Eide D. The ZRT2 gene encodes the low affinity zinc transporter in Saccharomyces cerevisiae. J Biol Chem. 1996;271:23203–23210. doi: 10.1074/jbc.271.38.23203. [DOI] [PubMed] [Google Scholar]