Abstract
The obligate parasitic bacterium Streptococcus equi subsp. equi is the causative agent of strangles, a serious disease of the upper respiratory tract in horses. In this study we have, using shotgun phage display, cloned from S. equi subsp. equi and characterized a gene, called sfs, encoding a protein termed SFS, representing a new type of fibronectin (Fn)-binding protein. The sfs gene was found to be present in all 50 isolates of S. equi subsp. equi tested and in 41 of 48 S. equi subsp. zooepidemicus isolates tested. The sfs gene is down-regulated during growth in vitro compared to fnz, a previously characterized gene encoding an Fn-binding protein from S. equi subsp. zooepidemicus. Sequence comparisons revealed no similarities to previously characterized Fn-binding proteins, but high scores were obtained against collagen. Besides similarity due to the high content of glycine, serine, and proline residues present in both proteins, there was a nine-residue motif present both in collagen and in the Fn-binding domain of SFS. By searching the Oklahoma S. pyogenes database, we found that this motif is also present in a potential cell surface protein from S. pyogenes. Protein SFS was found to inhibit the binding between Fn and collagen in a concentration-dependent way.
Streptococcus equi (Lancefield group C) comprises the two subspecies, S. equi subsp. equi and S. equi subsp. zooepidemicus. Subspecies equi is the causative agent of strangles, a worldwide-distributed and serious disease of the equine upper respiratory tract. Subspecies zooepidemicus is considered an opportunistic commensal, often occurring in the upper respiratory tract of healthy horses; however, after stress or a virus infection, it can cause a secondary infection, which results in strangles-like symptoms. Subspecies equi is virtually confined to horses, whereas subspecies zooepidemicus also infects a wide range of other animals, such as pigs, dogs, cats, and cows. Human cases with infection due to subspecies zooepidemicus have also been reported (1). Isolates of subspecies equi are serologically and genetically very homogeneous, whereas isolates of subspecies zooepidemicus display a high degree of heterogeneity (7, 14, 17, 24).
Attachment to eucaryotic cell surfaces is an essential step in the establishment of infection and colonization by bacterial pathogens. Binding to fibronectin (Fn) has been shown to be one of the mechanisms used by streptococci for attachment to host cells (8, 9). Binding between bacterial cell surface Fn-binding proteins and immobilized Fn promotes internalization of streptococci by epithelial cells (4, 13, 18). Fn is a dimeric glycoprotein found both in plasma and in a fibrillar form in the extracellular matrix. The main function of Fn is to mediate substrate adhesion of eucaryotic cells, which involves the binding of specific cell surface receptors to certain domains of the Fn molecule (10). The protein also interacts with several other macromolecules, such as DNA, heparin, fibrin, and collagen (10).
We previously cloned and characterized a Fn-binding cell surface protein of subspecies zooepidemicus, designated FNZ (16). This protein, like several Fn-binding proteins of gram-positive cocci, exhibits typical features of cell wall-associated proteins, i.e., a signal peptide, a C-terminal cell wall-spanning domain containing the cell wall anchoring motif LPXTG, and a hydrophobic membrane-spanning domain, followed by a short stretch of mainly positively charged residues. In addition, a repetitive region, located in the C-terminal half of these Fn-binding proteins, binds the 29-kDa N-terminal fragment of Fn generated by trypsin digestion. In some proteins, including FNZ, there is a nonrepetitive domain which binds to a second site on the Fn molecule, other than the 29-kDa fragment (16, 20). Both subspecies of S. equi bind native Fn. However, in contrast to subspecies zooepidemicus, subspecies equi does not bind the 29-kDa fragment of Fn (2, 17). This is interesting since Southern blot analyses have shown that the gene fnz is generally present in both subspecies of S. equi (17). The aim of this study was to further characterize the Fn-binding properties of subspecies equi.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Ninety-eight clinical isolates of S. equi (50 subsp. equi and 48 subsp. zooepidemicus) collected from different parts of Sweden between 1982 and 1996 were together with the streptococcal control strains S. dysgalactiae S2 and S. equisimilis 172, obtained from the National Veterinary Institute, Uppsala, Sweden. S. pyogenes AW-43 was a kind gift from G. Lindahl, Lund University. Plasmid pUC19 was used for cloning purposes, and pGEX-5X-2 (Pharmacia Biotech, Uppsala, Sweden) was used to facilitate purification of protein FNZ. Phagemid pG8SAET (19) was used for purification of protein SFS and for construction of the phage display library. Streptococcal strains were grown on horse blood agar plates or in Todd-Hewitt broth (Oxoid, Basingstoke, England) supplemented with 0.5% yeast extract (THY). Escherichia coli strains were cultured in Luria-Bertani (LB) medium supplemented in appropriate cases with 50 μg of ampicillin per ml.
Construction of phagemid library.
Shotgun phage display libraries were constructed essentially as described by Jacobsson and Frykberg (12). Briefly, chromosomal DNA of S. equi subsp. equi Bd 3221 was purified and fragmented by sonication. The obtained fragments were treated with T4 DNA polymerase to generate blunt ends and subsequently ligated into SnaBI-digested and dephosphorylated pG8SAET vector. Approximately 4.5 × 106 ampicillin-resistant transformants were obtained after electrotransformation of the ligated material into E. coli TG1 cells. Twenty randomly picked transformants were all shown to contain inserts. Cells from an overnight culture of the transformants were infected with helper phage R408 and poured together with soft agar onto L-agar (LA) plates containing ampicillin and incubated overnight. Phage particles were eluated from the soft agar by addition of LB and vigorous shaking. The suspension was centrifuged, and the supernatant was sterile filtrated. The titer of the library was determined to 7 × 1010 CFU/ml.
Panning of the phagemid library.
Microtiter wells (Maxisorp; Nunc, Copenhagen, Denmark) were coated with human Fn (Sigma, St. Louis, Mo.) at a concentration of 100 μg/ml in 50 mM sodium carbonate (pH 9.7). The wells were blocked with phosphate-buffered saline (PBS)–0.05% Tween 20 (PBS-T) containing casein (0.1 mg/ml). After washing with PBS-T, the library was added to the wells. Before elution, the wells were extensively washed with PBS-T and then eluted with 140 mM NaCl–50 mM sodium citrate (pH 2.0). Neutralized eluate was infected with E. coli TG1 cells and spread on LA plates containing ampicillin. The next day, approximately 1,500 colonies were pooled; after infection with helper phage R408, the sample was mixed with soft agar and poured out on LA plates. After incubation overnight, the phagemid particles were extracted and subjected to another round of panning.
Screening for Fn-binding and fnz-unrelated clones.
From each panning, 180 colonies were transferred in triplicate to LA plates and incubated overnight. The following day, one plate (master plate) was stored, and the colonies from the two remaining plates were transferred to nitrocellulose filters and incubated for 2 h. Cells from one filter were lysed with chloroform vapor; after blocking of the filter with PBS-T supplemented with casein (0.1 mg/ml), it was incubated with Fn (1 μg/ml; Sigma) for 2 h; after washing, a rabbit anti-Fn antibody (diluted 1/1,000; Sigma) was added. After 1 h of incubation and washing, the filter was incubated for an additional hour with a horseradish peroxidase-labeled secondary antibody (diluted 1/1,000; Bio-Rad, Richmond, Calif.). Reactive bands were visualized by using 4-chloro-1-naphthol (Serva, Heidelberg, Germany). The second filter was subjected to colony hybridization essentially as described by Sambrook et al. (22). The radioactively labeled probe covered the entire fnz gene and was generated by PCR amplification of chromosomal DNA from S. equi subsp. zooepidemicus ZV, using primers 5-fnz (5′-CGGGATCCCTATTACACATTCTCATCTCATAT [positions 19 to 42]) and 3-fnz (5′-GGAATTCCAGAAAGCCCGCCTGTAAAC [positions 1954 to 1935]). The underlined nucleotides and positions in the primers correspond to the published sequence of the gene fnz (16). Clones displaying Fn-binding activity and negative in the colony hybridization assay were sequenced by using a Thermo Sequence dye terminator cycle sequencing premix kit (Amersham) and ABI model 377XL DNA sequencer. Computer programs from the PCGENE, DNA, and protein sequence analysis software package (Intelligenetics, Inc., Mountain View, Calif.) were used to record and analyze the sequence data.
Isolation of sfs.
Southern blot analysis of restriction enzyme-digested chromosomal DNA of S. equi subsp. equi Bd 3221 revealed that a 2.6-kb SspI fragment contained sfs. Fragments of this size were purified from a preparative agarose gel and ligated into pUC19. The ligation mix was electroporated into E. coli, and transformants were screened for Fn-binding activity as described above. Among several positive clones, we selected one, designated pSFS62, and sequenced the insert.
Southern blots.
Agarose-embedded chromosomal DNA digested with ApaI was resolved on 1.2% SeaKem GTG agarose gel (FMC, Rockland, Maine) in 0.5× Tris-borate-EDTA buffer by pulsed-field gel electrophoresis using a Gene Navigator (Pharmacia Biotech) as described previously (17). The DNA was transferred to nylon filters (Hybond N+; Amersham) by vacuum blotting (VacuGene XL; Pharmacia Biotech) in accordance with the manufacturer’s protocol. After cross-linking, the filters were prehybridized for 2 h at 65°C in 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–3× Denhardt’s solution–0.5% sodium dodecyl sulfate and subsequently incubated with the radioactively labeled sfs probe overnight, using the same conditions. The membranes were washed three times for 20 min each at 65°C with 0.2× SSC–0.1% sodium dodecyl sulfate and subjected to autoradiography. The sfs probe was generated by PCR amplification of chromosomal DNA from S. equi subsp. equi Bd 3221 by using primers fs5 (5′-ACAAGCCATGGAGCACTTGTCTTTGGAGGT [positions 205 to 222]) and fr4 (5′-GTCGGGATTGTAAGAATAGCC [positions 1224 to 1204]). The underlined nucleotides and positions in the primers correspond to the sequence in Fig. 2. The single band obtained after agarose gel electrophoresis was purified and random primed.
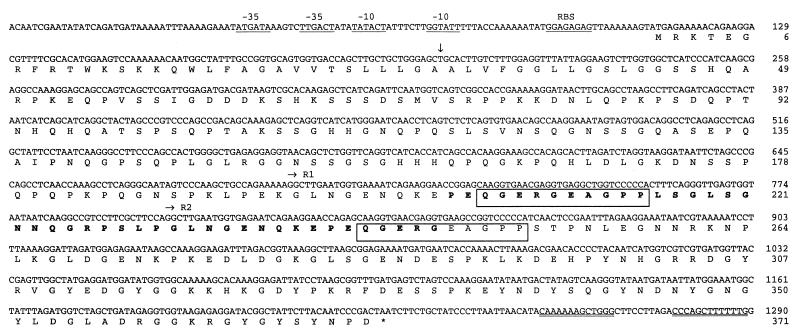
FIG. 2.
Complete nucleotide sequence of the sfs gene from S. equi subsp. equi Bd 3221 and deduced amino acid sequence of SFS. A putative ribosome-binding site (RBS) and possible −10 and −35 promoter sequences are underlined. A putative signal sequence cleavage site is marked with a vertical arrow, the translational stop codon is marked with an asterisk, and a possible transcription termination hairpin loop is double underlined. The two iterations of a 21-amino-acid-long motif situated in the Fn-binding domain are marked R1 and R2, and the 10 amino acids with homology to sequences in collagen and a potential cell surface protein from S. pyogenes are boxed. Bold letters indicate the amino acid sequence of phagemid clone S4.
Construction and purification of fusion proteins.
The purified PCR fragment corresponding to the mature protein of SFS, described above, was digested with NcoI and ligated into SnaBI-NcoI-cleaved pG8SAET. This vector encodes a 13-amino-acid peptide tag (E tag) which facilitates purification of the recombinant protein on a HiTrap Anti-E-tag column (Pharmacia Biotech). The recombinant protein SFS-E was purified from the periplasmic space according to the manufacturer’s protocol.
A fragment covering a major part of fnz was amplified by PCR, using primers Lages-3 (5′-TAGAATTCTTGTGCTGGCAACAAGCT [positions 157 to 174]) and Lages-4X (5′-ATCCACTCGAGTGGCGCAGGTGCAGGT [positions 1754 to 1740]) and chromosomal DNA from S. equi subsp. zooepidemicus ZV as the template. The underlined nucleotides and positions in the primers correspond to the published sequence of the gene fnz (16). The obtained fragment was ligated into pGEX-5X-2 (Pharmacia Biotech). In this vector, the insert is fused with a part of the plasmid encoding glutathione S-transferase (GST) which facilitates purification using glutathione-Sepharose 4B. The fusion protein (GST-FNZ) was purified according to the manufacturer’s protocol.
Inhibition assays.
Cells from overnight cultures of streptococci were collected by centrifugation, washed in PBS, and suspended in PBS–0.2% Tween 20 to an optical density at 600 nm (OD600) of 0.2. In cases of inhibition, 25 nM affinity purified fusion protein SFS-E and/or GST-FNZ was preincubated 15 min with 16 pM 125I-labeled human Fn (91,061 cpm), and then bacteria (500 μl) were added. After 2 h of incubation at room temperature, the mixtures were centrifuged and the supernatants were removed. The radioactivity associated with the pellets was quantified in a gamma counter (LKB Wallac, Turku, Finland). Radioactivity (808 cpm) recovered from a control (tubes that contained no streptococci) was subtracted from each test.
Expression of sfs and fnz.
RNA was extracted from S. equi cells by using a Blue FastRNA kit (Bio 101, Vista, Calif.) according to the manufacturer’s protocol. RNA concentration was determined spectrophotometrically and by visual estimation of the rRNA bands on an agarose gel. RNA (10 μg) was loaded on a formaldehyde-containing agarose gel. RNA was transferred by vacuum blotting to a positively charged nylon filter (Hybond N+; Amersham) and cross-linked. Further steps were performed as described for Southern blots with the exception that single-stranded DNA was added to the prehybridization and hybridization solutions.
The ability of SFS to inhibit the binding between collagen and Fn.
For the enzyme-linked immunosorbent assay (ELISA), polystyrene 96-well microtiter plates were coated for 1 h with collagen type I from calf skin (Boehringer, Mannheim, Germany) in PBS. The wells were blocked for 1 h with PBS-T supplemented with casein (0.1 mg/ml) and then washed four times with PBS-T. The fusion proteins GST-FNZ and SFS-E were diluted in a twofold series and added to the wells together with 0.2 ng of Fn. After 2 h of incubation, the wells were washed and a rabbit anti-Fn antibody was added and allowed to bind for 1 h. Finally, the wells were incubated for 1 h with a secondary horseradish peroxidase-labeled antibody. After washing, bound material was quantified by using tetramethylbenzidine (Boehringer) and a microplate reader (Bio-Tek Instruments, Vinooski, Vt.). Measurement was done at a wavelength of 450 nm. Absorbancy in wells without added fusion protein was set to 100%, and absorbancy in wells where Fn had been excluded was set to 0%.
Nucleotide sequence accession number.
The GenBank accession number for the nucleotide sequence of sfs is AF136451.
RESULTS
Cloning of a Fn-binding protein by using phage display.
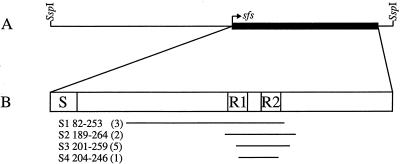
A shotgun phage display library, made from S. equi subsp. equi Bd 3221, was panned against human Fn. After the first panning, 41% of the colonies were found to bind Fn; after the second panning, all 180 tested colonies were positive for Fn binding. Colonies from the two pannings were transferred to nitrocellulose filters and subjected to colony hybridization using the fnz gene from S. equi subsp. zooepidemicus ZV as the probe. The percentage of Fn-binding clones that hybridized against fnz was in the first panning 40 and in the second panning 30. Sequence analysis of 11 Fn-binding and fnz-negative clones revealed that all inserts were identical to one of four different types of inserts, all with overlapping sequences and open reading frame (Fig. 1). To isolate the complete gene encoding the Fn-binding activity, a 2.6-kb fragment, containing the sequence of the characterized phagemid clones, was cloned and sequenced. This clone, designated pSFS62, had an open reading frame of 1,035 bp, from which the phagemid sequences were found to originate (Fig. 1 and 2). The open reading frame is preceded by sequences typical for promoter and ribosome-binding sites and is followed by sequences resembling a transcriptional termination, suggesting that the gene is translated from a monocistronic messenger. The gene, designated sfs, encodes a protein, named SFS, with a calculated molecular mass of 40 kDa. The charged amino acids, followed by a stretch of hydrophobic residues in the N-terminal end of the protein, indicate a signal sequence; by the method of von Heijne (27), a possible signal sequence cleavage site was found between amino acids 29 and 30, resulting in a mature protein with a calculated molecular mass of 36 kDa. The isolated Fn-binding phagemid clones contained inserts originating from the central part of the protein, where two repetitive sequences of 21 residues, called R1 and R2, are situated (Fig. 1 and 2). Three amino acids were found to dominate the composition of protein SFS; 53 residues (14.4%) are glycines, 39 (10.6%) are serines and 38 (10.3%) are prolines. These three amino acids are evenly distributed in the protein, in contrast to the 13 tyrosine residues, which occur only in the C-terminal part of the protein. Protein SFS does not contain any sequence motifs known to mediate attachment to the bacterial cell wall.
FIG. 1.
(A) Map of pSFS62 with the gene sfs indicated. (B) Schematic presentation of protein SFS with the functional domains indicated. The bars correspond to the amino acid sequences of phagemid clones isolated by panning against Fn (S1 to S4). Numbers refer to amino acid positions in protein SFS as shown in Fig. 2; the numbers of identical clones that were isolated and sequenced are given in parentheses.
The gene sfs is generally present in isolates of S. equi subsp. equi.
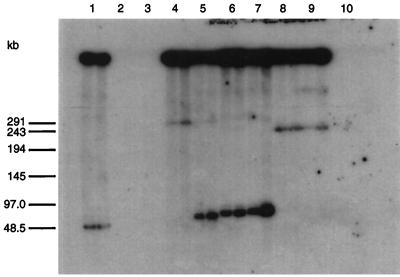
Southern blots revealed that a [32P]dATP-labeled probe, corresponding to the gene sfs, hybridized to all the 50 S. equi subsp. equi and to 41 of 48 S. equi subsp. zooepidemicus isolates tested. The results from the hybridization analysis are shown for a selected number of strains in Fig. 3. No significantly weak signal that could not be explained by less chromosomal DNA on the gel was detected for any of the positive S. equi isolates. The seven S. equi subsp. zooepidemicus isolates that were sfs negative could not be related to each other, considering symptoms, time of isolation, and geographic origin. Furthermore, the seven negative isolates were obtained from different species, four from horses, two from cows, and one from a dog. The sfs probe did not hybridize to any of the three control strains of other streptococcal species (Fig. 3).
FIG. 3.
Southern blot of chromosomal DNA from 10 streptococcal isolates. The DNA was digested with ApaI and separated by pulsed-field gel electrophoresis in duplicate. The radioactively labeled probe used corresponds to the gene sfs. Lanes: 1, S. equi subsp. zooepidemicus ZV; 2, S. dysgalactiae S2; 3, S. equisimilis 172; 4, S. equi subsp. equi Bd 3221; 5, S. equi subsp. equi Bd 995; 6, S. equi subsp. zooepidemicus DSM 20727T; 7, S. equi subsp. zooepidemicus ATCC 53698; 8, S. equi subsp. equi CCUG 11664; 9, S. equi subsp. equi NCTC 9682T; 10, S. pyogenes AW43. Positions of molecular weight marker (concatemers of lambda) are indicated at the left.
Protein SFS displays sequence similarity to both collagen and a potential cell-wall protein of S. pyogenes.
Collagen sequences gave highest scores when the database Swissprot for SFS-like sequences was searched. The similarity was evenly distributed through protein SFS, and the main reason for the high score is the high content of glycine, serine, and proline, residues which are also common in collagen. However, a more pronounced similarity was seen for the Fn-binding domain of SFS against collagen. A sequence comparison was also done against the Oklahoma S. pyogenes genomic sequence database, which at the time of search consisted of 98% of the S. pyogenes genome. SFS aligned best against a database sequence which besides a high content of glycine and proline residues also displayed the motif QGERGETGP. Eight of these nine residues are present in the Fn-binding domain of SFS (Fig. 2 and 4). Similar motifs are also present in chains of collagen, and alignment of these sequences is shown in Fig. 4. Upon closer study of the aligned S. pyogenes sequence, it was found that the aligned motif is situated in the middle of a potential gene encoding a typical streptococcal cell surface protein. This statement is based on the following: (i) promoter sequences and a putative ribosome-binding site are present adjacent to an open reading frame; (ii) in the C-terminal part there is a proline-rich domain with the cell wall-anchoring motif LPXTGX; (iii) the LPXTGX motif is directly followed by a stretch of 23 hydrophobic residues, and the open reading frame is terminated by six residues, three of which are charged; and (iv) a potential hairpin loop is situated 38 bp downstream the stop codon. However, a start codon at an acceptable distance from the ribosome-binding site could not be found.
FIG. 4.
Alignment of amino acid sequences from different types of collagen and a potential cell surface protein from S. pyogenes with that of a motif present in the Fn-binding domain of SFS. Numbers indicate the position of the first amino acid from the collagen sequences. Bold letters indicate residues identical to those in the SFS motif.
Inhibition of binding between Fn and cells of S. equi.
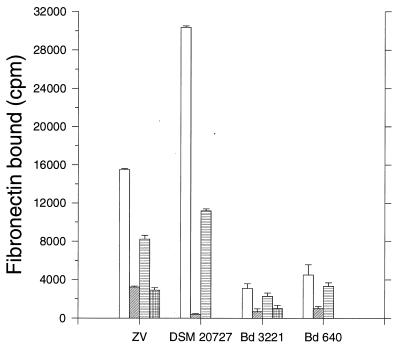
Recombinant proteins SFS and FNZ were purified by using affinity tails, and the purified proteins were found to bind Fn in a Western blot assay (data not shown). Before addition of iodinated Fn to cells of S. equi, the labeled Fn was, in appropriate cases, preincubated with FNZ and/or SFS in a molar ratio of 1:1,500. After incubation, the cells were collected by centrifugation; after removal of the supernatant, the radioactivity bound to the pellets was measured. The results from the inhibition experiments showed that protein FNZ had, for both subspecies, a significant stronger inhibitory effect than protein SFS (Fig. 5). An increase in the inhibitory effect was not seen when SFS and FNZ were added together compared to the effect of FNZ alone. That protein FNZ totally inhibits the binding between Fn and S. equi subsp. zooepidemicus DSM 20727T is interesting since this strain has earlier been shown to be the best Fn-binding strain among the 98 S. equi strains tested for presence of the sfs gene (17). Comparison of the effects of FNZ on the two S. equi subsp. zooepidemicus strains shows clearly that S. equi subsp. zooepidemicus ZV has besides FNZ an additional Fn-binding factor with a binding site separate from those for both FNZ and SFS. The two S. equi subsp. equi strains bind considerable less Fn than the two S. equi subsp. zooepidemicus strains, but the general pattern, that FNZ inhibits more efficiently than SFS and that a combination of the two proteins does not increase the inhibitory effect, is the same.
FIG. 5.
Inhibition of Fn binding. Cells of S. equi subsp. zooepidemicus ZV, subsp. zooepidemicus DSM 20727, subsp. equi Bd3221, and subsp. equi 640 were incubated with iodine-labeled Fn (hatched bars), with a mixture of iodine-labeled Fn and protein GST-FNZ (open bars), and with a mixture of iodine-labeled Fn and protein SFS-E (hatched bars). S. equi subsp. zooepidemicus ZV and subsp. equi Bd3221 were also incubated with labeled Fn together with a mixture of GST-FNZ and SFS-E (checked bars). The bars represent means of duplicates, and standard deviations are indicated.
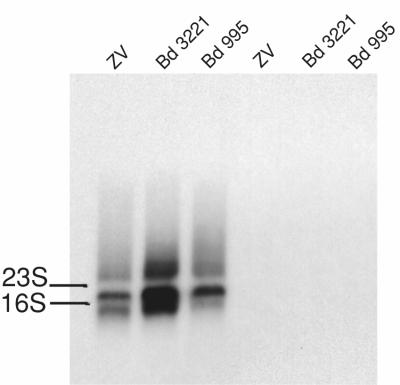
Expression of sfs is down-regulated during in vitro cultivation.
For Northern blot analysis, cells of S. equi subsp. equi Bd 3221 and Bd 995 and of S. equi subsp. zooepidemicus ZV, cultivated in THY, were harvested at an OD600 of 0.5, and RNA was extracted. The RNA preparations were loaded in duplicate on a gel, and after transfer, one half of the filter was incubated with a radiolabeled fragment of sfs and the other half was incubated with a fragment covering the fnz gene from S. equi subsp. zooepidemicus ZV. The fnz probe gave a strong signal for all three isolates when hybridized to the RNA filter, whereas no signals were detected for the sfs probe (Fig. 6). The fnz probe gave a stronger signal than the sfs probe when hybridized to chromosomal DNA from S. equi subsp. equi Bd 3221 (data not shown). However, this difference in signal intensity between the two probes was much less than that seen in Fig. 6, and even after prolonged exposure (3 days) no band could be detected for the sfs probe. Filters with RNA, extracted after harvesting S. equi subsp. equi Bd 3221 at OD600 of 0.2 and 1.0 grown in THY and a culture of the same strain harvested at OD600 of 0.5 grown in THY supplemented with horse serum, were also tested against the sfs probe, with negative result (data not shown).
FIG. 6.
Northern blots. Total RNA from S. equi subsp. zooepidemicus ZV (lanes 1 and 4), subsp. equi Bd 3221 (lanes 2 and 5), and subsp. equi Bd 995 (lanes 3 and 6) were subjected to hybridization with a radioactively labeled probe derived from fnz (lanes 1 to 3) or sfs (lanes 4 to 6).
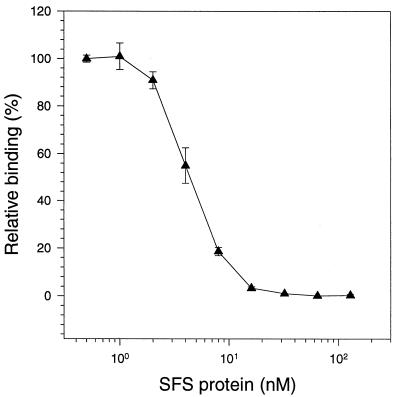
Protein SFS inhibits the binding between Fn and collagen.
The similarity between protein SFS and collagen suggested that these proteins might bind to the same site on the Fn molecule. To investigate this, microtiter wells coated with collagen were incubated with a mixture of Fn and a serial dilution of protein SFS. Bound Fn was detected by an anti-Fn antibody, and as seen in Fig. 7, protein SFS inhibited the binding in a concentration-dependent way. In a similar assay, protein FNZ did not inhibit the binding between Fn and collagen (data not shown). Furthermore, protein SFS did not inhibit the binding between protein FNZ and Fn, and protein FNZ did not inhibit the binding between protein SFS and Fn. Protein SFS does not bind collagen. This was tested in order to confirm that the inhibition of binding between Fn and collagen by protein SFS is dependent on the binding of protein SFS to Fn and not to collagen. Taken together, the results suggest that protein SFS and protein FNZ have clearly separate binding sites on the Fn molecule and that protein SFS binds to the 30- to 40-kDa collagen-binding domain of Fn.
FIG. 7.
Inhibition of binding between collagen and Fn with protein SFS. Collagen type I-coated microtiter wells were incubated with Fn and a twofold serial dilution of SFS. Bound Fn was detected by antibodies as described in Materials and Methods. Points represent means of duplicates, and standard deviations are indicated.
DISCUSSION
The deduced amino acid sequence of protein SFS diverges strongly from the sequence of the most studied class of Fn-binding proteins (see Introduction). However, among the dozen Fn-binding proteins cloned and sequenced from different streptococcal species, there are several that do not fall into the group of typical Fn-binding proteins, i.e., protein H (6), M1 (4), M3 (23), FBP54 (3), and SDH, a glyceraldehyde-3-phosphate dehydrogenase (21). SDH and FBP54 are bound to the cell wall although they, like SFS, lack typical cell wall-anchoring motifs. This and the finding that SFS displays, in its Fn-binding domain, a sequence motif which is also present in a potential cell wall protein from S. pyogenes led us to investigate if protein SFS is the cell wall-associated factor in S. equi subsp. equi that binds Fn. The results from the inhibition assay showed that addition of protein SFS had a limited effect on the binding between cells of S. equi subsp. equi and Fn, suggesting that SFS is secreted or that sfs is strongly down-regulated during growth in vitro. The Northern blot data showed that expression of sfs is strongly down-regulated in S. equi compared to fnz. Thus, it may be that SFS is cell wall associated, and during growth in vitro, fewer SFS than FNZ molecules are produced in S. equi subsp. zooepidemicus ZV. The finding that addition of SFS together with FNZ does not inhibit more than FNZ alone indicates that the proteins to some extent compete for the same site. Another explanation could be that SFS bound to Fn sterically hinders Fn from coming in close contact with some of the FNZ molecules displayed on the bacterial surface. This explanation fits better with the results from the ELISA inhibition assay, where SFS had no effect on the binding between FNZ and Fn and vice versa.
Protein FNZ had a strong inhibitory effect on the binding between Fn and the two strains of S. equi subsp. equi, indicating the presence of an FNZ-like protein on the bacterial cell surface. Southern blots have revealed that a gene analogous to fnz is generally present in isolates of S. equi subsp. equi (17), and the present study showed that clones with fnz-related fragments were selected during panning against Fn. The results from the Northern blots showed that the amounts and sizes of fnz-related transcripts are similar for both subspecies. Surprisingly, in contrast to protein FNZ derived from S. equi subsp. zooepidemicus ZV, cells of S. equi subsp. equi do not bind the 29-kDa fragment of Fn, and as seen in Fig. 5, S. equi subsp. equi also binds considerably less native Fn than does S. equi subsp. zooepidemicus ZV. To clarify these contradictory results, we intend to clone and sequence the fnz-related gene from S. equi subsp. equi.
Collagens of types I to V have all been shown to bind Fn, but with the highest affinity for type III (5). By inhibition assays, certain cyanogen bromide fragments have been shown to inhibit the binding between Fn and collagen, whereas other fragments had no inhibitory effect (for a review, see reference 15). The sequences from collagen chains aligned in Fig. 4 are in some cases part of the fragments with inhibitory effect and in some cases not. However, from these data we cannot exclude that the SFS motif mediates binding to Fn, since the sequences in Fig. 4 are those from each chain that aligned best to the SFS sequence and motifs similar to these are present along the various collagen chains. Interestingly, Ingham et al. (11) reported that besides a major Fn-binding site, the type I collagen α1 chain has one or more lower-affinity binding sites.
We could not conclude whether SFS is cell bound or secreted since there is no detectable expression of sfs in vitro. However, the presence of an FNZ-like protein bound to the cell surface of S. equi subsp. equi, with a binding site on Fn separate from that for SFS, suggests that protein SFS can be bound to the bacterial surface by using bound Fn as a receptor. One potential benefit for a pathogenic bacterium to produce a protein that restrains the binding between Fn and collagen can be disturbance of a process like wound healing where the interaction between collagen and Fn constitutes an important step. C1q, a component in the classical complement system, consists of one globular region and one collagen-like region. The latter region binds Fn, and the binding site on the Fn molecule has been mapped to the 30- to 40-kDa collagen-binding domain (25). It is thought that the biological consequence of the Fn-C1q interaction may be to enhance clearance of C1q-coated immune complexes by phagocytic cells (26); hence, there is an obvious reason for the bacterium to inhibit this binding.
The finding that within the Fn-binding domain of SFS there is a sequence motif also present in a potential cell surface protein from S. pyogenes is interesting. The motif from S. pyogenes is located in the middle of the protein, within a domain of 105 amino acid residues where every third amino acid is a glycine, as in collagens. It is tempting to speculate that this hypothetical cell surface protein can partially assume a triple-helical conformation composed of three chains and that this domain binds Fn. Future work will show whether the aligned S. pyogenes sequence is part of an Fn-binding cell surface protein.
ACKNOWLEDGMENTS
We thank Sussie Stier for skillful technical assistance, Lars Frykberg and Karin Jacobsson for the pG8SAET vector and help during construction of the phage display library, and Martin Lindberg for critical comments and advice.
This investigation was supported by grants from the Swedish Horserace Totalizator Board and the Swedish Council for Forestry and Agricultural Research (grants 32.0370/96 and 32.0646/97).
REFERENCES
- 1.Barnham M, Ljunggren A, McIntyre M. Human infection with Streptococcus zooepidemicus (Lancefield group C): three case reports. Epidemiol Infect. 1987;98:183–190. doi: 10.1017/s0950268800061896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chhatwal G S, Blobel H. Heterogeneity of fibronectin reactivity among streptococci as revealed by binding of fibronectin fragments. Comp Immunol Microbiol Infect Dis. 1987;10:99–108. doi: 10.1016/0147-9571(87)90003-8. [DOI] [PubMed] [Google Scholar]
- 3.Courtney H S, Li Y, Dale J B, Hasty D L. Cloning, sequencing and expression of a fibronectin/fibrinogen-binding protein from group A streptococci. Infect Immun. 1994;62:3937–3946. doi: 10.1128/iai.62.9.3937-3946.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cue D, Dombek P E, Lam H, Cleary P P. Streptococcus pyogenes serotype M1 encodes multiple pathways for entry into human epithelial cells. Infect Immun. 1998;66:4593–4601. doi: 10.1128/iai.66.10.4593-4601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engvall E, Ruoslahti E, Miller J M. Affinity of fibronectin to collagen of different genetic types and to fibrinogen. J Exp Med. 1978;147:1584–1595. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frick I-M, Crossin K L, Edelman G M, Björck L. Protein H-a bacterial surface protein with affinity for both immunoglobulin and fibronectin type III domains. EMBO J. 1995;14:1674–1679. doi: 10.1002/j.1460-2075.1995.tb07156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galán J E, Timoney J F. Immunologic and genetic comparison of Streptococcus equi isolates from the United States and Europe. J Clin Microbiol. 1988;26:1142–1146. doi: 10.1128/jcm.26.6.1142-1146.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanski E, Caparon M G. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci USA. 1992;89:6172–6176. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanski E, Horwitz P A, Caparon M G. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect Immun. 1992;60:5119–5125. doi: 10.1128/iai.60.12.5119-5125.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hynes R O. Fibronectins. New York, N.Y: Springer-Verlag; 1990. [Google Scholar]
- 11.Ingham K C, Brew S A, Isaacs B S. Interaction of fibronectin and its gelatin-binding domains with fluorescent-labeled chains of type I collagen. J Biol Chem. 1988;263:4624–4628. [PubMed] [Google Scholar]
- 12.Jacobsson K, Frykberg L. Phage display shot-gun cloning of ligand-binding domains of procaryotic receptors approaches 100% correct clones. BioTechniques. 1996;20:1070–1081. doi: 10.2144/96206rr04. [DOI] [PubMed] [Google Scholar]
- 13.Jadoun J, Ozeri V, Burstein E, Skutelsky E, Hanski E, Sela S. Protein F1 is required for efficient entry of Streptococcus pyogenes into epithelial cells. J Infect Dis. 1998;178:147–158. doi: 10.1086/515589. [DOI] [PubMed] [Google Scholar]
- 14.Jorm L R, Love D N, Bailey G D, Bailey G M, Briscoe D A. Genetic structure of populations of β-haemolytic Lancefield group C streptococci from horses and their association with disease. Res Vet Sci. 1994;57:292–299. doi: 10.1016/0034-5288(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 15.Kleinman H K, Klebe R J, Martin G R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981;88:473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindmark H, Jacobsson K, Frykberg L, Guss B. Fibronectin-binding protein of Streptococcus equi subsp. zooepidemicus. Infect Immun. 1996;64:3993–3999. doi: 10.1128/iai.64.10.3993-3999.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindmark, H., P. Jonsson, E. Olsson Engvall, and B. Guss. Pulsed-field gel electrophoresis and distribution of the genes zag and fnz in isolates of Streptococcus equi. Res. Vet. Sci., in press.
- 18.Molinari G, Talay S R, Valentin-Weigand P, Rohde M, Chhatwal G S. The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A streptococci by epithelial cells. Infect Immun. 1997;65:1357–1363. doi: 10.1128/iai.65.4.1357-1363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilsson M, Frykberg L, Flock J-I, Pei L, Lindberg M, Guss B. A fibrinogen-binding protein of Staphylococcus epidermidis. Infect Immun. 1998;66:2666–2673. doi: 10.1128/iai.66.6.2666-2673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozeri V, Tovi A, Burstein I, Natanson-Yaron S, Caparon M G, Yamada K M, Akiyama S K, Vlodavsky I, Hanski E. A two-domain mechanism for group A streptococcal adherence through protein F to the extracellular matrix. EMBO J. 1996;15:989–998. [PMC free article] [PubMed] [Google Scholar]
- 21.Pancholi V, Fischetti V A. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J Exp Med. 1992;176:415–426. doi: 10.1084/jem.176.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 23.Schmidt K-H, Mann K, Cooney J, Köhler W. Multiple binding of type 3 streptococcal M-protein to human fibrinogen, albumin and fibronectin. FEMS Immunol Med Microbiol. 1993;7:135–144. doi: 10.1111/j.1574-695X.1993.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 24.Skjold S A, Quie P G, Fries L A, Barnham M, Cleary P P. DNA fingerprinting of Streptococcus zooepidemicus (Lancefield group C) as an aid to epidemiological study. J Infect Dis. 1987;155:1145–1150. doi: 10.1093/infdis/155.6.1145. [DOI] [PubMed] [Google Scholar]
- 25.Sorvillo J M, Gigli I, Pearlstein E. Fibronectin binding to complement subcomponent C1q. Biochem J. 1985;226:207–215. doi: 10.1042/bj2260207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorvillo J M, Gigli I, Pearlstein E. The effect of fibronectin on the processing of C1q- and C3b/bi-coated immune complexes by peripheral blood monocytes. J Immunol. 1986;136:1023–1026. [PubMed] [Google Scholar]
- 27.von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]