Abstract
Intimate attachment to the host cell leading to the formation of attaching and effacing (A/E) lesions is an essential feature of enterohemorrhagic Escherichia coli (EHEC) O157:H7 pathogenesis. In a related pathogen, enteropathogenic E. coli (EPEC), this activity is dependent upon translocation of the intimin receptor, Tir, which becomes tyrosine phosphorylated within the host cell membrane. In contrast, the accumulation of tyrosine-phosphorylated proteins beneath adherent EHEC bacteria does not occur, leading to questions about whether EHEC uses a Tir-based mechanism for adherence and A/E lesion formation. In this report, we demonstrate that EHEC produces a functional Tir that is inserted into host cell membranes, where it serves as an intimin receptor. However, unlike in EPEC, in EHEC Tir is not tyrosine phosphorylated yet plays a key role in both bacterial adherence to epithelial cells and pedestal formation. EHEC, but not EPEC, was unable to synthesize Tir in Luria-Bertani medium but was able to secrete Tir into M9 medium, suggesting that Tir synthesis and secretion may be regulated differently in these two pathogens. EHEC Tir and EPEC Tir both bind intimin and focus cytoskeletal rearrangements, indicating that tyrosine phosphorylation is not needed for pedestal formation. EHEC and EPEC intimins are functionally interchangeable, but EHEC Tir shows a much greater affinity for EHEC intimin than for EPEC intimin. These findings highlight some of the differences and similarities between EHEC and EPEC virulence mechanisms, which can be exploited to further define the molecular basis of pedestal formation.
Enterohemorrhagic Escherichia coli (EHEC) O157:H7 is a major cause of serious diarrhea in North America. In young children and the elderly, EHEC can cause a serious systemic complication, known as hemolytic-uremic syndrome, characterized by hemolytic anemia, thrombocytopenia, and renal failure (36). The mechanism by which EHEC causes disease is not well understood. EHEC belongs to a family of pathogens that cause attaching and effacing (A/E) lesions. A/E lesion formation has also been characterized in enteropathogenic E. coli (EPEC) and involves the degeneration of the epithelial brush border microvilli and the formation of actin-rich pedestals within the host cell beneath the adherent bacteria (32, 35).
Intimin, a 94-kDa outer membrane protein expressed by EPEC and EHEC, is required for intimate attachment to the host cell and A/E lesion formation. Deletions in intimin render EPEC unable to focus the actin cytoskeleton, and in vivo studies have shown that both EPEC and EHEC require intimin for full virulence (6, 9, 10, 13, 44). Sequence analysis of intimins from several species has revealed homology at their amino termini but only about 50% identity at their carboxy-terminal, cell surface binding domains (18). These findings have led to speculation that EPEC and EHEC intimins bind to different receptors on the host cell. Experiments comparing the adhesion patterns of an EHEC intimin mutant expressing EPEC intimin and a wild-type EHEC strain have been done with a gnotobiotic piglet model (44). Unlike wild-type EHEC, which adheres to the large intestine, the EHEC strain expressing EPEC intimin adhered to the small and large intestines in a manner typical of EPEC in this animal model, again indicating that EHEC and EPEC intimins may bind to different receptors.
In EPEC, intimin binds to its receptor, Tir (translocated intimin receptor) (28), which was initially believed to be a mammalian membrane protein that was originally called Hp90 and that was tyrosine phosphorylated in response to EPEC infection (40). Recent work revealed that Hp90 is not a mammalian protein but is a previously uncharacterized EPEC-secreted protein which is translocated to the host cell and is essential for pedestal formation. EPEC Tir is tyrosine phosphorylated within the host cell, although the role that phosphorylation plays in pedestal formation is unclear. Intimin binding to Tir induces extensive cytoskeletal rearrangements within the host cell and modulates signal transduction pathways, including the activation of phospholipase C-γ (29).
Although EHEC and EPEC share the ability to form A/E lesions within the host cell, there are important differences between these two pathogens. One major difference is that EHEC does not accumulate tyrosine-phosphorylated proteins beneath adherent bacteria (25). In EPEC, these proteins correspond primarily to tyrosine-phosphoryled Tir (28), yet both pathogens cause similar cytoskeletal rearrangements. This feature, along with differences in both the intestinal colonization sites and the primary sequence of intimin, has led to speculation about whether EHEC, like EPEC, uses a Tir-based mechanism to form A/E lesions. To address this issue, we set out to determine whether EHEC O157:H7 actually produces Tir and, if so, whether it functions as the EHEC intimin receptor. In this report, we describe the identification, cloning, and characterization of EHEC O157:H7 Tir and show that, unlike EPEC Tir, it is not tyrosine phosphorylated in the host cell. We investigated the role of Tir phosphorylation in intimin binding and the binding affinity of EHEC and EPEC intimins for EHEC Tir and EPEC Tir in both in vitro and in vivo conditions.
MATERIALS AND METHODS
Bacterial strains and HeLa cell cultures.
The strains used in this study are listed in Table 1. HeLa cells (CCL2; American Type Culture Collection) were cultured in minimal essential medium (MEM) supplemented with 10% fetal calf serum, grown at 37°C in 5% CO2, and used at 80 to 90% confluence.
TABLE 1.
EHEC and EPEC strains used in this study
| Strain | Relevant characteristic(s) | Reference(s) or source |
|---|---|---|
| 86-24 | EHEC O157:H7 wild type | 23 |
| UMD619 | EHEC 86-24 intimin mutant | 10 |
| CVD451 | EHEC 86-24 sepB mutant | 26 |
| EHECΔtir | EHEC 86-24 tir deletion mutant | This study |
| EHECΔtir/T7-Tir | EHEC 86-24 tir1 deletion mutant carrying pEHEC-T7-Tir | This study |
| UMD619Δtir | EHEC 86-24 intimin-tir double mutant | This study |
| E2348/69 | EPEC O137:H6 wild type | 12 |
| EPECΔtir | EPEC E2348/69 tir deletion mutant | 28 |
| CVD206 | EPEC E2348/69 intimin mutant | 9 |
| UMD207 | EPEC intimin mutant of plasmid-cured E2348/69 | 9 |
| EPEC/pCVD540 | EPEC E2348/69 containing per regulon in pACYC184 | 22, 27 |
Cloning and DNA sequence analysis.
The oligonucleotides MS108+ (5′-AAAAG ATCTA TGCCT ATTGG TAACC TT-3′) and MS201− (5′-AAAGT CGACG TTCAG ATCTT GATGA CAT-3′) were used to amplify EHEC tir with chromosomal DNA from EHEC O157:H7. MS108+ hybridized to the minus strand of the 5′ end of EPEC tir, while MS201− hybridized to the plus strand of EHEC orfU, located directly downstream from tir sequences in EPEC and EHEC. The PCR product was cloned into pBluescript SK(+), generating plasmid pMS60a. Clustal W was used for alignments of the predicted protein sequences.
Construction of a nonpolar tir deletion mutant.
Primers RD101+ (5′-TCCCC CGGGT TAAAT CAGTG TTATC TCAGA-3′) and RD102− (5′-TCCCC CGGGC GGCGC ACGGT TATTA GGAA-3′) were used to create a deletion of 1,257 bp between positions 251 and 1508 of the tir gene in pMS60a by inverse PCR amplification. Both oligonucleotides RD101 and RD102 introduced a SmaI restriction site, and RD101 introduced an in-frame stop codon. The 633-bp SacI-SalI tir deletion fragment was cloned into the positive-selection suicide vector pCVD442 (9), also digested with SacI-SalI. The resulting plasmid was used to construct by allelic exchange a tir deletion in wild-type EHEC 86-24 and its eae deletion mutant derivative UMD619 as described previously (42), generating strains EHECΔtir and UMD619Δtir, respectively.
Construction of plasmids expressing fusion proteins.
The coding region of EHEC tir was initially cloned into expression vector pET28a (Novagen), which contains amino-terminal six-His and T7 epitope tags. For expression in EHECΔtir, the tir gene, including the vector-derived ribosome binding site and the six-His and T7 tags, was excised from pET28a and cloned into pACYC184 under the control of the Tetr promoter, generating plasmid pEHEC-T7-Tir. This plasmid was transformed into EHECΔtir to generate strain EHECΔtir/T7-Tir.
The T7-intimin and maltose-binding protein (MBP)–intimin fusion proteins for EPEC were constructed as previously described (19, 28). To create a T7-intimin fusion protein for EHEC, 831 bp encoding the carboxy terminus of EHEC intimin was amplified with primers DJR+ (5′-CACCC GGGGG ATCCG CCAGC ATTAC TGAGA TTAAG-3′) and DJR− (5′-TTGGA GCTCC GTCGA CGGAA TTATT CTACA CAAAC CGCA-3′) and cloned into pET28a. Both T7-intimin fusion proteins were purified by agarose-nickel chromatography by following the manufacturer’s directions (Qiagen).
EHEC and EPEC protein secretion.
High levels of protein secretion by EHEC 86-24 and EPEC E2348/69 carrying plasmid pCVD450 were obtained by diluting Luria-Bertani (LB) medium-grown overnight cultures 1:100 in M9 minimal medium supplemented with 44 mM NaHCO3, 0.4% glucose, and 0.1% Casamino Acids and allowing them to grow as standing cultures at 37°C in 5% CO2 until the optical density at 600 nm (OD600) reached 0.7 to 0.8. Ten milliliters of culture supernatant was concentrated by tricholoracetic acid (TCA; 10% [vol/vol]) precipitation, and the pellets were washed with 90% acetone and analyzed by sodium dodecyl sulfate (SDS)–12% polyacrylamide gel electrophoresis (PAGE). Culture supernatants used for enzyme-linked immunosorbent assays (ELISAs) were used undiluted (EPEC) or concentrated 10-fold by ultrafiltration (EHEC) (Amicon-30 apparatus; Amicon).
EHEC Tir antibody production and protein sequencing.
Concentrated EHEC-secreted proteins were resolved by SDS–12% PAGE and transferred to nitrocellulose. Bands were visualized by Ponceau S staining, and the 72-kDa Tir band was excised, fragmented, and used to immunize rats. The titer was assessed by immunoblot analysis of EHEC-secreted proteins; a titer of 1:2,000 to 1:5,000 was used for immunoblotting. For amino-terminal protein sequencing, proteins were transferred to polyvinylidene difluoride membranes, stained with Ponceau S, and excised for analysis.
Cellular fractionation and immunoblotting.
Cellular fractionation was performed as described previously (40). Briefly, cultured HeLa cells were infected with EPEC CVD206 for 3 h or EHEC UMD619 for 6 hr, with the culture media being replaced after 3 h, washed, and solubilized with 50 mM Tris (pH 7.4)–1% Triton X-100 supplemented with protease inhibitors (10 μg of leupeptin per ml, 1 mM phenylmethylsulfonyl fluoride, 1 mM EDTA) and phosphatase inhibitors (1 mM NaVO4, 1 mM NaF, 100 mM Microcystin LR). Triton X-100-soluble membrane fractions were separated from the insoluble fraction containing adherent bacteria and host cytoskeleton by centrifugation. Samples for alkaline phosphatase treatment were prepared with 50 mM Tris (pH 8.0)–1% Triton X-100 without phosphatase inhibitors and treated with 2 U of alkaline phosphatase (New England Biolabs) for 1 h at 37°C. Samples were analyzed by immunoblotting with anti-Tir, anti-phosphotyrosine (anti-PY; clone 4G10; Upstate Biotechnology, Inc.), and anti-T7 (Novagen) antisera as described previously (40).
Immunofluorescence microscopy.
One milliliter of 5 × 104 HeLa cells was added to each well of a 24-well plate containing a 12-mm round glass coverslip. For single infections, monolayers were infected with 5 μl of a standing overnight culture and incubated for 3 h (EPEC) or 6 h (EHEC) at 37°C in 5% CO2. Monolayers for coinfection experiments were infected with equal amounts of the two strains used and incubated for 6 h. Samples were fixed in 2.5% paraformaldehyde and permeabilized with 0.1% Triton X-100. Antisera were used at the following dilutions: rat anti-EPEC Tir, 1:500; mouse anti-PY, 1:100; mouse anti-T7, 1:100 (Novagen); rabbit anti-EHEC O157, 1:10 (Difco); fluorescein isothiocyanate-phalloidin or Texas red-phalloidin, 1:800 (Molecular Probes); donkey anti-rat Cy3, 1:1,600 (Jackson Laboratory); and goat anti-mouse Alexa 488 (Molecular Probes) or Texas red, 1:400 (Jackson Laboratory).
Intimin binding to epithelial cells.
One milliliter of 105 HeLa cells was added to each well of a 24-well plate containing a 12-mm glass coverslip and grown overnight. Monolayers were infected with broth-grown CVD206 or UMD619 (10 μl/well) for 3 or 6 h, followed by the addition of gentamicin (1 h) and washing as previously described (40). MBP-EPEC intimin and T7-EHEC intimin were added (5 μg/well), and the plates were incubated for 45 min at 37°C. Cells were fixed in 2.5% paraformaldehyde and prepared for immunofluorescence microscopy as described above. For these experiments, anti-T7 antiserum was used at 1:1,000 and anti-MBP antiserum was used at 1:1,000.
Intimin binding ELISAs.
ELISAs measuring intimin binding to Tir secreted by EPEC or EHEC were carried out as previously described (28). Briefly, 50 μl of EPEC or 10-fold-concentrated EHEC culture supernatant was added to Immulon-4 96-well plates, blocked with 0.1% Tween 20–phosphate-buffered saline, and incubated with T7-intimin (from EPEC or EHEC) (0.3 to 300 nM). T7-intimin binding was detected with mouse anti-T7 antiserum (1:5,000) followed by goat anti-mouse horseradish peroxidase (1:2,000) and visualized with o-phenylenediamine (Sigma) as a substrate at OD490.
Gel overlays.
Overlays were performed as previously described (28). Briefly, concentrated secreted proteins were resolved by SDS–12% PAGE, transferred to nitrocellulose, and incubated with T7-EHEC intimin followed by anti-T7 antiserum. Blots were reprobed with polyclonal rat anti-Tir antiserum.
Nucleotide sequence accession number.
The nucleotide sequence of the EHEC tir gene has been deposited in GenBank under accession no. AF125993.
RESULTS
EHEC O157:H7 secretes a homologue of Tir.
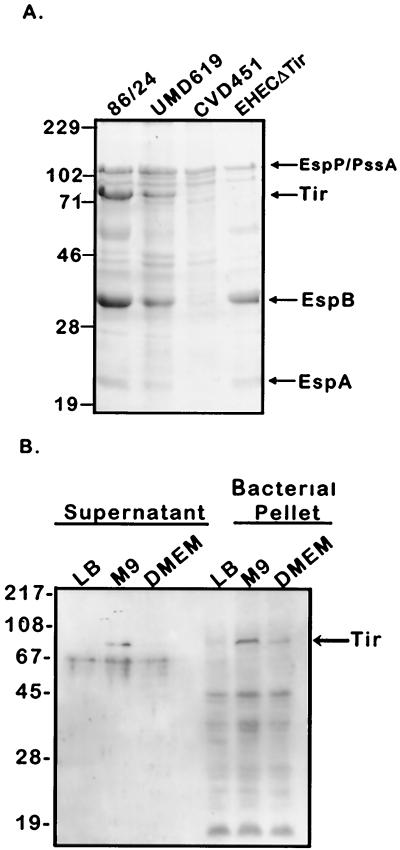
To determine whether EHEC secretes a Tir homologue, we analyzed TCA precipitates of culture supernatants from different EHEC strains grown in M9 minimal medium. A 72-kDa protein was found to be secreted by both wild-type EHEC O157:H7 strain 86-24 and its intimin mutant derivative UMD619 but not by the type III secretion mutant CVD451 (Fig. 1A). Secretion of other EHEC virulence proteins, including EspA and EspB (26) and EspP/PssA (5, 8), was also observed under these conditions (Fig. 1A). The 72-kDa band also cross-reacted with anti-EPEC Tir antisera on an immunoblot (data not shown). To further investigate this protein, we transferred the 72-kDa band to polyvinylidene difluoride paper and obtained its amino-terminal sequence, which was identical to that of EPEC Tir, with the exception of 4 residues (Fig. 2). These results indicate that, like EPEC, EHEC produces and secretes a Tir protein, albeit of a different size (EHEC Tir, 72 kDa; EPEC Tir, 78 kDa) (28).
FIG. 1.
Protein secretion profiles of EHEC O157:H7 strains. (A) Secreted proteins derived from equal amounts of EHEC 86-24 (wild type), UMD619 (intimin mutant), CVD451 (SepB mutant), and EHECΔtir standing cultures grown to an OD600 of 0.8 were analyzed by SDS–12% PAGE and stained with Coomassie blue. Arrows mark the positions of known EHEC-secreted proteins. (B) EHEC O157:H7 strain 86-24 was cultured in LB medium, Dulbecco MEM (DMEM), or M9 minimal medium and grown to an OD600 of 0.7 to 0.8. TCA-precipitated culture supernatants or bacterial cell lysates derived from equivalent amounts of bacteria were resolved by SDS–12% PAGE, transferred to nitrocellulose, and probed with polyclonal rat anti-EHEC Tir antisera. Molecular mass markers are in kilodaltons.
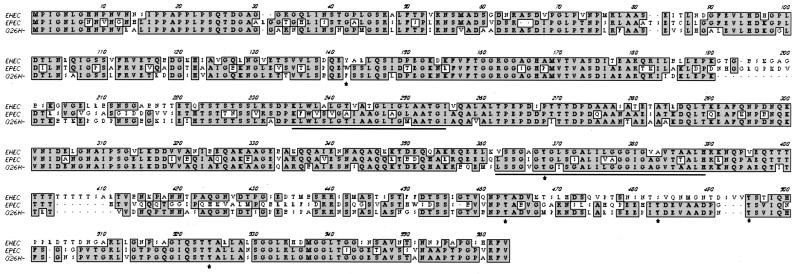
FIG. 2.
Clustal W alignment of the EHEC O157:H7 strain 86-24 Tir protein sequence (AF125993) with those of EPEC O127:H6 strain E2348/69 Tir (AF013122) and EHEC O26:− Tir (AJ223063). Amino acid residues identical in all three proteins are indicated by shading, the two predicted transmembrane domains are underlined, and the tyrosine residues are indicated by asterisks.
Cloning and characterization of EHEC tir.
Based on the above evidence, we cloned the corresponding gene by PCR amplification from EHEC chromosomal DNA. Primers were designed to hybridize with the start of orfU, directly downstream from EPEC tir and, because of the amino-terminal protein sequence similarity, with the 5′ end of EPEC tir. Once this region was cloned and sequenced, an open reading frame encoding a predicted protein of 558 amino acids was found. We compared the EHEC O157:H7 Tir protein sequence to the sequences of EPEC O127:H6 strain E2348/69 Tir and E. coli O26:H− Tir, two other functionally characterized Tir proteins reported to become tyrosine phosphorylated upon translocation to the host cell (7). Figure 2 shows a Clustal W alignment of these sequences. EHEC Tir was 58% identical to EPEC Tir and 68% identical to O26:H− Tir. All three Tir sequences contain two putative transmembrane domains (TM predict; ISREC, Epalinges, Switzerland). EHEC Tir and EPEC Tir were most homologous from the amino terminus through the second predicted transmembrane domain (65% identity) but showed only 41% identity over the carboxy-terminal 200 amino acids. This region contains the tyrosine residues that in EPEC are potential substrates for phosphorylation, due to their predicted intracellular location (28, 40). Interestingly, although all three sequences contain 6 tyrosine residues, the positions of these tyrosines are not identical. EHEC Tir lacks one tyrosine (position 473 in EPEC) present in both EPEC Tir and O26:H− Tir, and a second tyrosine in EHEC Tir (position 490 in EHEC) is in an entirely different primary sequence context.
EHEC and EPEC secrete Tir under different conditions.
Our data demonstrating that EHEC Tir is secreted in M9 medium suggested that conditions for Tir secretion from EHEC are different from those observed with EPEC. EPEC does not secrete Tir in M9 medium unless provided with extra copies of the per regulator, which is not present in EHEC O157:H7 (28). To further investigate the conditions necessary for EHEC Tir secretion, EHEC 86-24 was grown as a standing culture for 7 h at 37°C in either LB medium, M9 medium, or Dulbecco MEM, and Tir synthesis and secretion were examined by Western blotting with polyclonal rat anti-EHEC Tir antisera. Unlike that in EPEC, EHEC Tir synthesis was stimulated in bacteria grown in M9 medium or Dulbecco MEM but not in LB medium (Fig. 1B and data not shown). Detectable EHEC Tir secretion occurred only from bacteria cultured in M9 medium.
EHEC Tir is necessary for cytoskeletal rearrangements and bacterial adhesion.
In order to evaluate the role of EHEC Tir in A/E lesion formation, we next constructed an EHEC tir deletion mutant by allelic exchange as described in Materials and Methods. The chromosomal deletion was confirmed by the absence of Tir protein on both Coomassie blue-stained gels (Fig. 1A) and anti-Tir antiserum immunoblots and by the PCR amplification of a smaller DNA fragment with primers RD101+ and RD102− (data not shown). Secretion of EspA, EspB, and EspP/PssA was unaffected by the tir deletion (Fig. 1A).
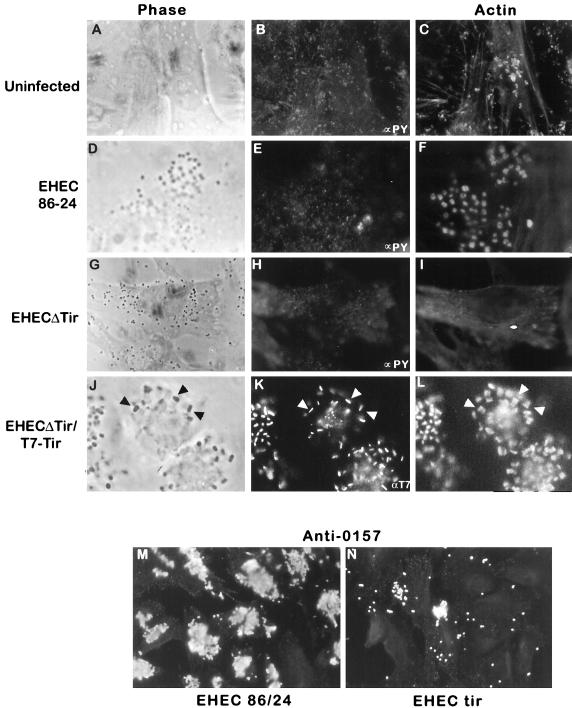
HeLa cells infected with either wild-type EHEC or EHECΔtir for 6 h were fixed and stained for immunofluorescence microscopy. Wild-type EHEC formed microcolonies, as shown by phase microscopy and staining with anti-O157 antisera (Fig. 3D and M). Actin, but not phosphotyrosine, was observed beneath adherent EHEC (Fig. 3E and F), confirming previous results reported from other laboratories (25). Infection with the tir deletion strain EHECΔtir resulted in a loss of actin condensation beneath adherent bacteria, with no change in host cell phosphotyrosine (Fig. 3G to I). Additionally, deletion of tir significantly altered the pattern of EHEC adhesion to HeLa cells. Bacterial adhesion was greatly reduced, and the few remaining EHEC adhered diffusely, rather than as microcolonies (Fig. 3G and N). To further determine whether the microcolony adhesion pattern in EHEC was mediated by Tir binding to its ligand intimin, we examined the adherence patterns of the EHEC intimin mutant UMD619 and a UMD619Δtir double mutant. Both strains showed reduced, diffuse adhesion, in a pattern identical to that of strain EHECΔtir (see Fig. 8C) (data not shown). These data suggest that EHEC Tir plays an important role in promoting bacterial adherence to the host cell.
FIG. 3.
Immunofluorescence microscopy of HeLa cells following a 6-h infection with wild-type EHEC O157:H7 strain 86-24 or EHECΔtir or a 9 h infection with EHECΔtir/T7-Tir. Fixed and permeabilized cells were colabelled with either anti-PY antisera and Texas red-phalloidin (B, C, E, F, H, and I) or anti-T7 antisera and Texas red-phalloidin (K and K) or singly labelled with anti-O157 antisera (M and N) to illustrate bacterial adherence. Arrowheads in J, K, and L indicate the localization of anti-T7 antisera with the actin pedestals. (A, D, G, and J) Phase-contrast images of the sections used for immunofluorescence.
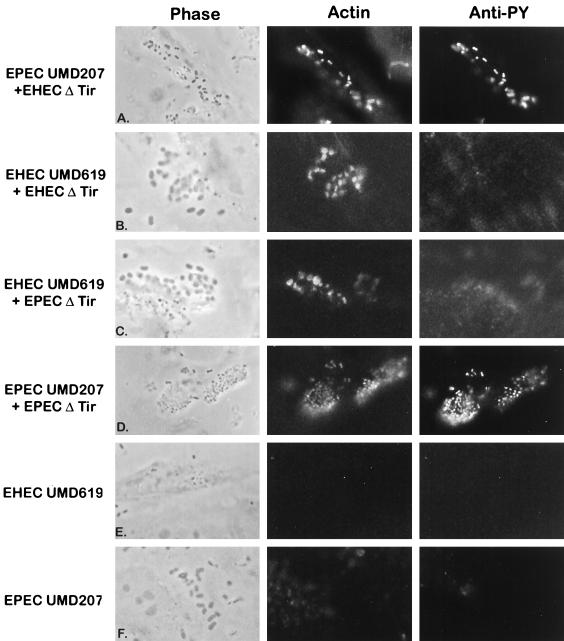
FIG. 8.
Heterologous Tir-intimin binding results in A/E lesion formation. HeLa cells were infected with either the EHEC or the EPEC intimin mutant UMD619 or UMD207 alone (E and F) or coinfected for 6 h with EPEC UMD207 plus EHECΔtir (A), EHEC UMD619 plus EHECΔtir (B), EHEC UMD619 plus EPECΔtir (C), or EPEC UMD207 plus EPECΔtir (D). Cells were fixed for fluorescence microscopy and labelled with either Texas red-phalloidin to visualize actin rearrangements (middle panels) or anti-PY antisera (right panels). (Left panels) Phase-contrast images of the same sections as those used for immunofluorescence.
EHEC Tir is translocated to the host cell but is not tyrosine phosphorylated.
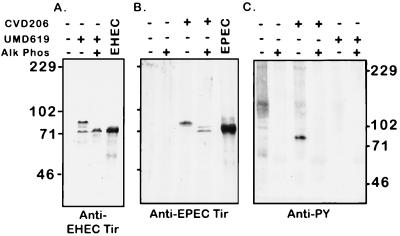
We next determined whether EHEC Tir is translocated to the host cell membrane and its location in the actin pedestal. EHECΔtir was transformed with plasmid pEHEC-T7-Tir (a pACYC184 derivative encoding the T7-His-Tir fusion protein) to create EHECΔtir/T7-Tir. HeLa cells were infected with this strain for 6 h, after which samples were prepared for immunofluorescence microscopy or bacteria were killed with gentamicin and incubated for a further 3 h in order to enhance pedestal formation (40). Expression of the T7-Tir fusion protein in EHECΔTir restored both bacterial adhesion and actin accumulation, which was evident after 6 h of infection. After 9 h, cells infected with EHECΔtir/T7-Tir were strongly labelled with anti-T7 antisera (Fig. 3K). Anti-T7 antiserum staining was localized to the tip of the EHEC pedestal (Fig. 3J to L) and was not observed in bacteria not associated with HeLa cells. Since anti-T7 antisera only recognize the amino-terminal T7 epitope fused to Tir in cells permeabilized with Triton X-100, this pattern of labelling strongly suggests that the amino terminus is inside the host cell. Collectively, these data indicate that Tir is translocated to the host cell, where it resides at the tip of the pedestal beneath adherent EHEC.
The absence of anti-PY antiserum labelling beneath adherent EHEC suggested that Tir is not tyrosine phosphorylated. To determine the size and possible phosphorylation of the translocated form of EHEC Tir, we prepared membranes from HeLa cells infected with the EHEC intimin mutant UMD619. Under these conditions, Tir should become phosphorylated and remain in the Triton X-100-soluble fraction, facilitating further analysis (38). Triton X-100-soluble membranes were incubated in the presence or absence of the nonspecific phosphatase calf intestinal alkaline phosphatase prior to resolution by SDS–8% PAGE and immunoblotting with anti-EHEC Tir and anti-PY antisera. Membranes from EPEC CVD206-infected HeLa cells were used for comparison. Anti-EHEC Tir antiserum recognized two bands (88 and 72 kDa) in membranes from UMD619-infected HeLa cells but not uninfected cells (Fig. 4A). The 88-kDa form was found only with the membrane fractions from UMD619-infected HeLa cells, while the 72-kDa band comigrated with the secreted form of EHEC Tir. Alkaline phosphatase treatment decreased the apparent size of the 88-kDa band to 72 kDa, suggesting that EHEC Tir is posttranslationally modified in the host cell, most likely by phosphorylation. However, there was no evidence for EHEC Tir tyrosine phosphorylation, as no bands of the appropriate molecular weight were observed on an an anti-PY antiserum blot (Fig. 4C), confirming the results obtained by immunofluorescence microscopy (Fig. 3). The 72-kDa band observed in the HeLa cell extract may represent translocated but unmodified Tir or Tir that became dephosphorylated during the process of membrane preparation. Thus, the posttranslational modification of EHEC Tir differs from that of EPEC Tir, which is tyrosine phosphorylated upon translocation to the host cell (Fig. 4B and C) (28, 38, 40).
FIG. 4.
EHEC Tir is translocated to the host cell but not tyrosine phosphorylated. HeLa cells were infected with either EHEC UMD619 or EPEC CVD206, and Triton X-100-soluble membrane fractions were prepared, treated with alkaline phosphatase (Alk Phos), and resolved by SDS–8% PAGE. After being blotted to nitrocellulose, the samples were probed with anti-EHEC Tir (a), anti-EPEC Tir (b), or anti-PY (c) antisera. Molecular mass markers are in kilodaltons.
Intimin binding to EHEC Tir and EPEC Tir.
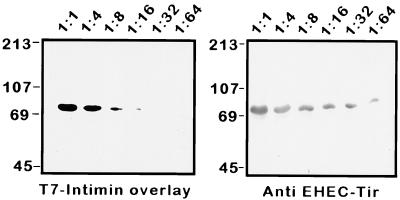
An important function of EPEC Tir is to bind EPEC intimin. We have previously shown that EPEC intimin can bind to both the unphosphorylated, bacterially secreted form of Tir (28) and the larger, phosphorylated form isolated from host cell membranes (40). We examined whether EHEC Tir is the EHEC intimin receptor and whether posttranslational modification is necessary for intimin binding by using gel overlay experiments. EHEC culture supernatants were diluted in the presence of a constant amount of HeLa cell membrane extract to assess binding specificity, resolved by SDS–12% PAGE, transferred to nitrocellulose, and probed with purified T7-intimin fusion protein. As shown in Fig. 5, intimin bound in a concentration-dependent manner, to a single 72-kDa band which was identified as EHEC Tir after reprobing of the blot with anti-EHEC Tir antisera. These data suggest that, as in EPEC, posttranslational modification is not necessary for EHEC intimin binding in vitro and that other EHEC-secreted proteins are not needed to facilitate this binding. Additionally, the binding of EHEC intimin to proteins contained in the HeLa cell extract was not detected.
FIG. 5.
Intimin binds to secreted EHEC Tir. Gel overlay of diluted EHEC culture supernatants treated with equal amounts of HeLa cell membrane extracts. (Left) Samples were resolved by SDS–12% PAGE, transferred to nitrocellulose, overlaid with 100 μg of EHEC T7-intimin fusion protein, and probed with anti-T7 antisera. (Right) The same blot was reproved with anti-EHEC Tir antisera. Dilutions of EHEC culture supernatants are indicated above the panels, and molecular mass markers are in kilodaltons.
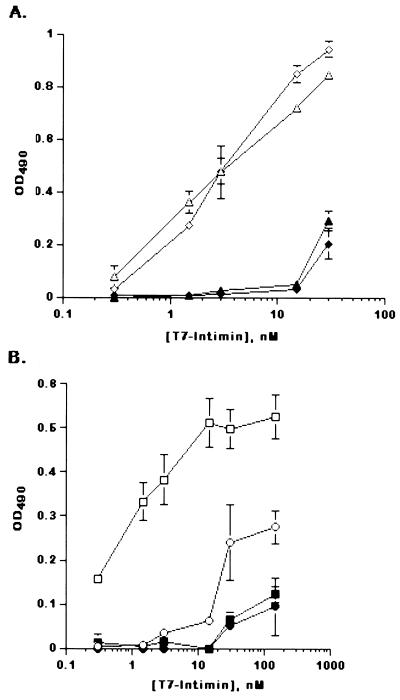
Our data demonstrating that Tir phosphorylation is not necessary for in vitro intimin binding suggested that EHEC and EPEC intimins may thus bind interchangeably to EPEC Tir and EHEC Tir, which are not equally posttranslationally modified in the host cell. We therefore used an ELISA to measure the relative affinities of these Tir-intimin interactions. ELISA wells were coated with culture supernatants from either EHEC or EPEC/pCVD450 (28) and incubated with increasing concentrations of either T7-EHEC intimin or T7-EPEC intimin. Both T7-EHEC intimin and T7-EPEC intimin were ligands for EPEC Tir and bound with similar affinities, with a half-maximal concentration in the low nanomolar range (1 to 10 nM) (Fig. 6A). Surprisingly, this was not the case for EHEC Tir, which had an approximately 20-fold-higher affinity for its own T7-intimin than for T7-EPEC intimin (Fig. 6B). No specific intimin binding was observed with supernatants from EHEC or EPEC tir deletion strains, suggesting that both EHEC and EPEC intimins bound to the same protein (Tir) in the supernatants. These data indicate that EHEC and EPEC intimins can cross complement each other in vitro, albeit with different binding affinities.
FIG. 6.
Intimin binds interchangeably to EHEC Tir and EPEC Tir. ELISA wells were coated with EPEC- or EHEC-secreted proteins, incubated with increasing concentrations of intimin, and visualized with o-phenylenediamine. (A) EPEC-T7 intimin (triangles) or EHEC-T7 intimin (diamonds) binding to culture supernatants from EPEC/pCVD450 (open symbols) or EPECΔtir (filled symbols). (B) EHEC-T7 intimin (squares) or EPEC-T7 intimin (circles) binding to EHEC 86-24 (open symbols) or EHECΔtir (filled symbols). Data are presented as means ± standard deviations for triplicate points from one representative experiment.
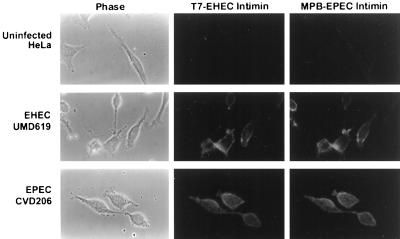
We next investigated the ability of EHEC and EPEC intimins to bind to EHEC Tir and EPEC Tir delivered to HeLa cell membranes. Tir was delivered to HeLa cells in the absence of intimin by use of the intimin mutants UMD619 (EHEC) and CVD206 (EPEC). The bacteria were killed with gentamicin, followed by the addition of equal amounts of T7-EHEC intimin and MPB-EPEC intimin fusion proteins. Bound fusion protein was detected by immunofluorescence microscopy. No binding of either fusion protein to uninfected cells was detected (Fig. 7, top panels). However, both T7-EHEC intimin and MBP-EPEC intimin bound to UMD619- and CVD206-infected cells (Fig. 7, middle and bottom panels). These data suggest that EHEC and EPEC intimins can bind to both EHEC Tir and EPEC Tir residing in the host cell membrane.
FIG. 7.
Immunofluorescence microscopy of epitope-tagged intimin binding to EHEC- or EPEC-infected cells. HeLa cells infected with either EPEC CVD206 or EHEC UMD619 were incubated with equal amounts of both T7-EHEC intimin and MBP-EPEC intimin. Bound fusion proteins were visualized with anti-T7 and anti-MBP antisera labelled with either fluorescein isothiocyanate (EHEC intimin) or Texas red (EPEC intimin).
The above experiments demonstrated that EHEC and EPEC intimins can bind to EHEC Tir and EPEC Tir independently of their phosphorylation states, but did not address whether actin rearrangements still occurred. To address this question, HeLa cells were coinfected with the EHEC or EPEC intimin mutants (to deliver Tir) and EHECΔtir or EPECΔtir (to provide intimin). We used the EPEC intimin mutant UMD207, which lacks the bundle-forming pilus, as it adheres to the host cell in a diffuse, EHEC-like adhesion pattern. Under these conditions, actin structures should be evident beneath EHECΔtir or EPECΔtir only if the intimin they provide can interact functionally with the Tir delivered by the intimin mutants. When used alone, none of the strains focused actin or tyrosine phosphorylated proteins beneath adherent bacteria (Fig. 3G and H and Fig. 8E and F) (28). In all cases, coinfection with tir and intimin deletion strains restored the ability to induce cytoskeletal rearrangements and pedestal formation. Coinfection with EHECΔtir and either the EPEC or the EHEC intimin mutant resulted in the formation of small bacterial colonies on the HeLa cell surface (Fig. 8A and B), in a manner similar to that for wild-type EHEC. This result is consistent with our observation that EHEC can adhere to the host cell only if a functional Tir-intimin interaction takes place. Actin structures were evident directly beneath all adherent EHECΔtir bacteria and were indistinguishable from those formed when wild-type EHEC was used.
Coinfection with EPECΔtir to provide intimin and either EHEC UMD619 or EPEC UMD207 to deliver Tir resulted in actin rearrangements beneath the adherent bacteria, but the adherence patterns were not identical. Coinfection with EPECΔtir and EHEC UMD619 resulted in the formation of microcolonies on the HeLa cell surface, with rearranged actin evident beneath some but not all microcolonies (Fig. 8C). In contrast, when HeLa cells were infected with EPECΔtir and EPEC UMD207, actin rearrangements were observed beneath adherent bacteria within each microcolony, in a manner similar to that seen with wild-type EPEC (Fig. 8D). These differences in actin rearrangements may be due to either less efficient Tir delivery by EHEC or lower-affinity binding of EPEC intimin to EHEC Tir (Fig. 6B).
Tir tyrosine phosphorylation was examined by labelling with anti-PY antiserum and was only evident beneath adherent bacteria when EPEC Tir was delivered. Anti-PY antiserum labelling was observed beneath both EHECΔtir and EPECΔtir when coinfected with EPEC UMD207 (Fig. 8A and D), suggesting that EPEC Tir tyrosine phosphorylation is not altered by binding EHEC intimin. Additionally, we were unable to stimulate EHEC Tir tyrosine phosphorylation with any coinfection conditions tested. In HeLa cells coinfected with EHEC UMD619 and EPECΔtir, there was no accumulation of phosphotyrosine beneath adherent bacteria (Fig. 8C). These data also indicate that EPEC signalling to the host cell does not stimulate the tyrosine phosphorylation of EHEC Tir and that EHEC Tir is not a substrate for tyrosine phosphorylation.
DISCUSSION
Intimate attachment to the host cell leading to the formation of A/E lesions is an essential feature of EHEC pathogenesis. This feature has been shown in the related pathogen EPEC to be dependent upon translocation of the intimin receptor, Tir, which becomes tyrosine phosphorylated within the host cell. In EHEC, the accumulation of tyrosine-phosphorylated proteins beneath adherent bacteria does not occur, leading to questions about whether EHEC uses the same Tir-based mechanism for adherence and A/E lesion formation as EPEC. In this report, we demonstrate that EHEC O157:H7, like EPEC, produces a Tir protein which is translocated to the host cell membrane, where it binds intimin and focuses the cytoskeleton beneath the pathogen. Although EHEC Tir shares these functions with EPEC Tir, there are some significant differences between these two proteins. EHEC Tir is not tyrosine phosphorylated, and it is required for adherence to the host cell. Additionally, we show that both EHEC and EPEC intimins are functionally interchangeable with regard to Tir binding and the induction of cytoskeletal rearrangements in epithelial cells.
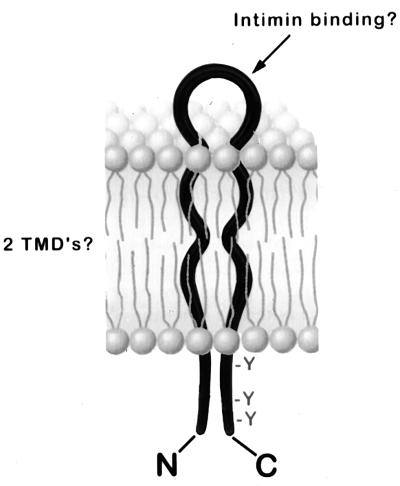
Tir proteins from EHEC and EPEC share several common features. Secretion and translocation of both EHEC Tir and EPEC Tir are facilitated by an intact type III secretory apparatus (Fig. 1) (28). Both EHEC Tir and EPEC Tir are predicted to be integral membrane proteins that contain two transmembrane domains. The proteins show a high degree of amino acid identity, particularly in their amino termini, but are most divergent in their carboxy-terminal domains. This region of the protein contains tyrosine residues that in EPEC Tir are potential substrates for phosphorylation. EHEC Tir, which is not tyrosine phosphorylated, lacks one of these residues. In EPEC Tir, these residues are predicted to reside in an intracellular domain due to their inaccessibility to labelling with anti-PY antiserum unless cells are permeabilized in immunofluorescence microscopy experiments (40). Based on this information, the prediction of two transmembrane domains, and our observations suggesting that the EHEC Tir amino terminus may be intracellular, we propose a “hairpin” model for Tir topology (Fig. 9). In this model, the amino and carboxy termini are intracellular, and the intimin binding domain is predicted to be the extracellular loop. We are presently undertaking experiments to test the validity of this model and to explore the role of the type III secretion system in Tir delivery to the host cell.
FIG. 9.
Hairpin model for EHEC Tir structure. EHEC Tir is predicted to be an integral membrane protein with two transmembrane domains (TMD’s). Both the amino and carboxy termini are predicted to reside within the host cell, and intimin is thought to bind to a putative extracellular loop.
Tir is probably a common feature of A/E pathogens. Sequences encoding tir have been identified from a variety of A/E pathogens, including several strains of Shiga toxin-producing E. coli (STEC), Hafnia alvei, and the rabbit pathogen RDEC-1 (7, 37) (GenBank accession no. AF045568). Tir translocation to the host membrane has been described for another A/E pathogen, a STEC O26:H− strain (7). Unlike EHEC O157:H7 Tir, STEC O26:H− Tir is tyrosine phosphorylated and contains the same number and sequence distribution of tyrosine residues as does EPEC Tir.
A role for Tir tyrosine phosphorylation is not immediately obvious. Tir phosphorylation is not necessary for in vitro intimin binding, as indicated by our ELISA and gel overlay results demonstrating that EHEC and EPEC intimins can bind to the unphosphorylated forms of EHEC Tir and EPEC Tir. Although EHEC Tir is not tyrosine phosphorylated, infection with EHEC induces the formation of pedestal structures that are indistinguishable from those elicited by EPEC. Under no conditions did we observe the accumulation of tyrosine-phosphorylated proteins within the actin structures beneath adherent EHEC, even when we coinfected them with an EPEC tir deletion strain, suggesting that EHEC Tir is not a substrate for tyrosine phosphorylation. This result is in contrast to work by Ismaili and coworkers, who reported that phosphotyrosine-containing proteins accumulate beneath EHEC when HEp-2 cells are coinfected with an EPEC intimin mutant strain (24). As the study was performed before the identification of EHEC Tir and EPEC Tir, a possible explanation for the data is that EHEC intimin binds to Tir translocated by the EPEC intimin mutant strain, which is tyrosine phosphorylated. Taken together, the results from this and other studies strongly suggest that Tir tyrosine phosphorylation is not essential for intimin binding and pedestal formation.
EHEC forms actin-rich pedestals that are indistinguishable from the ones elicited by EPEC, although they appear to form at a slower rate. In EPEC, Tir translocation is evident 2 to 3 h postinfection, and pseudopods form after 6 h (28, 40); in EHEC, Tir translocation is not observed until 5 to 6 h postinfection, and pseudopods appear after 9 h. These results are most likely due to differences in the regulation of Tir expression and translocation observed between EHEC and EPEC, rather than to differences in Tir phosphorylation. Unlike EPEC, EHEC cannot be preinduced to rapidly form A/E lesions by subculturing in tissue culture medium for several hours prior to infection of host cells (39; this study; and data not shown), suggesting that conditions regulating gene expression or translocation of proteins involved in A/E lesion formation differ between EHEC and EPEC. Once Tir is translocated to the host cell, pedestal elongation occurs over the same time frame in both pathogens. Although the initiation of pedestal formation requires de novo bacterial protein synthesis, bacterial viability is not required for pedestal elongation in both EPEC and EHEC, suggesting that these events are wholly dependent on host cell processes (40).
It is not surprising that EHEC Tir and EPEC Tir expression and secretion may be regulated differently. EHEC Tir secretion can be induced by culturing in M9 medium, while that of EPEC cannot (this study; 28). EPEC, but not EHEC, contains the pEAF virulence plasmid encoding the per/bfpTVW genes, one of which encodes a member of the AraC family of transcriptional regulators (22, 43). Overexpression of the per/bfpTVW genes in EPEC enhances Tir and Esp secretion (28, 30). The sequence of the EHEC large virulence plasmid has just recently been published (GenBank accession no. AB011549) and does not appear to encode genes having homology to known transcriptional regulator genes. Protein secretion from EPEC, STEC, and the rabbit pathogen RDEC-1 is known to be tightly controlled by environmental conditions. Secretion of EspA and EspB from all three bacterial species is greatly enhanced at the normal host body temperature (2, 14, 30) and, for EPEC, induced by conditions similar to those found in the host gastrointestinal tract (27). As EHEC and EPEC colonize different regions of the intestine (36), the regulation of protein secretion may be fine-tuned to the different microenvironments encountered by these pathogens.
EHEC Tir plays an essential role in promoting bacterial adhesion by binding its ligand intimin. Deletion of Tir, intimin, or both results in a profound decrease in bacterial adhesion to the host cell, suggesting that EHEC requires an intact Tir-intimin pair for adhesion to HeLa cells. Our studies examining EHEC adherence in the absence of the receptor, Tir, complement work examining the effect of deletion of the ligand, intimin (10, 34). McKee and coworkers demonstrated that an EHEC O157:H7 intimin deletion strain did not adhere either to HEp-2 cultured epithelial cells or to the intestines of gnotobiotic piglets, nor did it form A/E lesions (34). Transformation with a plasmid encoding EHEC intimin restored the wild-type phenotype. This result differs from what has been observed with other A/E pathogens, where adherence in vitro is not affected by mutations in the intimin gene, tir, or genes required for Tir delivery. For example, in EPEC, RDEC-1, and rabbit O103 EPEC, strains containing mutations in espA, espB, or the type III secretory apparatus genes adhere normally to epithelial cells both in vitro and in vivo without forming A/E lesions (1, 2, 11, 28, 31). In these pathogens, initial adherence is believed to be mediated by other adhesins, such as BFP (EPEC), AF/R1 (RDEC-1), and AF/R2 (rabbit O103 EPEC) (3, 4, 16, 21, 45), whereas Tir and intimin mediate intimate adherence and pedestal formation. To date, no adhesins of this type have been identified for EHEC O157:H7.
Recently, novel filamentous organelles containing the secreted protein EspA have been identified in both EPEC and STEC O26:H− (15, 33). These organelles form a bridge between the bacterium and the host cell and are hypothesized to be involved in the translocation of EPEC and STEC virulence proteins into the host cell. Although these structures have not yet been reported for EHEC O157:H7, an EHEC EspA homologue has been identified (14). espA mutant strains do not translocate Tir or form A/E lesions (28) and, in the case of the STEC strain, no longer adhere to the host cell (15). Whether the lack of adherence observed is due to the inability of espA mutant strains to translocate Tir or to a role played by the EspA filaments is unknown.
We have shown that actin pedestals elicited by EHEC and EPEC appear similar and that EHEC and EPEC intimins are functionally interchangeable with regard to Tir binding and pedestal formation. Heterologous Tir-intimin binding elicited actin pedestals that were indistinguishable from those formed by same-species Tir-intimin binding pairs, despite the differences observed in the in vitro binding affinities. However, one major difficulty in fully assessing differences in the pedestals formed in response to EHEC or EPEC binding either their own or each other’s intimin is that we only examined the recruitment of one cytoskeletal component (actin) to the pedestal structure. We are just beginning to determine the composition of the EPEC pedestal which, along with actin, contains at least ezrin, tailin, α-actinin, myosin light chain, and villin (17, 41). The cytoskeletal composition of the EHEC pedestal is still uncharacterized, there still may be differences in cytoskeletal proteins recruited to EHEC and EPEC pedestals, and these proteins may be differentially recruited to the pedestal in response to heterologous intimin binding. However, we conclude that both EHEC and EPEC, despite their differences in Tir tyrosine phosphorylation, form actin-rich pedestals and that heterologous intimin binding does not affect pedestal formation.
The in vivo consequences of heterologous Tir-intimin binding are unclear. EPEC intimin has been shown to functionally complement the EHEC intimin mutant UMD619 in adherence to the piglet intestine and in eliciting diarrhea (44). Surprisingly, the localization of the bacteria within the intestine was found to be directed by the species from which intimin was expressed (44). Wild-type EHEC adheres to the large intestine, whereas EHEC UMD619, expressing plasmid-encoded EPEC intimin, adheres to both the large and the small intestines, in a manner representative of that of wild-type EPEC. The localization differences were attributed to the sequence divergence in the carboxy-terminal cell binding domains of EHEC and EPEC intimins; it was hypothesized that this divergence may direct intimin binding to different host receptors (44). It is difficult to reconcile this idea with our knowledge that both EHEC and EPEC insert their own intimin receptors into the host cell and that EHEC and EPEC intimins can bind interchangeably to both EHEC Tir and EPEC Tir. Possibly, the tissue specificity observed is due to an additional intimin receptor. EPEC intimin has been reported to bind to β1 integrins in vitro (20), although the contribution of this binding event to adhesion to host tissues is unknown.
We have shown that E. coli O157:H7 produces a functional Tir that is inserted into host cell membranes, where it then functions as an intimin receptor. EHEC Tir is not tyrosine phosphorylated and plays a role in bacterial adherence to epithelial cells. Intimin binding to EHEC Tir and EPEC Tir results in cytoskeletal rearrangements within the host cell, indicating that tyrosine phosphorylation is not needed for pedestal formation. EHEC and EPEC intimins can functionally cross complement each other by binding to Tir from either EHEC or EPEC and inducing A/E lesion formation, although EHEC Tir has a higher affinity for EHEC intimin than for EPEC intimin. These findings highlight some of the differences between EHEC and EPEC adherence mechanisms and pedestal formation.
ACKNOWLEDGMENTS
We thank Annick Gauthier, Sandra Marcus, and Jose Luis Puente for critical reading of the manuscript and helpful discussions. We also thank the members of the NAPS unit at UBC for excellent protein and DNA sequencing.
This work was supported by a Howard Hughes International Research Scholar Award and an operating grant to B.B.F. from the Medical Research Council of Canada (MRC). B.B.F. is an MRC scientist.
REFERENCES
- 1.Abe A, Heczko U, Hegele R G, Finlay B B. Two enteropathogenic Escherichia coli type III secreted proteins, EspA and EspB, are virulence factors. J Exp Med. 1998;188:1–10. doi: 10.1084/jem.188.10.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe A, Kenny B, Stein M, Finlay B B. Characterization of two virulence proteins secreted by rabbit enteropathogenic Echerichia coli, EspA and EspB, whose maximal expression is sensitive to host body temperature. Infect Immun. 1997;65:3547–3555. doi: 10.1128/iai.65.9.3547-3555.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berendson R, Cheney C P, Schad P A, Boedeker E C. Species-specific binding of purified pili (AF/R1) from Escherichia coli RDEC-1 to rabbit intestinal mucosa. Gastroenterology. 1983;85:837–845. [PubMed] [Google Scholar]
- 4.Bieber D, Ramer S W, Wu C Y, Murray W J, Tobe T, Fernandez R, Schoolnik G K. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science. 1998;280:2114–2118. doi: 10.1126/science.280.5372.2114. [DOI] [PubMed] [Google Scholar]
- 5.Brunder W, Schmidt H, Karsh H. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7, cleaves human coagulation factor. V Mol Microbiol. 1997;24:767–778. doi: 10.1046/j.1365-2958.1997.3871751.x. [DOI] [PubMed] [Google Scholar]
- 6.Dean-Nystrom E, Bosworth B, Moon H, O’Brien A. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect Immun. 1998;66:4560–4563. doi: 10.1128/iai.66.9.4560-4563.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deibel C, Kramer S, Chakraborty T, Ebel F. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells, where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol Microbiol. 1998;28:463–474. doi: 10.1046/j.1365-2958.1998.00798.x. [DOI] [PubMed] [Google Scholar]
- 8.Djafari S, Ebel F, Diebel C, Kramer S, Hudel M, Chakraborty T. Characterization of an exported protease from Shiga toxin-producing Escherichia coli. Mol Microbiol. 1997;25:771–784. doi: 10.1046/j.1365-2958.1997.5141874.x. [DOI] [PubMed] [Google Scholar]
- 9.Donnenberg M, Kaper J. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnenberg M, Tzipori S, McKee M, O’Brien A, Alroy J, Kaper J. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J Clin Investig. 1993;92:1418–1424. doi: 10.1172/JCI116718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnenberg M, Yu J, Kaper J. A second chromosomal gene necessary for intimate adherence of enteropathogenic Escherichia coli to epithelial cells. J Bacteriol. 1993;175:4670–4680. doi: 10.1128/jb.175.15.4670-4680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnenberg M S, Donohue-Rolfe A, Keusch G T. A comparison of HEp-2 cell invasion by enteropathogenic and enteroinvasive Escherichia coli. FEMS Microbiol Lett. 1990;57:83–86. doi: 10.1016/0378-1097(90)90417-o. [DOI] [PubMed] [Google Scholar]
- 13.Donnenberg M S, Tacket C O, James S P, Losonsky G, Nataro J P, Wasserman S S, Kaper J B, Levine M M. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J Clin Investig. 1993;92:1412–1417. doi: 10.1172/JCI116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebel F, Diebel C, Kresse A, Guzman C, Chakraborty T. Temperature- and medium-dependent secretion of proteins by Shiga toxin-producing Escherichia coli. Infect Immun. 1996;64:4472–4479. doi: 10.1128/iai.64.11.4472-4479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebel F, Podzadel T, Rohde M, Kresse A, Kramer S, Deibel C, Guzman C, Chakraborty T. Initial binding of Shiga toxin-producing Escherichia coli to host cells and subsequent induction of actin rearrangements depend on filamentous EspA-containing surface appendages. Mol Microbiol. 1998;30:147–161. doi: 10.1046/j.1365-2958.1998.01046.x. [DOI] [PubMed] [Google Scholar]
- 16.Fiederling F, Boury M, Petit C, Milon A. Adhesive factor/rabbit 2, a new fimbrial adhesion and a virulence factor from Escherichia coli O103, a serogroup enteropathogenic for rabbits. Infect Immun. 1997;65:847–851. doi: 10.1128/iai.65.2.847-851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finlay B B, Rosenshine I, Donnenberg M S, Kaper J B. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun. 1992;60:2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frankel G, Candy D, Fabiani E, Adu-Bobie J, Gil S, Novakova M, Phillips A, Dougan G. Molecular characterization of a carboxy-terminal eukaryotic-cell-binding domain of intimin from enteropathogenic Escherichia coli. Infect Immun. 1995;63:4323–4328. doi: 10.1128/iai.63.11.4323-4328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frankel G, Candy D C, Everest P, Dougan G. Characterization of the C-terminal domains of intimin-like proteins of enteropathogenic and enterohemorrhagic Escherichia coli, Citrobacter freundii, and Hafnia alvei. Infect Immun. 1994;62:1835–1842. doi: 10.1128/iai.62.5.1835-1842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frankel G, Lider O, Hershkoviz R, Mould A P, Kachalsky S G, Candy D, Cahalon L, Humphries M J, Dougan G. The cell-binding domain of intimin from enteropathogenic Escherichia coli binds to β1 integrins. J Biol Chem. 1996;271:20359–20364. doi: 10.1074/jbc.271.34.20359. [DOI] [PubMed] [Google Scholar]
- 21.Giron J A, Ho A S, Schoolnik G K. Characterization of fimbriae produced by enteropathogenic Escherichia coli. J Bacteriol. 1993;175:7391–7403. doi: 10.1128/jb.175.22.7391-7403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Duarte O G, Kaper J B. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect Immun. 1995;63:1767–1676. doi: 10.1128/iai.63.5.1767-1776.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin P, Ostroff S, Tauxe R, Greene K, Wells J, Lewis J, Blake P. Illnesses associated with Escherichia coli O157:H7 infections. Ann Intern Med. 1988;109:705–712. doi: 10.7326/0003-4819-109-9-705. [DOI] [PubMed] [Google Scholar]
- 24.Ismaili A, McWhirter E, Handelsman M, Brunton J, Sherman P. Divergent signal transduction responses to infection with attaching and effacing Escherichia coli. Infect Immun. 1998;66:1688–1696. doi: 10.1128/iai.66.4.1688-1696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ismaili A, Philpott D J, Dytoc M T, Sherman P M. Signal transduction responses following adhesion of verocytotoxin-producing Escherichia coli. Infect Immun. 1995;63:3316–3326. doi: 10.1128/iai.63.9.3316-3326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarvis K, Kaper J. Secretion of extracellular proteins by enterohemorrhagic Escherichia coli via a putative type III secretion system. Infect Immun. 1996;64:4826–4829. doi: 10.1128/iai.64.11.4826-4829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenny B, Abe A, Stein M, Finlay B B. Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infect Immun. 1997;65:2606–2612. doi: 10.1128/iai.65.7.2606-2612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 29.Kenny B, Finlay B. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-γ1. Infect Immun. 1997;65:2528–2536. doi: 10.1128/iai.65.7.2528-2536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenny B, Finlay B B. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals to epithelial cells. Proc Natl Acad Sci USA. 1995;92:7991–7995. doi: 10.1073/pnas.92.17.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenny B, Lai L C, Finlay B B, Donnenberg M S. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol Microbiol. 1996;20:313–323. doi: 10.1111/j.1365-2958.1996.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 32.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knutton S, Rosenshine I, Pallen M J, Nisan I, Neves B C, Bain C, Wolff C, Dougan G, Frankel G. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998;17:2166–2176. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKee M, Melton-Celsa A, Moxley R, Francis D, O’Brien A. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect Immun. 1995;63:3739–3744. doi: 10.1128/iai.63.9.3739-3744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moon H W, Whipp S C, Argenzio R A, Levine M M, Giannella R A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nataro J, Kaper J. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paton A, Manning P, Woodrow M, Paton J. Translocated intimin receptors (Tir) of Shiga-toxigenic Escherichia coli isolates belonging to serogroups O26, O111, and O157 react with sera from patients with hemolytic-uremic syndrome and exhibit marked sequence homology. Infect Immun. 1998;66:5580–5586. doi: 10.1128/iai.66.11.5580-5586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenshine I, Ruschkowski S, Finlay B B. Expression of attaching/effacing activity by enteropathogenic Escherichia coli depends on growth phase, temperature, and protein synthesis upon contact with epithelial cells. Infect Immun. 1996;64:966–973. doi: 10.1128/iai.64.3.966-973.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenshine I, Ruschkowski S, Stein M, Reinscheid D J, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 41.Sanger J, Chang R, Ashton F, Kaper J, Sanger J. Novel form of actin-based motility transports bacteria on the surfaces of infected cells. Cell Mot Cytoskeleton. 1996;34:279–287. doi: 10.1002/(SICI)1097-0169(1996)34:4<279::AID-CM3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 42.Stein M, Kenny B, Stein M A, Finlay B B. Characterization of EspC, a 110-kDa protein secreted by enteropathogenic Escherichia coli which is homologous to members of the immunoglobulin A protease-like family of secreted proteins. J Bacteriol. 1996;178:6546–6554. doi: 10.1128/jb.178.22.6546-6554.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tobe T, Schoolnik G, Sohel I, Bustamante V, Puente J. Cloning and characterization of bfpTVW, genes required for the transcriptional activation of bfpA in enteropathogenic Escherichia coli. Mol Microbiol. 1996;21:963–975. doi: 10.1046/j.1365-2958.1996.531415.x. [DOI] [PubMed] [Google Scholar]
- 44.Tzipori S, Gunzer F, Donnenberg M, de Montigny L, Kaper J, Donohue-Rolfe A. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infect Immun. 1995;63:3621–3627. doi: 10.1128/iai.63.9.3621-3627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolf M K, Boedeker E C. Cloning of the genes for AF/P1 pili from rabbit enteroadherent Escherichia coli REDC-1 and DNA sequence of the major structural subunit. Infect Immun. 1990;58:1124–1128. doi: 10.1128/iai.58.4.1124-1128.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]