Abstract
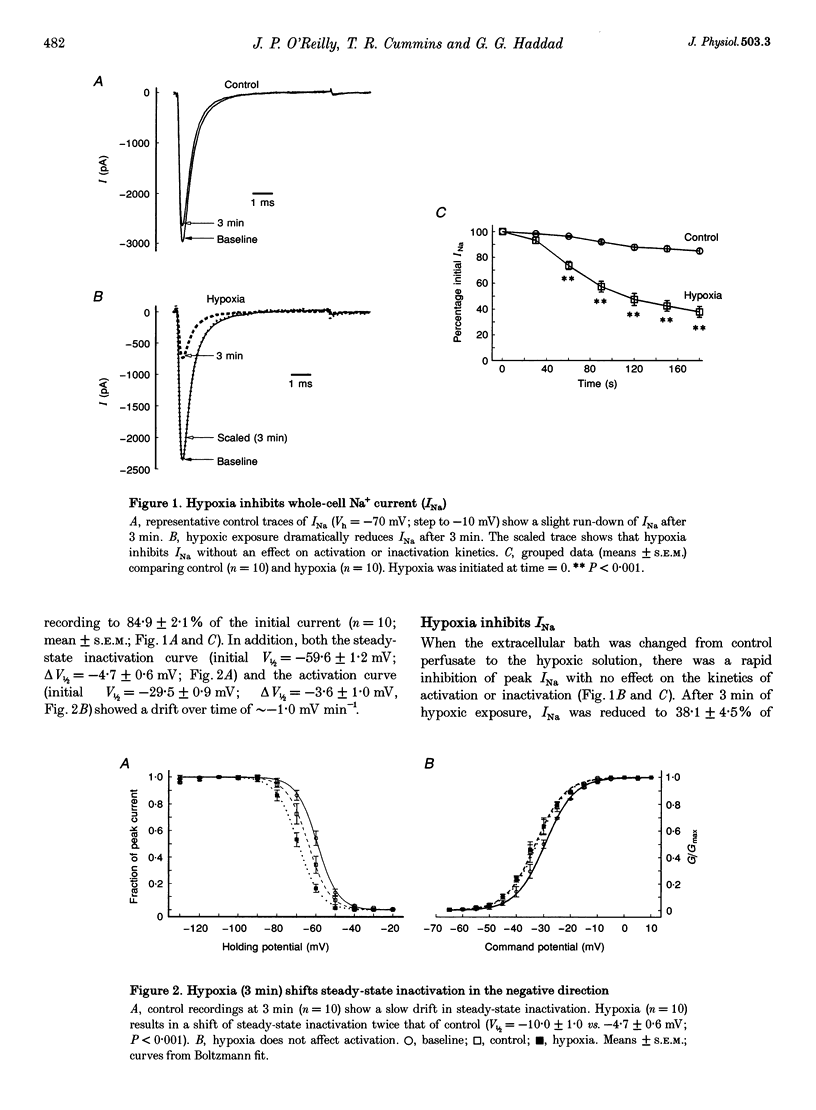
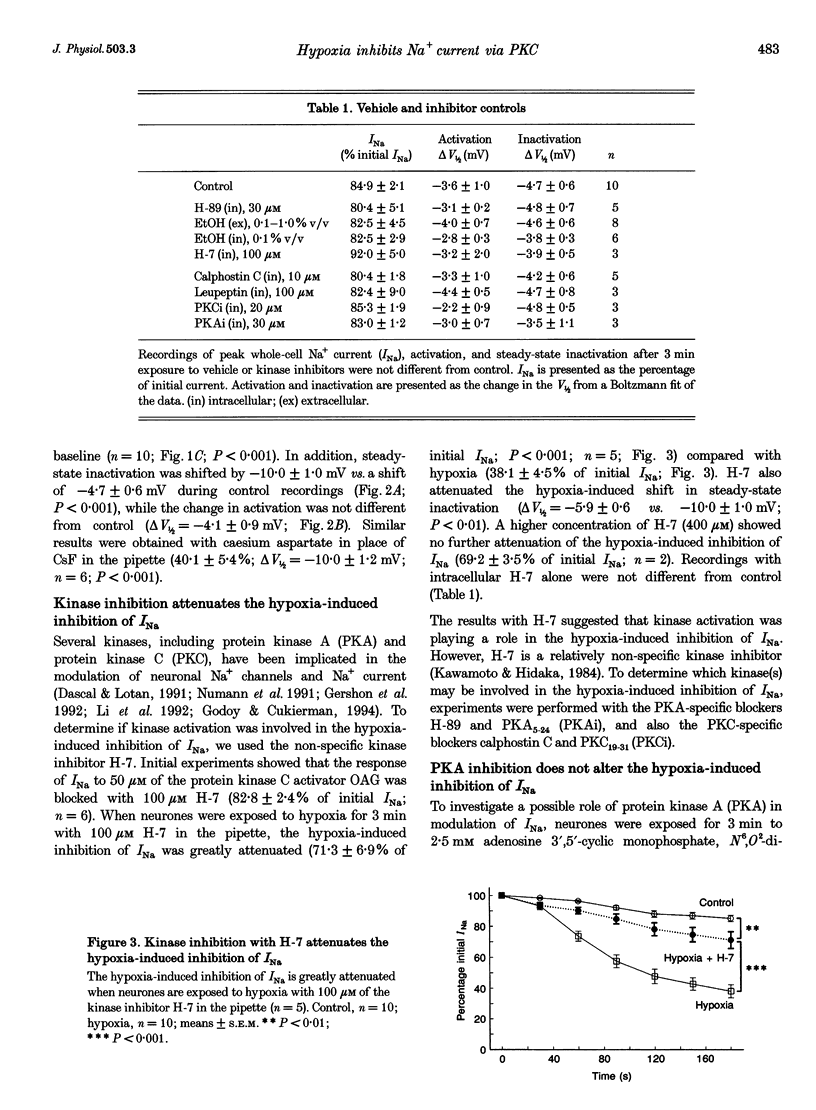
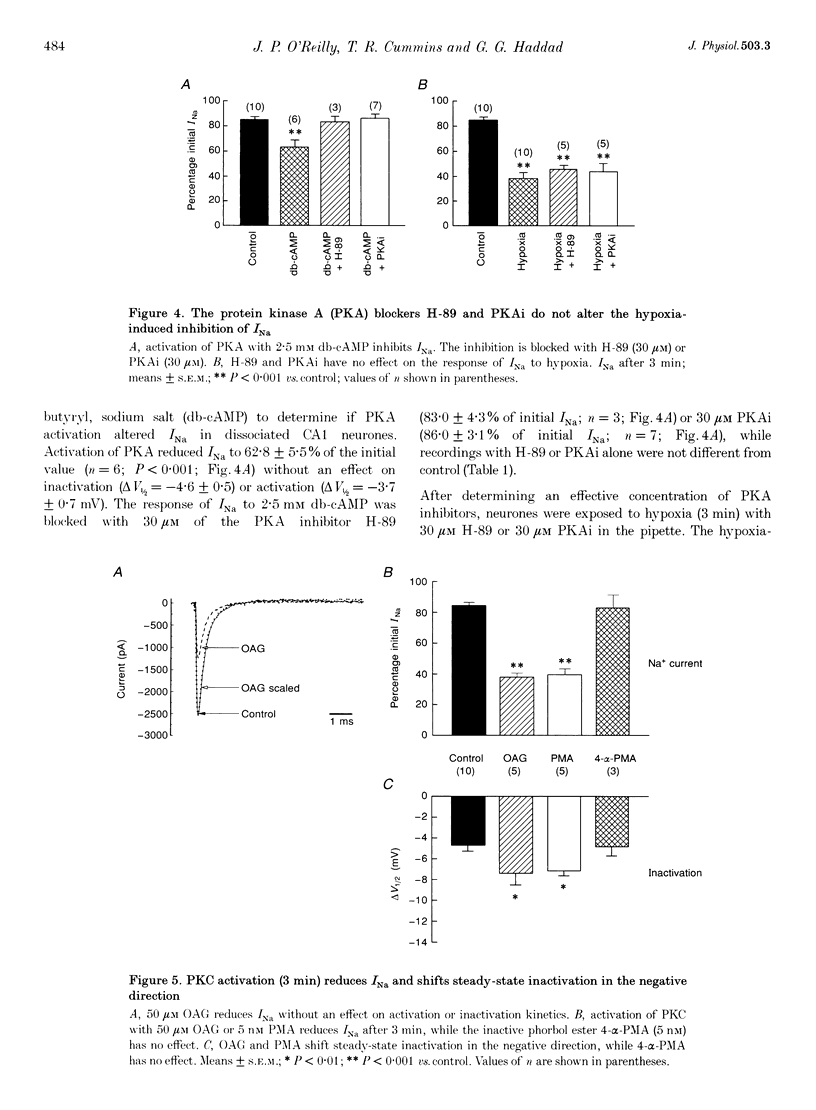
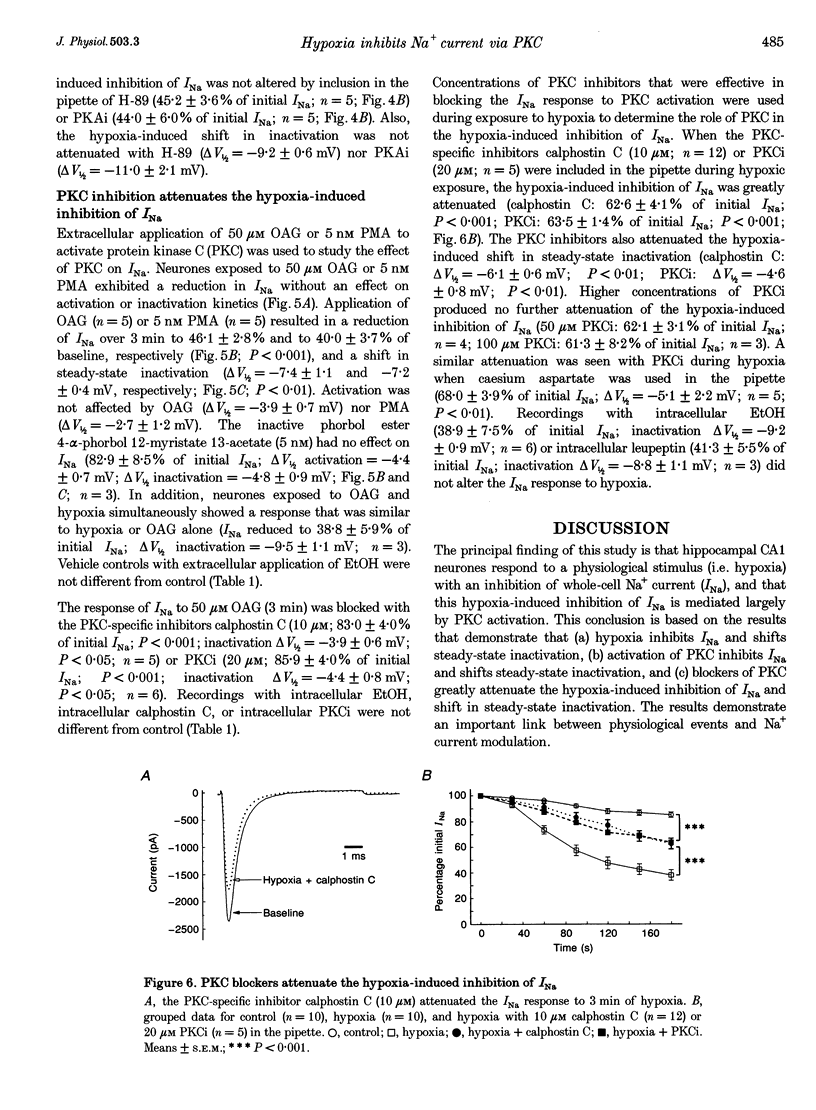
1. Hippocampal neurones respond to acute oxygen deprivation (hypoxia) with an inhibition of whole-cell Na+ current (INa), although the mechanism of the inhibition is unknown. Kinases can modulate INa and kinases are activated during hypoxia. We hypothesized that kinase activation may play a role in the hypoxia-induced inhibition of INa. 2. Single electrode patch clamp techniques were used in dissociated hippocampal CA1 neurones from the rat. INa was recorded at baseline, during exposure to kinase activators (with and without kinase inhibitors), and during acute hypoxia (with and without kinase inhibitors). 3. Hypoxia (3 min) reduced INa to 38.1 +/- 4.5% of initial values, and shifted steady-state inactivation in the negative direction. Hypoxia produced no effect on activation or fast inactivation. 4. Protein kinase A (PKA) activation with 2.5 mM adenosine 3',5'-cyclic adenosine monophosphate, N6,O2-dibutyryl, sodium salt (db-cAMP) resulted in reduction of INa to 62.8 +/- 5.5% without an effect on activation or steady-state inactivation. INa was also reduced by activation of protein kinase C (PKC) with 5 nM phorbol 12-myristate 13-acetate (PMA; to 40.0 +/- 3.7%) or 50 microM 1-oleoyl-2-acetyl-sn-glycerol (OAG; to 46.1 +/- 2.8%). In addition, steady-state inactivation was shifted in the negative direction by PKC activation. Neither the activation curve nor the kinetics of fast inactivation was altered by PKC activation. 5. The response to PKA activation was blocked by the PKA inhibitor N-[2-p-bromocinnamyl-amino) ethyl]-5-isoquinolinesulphonamide (H-89; 30 microM) and by 30 microM of PKA inhibitory peptide PKA5-24 (PKAi). PKC activation was blocked by the kinase inhibitor 1-(5-isoquinolinesulphonyl)-2-methylpiperazine (H-7; 100 microM), by the PKC inhibitor calphostin C (10 microM) and by 20 microM of the inhibitory peptide PKC19-31 (PKCi). 6. The hypoxia-induced inhibition of INa and shift in steady-state inactivation were greatly attenuated with H-7, calphostin C, or PKCi, but not with H-89 or PKAi. 7. We conclude that hypoxia activates PKC in rat CA1 neurones, and that PKC activation leads to the hypoxia-induced inhibition of INa.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrup J., Sørensen P. M., Sørensen H. R. Oxygen and glucose consumption related to Na+-K+ transport in canine brain. Stroke. 1981 Nov-Dec;12(6):726–730. doi: 10.1161/01.str.12.6.726. [DOI] [PubMed] [Google Scholar]
- Bazán N. G., Jr Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochim Biophys Acta. 1970 Oct 6;218(1):1–10. doi: 10.1016/0005-2760(70)90086-x. [DOI] [PubMed] [Google Scholar]
- Bell R. M. Protein kinase C activation by diacylglycerol second messengers. Cell. 1986 Jun 6;45(5):631–632. doi: 10.1016/0092-8674(86)90774-9. [DOI] [PubMed] [Google Scholar]
- Budworth J., Gescher A. Differential inhibition of cytosolic and membrane-derived protein kinase C activity by staurosporine and other kinase inhibitors. FEBS Lett. 1995 Apr 3;362(2):139–142. doi: 10.1016/0014-5793(95)00227-z. [DOI] [PubMed] [Google Scholar]
- Cantrell A. R., Ma J. Y., Scheuer T., Catterall W. A. Muscarinic modulation of sodium current by activation of protein kinase C in rat hippocampal neurons. Neuron. 1996 May;16(5):1019–1026. doi: 10.1016/s0896-6273(00)80125-7. [DOI] [PubMed] [Google Scholar]
- Cardell M., Wieloch T. Time course of the translocation and inhibition of protein kinase C during complete cerebral ischemia in the rat. J Neurochem. 1993 Oct;61(4):1308–1314. doi: 10.1111/j.1471-4159.1993.tb13623.x. [DOI] [PubMed] [Google Scholar]
- Chijiwa T., Mishima A., Hagiwara M., Sano M., Hayashi K., Inoue T., Naito K., Toshioka T., Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990 Mar 25;265(9):5267–5272. [PubMed] [Google Scholar]
- Costa M. R., Catterall W. A. Phosphorylation of the alpha subunit of the sodium channel by protein kinase C. Cell Mol Neurobiol. 1984 Sep;4(3):291–297. doi: 10.1007/BF00733592. [DOI] [PubMed] [Google Scholar]
- Cukierman S. Regulation of voltage-dependent sodium channels. J Membr Biol. 1996 Jun;151(3):203–214. doi: 10.1007/s002329900071. [DOI] [PubMed] [Google Scholar]
- Cummins T. R., Donnelly D. F., Haddad G. G. Effect of metabolic inhibition on the excitability of isolated hippocampal CA1 neurons: developmental aspects. J Neurophysiol. 1991 Nov;66(5):1471–1482. doi: 10.1152/jn.1991.66.5.1471. [DOI] [PubMed] [Google Scholar]
- Cummins T. R., Jiang C., Haddad G. G. Human neocortical excitability is decreased during anoxia via sodium channel modulation. J Clin Invest. 1993 Feb;91(2):608–615. doi: 10.1172/JCI116241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N., Lotan I. Activation of protein kinase C alters voltage dependence of a Na+ channel. Neuron. 1991 Jan;6(1):165–175. doi: 10.1016/0896-6273(91)90131-i. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Silver I. A. ATP and brain function. J Cereb Blood Flow Metab. 1989 Feb;9(1):2–19. doi: 10.1038/jcbfm.1989.2. [DOI] [PubMed] [Google Scholar]
- Fraser D. D., Hoehn K., Weiss S., MacVicar B. A. Arachidonic acid inhibits sodium currents and synaptic transmission in cultured striatal neurons. Neuron. 1993 Oct;11(4):633–644. doi: 10.1016/0896-6273(93)90075-3. [DOI] [PubMed] [Google Scholar]
- Gershon E., Weigl L., Lotan I., Schreibmayer W., Dascal N. Protein kinase A reduces voltage-dependent Na+ current in Xenopus oocytes. J Neurosci. 1992 Oct;12(10):3743–3752. doi: 10.1523/JNEUROSCI.12-10-03743.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy C. M., Cukierman S. Multiple effects of protein kinase C activators on Na+ currents in mouse neuroblastoma cells. J Membr Biol. 1994 Jun;140(2):101–110. doi: 10.1007/BF00232898. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- House C., Kemp B. E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987 Dec 18;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- Jiang C., Haddad G. G. A direct mechanism for sensing low oxygen levels by central neurons. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7198–7201. doi: 10.1073/pnas.91.15.7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur J., Macdonald R. L. Cyclic AMP-dependent protein kinase enhances hippocampal dentate granule cell GABAA receptor currents. J Neurophysiol. 1996 Oct;76(4):2626–2634. doi: 10.1152/jn.1996.76.4.2626. [DOI] [PubMed] [Google Scholar]
- Kawamoto S., Hidaka H. 1-(5-Isoquinolinesulfonyl)-2-methylpiperazine (H-7) is a selective inhibitor of protein kinase C in rabbit platelets. Biochem Biophys Res Commun. 1984 Nov 30;125(1):258–264. doi: 10.1016/s0006-291x(84)80362-9. [DOI] [PubMed] [Google Scholar]
- Kay A. R. An intracellular medium formulary. J Neurosci Methods. 1992 Sep;44(2-3):91–100. doi: 10.1016/0165-0270(92)90002-u. [DOI] [PubMed] [Google Scholar]
- Kay A. R., Wong R. K. Isolation of neurons suitable for patch-clamping from adult mammalian central nervous systems. J Neurosci Methods. 1986 May;16(3):227–238. doi: 10.1016/0165-0270(86)90040-3. [DOI] [PubMed] [Google Scholar]
- Kobayashi E., Nakano H., Morimoto M., Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989 Mar 15;159(2):548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Lukyanetz E. A., Ter-Markosyan A. S. Parathyroid hormone enhances calcium current in snail neurones--simulation of the effect by phorbol esters. Pflugers Arch. 1992 Feb;420(2):146–152. doi: 10.1007/BF00374983. [DOI] [PubMed] [Google Scholar]
- Li M., West J. W., Lai Y., Scheuer T., Catterall W. A. Functional modulation of brain sodium channels by cAMP-dependent phosphorylation. Neuron. 1992 Jun;8(6):1151–1159. doi: 10.1016/0896-6273(92)90135-z. [DOI] [PubMed] [Google Scholar]
- Madden K. P., Clark W. M., Kochhar A., Zivin J. A. Effect of protein kinase C modulation on outcome of experimental CNS ischemia. Brain Res. 1991 May 3;547(2):193–198. doi: 10.1016/0006-8993(91)90962-u. [DOI] [PubMed] [Google Scholar]
- Marchetti C., Brown A. M. Protein kinase activator 1-oleoyl-2-acetyl-sn-glycerol inhibits two types of calcium currents in GH3 cells. Am J Physiol. 1988 Jan;254(1 Pt 1):C206–C210. doi: 10.1152/ajpcell.1988.254.1.C206. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Post-natal development of electrophysiological properties of rat cerebral cortical pyramidal neurones. J Physiol. 1987 Dec;393:743–762. doi: 10.1113/jphysiol.1987.sp016851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H. Modulation of ion channels by arachidonic acid. Prog Neurobiol. 1994 Jun;43(2):175–186. doi: 10.1016/0301-0082(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Numann R., Catterall W. A., Scheuer T. Functional modulation of brain sodium channels by protein kinase C phosphorylation. Science. 1991 Oct 4;254(5028):115–118. doi: 10.1126/science.1656525. [DOI] [PubMed] [Google Scholar]
- Ono K., Kiyosue T., Arita M. Isoproterenol, DBcAMP, and forskolin inhibit cardiac sodium current. Am J Physiol. 1989 Jun;256(6 Pt 1):C1131–C1137. doi: 10.1152/ajpcell.1989.256.6.C1131. [DOI] [PubMed] [Google Scholar]
- Prenen G. H., Go K. G., Postema F., Zuiderveen F., Korf J. Cerebral cation shifts in hypoxic-ischemic brain damage are prevented by the sodium channel blocker tetrodotoxin. Exp Neurol. 1988 Jan;99(1):118–132. doi: 10.1016/0014-4886(88)90132-x. [DOI] [PubMed] [Google Scholar]
- Pérez-Pinzón M. A., Rosenthal M., Sick T. J., Lutz P. L., Pablo J., Mash D. Downregulation of sodium channels during anoxia: a putative survival strategy of turtle brain. Am J Physiol. 1992 Apr;262(4 Pt 2):R712–R715. doi: 10.1152/ajpregu.1992.262.4.R712. [DOI] [PubMed] [Google Scholar]
- Rossie S., Catterall W. A. Cyclic-AMP-dependent phosphorylation of voltage-sensitive sodium channels in primary cultures of rat brain neurons. J Biol Chem. 1987 Sep 15;262(26):12735–12744. [PubMed] [Google Scholar]
- Takano K., Stanfield P. R., Nakajima S., Nakajima Y. Protein kinase C-mediated inhibition of an inward rectifier potassium channel by substance P in nucleus basalis neurons. Neuron. 1995 May;14(5):999–1008. doi: 10.1016/0896-6273(95)90338-0. [DOI] [PubMed] [Google Scholar]
- Urenjak J., Obrenovitch T. P. Pharmacological modulation of voltage-gated Na+ channels: a rational and effective strategy against ischemic brain damage. Pharmacol Rev. 1996 Mar;48(1):21–67. [PubMed] [Google Scholar]
- Weber M. L., Taylor C. P. Damage from oxygen and glucose deprivation in hippocampal slices is prevented by tetrodotoxin, lidocaine and phenytoin without blockade of action potentials. Brain Res. 1994 Nov 21;664(1-2):167–177. doi: 10.1016/0006-8993(94)91967-4. [DOI] [PubMed] [Google Scholar]
- West J. W., Numann R., Murphy B. J., Scheuer T., Catterall W. A. A phosphorylation site in the Na+ channel required for modulation by protein kinase C. Science. 1991 Nov 8;254(5033):866–868. doi: 10.1126/science.1658937. [DOI] [PubMed] [Google Scholar]
- Wieloch T., Bergstedt K., Hu B. R. Protein phosphorylation and the regulation of mRNA translation following cerebral ischemia. Prog Brain Res. 1993;96:179–191. doi: 10.1016/s0079-6123(08)63266-5. [DOI] [PubMed] [Google Scholar]


