Abstract
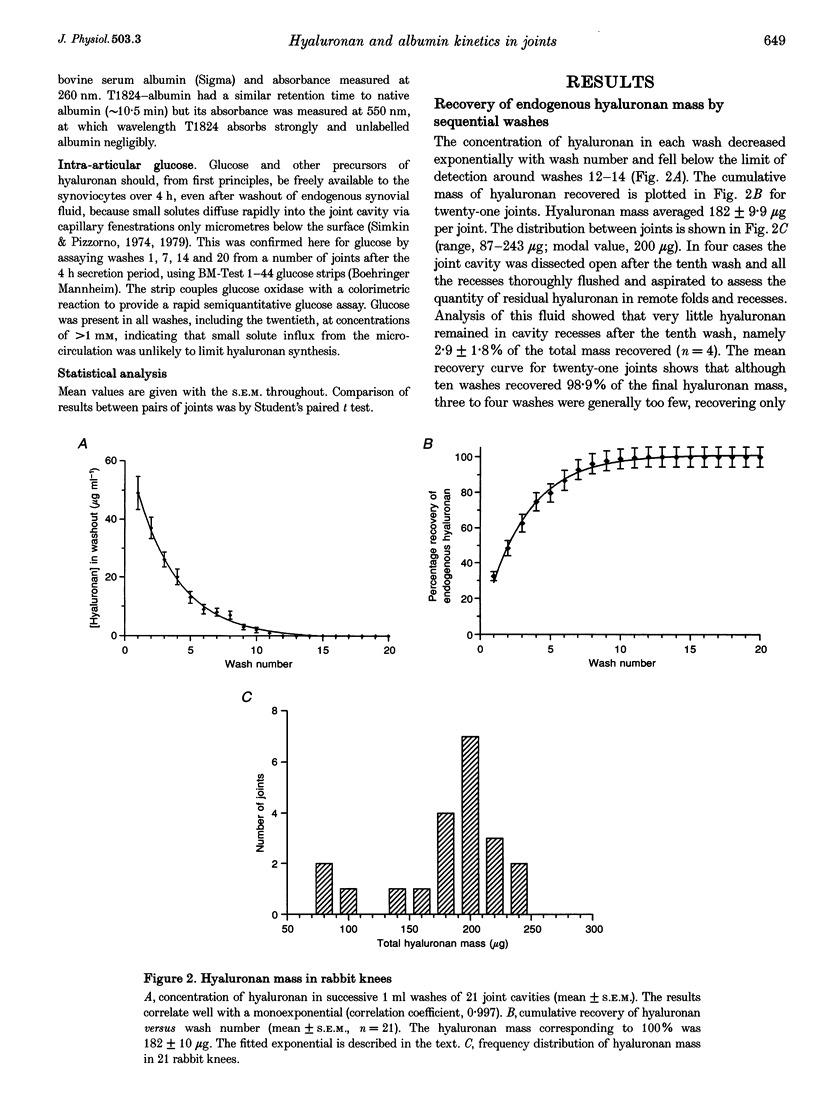
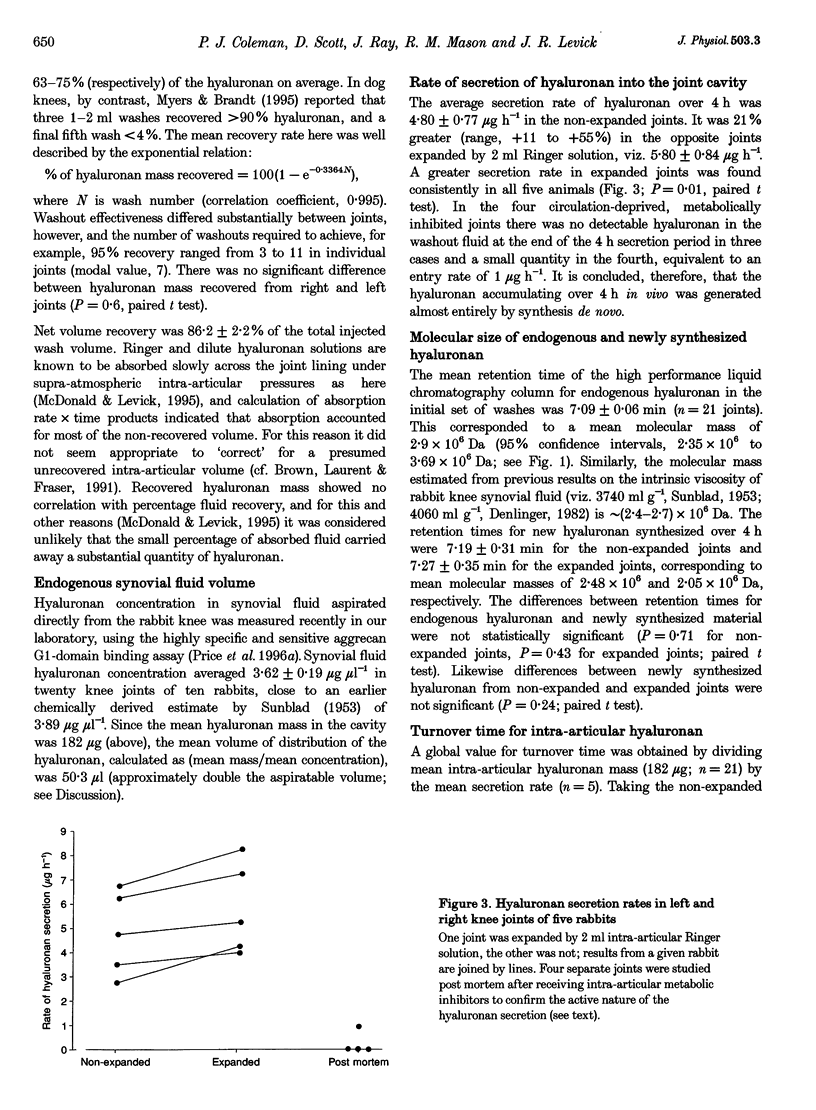
1. Hyaluronan is not only a lubricant but also enhances the synovial lining's resistance to fluid outflow. This finding led to the proposal that hyaluronan (> 2 x 10(6) Da, approximately 210 nm radius) may escape across the synovial lining less freely than smaller solutes (e.g. albumin, 6.7 x 10(4) Da, 3.6 nm radius) or water. Here multiple washouts were used to measure intraarticular hyaluronan mass and secretion rate in rabbit knees, leading to an estimate of hyaluronan turnover time. Plasma albumin permeation into the joint cavity was also measured to enable comparison of turnover times between molecules of very disparate size. 2. Endogenous hyaluronan mass in the joint cavity, analysed by high performance liquid chromatography of joint washes, was 182 +/- 9.9 micrograms (mean +/- S.E.M; n = 21). Since hyaluronan concentration in synovial fluid averages 3.62 +/- 0.19 micrograms microliters-1, the endogenous synovial fluid volume was calculated to be 50 microliters (mass/concentration), about double the aspiratable volume. 3. The hyaluronan secretion rate over 4 h was 4.80 +/- 0.77 micrograms h-1 (n = 5). The rate was significantly higher in contralateral joints expanded by 2 ml Ringer solution (5.80 +/- 0.84 micrograms h-1, n = 5, P = 0.01, Student's paired t test), indicating a stretch/hydration sensitive secretory mechanism. The newly secreted chains ((2.05-2.48) x 10(6) Da) were not significantly different in length from the endogenous chains (2.95 x 10(6) Da). 4. Hyaluronan turnover time, calculated as mass/secretion rate, was 31.4-37.9 h. This is more than an order of magnitude longer than turnover time for intra-articular albumin. The latter, determined from the intra-articular albumin mass and plasma-to-cavity permeation rate was 1.8 h (95% confidence intervals 1.2-3.5 h, n = 9). The big difference in turnover times support the view that, relative to albumin and water, hyaluronan is partially sieved out and retained in the joint cavity by the synovial lining. The lining cell layer is discontinuous, so it appears that interstitial matrix itself acts as a leaky size-selective molecular filter.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bansal M. K., Mason R. M. Evidence for rapid metabolic turnover of hyaluronate synthetase in Swarm rat chondrosarcoma chondrocytes. Biochem J. 1986 Jun 1;236(2):515–519. doi: 10.1042/bj2360515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanckaert N., Kabra P. M., Farina F. A., Stafford B. E., Marton L. J., Schmid R. Measurement of bilirubin and its monoconjugates and diconjugates in human serum by alkaline methanolysis and high-performance liquid chromatography. J Lab Clin Med. 1980 Aug;96(2):198–212. [PubMed] [Google Scholar]
- Brown T. J., Laurent U. B., Fraser J. R. Turnover of hyaluronan in synovial joints: elimination of labelled hyaluronan from the knee joint of the rabbit. Exp Physiol. 1991 Jan;76(1):125–134. doi: 10.1113/expphysiol.1991.sp003474. [DOI] [PubMed] [Google Scholar]
- Delecrin J., Oka M., Kumar P., Takahashi S., Kotoura Y., Yamamuro T., Daculsi G. Measurement of synovial fluid volume: a new dilution method adapted to fluid permeation from the synovial cavity. J Rheumatol. 1992 Nov;19(11):1746–1752. [PubMed] [Google Scholar]
- Edwards J. C. The nature and origins of synovium: experimental approaches to the study of synoviocyte differentiation. J Anat. 1994 Jun;184(Pt 3):493–501. [PMC free article] [PubMed] [Google Scholar]
- Fraser J. R., Kimpton W. G., Pierscionek B. K., Cahill R. N. The kinetics of hyaluronan in normal and acutely inflamed synovial joints: observations with experimental arthritis in sheep. Semin Arthritis Rheum. 1993 Jun;22(6 Suppl 1):9–17. doi: 10.1016/s0049-0172(10)80015-0. [DOI] [PubMed] [Google Scholar]
- Granger H. J., Taylor A. E. Permeability of connective tissue linings isolated from implanted capsules; implications for interstitial pressure measurements. Circ Res. 1975 Jan;36(1):222–228. doi: 10.1161/01.res.36.1.222. [DOI] [PubMed] [Google Scholar]
- Jensen L. T., Henriksen J. H., Olesen H. P., Risteli J., Lorenzen I. Lymphatic clearance of synovial fluid in conscious pigs: the aminoterminal propeptide of type III procollagen. Eur J Clin Invest. 1993 Dec;23(12):778–784. doi: 10.1111/j.1365-2362.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Knight A. D., Levick J. R. Physiological compartmentation of fluid within the synovial cavity of the rabbit knee. J Physiol. 1982 Oct;331:1–15. doi: 10.1113/jphysiol.1982.sp014361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A. D., Levick J. R. The density and distribution of capillaries around a synovial cavity. Q J Exp Physiol. 1983 Oct;68(4):629–644. doi: 10.1113/expphysiol.1983.sp002753. [DOI] [PubMed] [Google Scholar]
- Knox P., Levick J. R., McDonald J. N. Synovial fluid--its mass, macromolecular content and pressure in major limb joints of the rabbit. Q J Exp Physiol. 1988 Jan;73(1):33–45. doi: 10.1113/expphysiol.1988.sp003121. [DOI] [PubMed] [Google Scholar]
- Laurent T. C. The interaction between polysaccharides and other macromolecules. 9. The exclusion of molecules from hyaluronic acid gels and solutions. Biochem J. 1964 Oct;93(1):106–112. doi: 10.1042/bj0930106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick J. R. An analysis of the interaction between interstitial plasma protein, interstitial flow, and fenestral filtration and its application to synovium. Microvasc Res. 1994 Jan;47(1):90–125. doi: 10.1006/mvre.1994.1007. [DOI] [PubMed] [Google Scholar]
- Levick J. R. An investigation into the validity of subatmospheric pressure recordings from synovial fluid and their dependence on joint angle. J Physiol. 1979 Apr;289:55–67. doi: 10.1113/jphysiol.1979.sp012724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick J. R., Michel C. C. The permeability of individually perfused frog mesenteric capillaries to T1824 and T1824-albumin as evidence for a large pore system. Q J Exp Physiol Cogn Med Sci. 1973 Jan;58(1):67–85. doi: 10.1113/expphysiol.1973.sp002192. [DOI] [PubMed] [Google Scholar]
- Lindenhayn K., Heilmann H. H., Niederhausen T., Walther H. U., Pohlenz K. Elimination of tritium-labelled hyaluronic acid from normal and osteoarthritic rabbit knee joints. Eur J Clin Chem Clin Biochem. 1997 May;35(5):355–363. doi: 10.1515/cclm.1997.35.5.355. [DOI] [PubMed] [Google Scholar]
- Maroudas A. Distribution and diffusion of solutes in articular cartilage. Biophys J. 1970 May;10(5):365–379. doi: 10.1016/S0006-3495(70)86307-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. N., Levick J. R. Effect of intra-articular hyaluronan on pressure-flow relation across synovium in anaesthetized rabbits. J Physiol. 1995 May 15;485(Pt 1):179–193. doi: 10.1113/jphysiol.1995.sp020722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. N., Levick J. R. Hyaluronan reduces fluid escape rate from rabbit knee joints disparately from its effect on fluidity. Exp Physiol. 1994 Jan;79(1):103–106. doi: 10.1113/expphysiol.1994.sp003736. [DOI] [PubMed] [Google Scholar]
- Myers S. L., Brandt K. D. Effects of synovial fluid hyaluronan concentration and molecular size on clearance of protein from the canine knee. J Rheumatol. 1995 Sep;22(9):1732–1739. [PubMed] [Google Scholar]
- Nagata Y., Matsumura F., Motoyoshi H., Yamasaki H., Fukuda K., Tanaka S. Secretion of hyaluronic acid from synovial fibroblasts is enhanced by histamine: a newly observed metabolic effect of histamine. J Lab Clin Med. 1992 Nov;120(5):707–712. [PubMed] [Google Scholar]
- Ostgaard G., Reed R. K. Increased lymphatic hyaluronan output and preserved hyaluronan content of the rat small intestine in prolonged hypoproteinaemia. Acta Physiol Scand. 1994 Sep;152(1):51–56. doi: 10.1111/j.1748-1716.1994.tb09783.x. [DOI] [PubMed] [Google Scholar]
- Page-Thomas D. P., Bard D., King B., Dingle J. T. Clearance of proteoglycan from joint cavities. Ann Rheum Dis. 1987 Dec;46(12):934–937. doi: 10.1136/ard.46.12.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K. H., Winlove C. P. The macromolecular basis of the hydraulic conductivity of the arterial wall. Biorheology. 1984;21(1-2):181–196. doi: 10.3233/bir-1984-211-221. [DOI] [PubMed] [Google Scholar]
- Pitsillides A. A., Wilkinson L. S., Mehdizadeh S., Bayliss M. T., Edwards J. C. Uridine diphosphoglucose dehydrogenase activity in normal and rheumatoid synovium: the description of a specialized synovial lining cell. Int J Exp Pathol. 1993 Feb;74(1):27–34. [PMC free article] [PubMed] [Google Scholar]
- Prehm P. Synovial hyaluronate in rheumatoid arthritis binds C1q and is covalently bound to antibodies: a model for chronicity. Ann Rheum Dis. 1995 May;54(5):408–412. doi: 10.1136/ard.54.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price F. M., Levick J. R., Mason R. M. Changes in glycosaminoglycan concentration and synovial permeability at raised intra-articular pressure in rabbit knees. J Physiol. 1996 Sep 15;495(Pt 3):821–833. doi: 10.1113/jphysiol.1996.sp021635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price F. M., Levick J. R., Mason R. M. Glycosaminoglycan concentration in synovium and other tissues of rabbit knee in relation to synovial hydraulic resistance. J Physiol. 1996 Sep 15;495(Pt 3):803–820. doi: 10.1113/jphysiol.1996.sp021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. K., Townsley M. I., Zhao Z., Ishibashi M., Laurent T. C., Taylor A. E. Lymphatic hyaluronan flux from skin increases during increased lymph flow induced by intravenous saline loading. Int J Microcirc Clin Exp. 1994 Jan-Apr;14(1-2):56–61. doi: 10.1159/000178207. [DOI] [PubMed] [Google Scholar]
- SUNBLAD L. Studies on hyaluronic acid in synovial fluids. Acta Soc Med Ups. 1953 Apr 29;58(3-4):113–238. [PubMed] [Google Scholar]
- Simkin P. A., Benedict R. S. Iodide and albumin kinetics in normal canine wrists and knees. Arthritis Rheum. 1990 Jan;33(1):73–79. doi: 10.1002/art.1780330109. [DOI] [PubMed] [Google Scholar]
- Simkin P. A., Pizzorno J. E. Transynovial exchange of small molecules in normal human subjects. J Appl Physiol. 1974 May;36(5):581–587. doi: 10.1152/jappl.1974.36.5.581. [DOI] [PubMed] [Google Scholar]
- Simkin P. A. Synovial permeability in rheumatoid arthritis. Arthritis Rheum. 1979 Jul;22(7):689–696. doi: 10.1002/art.1780220701. [DOI] [PubMed] [Google Scholar]
- Stevens C. R., Blake D. R., Merry P., Revell P. A., Levick J. R. A comparative study by morphometry of the microvasculature in normal and rheumatoid synovium. Arthritis Rheum. 1991 Dec;34(12):1508–1513. doi: 10.1002/art.1780341206. [DOI] [PubMed] [Google Scholar]
- Townsley M. I., Reed R. K., Ishibashi M., Parker J. C., Laurent T. C., Taylor A. E. Hyaluronan efflux from canine lung with increased hydrostatic pressure and saline loading. Am J Respir Crit Care Med. 1994 Dec;150(6 Pt 1):1605–1611. doi: 10.1164/ajrccm.150.6.7952622. [DOI] [PubMed] [Google Scholar]


