Abstract
We studied serum antibodies against gangliosides GQ1b and GM1 in 13 patients with Miller Fisher syndrome (MFS) and in 18 patients with Guillain-Barré syndrome (GBS) with cranial nerve involvement. Anti-GQ1b titers were elevated in all patients with MFS cases (immunoglobulin G [IgG] > IgA, IgM), and in 8 of the 18 with GBS. Lower frequencies of increased anti-GM1 titers were observed in MFS patients (3 of 13), as well as in GBS patients (5 of 18). During the course of MFS, anti-GQ1b titers of all Ig classes decreased within 3 weeks after onset. By contrast, anti-GM1 titers (mainly IgM) transiently increased during the course of MFS in five of six patients, suggesting a nonspecific secondary immune response. In patients with MFS following respiratory infections, IgG was the major anti-GQ1b Ig class (six of six patients) and IgG3 was the major subclass (five of six). In contrast, four of five patients with MFS following gastrointestinal infections showed predominance of anti-GQ1b IgA or IgM over IgG and predominance of the IgG2 subclass; anti-GQ1b IgG (IgG3) prevailed in one patient only. These distinct Ig patterns strongly suggest that different infections may trigger different mechanisms of anti-GQ1b production, such as via T-cell-dependent as opposed to T-cell-independent pathways. Thus, the origin of antibodies against GQ1b in MFS may be determined by the type of infectious agent that precipitates the disease.
Miller Fisher syndrome (MFS) is regarded as a rare clinical variant of Guillain-Barré syndrome (GBS), an inflammatory demyelinating disease of the peripheral nervous system, and is characterized by cranial nerve involvement typically leading to ophthalmoplegia (5). Both MFS and GBS are associated with serum antibodies against gangliosides, which may contribute to an autoimmune pathogenesis of these diseases. More than 90% of MFS patients show immunoglobulin G (IgG) antibodies against gangliosides GQ1b and GT1a (8, 35, 36, 38). Anti-GQ1b and/or anti-GT1a antibodies are also present in patients with Bickerstaff’s encephalitis (37) as well as in GBS patients who exhibit ophthalmoplegia (8) or oropharyngeal palsy (19, 22), suggesting a specific role in cranial nerve impairment. On the other hand, a considerably lower incidence (20 to 30%) of antibodies against gangliosides, predominantly GM1 and GD1b, is found in classical GBS (for reviews, see references 13 and 32).
Both MFS and GBS develop following various infections of the respiratory or gastrointestinal tract in around two-thirds of patients (5, 14), justifying the former term, “postinfectious polyneuritis.” Identified associated agents are cytomegalovirus and Epstein-Barr virus (10), as well as Mycoplasma pneumoniae (11); the most common pathogen linked to MFS and GBS (in roughly 30% of cases) is Campylobacter jejuni (24), a gram-negative bacterium that frequently causes enteritis. Specific associations between MFS or GBS and certain C. jejuni serotypes (17, 42) and particular structural homologies between lipopolysaccharides (LPS) from these serotypes and gangliosides (2, 3, 26, 39) suggest molecular mimicry as a trigger for the production of antibodies against gangliosides (reviewed in reference 32). The issue of ganglioside-specific antibodies and infection with C. jejuni has been studied extensively in GBS patients (for reviews, see references 13 and 32), and significant associations between the presence of anti-GM1 antibodies and preceding C. jejuni infection have recently been reported (15, 23, 42).
Studies on the IgG subclass distribution of antibodies against gangliosides have demonstrated mainly IgG1 and IgG3 among anti-GM1 antibodies in patients with GBS, including patients after C. jejuni infection (9, 21, 41), as well as among anti-GQ1b antibodies in MFS patients (36). This subclass pattern suggests a recruitment of T-cell help in antibody generation and appears unusual, since IgG1 and IgG3 are generally associated with T-cell-dependent responses to protein antigens whereas IgG2 is characteristically induced by T-cell-independent carbohydrate antigens (reviewed in reference 16).
Whereas many reports have focused on anti-GM1 antibodies in GBS patients with respect to preceding infection, anti-GQ1b antibodies in MFS patients have, to our knowledge, not been studied with regard to possible differences in Ig patterns after different infections. We investigated the Ig class and IgG subclass of antibodies against GQ1b in MFS patients following respiratory and gastrointestinal infections. The specificity of the immune response against GQ1b was assessed by comparison of anti-GQ1b and anti-GM1 antibodies in MFS patients as well as GBS patients showing involvement of cranial nerves (GBS/cra patients). In particular, we monitored the titers of antibodies against GQ1b and GM1 in parallel over the course of disease in several MFS patients.
MATERIALS AND METHODS
Patients.
Serum and cerebrospinal fluid (CSF) were obtained from patients admitted to Austrian neurological hospital departments and fulfilling the current diagnostic criteria for MFS (25) or GBS (1). The clinical characteristics of 13 MFS and 18 GBS patients are given in Table 1. MFS patients showed the typical triad of areflexia, ataxia, and ophthalmoplegia without major limb weakness. Except for two cases, MFS followed either upper respiratory or gastrointestinal infections. In three of the latter cases (patients 7 to 9 [see Table 3]), C. jejuni was demonstrated by conventional bacteriological cultivation; however, serotyping of these C. jejuni strains was not performed. The causative agents for the other cases of antecedent respiratory or gastrointestinal infection were not identified. GBS patients showed deficits of one or more cranial nerves (facial and/or abducens palsy, dysarthria, and dysphagia). In all but two cases, first serum samples were collected in the acute phase of the disease, before specific treatment started; consecutive samples were studied in six MFS patients. Serum from 20 healthy individuals and CSF from 30 patients with other neurological disorders, such as motor neuron disease or multiple sclerosis, served as controls.
TABLE 1.
Clinical characteristics of patient groups
| Characteristic | MFS patients | GBS patients |
|---|---|---|
| No. | 13 | 18 |
| Age range (yr) | 12–80, mean 49 | 18–86, mean 48 |
| Sex: female, male | 4f, 9m | 8f, 10m |
| Preceding infection (daysa) | ||
| Respiratory | 6/13 (4–14) | 3/18 (2–7) |
| Gastrointestinal | 5/13 (6–21) | 6/18 (3–17) |
| Unknown | 2/13 | 9/18 |
| Hypo-or areflexia | 13/13 | 18/18 |
| Ataxia | 13/13 | 9/18 |
| Ophthalmoplegia | 13/13 | 0/18 |
| Abducens palsy | 5/18 | |
| Facial palsy | 6/13 | 14/18 |
| Dysarthria | 8/13 | 2/18 |
| Dysphagia | 5/13 | 3/18 |
| First serum sampling (daysb) | ||
| Acute phase | 12/13 (2–18, mean 7) | 17/18 (2–21, mean 11) |
| Recovery phase | 1/13 (61) | 1/18 (38) |
| CSF sampling (daysb) | 3/13 (5–6) | 4/18 (2–15) |
Days (range) between onset of preceding infection and onset of neurological symptoms.
Days (range) between onset of neurological symptoms and serum sampling.
TABLE 3.
Anti-GQ1b Ig class and IgG subclass titers in serum of MFS patients
| Patient | Preceding infectionb | DAOc | Titer of anti-GQ1b Ig classa:
|
Titer of anti-GQ1b IgG subclassa:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| IgA | IgG | IgM | IgG1 | IgG2 | IgG3 | IgG4 | |||
| 1 | Resp | 6 | 3.24 | 64.06 | 3.73 | 1.40 | 2.70 | 29.70 | 0.00 |
| 2 | Resp | 7 | 30.00 | 1.67 | 5.10 | 3.80 | 17.50 | 0.50 | |
| 3 | Resp | 6 | 1.35 | 17.50 | 2.97 | 0.43 | 0.60 | 7.57 | 0.00 |
| 4 | Resp | 7 | 4.71 | 15.50 | 1.10 | 0.17 | 0.50 | 7.63 | 0.00 |
| 5 | Resp | 10 | 2.76 | 3.56 | 2.53 | 1.90 | 0.31 | 0.00 | 0.02 |
| 6 | Resp | 2 | 2.38 | 0.00 | 0.04 | 1.27 | 0.01 | ||
| 7 | Gast/C.j. | 61 | 12.63 | 1.33 | 2.35 | 0.49 | 9.40 | 0.12 | |
| 8 | Gast/C.j. | 18 | 3.71 | 2.56 | 5.00 | 0.11 | 1.37 | 0.05 | 0.00 |
| 9 | Gast/C.j. | 9 | 4.00 | 1.09 | 2.13 | 0.13 | 0.54 | 0.02 | 0.01 |
| 10 | Gast | 5 | 1.35 | 2.00 | 17.07 | 0.17 | 1.16 | 0.00 | 0.00 |
| 11 | Gast | 11 | 1.47 | 1.38 | 0.17 | 1.38 | 0.02 | 0.00 | |
| 12 | Unknown | 5 | 1.59 | 6,625.00 | 0.00 | 40.00 | 4,330.00 | 0.00 | |
| 13 | Unknown | 7 | 21.65 | 6.31 | 4.30 | 2.13 | 1.64 | 0.25 | 0.08 |
Anti-GQ1b titers as determined with the earliest serum samples available, diluted 1:20 and 1:2 for Ig class and IgG subclass determinations, respectively. Ig class titers are given as ΔE/cutoff ratios. Bold type indicates the predominant Ig class or IgG subclass.
Resp, respiratory; Gast, gastrointestinal; C.j., C. jejuni infection.
DAO, day after onset of neurological symptoms.
Ig classes of antibodies against GQ1b and GM1.
Anti-ganglioside IgA, IgG, and IgM antibody levels in serum were determined by enzyme-linked immunosorbent assay (ELISA) as described by Marcus et al. (18) and modified as follows. Polystyrene microtiter plates (Nunc, Kamstrup, Denmark) were coated with 1.8 mM GQ1b (IsoSep AB, Tullinge, Sweden) or GM1 (Sigma, St Louis, Mo.) in ethanol or with ethanol alone. The plates were dried and then saturated for 2 h at room temperature with 1% fetal calf serum (FCS [Gibco, Paisley, Scotland]) in phosphate-buffered saline (pH 7.2) (PBS) containing 0.005% Tween 20 (FCS-PBST). Test serum was applied in serial twofold dilutions starting at 1:20 (for anti-GQ1b) or 1:10 (for anti-GM1) in FCS-PBST for an 18-h incubation at 4°C, including a positive standard serum as the internal reference on each plate. Washing with PBST was followed by incubation with peroxidase-conjugated rabbit anti-human IgA, IgG, or IgM (Dako, Glostrup, Denmark), diluted 1:500 in FCS-PBST, for 2 h at 4°C. Standardized peroxidase substrate and extinction (E) reading steps were performed as described previously (28).
Titers were expressed as ΔE (EGQ1b coat or EGM1 coat minus Eethanol coat) values. Coefficients of variation (CV) for interassay variance were determined in repetitive runs (n = 20) of the positive reference sera; CV for anti-GQ1b were 13.5% (IgA), 11.0% (IgG), and 15.7% (IgM); CV for anti-GM1 were 12.2% (IgA), 13.9% (IgG), and 11.6% (IgM). To minimize the influence of this variation, ΔE values obtained for test sera were corrected by being related to the reference ΔE on each plate. In addition, sequential samples for longitudinal monitoring were tested in the same ELISA run. Titers were calculated for a 1:20 (anti-GQ1b) or 1:10 (anti-GM1) serum dilution, and samples were considered positive when their mean titer (from at least duplicate independent assays) exceeded the cutoff, determined as the mean titer plus 2 standard deviations in groups of healthy controls. Serum anti-GQ1b cutoffs (1:20; 16 controls) were 0.17, 0.32, and 0.30 for IgA, IgG, and IgM, respectively, and serum anti-GM1 cutoffs (1:10; 20 controls) were 0.08, 0.25, and 0.18 for IgA, IgG, and IgM, respectively.
CSF samples were examined by using a 1:2 starting dilution for both anti-GQ1b and anti-GM1 antibodies. CSF cutoffs (1:2), determined in controls with other neurological disorders, were 0.08 (IgA), 0.14 (IgG), and 0.07 (IgM) for anti-GQ1b (10 controls) and 0.02 (IgA), 0.04 (IgG), and 0.02 (IgM) for anti-GM1 (30 controls).
IgG subclasses of antibodies against GQ1b.
The method outlined above was used to determine anti-GQ1b IgG subclasses in IgG-positive samples with dilutions starting between 1:2 and 1:10,000 (depending on the IgG titer). Peroxidase-conjugated monoclonal mouse anti-human IgG1, IgG2, IgG3, and IgG4 antibodies (Zymed, San Francisco, Calif.), diluted 1:1,000, were the second antibodies. The optimal dilution, sensitivity, and specificity of anti-IgG subclass conjugates were determined by ELISA with purified human kappa myeloma IgG1, IgG2, IgG3, and IgG4 (Sigma) as standards. Anti-IgG subclass antibodies at a 1:1,000 dilution detected less than 8 ng of each respective IgG subclass per well with virtually no cross-reactivity. Anti-GQ1b IgG subclass mean titers (duplicate assays) were expressed as ΔE values at a 1:2 sample dilution.
Statistical data analysis.
Comparison of anti-GQ1b titers between patient and control groups and assessment of significant differences were performed by the Mann-Whitney U test. The correlation between titers of different Ig classes or IgG subclasses was examined by linear regression analysis. Differences in anti-GQ1b and anti-GM1 frequencies among groups were tested for significance by Fisher’s exact test of probability. StatView II software was used for statistical calculations.
RESULTS
Anti-GQ1b and anti-GM1 antibodies in patients with MFS and GBS.
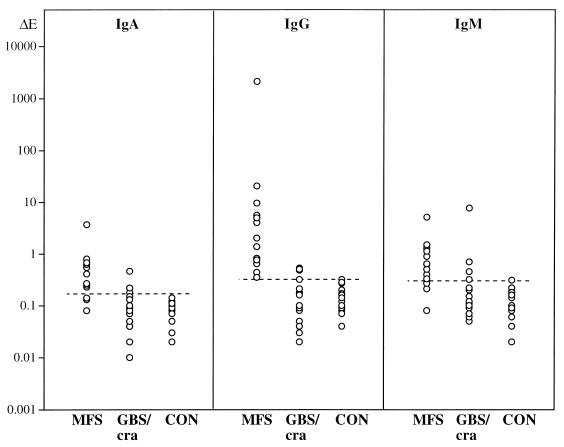
Serum IgA, IgG, and IgM titers against ganglioside GQ1b in MFS patients, GBS/cra patients, and healthy controls are presented in Fig. 1. MFS patients showed significantly higher anti-GQ1b titers of all Ig classes, predominantly IgG, than did controls (Mann-Whitney U test; P = 0.0001 for IgA, IgG, and IgM) or GBS/cra patients (P < 0.001 for IgA, P = 0.0001 for IgG, P < 0.01 for IgM). No significant differences in anti-GQ1b titers were detected between GBS/cra patients and controls.
FIG. 1.
IgA, IgG, and IgM anti-GQ1b titers (log10 scale) in serum of 13 MFS patients, 18 GBS/cra patients, and 16 healthy controls (CON). Each point represents one individual (earliest serum sample available, 1:20 dilution). Broken lines indicate the cutoffs (mean ΔE plus 2 standard deviations of control group) for IgA, IgG, and IgM, respectively.
Incidences of elevated serum antibody titers against gangliosides GQ1b and GM1 in patient groups and controls are compared in Table 2. Seropositivity for anti-GQ1b antibodies (IgA, IgG, and/or IgM) was demonstrated in 13 of 13 MFS patients (100%) (P < 0.0001, Fisher’s exact test) and in 8 of 18 GBS/cra patients (44%) (P < 0.05, Fisher’s exact test), versus 1 of 16 controls (6%). In contrast, frequencies of anti-GM1-seropositive cases were lower than those of anti-GQ1b-seropositive cases and were similar in the MFS (23%) and the GBS/cra (28%) patient groups but were not significantly different from controls (5%). However, the IgG class anti-GM1 titer was elevated in 4 of 18 GBS/cra patients versus 0 of 20 controls (P < 0.05, Fisher’s exact test), and in these 4 patients the GBS had followed gastrointestinal infections.
TABLE 2.
Incidence of elevated serum antibody titers against GQ1b and GM1
| Patient group | Incidence of elevated anti-GQ1b titera
|
Incidence of elevated anti-GM1 titera
|
||||||
|---|---|---|---|---|---|---|---|---|
| IgA | IgG | IgM | Totalb (%) | IgA | IgG | IgM | Totalb (%) | |
| MFS | 10**** | 13**** | 10*** | 13/13**** (100) | 2 | 1 | 1 | 3/13 (23) |
| GBS/cra | 3 | 3 | 4 | 8/18* (44) | 1 | 4* | 0 | 5/18 (28) |
| CONc | 0 | 0 | 1 | 1/16 (6) | 1 | 0 | 1 | 1/20 (5) |
The incidence of elevated titers is given as number of seropositive cases (above the respective cutoff), using the earliest serum samples available. Significant differences between patients and controls (Fisher’s exact test) are indicated as follows: ****, P < 0.0001; ***, P < 0.001; *, P < 0.05.
Total number of seropositive cases in any of the three Ig classes/total number of patients.
CON, healthy controls.
Anti-GQ1b Ig class and IgG subclass pattern in relation to preceding infection.
The Ig class and IgG subclass distributions of serum antibodies against GQ1b in MFS patients are given in Table 3. Overall, anti-GQ1b IgG was strongly associated with IgG3: there was a significant positive correlation between IgG and IgG3 titers (linear regression, r = 1, P = 0.0001); IgG3 was the predominating subclass in seven of eight MFS patients with mainly IgG antibodies (versus IgA and IgM), while IgG2 predominated in four of five patients with substantial IgA or IgM titers (P < 0.005, Fisher’s exact test).
The frequencies of predominant serum anti-GQ1b Ig classes and IgG subclasses in MFS patients following respiratory (MFS/resp) and gastrointestinal (MFS/gast) infections are compared in Table 4. IgG was the main anti-GQ1b Ig class in six of six MFS/resp patients, while IgA or IgM predominated over IgG in four of five MFS/gast patients (P < 0.02, Fisher’s exact test). The main anti-GQ1b IgG subclasses were IgG3 (five cases) or IgG1 (one case) in MFS/resp patients but IgG2 in four of five MFS/gast patients (P < 0.02, Fisher’s exact test). Anti-GQ1b IgG titers were higher in MFS/resp patients than MFS/gast patients (P = 0.0285, Mann-Whitney U test); however, differences between MFS/resp and MFS/gast patients with regard to anti-GQ1b IgA, IgM, and IgG subclass titers did not reach significance.
TABLE 4.
Incidence of predominant serum anti-GQ1b Ig classes and IgG subclasses in MFS patients
| Preceding infection | Incidence of anti-GQ1b Ig classa:
|
Incidence of anti-GQ1b IgG subclassa:
|
Total no. | ||||
|---|---|---|---|---|---|---|---|
| IgA | IgG | IgM | IgG1 | IgG2 | IgG3 | ||
| Respiratory | 0 | 6 | 0 | 1 | 0 | 5 | 6 |
| Gastrointestinal | 2 | 1 | 2 | 0 | 4 | 1 | 5 |
| Unknown | 1 | 1 | 0 | 1 | 0 | 1 | 2 |
| Total no. | 3 | 8 | 2 | 2 | 4 | 7 | 13 |
Incidence is given as number of patients with predominant anti-GQ1b Ig class or IgG subclass (Table 3).
Of the MFS patients after C. jejuni infections, two had anti-GQ1b IgA and IgM titers higher than IgG titers (patient 9, on day 9 after MFS onset, and patient 8, on day 18 [Table 3]); in one patient, IgG was the predominating Ig class by far (patient 7, on day 61). This pattern might be due to late serum sampling in patient 7 and to a disappearance of IgA or IgM earlier than IgG, thus closely resembling the immune response against C. jejuni antigens (6, 12, 15).
Three GBS/cra patients were seropositive for anti-GQ1b IgG (Table 2). Predominant IgG class and IgG2 or IgG3 subclass (one patient each) responses against GQ1b were observed in two GBS/cra patients with antecedent gastrointestinal infections; IgA predominated over IgG (IgG1) in one patient without known infection (results not shown). Summarizing data from MFS and GBS/cra patients for analysis of anti-GQ1b Ig classes or IgG subclasses with respect to infection did not significantly alter the results described above.
Anti-GQ1b and anti-GM1 antibodies during the course of MFS.
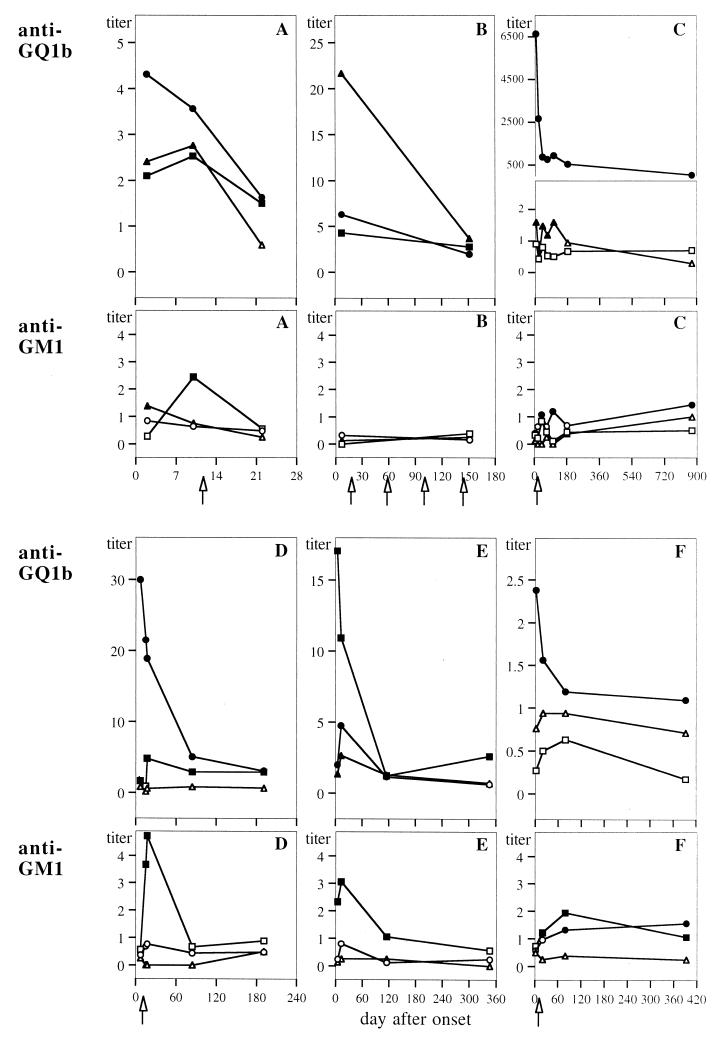
Antibodies against GQ1b and GM1 were monitored in sequential serum samples from six MFS patients over the course of disease, as demonstrated in Fig. 2. Anti-GQ1b IgA, IgG, and IgM titers decreased within 2 to 3 weeks after disease onset in all cases. Simultaneously, the patients showed a substantial clinical recovery of function without recurrence. However, five patients showed anti-GQ1b IgG and/or IgM titers still elevated above the cutoff level 5 to 29 months after onset (Fig. 2B to F).
FIG. 2.
Antibody titers against GQ1b and GM1 during the course of disease in sera of six MFS patients (A to F). Each point represents a sequential sample, diluted 1:20 and 1:10 for anti-GQ1b and anti-GM1, respectively. Titers of the IgA class (triangles), IgG class (circles), and IgM class (squares) are given as ΔE/cutoff ratios; values above the corresponding cutoff are given as solid symbols, and those below the cutoff as given as open symbols. Cases shown in panels A, B, C, D, E, and F correspond to patients 5, 13, 12, 2, 10, and 6, respectively (see Table 3). Arrows indicate the time points of treatment with intravenous Ig (B, C, D, and F) or plasmapheresis (A).
On the other hand, anti-GM1 titers increased during the course of MFS in five of six patients. Transient peaks of anti-GM1 IgM were seen around 2 weeks after onset in three patients (Fig. 2A, D, and E); IgM and IgG (Fig. 2F), and IgG only (Fig. 2C) elevated above the cutoff level were observed in two other patients. An anti-GM1 response may have been missed in the only negative patient (Fig. 2B), since no serum samples were obtained between days 7 and 151 after onset.
In the MFS patient with the highest serum anti-GQ1b IgG (IgG3) titer (Fig. 2C; patient 12 in Table 3) a high level of this antibody with the same subclass pattern was detected in CSF as well (results not shown). The anti-GQ1b IgG level in CSF was increased by a factor of 65 above cutoff; IgA and IgM were negative, as were anti-GM1 antibody titers. Calculation of anti-GQ1b IgG/total IgG as well as IgG/albumin ratios in CSF and serum suggested a peripheral origin rather than intrathecal synthesis of anti-GQ1b. Two of the three MFS patients tested did not show antibodies against GQ1b or GM1 in CSF, nor did the four GBS/cra patients.
DISCUSSION
Antibodies against ganglioside GQ1b were demonstrated in the serum of 100% of MFS patients and in the CSF of one patient with a remarkably high anti-GQ1b IgG titer in serum, thus confirming the very high incidence of anti-GQ1b antibodies in MFS (8, 33, 35, 36, 38). Antibodies against GQ1b were also observed in serum of 44% GBS/cra patients, which supports a role of anti-GQ1b in the pathogenesis of cranial nerve impairment (8, 22). The specificity of anti-GQ1b immunoreactivity is further substantiated by direct comparison to antibodies against GM1, which were found at considerably lower (and similar) frequencies among MFS and GBS/cra patients (23 and 28%, respectively). However, the anti-GM1 IgG titer was elevated in four GBS/cra patients with preceding gastrointestinal infections. These results agree very well with the reported incidence (20 to 30%) of anti-GM1 antibodies (13, 32) and with the association between anti-GM1 and enteritic infection in GBS (15, 23).
Anti-GQ1b titers (all Ig classes) decreased within 2 to 3 weeks after MFS onset in all six patients monitored over the course of disease, again indicating a specific pathogenetic involvement, as reported in earlier studies (7, 8, 36, 38). However, to our knowledge this is the first longitudinal study of MFS to examine antibody titers against GQ1b and GM1 in parallel. In contrast to the decrease in the anti-GQ1b titer, an increase in the anti-GM1 titer during the course of MFS was detected in five of six patients; this anti-GM1 response was predominantly IgM, with a transient peak around 2 weeks after disease onset. The appearance of antibodies against GM1 suggests a nonspecific immune response, possibly reflecting the release of GM1 as a major myelin ganglioside during the course of myelin destruction. That antibodies against gangliosides can be produced in response to nerve injury has been shown in an experimental system (27). Such a process of autoantibody formation secondary to tissue damage may make a considerable contribution to the frequent occurrence of anti-GM1 antibodies, particularly IgM, in many different types of neuropathy (15, 34). To what extent this might concern antibodies against GM1 in GBS, apart from their association with C. jejuni infection, remains an open question. Interestingly, early peaks in anti-GM1 IgM titers followed by a gradual decline were observed in a recent longitudinal study on GBS patients (4), thus supporting our results.
Ig classes of serum antibodies against GQ1b in MFS patients have been reported to be IgG more than IgM (8, 33, 38) and IgA (36); however, antecedent infections were either respiratory or not identified, and different types of infection were not compared. In our study, anti-GQ1b Ig class patterns were significantly different in MFS/resp and MFS/gast patients, although the number of patients studied was relatively small. MFS/resp patients showed higher anti-GQ1b IgG titers, and IgG predominated over IgA and IgM (six of six patients); in MFS/gast patients, IgA and IgM were the predominant anti-GQ1b Ig classes (four of five patients). The frequent presence of IgA class anti-GQ1b antibodies in MFS/gast patients, similar to IgA anti-GM1 in GBS patients after intestinal infection (15, 29–31), further strengthens the link between antibodies and enteritic processes.
IgG1 and IgG3 subclasses of anti-GQ1b have been observed in MFS patients; however, antecedent infections were not reported (36). Our study comparing MFS/resp and MFS/gast patients demonstrated anti-GQ1b IgG subclass patterns different between the two. The major subclasses were IgG3 (five of six) or IgG1 (one of six) in MFS/resp patients and IgG2 (four of five) or IgG3 (one of five) in MFS/gast patients. In addition, significant associations between predominant anti-GQ1b IgG and the IgG3 subclass, as well as between substantial IgA or IgM (versus IgG) and the IgG2 subclass, were detected. These are new findings, at variance with previous reports on IgG subclasses of anti-GQ1b antibodies in MFS (36) and of anti-GM1 antibodies in GBS (9, 21, 41). A possible explanation is that IgG subclasses against gangliosides have apparently not been examined in patients with considerable IgA and/or IgM responses until now.
Although causative agents were not positively identified, apart from in three patients with C. jejuni enteritis, the novel results of the present study revealed distinct distributions of Ig against GQ1b in MFS/resp and MFS/gast patients. This difference appears relevant to the question of anti-GQ1b origin and production mechanism (13, 32). The prominent IgG class and IgG3 or IgG1 subclass response against GQ1b in MFS/resp patients supports the conclusion that antibody generation is dependent on T-cell help, possibly induced by a glycoprotein antigen or mediated via noncognate mechanisms (36). On the other hand, the predominant anti-GQ1b IgG2 response in MFS/gast patients may favor T-cell-independent antibody formation, typically stimulated by carbohydrate antigens such as glycolipids or bacterial LPS (16). Consequently, the distinct anti-GQ1b Ig patterns might relate to carbohydrate epitopes in the context of different antigens expressed by infectious agents. Thus, Ig classes and IgG subclasses of antibodies against GQ1b may reflect the immune response initiated by the particular viruses or bacteria which precipitate the disease (10, 11, 24). LPS from C. jejuni have been suggested as candidate antigens for triggering the induction of antibodies against gangliosides through the mechanism of molecular mimicry by oligosaccharide epitopes (2, 3, 26, 39). With respect to MFS, this possibility has been supported by antibody cross-reactivity between GQ1b and LPS from MFS-linked C. jejuni serotypes, demonstrated with monoclonal anti-GQ1b antibodies (40) as well as with MFS patient antisera in our earlier study (20). The distinct patterns of antibody against GQ1b in MFS/resp and MFS/gast patients may argue in favor of a causal relationship between infectious illness and neurological disease via the priming of an anti-GQ1b immune response.
ACKNOWLEDGMENTS
This work was supported by grants from the Austrian Science Research Fund (project P 09506-MED), and the Austrian National Bank Fund (project 5757).
We thank Silvia Zimmermann for excellent technical assistance. We are particularly grateful to Maria Storch for valuable discussions.
REFERENCES
- 1.Asbury A K, Cornblath D R. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27(Suppl.):S21–S24. doi: 10.1002/ana.410270707. [DOI] [PubMed] [Google Scholar]
- 2.Aspinall G O, Fujimoto S, McDonald A G, Pang H, Kurjanczyk L A, Penner J L. Lipopolysaccharides from Campylobacter jejuni associated with Guillain-Barré syndrome patients mimic human gangliosides in structure. Infect Immun. 1994;62:2122–2125. doi: 10.1128/iai.62.5.2122-2125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aspinall G O, McDonald A G, Pang H, Kurjanczyk L A, Penner J L. Lipopolysaccharides of Campylobacter jejuni serotype O:19: structures of core oligosaccharide regions from the serostrain and two bacterial isolates from patients with the Guillain-Barré syndrome. Biochemistry. 1994;33:241–249. doi: 10.1021/bi00167a032. [DOI] [PubMed] [Google Scholar]
- 4.Bech E, Orntoft T F, Andersen L P, Skinhoj P, Jakobsen J. IgM anti-GM1 antibodies in the Guillain-Barré syndrome: a serological predictor of the clinical course. J Neuroimmunol. 1997;72:59–66. doi: 10.1016/s0165-5728(96)00145-2. [DOI] [PubMed] [Google Scholar]
- 5.Berlit P, Rakicky J. The Miller Fisher syndrome: review of the literature. J Clin Neuro-Ophthalmol. 1992;12:57–63. [PubMed] [Google Scholar]
- 6.Blaser M J, Duncan D J. Human serum antibody response to Campylobacter jejuni infection as measured in an enzyme-linked immunosorbent assay. Infect Immun. 1984;44:292–298. doi: 10.1128/iai.44.2.292-298.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiba A, Kusunoki S, Shimizu T, Kanazawa I. Serum IgG antibody to ganglioside GQ1b is a possible marker of Miller Fisher syndrome. Ann Neurol. 1992;31:677–679. doi: 10.1002/ana.410310619. [DOI] [PubMed] [Google Scholar]
- 8.Chiba A, Kusunoki S, Obata H, Machinami R, Kanazawa I. Serum anti-GQ1b IgG antibody is associated with ophthalmoplegia in Miller Fisher syndrome and Guillain-Barré syndrome: clinical and immunohistochemical studies. Neurology. 1993;43:1911–1917. doi: 10.1212/wnl.43.10.1911. [DOI] [PubMed] [Google Scholar]
- 9.Deisenhammer F, Keir G, Pfausler B, Thompson E J. Affinity of anti-GM1 antibodies in Guillain-Barré syndrome patients. J Neuroimmunol. 1996;66:85–93. doi: 10.1016/0165-5728(96)00029-x. [DOI] [PubMed] [Google Scholar]
- 10.Dowling P C, Cook S D. Role of infection in Guillain-Barré syndrome: laboratory confirmation of herpesviruses in 41 cases. Ann Neurol. 1981;9(Suppl.):44–55. doi: 10.1002/ana.410090709. [DOI] [PubMed] [Google Scholar]
- 11.Goldschmidt B, Menonna J, Fortunato J, Dowling P, Cook S. Mycoplasma antibody in Guillain-Barré syndrome and other neurological diseases. Ann Neurol. 1980;7:108–112. doi: 10.1002/ana.410070203. [DOI] [PubMed] [Google Scholar]
- 12.Griffin J W, Ho T W H. The Guillain-Barré syndrome at 75: the Campylobacter connection. Ann Neurol. 1993;34:125–127. doi: 10.1002/ana.410340204. [DOI] [PubMed] [Google Scholar]
- 13.Hartung H-P, Toyka K V, Griffin J W. Guillain-Barré syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. In: Antel J, Birnbaum G, Hartung H-P, editors. Clinical neuroimmunology. Malden, Mass: Blackwell Science; 1998. pp. 294–306. [Google Scholar]
- 14.Hughes R A C. The Guillain-Barré syndrome. London, United Kingdom: Springer-Verlag; 1990. [Google Scholar]
- 15.Jacobs B C, van Doorn P A, Schmitz P I M, Tio-Gillen A P, Herbrink P, Visser L H, Hooijkaas H, van der Meché F G A. Campylobacter jejuni infections and anti-GM1 antibodies in Guillain-Barré syndrome. Ann Neurol. 1996;40:181–187. doi: 10.1002/ana.410400209. [DOI] [PubMed] [Google Scholar]
- 16.Klaus G G B. B cell activation: induction of the primary immune response, 21–39. In: Male D, editor. B-lymphocytes. Oxford, United Kingdom: Oxford University Press; 1990. [Google Scholar]
- 17.Kuroki S, Saida T, Nukina M, Haruta T, Yoshioka M, Kobayashi Y, Nakanishi H. Campylobacter jejuni strains from patients with Guillain-Barré syndrome belong mostly to Penner serogroup 19 and contain β-N-acetylglucosamine residues. Ann Neurol. 1993;33:243–247. doi: 10.1002/ana.410330304. [DOI] [PubMed] [Google Scholar]
- 18.Marcus D M, Latov N, Hsi B P, Gillard B K participating laboratories. Measurement and significance of antibodies against GM1 ganglioside. J Neuroimmunol. 1989;25:255–259. doi: 10.1016/0165-5728(89)90144-6. [DOI] [PubMed] [Google Scholar]
- 19.Mizoguchi K, Hase A, Obi T, Matsuoka H, Takatsu M, Nishimura Y, Irie F, Seyama Y, Hirabayashi Y. Two species of antiganglioside antibodies in a patient with a pharyngeal-cervical-brachial variant of Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 1994;57:1121–1123. doi: 10.1136/jnnp.57.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neisser A, Bernheimer H, Berger T, Moran A P, Schwerer B. Serum antibodies against gangliosides and Campylobacter jejuni lipopolysaccharides in Miller Fisher syndrome. Infect Immun. 1997;65:4038–4042. doi: 10.1128/iai.65.10.4038-4042.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogino M, Nobile-Orazio E, Latov N. IgG anti-GM1 antibodies from patients with acute motor neuropathy are predominantly of the IgG1 and IgG3 subclasses. J Neuroimmunol. 1995;58:77–80. doi: 10.1016/0165-5728(94)00190-y. [DOI] [PubMed] [Google Scholar]
- 22.O’Leary C P, Veitch J, Durward W F, Thomas A M, Rees J H, Willison H J. Acute oropharyngeal palsy is associated with antibodies to GQ1b and GT1a gangliosides. J Neurol Neurosurg Psychiatry. 1996;61:649–651. doi: 10.1136/jnnp.61.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rees J H, Gregson N A, Hughes R A C. Anti-ganglioside GM1 antibodies in Guillain-Barré syndrome and their relationship to Campylobacter jejuni infection. Ann Neurol. 1995;38:809–816. doi: 10.1002/ana.410380516. [DOI] [PubMed] [Google Scholar]
- 24.Rees J H, Soudain S E, Gregson N A, Hughes R A C. Campylobacter jejuni infection and Guillain-Barré syndrome. N Engl J Med. 1995;333:1374–1379. doi: 10.1056/NEJM199511233332102. [DOI] [PubMed] [Google Scholar]
- 25.Ropper A H, Wijdicks E F M, Truax B T. Guillain-Barré syndrome. Philadelphia, Pa: F. A. Davis Co.; 1991. pp. 18–21. [Google Scholar]
- 26.Salloway S, Mermel L A, Seamans M, Aspinall G O, Nam Shin J E, Kurjanczyk L A, Penner J L. Miller-Fisher syndrome associated with Campylobacter jejuni bearing lipopolysaccharide molecules that mimic human ganglioside GD3. Infect Immun. 1996;64:2945–2949. doi: 10.1128/iai.64.8.2945-2949.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz M, Sela B A, Eshhar N. Antibodies to gangliosides and myelin autoantigens are produced in mice following sciatic nerve injury. J Neurochem. 1982;38:1192–1195. doi: 10.1111/j.1471-4159.1982.tb07890.x. [DOI] [PubMed] [Google Scholar]
- 28.Schwerer B, Lassmann H, Kitz K, Bernheimer H. Ganglioside GM1, a molecular target for immunological and toxic attacks: similarity of neuropathological lesions induced by ganglioside-antiserum and cholera toxin. Acta Neuropathol (Berlin) 1986;72:55–61. doi: 10.1007/BF00687947. [DOI] [PubMed] [Google Scholar]
- 29.Schwerer B, Neisser A, Polt R J, Bernheimer H, Moran A P. Antibody cross-reactivities between gangliosides and lipopolysaccharides of Campylobacter jejuni serotypes associated with Guillain-Barré syndrome. J Endotoxin Res. 1995;2:395–403. [Google Scholar]
- 30.van den Berg L H, Marrink J, de Jager A E J, de Jong H J, van Imhoff G W, Latov N, Sadiq S A. Anti-GM1 antibodies in patients with Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 1992;55:8–11. doi: 10.1136/jnnp.55.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Wulffen H, Hartard C, Scharein E. Seroreactivity to Campylobacter jejuni and gangliosides in patients with Guillain-Barré syndrome. J Infect Dis. 1994;170:828–833. doi: 10.1093/infdis/170.4.828. [DOI] [PubMed] [Google Scholar]
- 32.Willison H J, Kennedy P G E. Gangliosides and bacterial toxins in Guillain-Barré syndrome. J Neuroimmunol. 1993;46:105–112. doi: 10.1016/0165-5728(93)90239-u. [DOI] [PubMed] [Google Scholar]
- 33.Willison H J, Veitch J, Paterson G, Kennedy P G E. Miller Fisher syndrome is associated with serum antibodies to GQ1b ganglioside. J Neurol Neurosurg Psychiatry. 1993;56:204–206. doi: 10.1136/jnnp.56.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willison H J. Antiglycolipid antibodies in peripheral neuropathy: fact or fiction? J Neurol Neurosurg Psychiatry. 1994;57:1303–1307. doi: 10.1136/jnnp.57.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willison H J, Almemar A, Veitch J, Thrush D. Acute ataxic neuropathy with cross-reactive antibodies to GD1b and GD3 gangliosides. Neurology. 1994;44:2395–2397. doi: 10.1212/wnl.44.12.2395. [DOI] [PubMed] [Google Scholar]
- 36.Willison H J, Veitch J. Immunoglobulin subclass distribution and binding characteristics of anti-GQ1b antibodies in Miller Fisher syndrome. J Neuroimmunol. 1994;50:159–165. doi: 10.1016/0165-5728(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 37.Yuki N, Sato S, Tsuji S, Hozumi I, Miyatake T. An immunologic abnormality common to Bickerstaff’s brain stem encephalitis and Fisher’s syndrome. J Neurol Sci. 1993;118:83–87. doi: 10.1016/0022-510x(93)90250-3. [DOI] [PubMed] [Google Scholar]
- 38.Yuki N, Sato S, Tsuji S, Ohsawa T, Miyatake T. Frequent presence of anti-GQ1b antibody in Fisher’s syndrome. Neurology. 1993;43:414–417. doi: 10.1212/wnl.43.2.414. [DOI] [PubMed] [Google Scholar]
- 39.Yuki N, Taki T, Inagaki F, Kasama T, Takahashi M, Saito K, Handa S, Miyatake T. A bacterium lipopolysaccharide that elicits Guillain-Barré syndrome has a GM1 ganglioside-like structure. J Exp Med. 1993;178:1771–1775. doi: 10.1084/jem.178.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuki N, Taki T, Takahashi M, Saito K, Yoshino H, Tai T, Handa S, Miyatake T. Molecular mimicry between GQ1b ganglioside and lipopolysaccharides of Campylobacter jejuni isolated from patients with Fisher’s syndrome. Ann Neurol. 1994;36:791–793. doi: 10.1002/ana.410360517. [DOI] [PubMed] [Google Scholar]
- 41.Yuki N, Ichihashi Y, Taki T. Subclass of IgG antibody to GM1 epitope-bearing lipopolysaccharide of Campylobacter jejuni in patients with Guillain-Barré syndrome. J Neuroimmunol. 1995;60:161–164. doi: 10.1016/0165-5728(95)00052-4. [DOI] [PubMed] [Google Scholar]
- 42.Yuki N, Takahashi M, Tagawa Y, Kashiwase K, Tadokoro K, Saito K. Association of Campylobacter jejuni serotype with antiganglioside antibody in Guillain-Barré syndrome and Fisher’s syndrome. Ann Neurol. 1997;42:28–33. doi: 10.1002/ana.410420107. [DOI] [PubMed] [Google Scholar]