Abstract
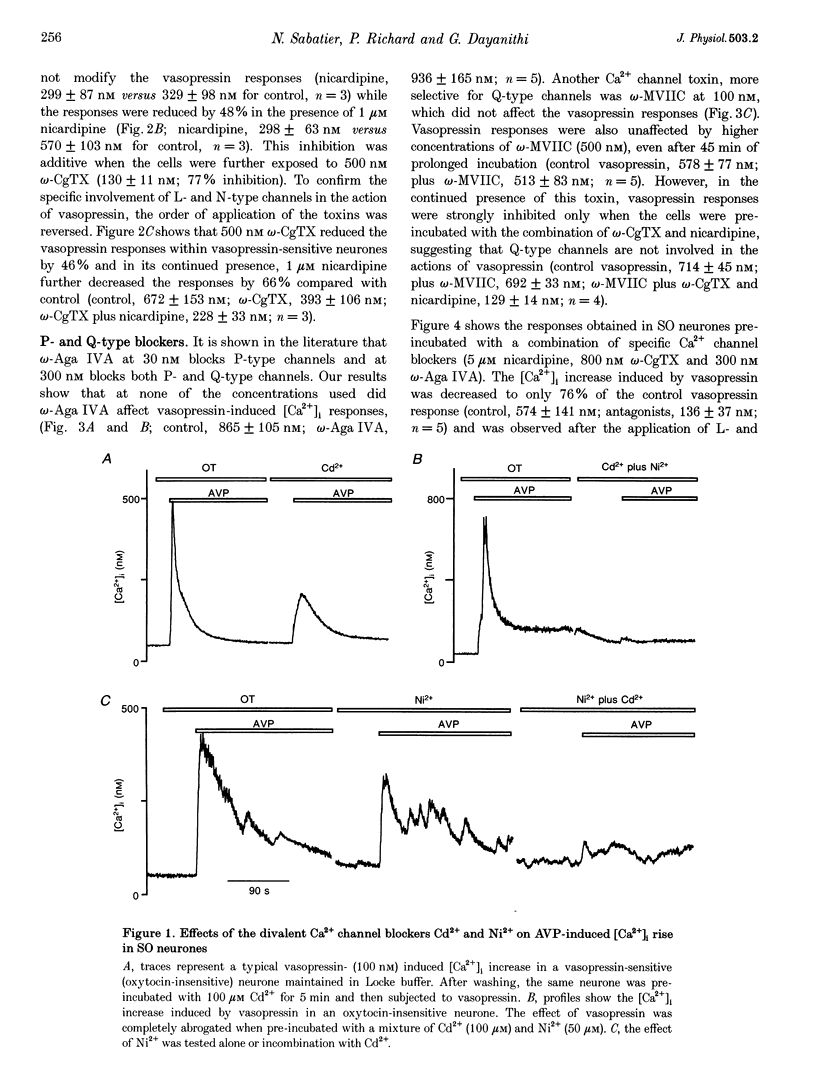
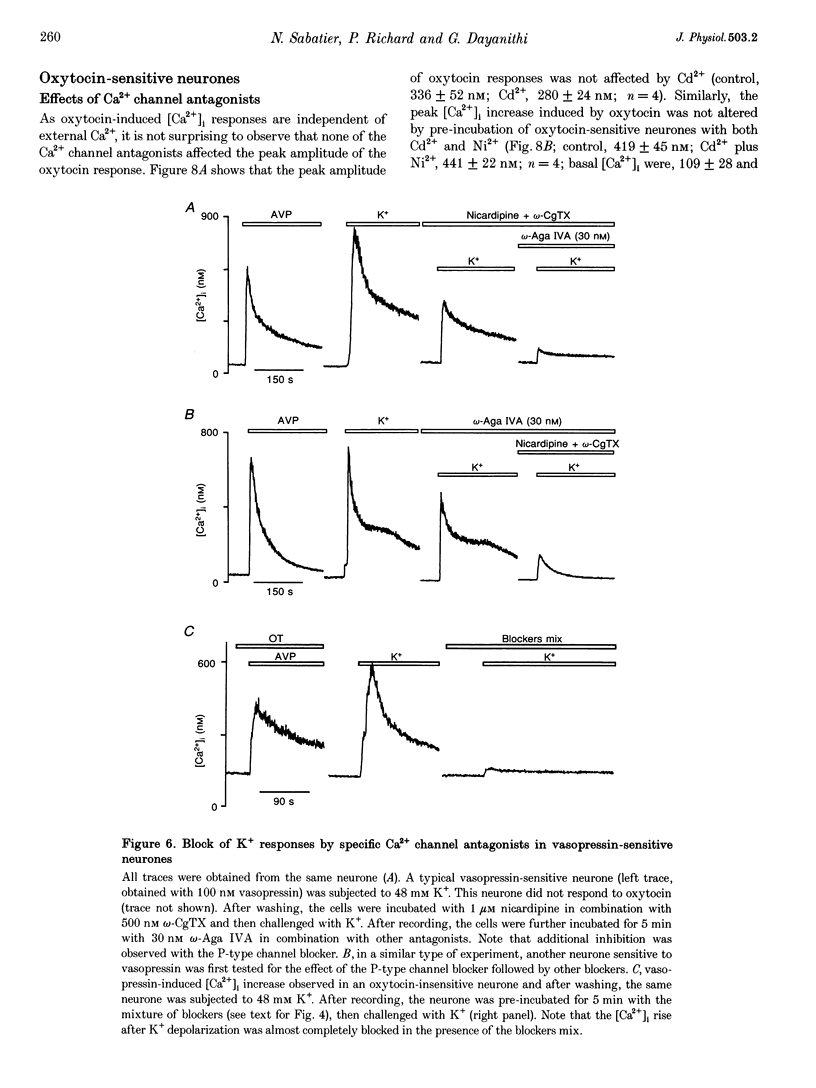
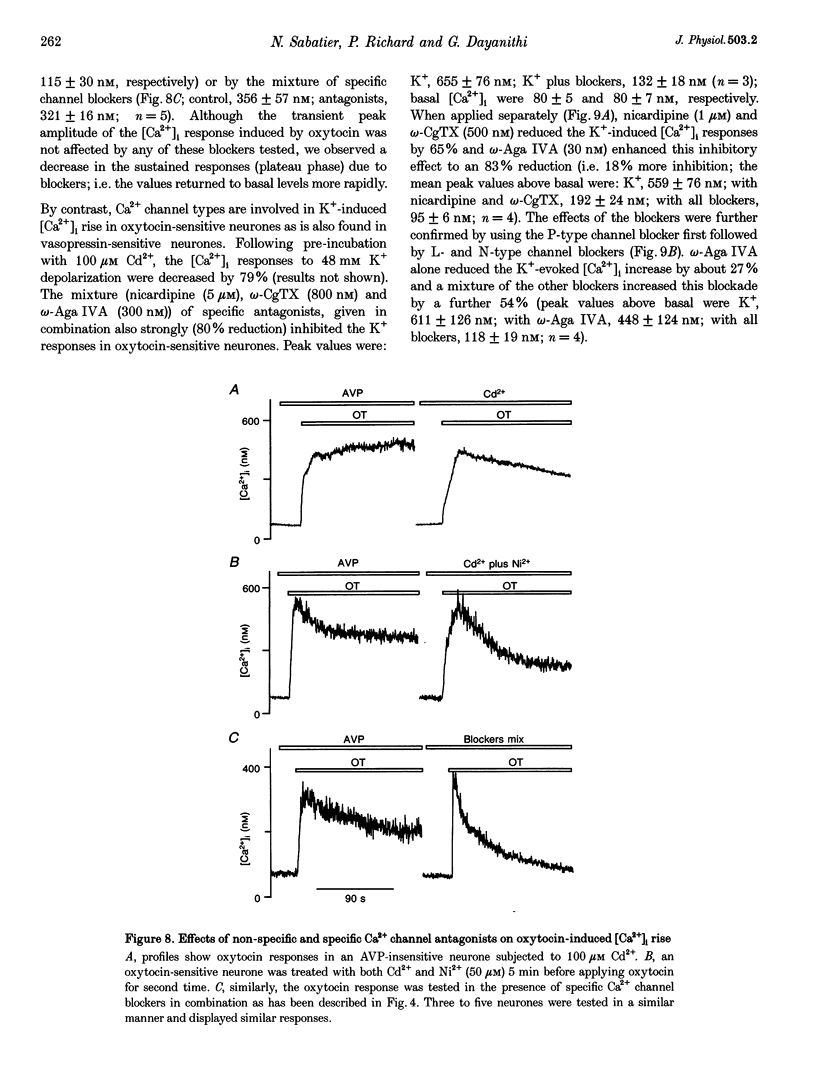
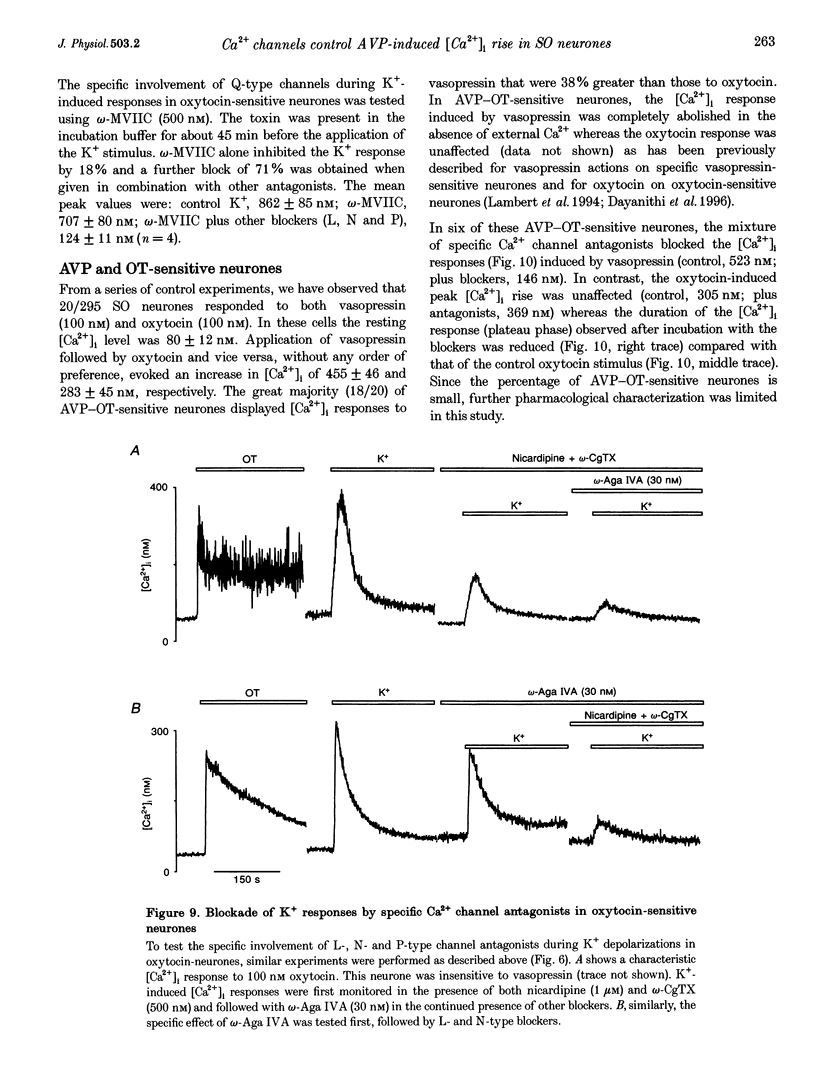
1. The role of voltage-dependent Ca2+ channels during vasopressin and oxytocin actions on their respective neurones has been analysed by measuring intracellular Ca2+ concentration ([Ca2+]i) in individual, freshly dissociated magnocellular neurones from rat supraoptic nucleus (SO) using microspectrofluorimetry. 2. Pre-incubation of vasopressin-sensitive neurones with Cd2+ (100 microM), a non-discriminatory high-voltage-activated Ca2+ channel antagonist, or Ni2+ (50 microM), a blocker of T-type Ca2+ current, reduced [Ca2+]i responses by 77 and 19%, respectively. When Cd2+ was given together with Ni2+, the response was blocked by 92%. Similarly, when Ni2+ was pre-incubated with Cd2+, the response was blocked by approximately 84%. 3. Exposure of vasopressin sensitive neurones to a specific Ca2+ channel blocker, nicardipine (L-type) reduced vasopressin responses by 48% at 1 microM and 62% at 5 microM. Similarly, omega-conotoxin GVIA (omega-CgTX, N-type; 500 nM) inhibited the response by 46% with a stronger inhibition (75%) at 800 nM. By contrast, neither omega-agatoxin IVA (omega-Aga IVA; 300 nM), which blocks both P- and Q-type channels, nor synthetic omega-conotoxin MVIIC (omega-MVIIC; 100 or 500 nM), a Q-type blocker, affected vasopressin-induced [Ca2+]i responses. These antagonists, given together (nicardipine 5 microM + omega-CgTX 800 nM + omega-Aga IVA 300 nM), decreased vasopressin-induced [Ca2+]i responses by 76%. 4. In vasopressin-sensitive neurones, the presence of both nicardipine and omega-CgTX, reduced the K(+)-evoked [Ca2+]i increase by 61%. This blockade was increased by a further 21% with omega-Aga IVA, suggesting that N-, L- and P-type channels contribute to the depolarization-induced [Ca2+]i rise. In addition, omega-MVIIC alone reduced the K(+)-evoked [Ca2+]i release by 24%. Also the remaining K+ responses were further reduced by 60% when pre-incubated with L-N- and P-type blockers, suggesting the involvement of Q-type channels. 5. In oxytocin-sensitive neurones, the peak amplitude of the [Ca2+]i response was not affected by Cd2+ alone, by combined Cd2+ and Ni2+, or by the mixture of nicardipine, omega-CgTX and omega-Aga IVA. By contrast, the responses evoked by depolarization with K+ were blocked by Cd2+. Both nicardipine and omega-CgTX blocked 65% of K+ response and an additional block of approximately 18% was obtained with omega-Aga IVA, suggesting the involvement of L-, N- and P-type channels. In combination, these antagonists strongly inhibited (approximately 80% reduction) the K+ responses. Further reduction to 18% was made by the Q-type blocker omega-MVIIC. Pre-incubation with L-, N- and P-type blockers caused an additional block of 71%. 6. Some supraoptic neurones (5-10%) responded to both vasopressin and oxytocin, with only the [Ca2+]i responses induced by vasopressin blocked (> 90% inhibition) by the mixture of Ca2+ channel antagonists. 7. In conclusion, both vasopressin and oxytocin magnocellular SO neurones have been shown to express T-, L-, N-, P-, Q- and R-type Ca2+ channels in their somata. Our results show that the vasopressin-induced [Ca2+]i increase in vasopressin-sensitive neurones is mediated by L-, N- and T-type Ca2+ channels and not by P- and Q-type channels; Ca2+ channels are not involved in oxytocin action on oxytocin-sensitive neurones and L-, N-, P- and Q-type channels control the K(+)-induced [Ca2+]i increase in SO neurones.
Full text
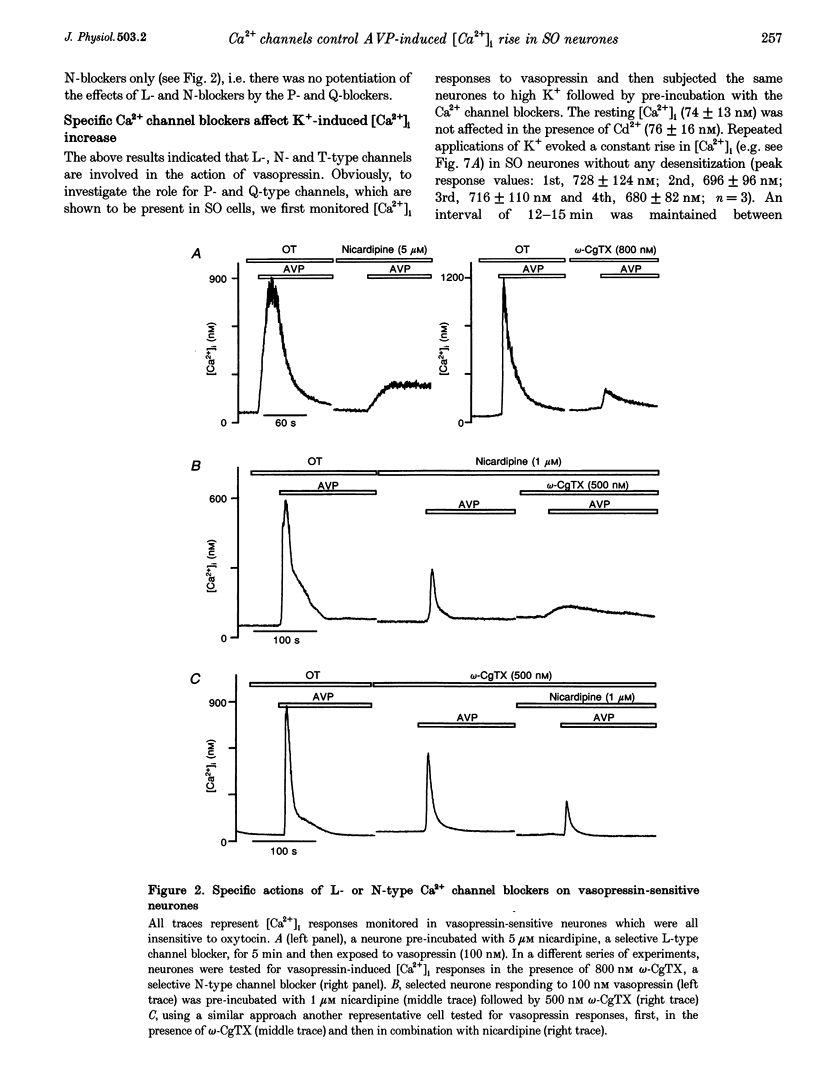
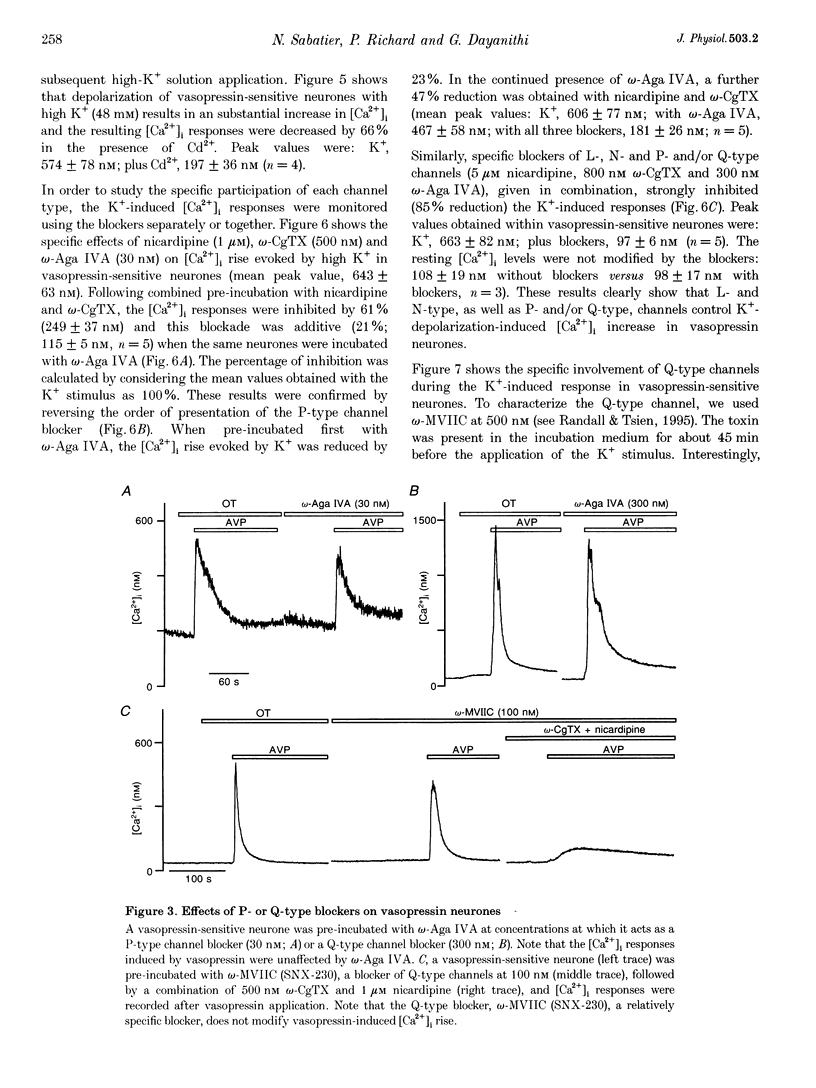
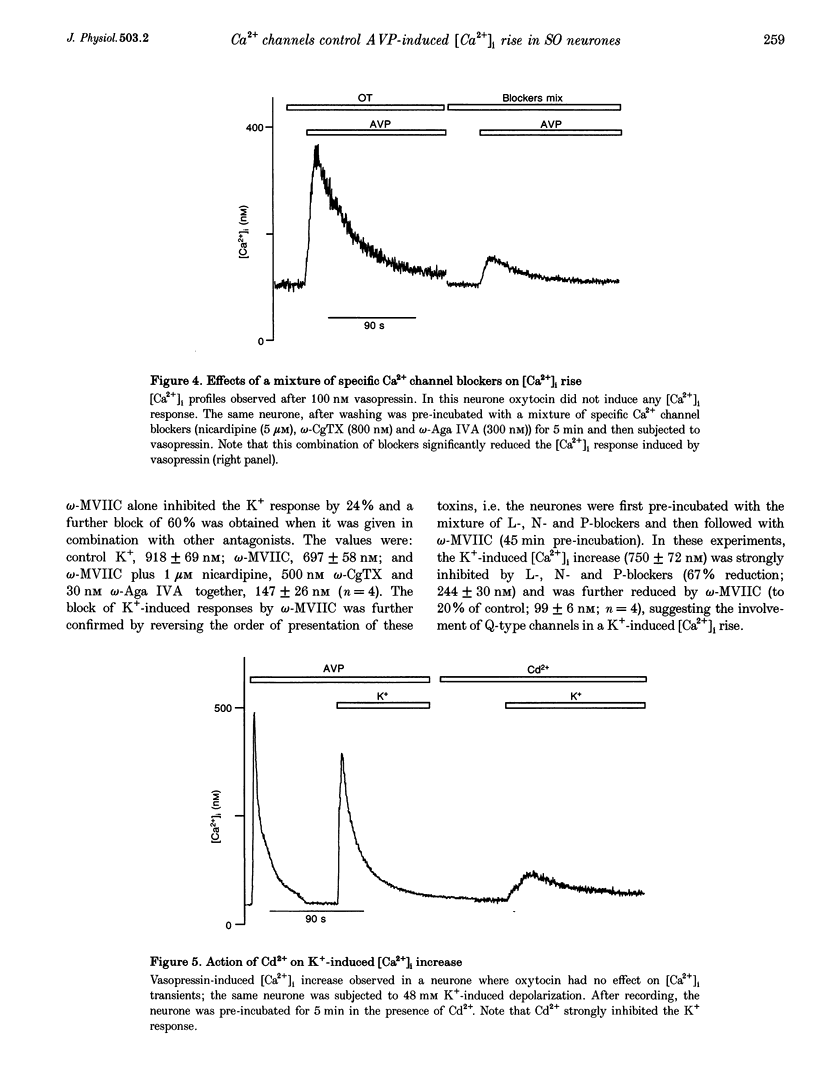
PDF


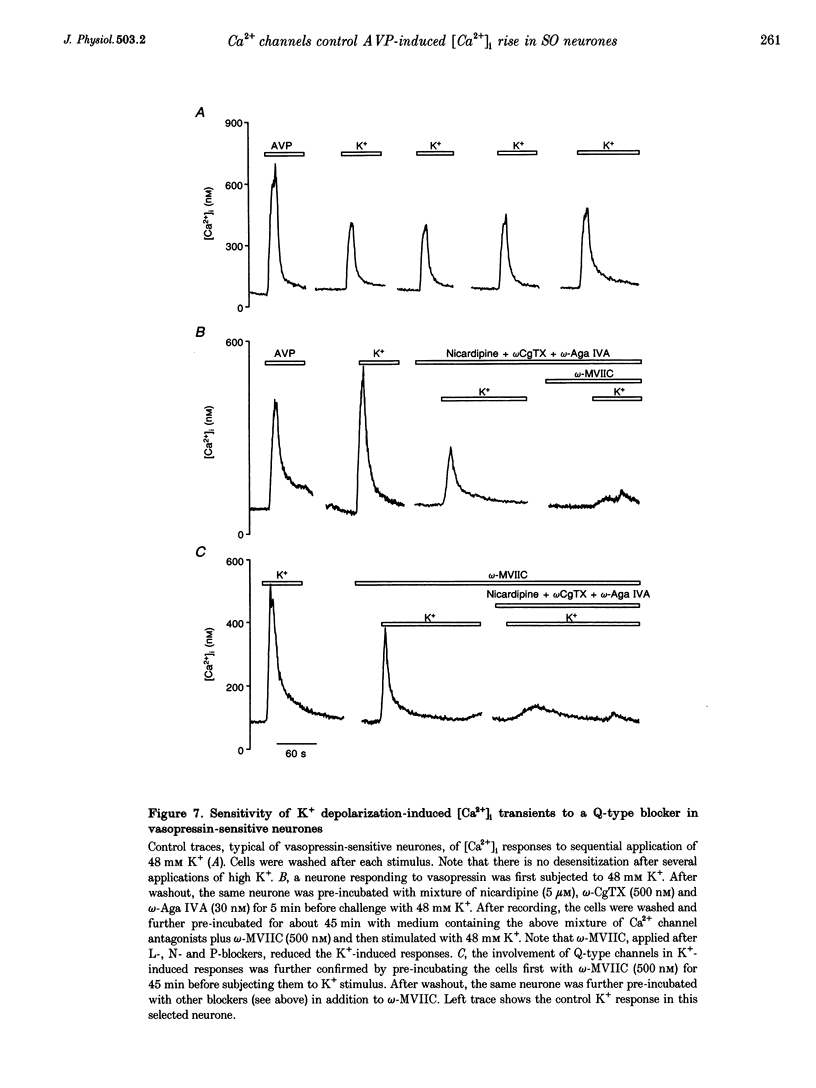




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe H., Inoue M., Matsuo T., Ogata N. The effects of vasopressin on electrical activity in the guinea-pig supraoptic nucleus in vitro. J Physiol. 1983 Apr;337:665–685. doi: 10.1113/jphysiol.1983.sp014648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew R. D., Dudek F. E. Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells. J Neurophysiol. 1984 Mar;51(3):552–566. doi: 10.1152/jn.1984.51.3.552. [DOI] [PubMed] [Google Scholar]
- Armstrong W. E., Smith B. N., Tian M. Electrophysiological characteristics of immunochemically identified rat oxytocin and vasopressin neurones in vitro. J Physiol. 1994 Feb 15;475(1):115–128. doi: 10.1113/jphysiol.1994.sp020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque C. W., Randle J. C., Renaud L. P. Calcium-dependent potassium conductance in rat supraoptic nucleus neurosecretory neurons. J Neurophysiol. 1985 Dec;54(6):1375–1382. doi: 10.1152/jn.1985.54.6.1375. [DOI] [PubMed] [Google Scholar]
- Brinton R. D., Gonzalez T. M., Cheung W. S. Vasopressin-induced calcium signaling in cultured hippocampal neurons. Brain Res. 1994 Oct 24;661(1-2):274–282. doi: 10.1016/0006-8993(94)91194-0. [DOI] [PubMed] [Google Scholar]
- Chen C., Díaz Brinton R. D., Shors T. J., Thompson R. F. Vasopressin induction of long-lasting potentiation of synaptic transmission in the dentate gyrus. Hippocampus. 1993 Apr;3(2):193–203. doi: 10.1002/hipo.450030211. [DOI] [PubMed] [Google Scholar]
- Dayanithi G., Martin-Moutot N., Barlier S., Colin D. A., Kretz-Zaepfel M., Couraud F., Nordmann J. J. The calcium channel antagonist omega-conotoxin inhibits secretion from peptidergic nerve terminals. Biochem Biophys Res Commun. 1988 Oct 14;156(1):255–262. doi: 10.1016/s0006-291x(88)80833-7. [DOI] [PubMed] [Google Scholar]
- Dayanithi G., Widmer H., Richard P. Vasopressin-induced intracellular Ca2+ increase in isolated rat supraoptic cells. J Physiol. 1996 Feb 1;490(Pt 3):713–727. doi: 10.1113/jphysiol.1996.sp021180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher T. E., Bourque C. W. Calcium-channel subtypes in the somata and axon terminals of magnocellular neurosecretory cells. Trends Neurosci. 1996 Oct;19(10):440–444. doi: 10.1016/0166-2236(96)10034-5. [DOI] [PubMed] [Google Scholar]
- Fisher T. E., Bourque C. W. Distinct omega-agatoxin-sensitive calcium currents in somata and axon terminals of rat supraoptic neurones. J Physiol. 1995 Dec 1;489(Pt 2):383–388. doi: 10.1113/jphysiol.1995.sp021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher T. E., Bourque C. W. Voltage-gated calcium currents in the magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 1995 Aug 1;486(Pt 3):571–580. doi: 10.1113/jphysiol.1995.sp020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foehring R. C., Armstrong W. E. Pharmacological dissection of high-voltage-activated Ca2+ current types in acutely dissociated rat supraoptic magnocellular neurons. J Neurophysiol. 1996 Aug;76(2):977–983. doi: 10.1152/jn.1996.76.2.977. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund-Mercier M. J., Richard P. Electrophysiological evidence for facilitatory control of oxytocin neurones by oxytocin during suckling in the rat. J Physiol. 1984 Jul;352:447–466. doi: 10.1113/jphysiol.1984.sp015302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund-Mercier M. J., Richard P. Excitatory effects of intraventricular injections of oxytocin on the milk ejection reflex in the rat. Neurosci Lett. 1981 May 6;23(2):193–198. doi: 10.1016/0304-3940(81)90039-2. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Inenaga K., Yamashita H. Excitation of neurones in the rat paraventricular nucleus in vitro by vasopressin and oxytocin. J Physiol. 1986 Jan;370:165–180. doi: 10.1113/jphysiol.1986.sp015928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick K., Bourque C. W. Activity dependence and functional role of the apamin-sensitive K+ current in rat supraoptic neurones in vitro. J Physiol. 1996 Jul 15;494(Pt 2):389–398. doi: 10.1113/jphysiol.1996.sp021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert R. C., Dayanithi G., Moos F. C., Richard P. A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. J Physiol. 1994 Jul 15;478(Pt 2):275–287. doi: 10.1113/jphysiol.1994.sp020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos J. R., Nowycky M. C. Two types of calcium channels coexist in peptide-releasing vertebrate nerve terminals. Neuron. 1989 May;2(5):1419–1426. doi: 10.1016/0896-6273(89)90187-6. [DOI] [PubMed] [Google Scholar]
- Leng G., Mason W. T. Influence of vasopressin upon firing patterns of supraoptic neurons: a comparison of normal and Brattleboro rats. Ann N Y Acad Sci. 1982;394:153–158. doi: 10.1111/j.1749-6632.1982.tb37422.x. [DOI] [PubMed] [Google Scholar]
- Ludwig M., Callahan M. F., Morris M. Effects of tetrodotoxin on osmotically stimulated central and peripheral vasopressin and oxytocin release. Neuroendocrinology. 1995 Dec;62(6):619–627. doi: 10.1159/000127058. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Leng G. Complex action potential waveform recorded from supraoptic and paraventricular neurones of the rat: evidence for sodium and calcium spike components at different membrane sites. Exp Brain Res. 1984;56(1):135–143. doi: 10.1007/BF00237449. [DOI] [PubMed] [Google Scholar]
- Mezey E., Kiss J. Z. Coexpression of vasopressin and oxytocin in hypothalamic supraoptic neurons of lactating rats. Endocrinology. 1991 Oct;129(4):1814–1820. doi: 10.1210/endo-129-4-1814. [DOI] [PubMed] [Google Scholar]
- Moos F. C., Ingram C. D. Electrical recordings of magnocellular supraoptic and paraventricular neurons displaying both oxytocin- and vasopressin-related activity. Brain Res. 1995 Jan 16;669(2):309–314. doi: 10.1016/0006-8993(94)01296-t. [DOI] [PubMed] [Google Scholar]
- Moos F., Freund-Mercier M. J., Guerné Y., Guerné J. M., Stoeckel M. E., Richard P. Release of oxytocin and vasopressin by magnocellular nuclei in vitro: specific facilitatory effect of oxytocin on its own release. J Endocrinol. 1984 Jul;102(1):63–72. doi: 10.1677/joe.0.1020063. [DOI] [PubMed] [Google Scholar]
- Oliet S. H., Bourque C. W. Properties of supraoptic magnocellular neurones isolated from the adult rat. J Physiol. 1992 Sep;455:291–306. doi: 10.1113/jphysiol.1992.sp019302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Poulain P., Carette B. Low-threshold calcium spikes in hypothalamic neurons recorded near the paraventricular nucleus in vitro. Brain Res Bull. 1987 Oct;19(4):453–460. doi: 10.1016/0361-9230(87)90149-3. [DOI] [PubMed] [Google Scholar]
- Pow D. V., Morris J. F. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32(2):435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Randall A., Tsien R. W. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995 Apr;15(4):2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard P., Moos F., Freund-Mercier M. J. Central effects of oxytocin. Physiol Rev. 1991 Apr;71(2):331–370. doi: 10.1152/physrev.1991.71.2.331. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Pearson H. A., Dolphin A. C. Aspects of vertebrate neuronal voltage-activated calcium currents and their regulation. Prog Neurobiol. 1991;36(6):485–520. doi: 10.1016/0301-0082(91)90014-r. [DOI] [PubMed] [Google Scholar]
- Wakerley J. B., Lincoln D. W. The milk-ejection reflex of the rat: a 20- to 40-fold acceleration in the firing of paraventricular neurones during oxytocin release. J Endocrinol. 1973 Jun;57(3):477–493. doi: 10.1677/joe.0.0570477. [DOI] [PubMed] [Google Scholar]
- Wang G., Dayanithi G., Kim S., Hom D., Nadasdi L., Kristipati R., Ramachandran J., Stuenkel E. L., Nordmann J. J., Newcomb R. Role of Q-type Ca2+ channels in vasopressin secretion from neurohypophysial terminals of the rat. J Physiol. 1997 Jul 15;502(Pt 2):351–363. doi: 10.1111/j.1469-7793.1997.351bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Treistman S. N., Lemos J. R. Single channel recordings of Nt- and L-type Ca2+ currents in rat neurohypophysial terminals. J Neurophysiol. 1993 Oct;70(4):1617–1628. doi: 10.1152/jn.1993.70.4.1617. [DOI] [PubMed] [Google Scholar]
- Wang X., Treistman S. N., Lemos J. R. Two types of high-threshold calcium currents inhibited by omega-conotoxin in nerve terminals of rat neurohypophysis. J Physiol. 1992 Jan;445:181–199. doi: 10.1113/jphysiol.1992.sp018919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D. B., Randall A., Tsien R. W. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994 Apr 1;264(5155):107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- Widmer H., Amerdeil H., Fontanaud P., Desarménien M. G. Postnatal maturation of rat hypothalamoneurohypophysial neurons: evidence for a developmental decrease in calcium entry during action potentials. J Neurophysiol. 1997 Jan;77(1):260–271. doi: 10.1152/jn.1997.77.1.260. [DOI] [PubMed] [Google Scholar]
- Zhang J. F., Randall A. D., Ellinor P. T., Horne W. A., Sather W. A., Tanabe T., Schwarz T. L., Tsien R. W. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993 Nov;32(11):1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]


